Inactivation Mechanisms of Escherichia coli in Simulants of Respiratory and Environmental Aerosol Droplets
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Method
2.2. Microbial Media
2.3. Sample Preparation for Studies of Bioaerosol Survival and Aerosol Dynamics
2.4. Complementary Aerosol Analysis Technique to Investigate the Interplay between Aerosol Microphysics and Bioaerosol Survival
- The comparative kinetic electrodynamic balance (CK-EDB) is used to study thermodynamically and kinetically driven changes in the properties of a single levitated aerosol particle [13]. Changes in the size, composition, hygroscopicity, morphology, and droplet formation process of aqueous aerosol droplets containing mixtures of organic and inorganic solutes can be measured with great accuracy over a short timescale at a high time resolution (~100 Hz) with this technique. A comprehensive description of the experimental setup and operation of the CK-EDB has been discussed previously [13].
- The determination of survival fractions for populations of airborne microorganisms as a function of environmental conditions, bioaerosol composition and other biological factors (e.g., microbe age) is performed with a novel instrument described as controlled electrodynamic levitation and extraction of bioaerosol onto a substrate (CELEBS). This instrument is also utilised in these studies as a particle preparation technique for SEM off-line microscopy, where particles are levitated and subsequently deposited into an empty petri dish containing a polycarbonate filter paper. Details on the set-up, operation and data analysis of CELEBS have been previously reported [3,10,11].
2.5. Bioaerosol Generation with Droplet-on-Demand Dispensers
2.6. Bioaerosol Microphysics
2.6.1. Determination of Aerosol Evaporation Kinetics
2.6.2. Determination of Hygroscopicity Properties
2.6.3. Simulations of Evaporation Kinetics
2.6.4. Determination of Particle Morphology
2.6.5. Measurements of Surface Enrichment
2.7. Bioaerosol Suspension, Sampling, and Survival Studies with the CELEBS System
2.8. Scanning Electron Microscopy (SEM) Analysis
2.9. Statistical Analysis
3. Results
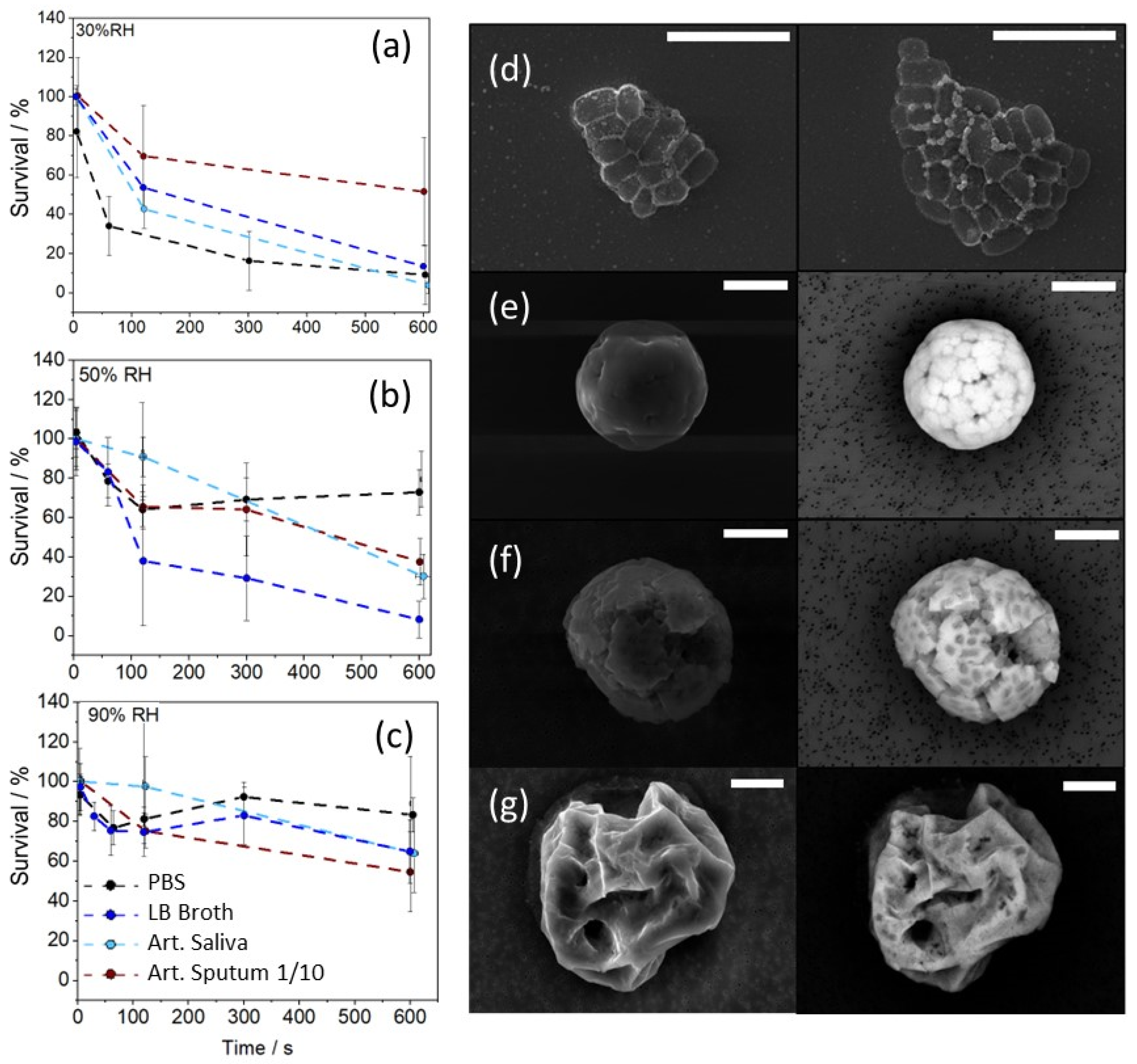
3.1. Measurements of Bacterial Longevity in Artificial Respiratory Droplets
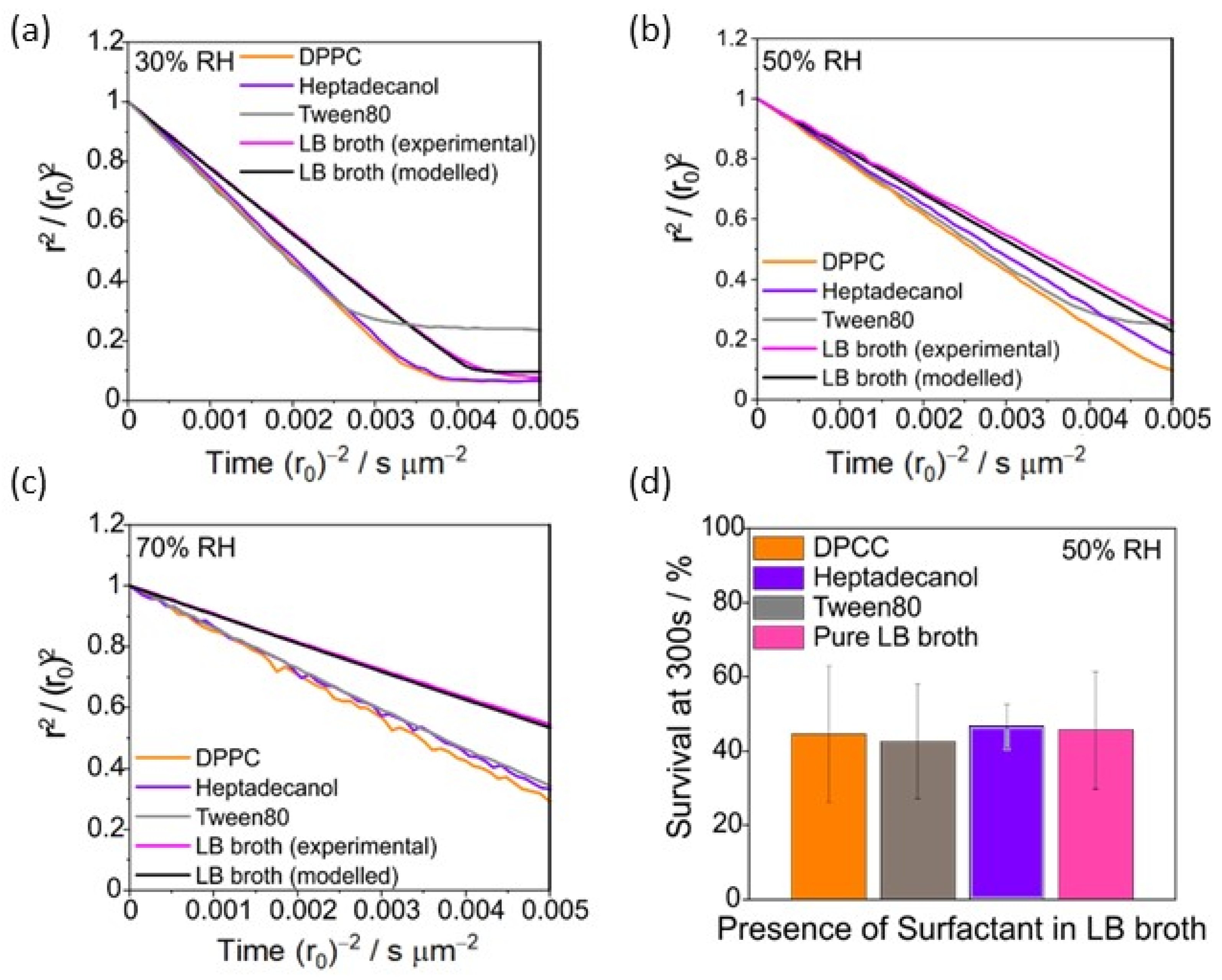
3.2. The Presence of Surfactants in LB Broth Droplets Has No Effect on Evaporation Kinetics or Airborne Bacterial Survival
3.3. The Effect of Microbial Load on Airborne Bacterial Viability
3.4. Mucin Concentration Has No Significant Effect on Bacteria Viability
3.5. The Effect of Gas-to-Particle Partitioning of Pyruvic Acid on Airborne Bacterial Viability
3.6. The Effect of Bacterial Physiology on Airborne Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duval, D.; Palmer, J.C.; Tudge, I.; Pearce-Smith, N.; O’Connell, E.; Bennett, A.; Clark, R. Long distance airborne transmission of SARS-CoV-2: Rapid systematic review. BMJ 2022, 377, e068743. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.O.; Thomas, R.J.; Oswin, H.; Haddrell, A.E.; Reid, J.P. Transformative Approach to Investigate the Microphysical Factors Influencing Airborne Transmission of Pathogens. Appl. Environ. Microbiol. 2020, 86, e01543-20. [Google Scholar] [CrossRef] [PubMed]
- Alsved, M.; Holm, S.; Christiansen, S.; Smidt, M.; Rosati, B.; Ling, M.; Boesen, T.; Finster, K.; Bilde, M.; Löndahl, J.; et al. Effect of Aerosolization and Drying on the Viability of Pseudomonas syringae Cells. Front. Microbiol. 2018, 9, 3086. [Google Scholar] [CrossRef] [PubMed]
- Gralton, J.; Tovey, E.; McLaws, M.L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.K.; Heinson, W.R. Scaling Laws for Light Absorption Enhancement Due to Nonrefractory Coating of Atmospheric Black Carbon Aerosol. Phys. Rev. Lett. 2018, 121, 218701. [Google Scholar] [CrossRef]
- Service, R.F. Climate change. Study fingers soot as a major player in global warming. Science 2008, 319, 1745. [Google Scholar] [CrossRef]
- Lee, A.K.; Zhao, R.; Li, R.; Liggio, J.; Li, S.M.; Abbatt, J.P. Formation of light absorbing organo-nitrogen species from evaporation of droplets containing glyoxal and ammonium sulfate. Environ. Sci. Technol. 2013, 47, 12819–12826. [Google Scholar] [CrossRef] [PubMed]
- Oswin, H.P.; Haddrell, A.E.; Hughes, C.; Otero-Fernandez, M.; Thomas, R.J.; Reid, J.P. Oxidative Stress Contributes to Bacterial Airborne Loss of Viability. Microbiol. Spectr. 2023, 11, e0334722. [Google Scholar] [CrossRef]
- Haddrell, A.; Otero-Fernandez, M.; Oswin, H.; Cogan, T.; Bazire, J.; Tian, J.; Alexander, R.; Mann, J.F.S.; Hill, D.; Finn, A.; et al. Differences in airborne stability of SARS-CoV-2 variants of concern is impacted by alkalinity of surrogates of respiratory aerosol. J. R. Soc. Interface 2023, 20, 20230062. [Google Scholar] [CrossRef]
- Oswin, H.P.; Haddrell, A.E.; Otero-Fernandez, M.; Mann, J.F.S.; Cogan, T.A.; Hilditch, T.G.; Tian, J.; Hardy, D.A.; Hill, D.J.; Finn, A.; et al. The dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc. Natl. Acad. Sci. USA 2022, 119, e2200109119. [Google Scholar] [CrossRef] [PubMed]
- Oswin, H.P.; Blake, E.; Haddrell, A.E.; Finn, A.; Sriskandan, S.; Reid, J.P.; Halliday, A.; Goenka, A. An assessment of the airborne longevity of group A Streptococcus. Microbiology 2024, 170, 001421. [Google Scholar] [CrossRef]
- Haddrell, A.; Rovelli, G.; Lewis, D.; Church, T.; Reid, J. Identifying time-dependent changes in the morphology of an individual aerosol particle from its light scattering pattern. Aerosol Sci. Technol. 2019, 53, 1334–1351. [Google Scholar] [CrossRef]
- Orr, R.J.S.; Littner, E.; Larigauderie, G.; Lonsdale, C.L.; Dybwad, M. Complete reference genome assemblies and annotations of three Escherichia coli MRE162 clones. Microbiol. Resour. Announc. 2023, 12, e0049023. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.; Fothergill, J.L.; Wright, E.A.; James, C.E.; Mowat, E.; Winstanley, C. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J. Vis. Exp. 2012, 64, e3857. [Google Scholar] [CrossRef]
- Woo, M.H.; Hsu, Y.M.; Wu, C.Y.; Heimbuch, B.; Wander, J. Method for contamination of filtering facepiece respirators by deposition of MS2 viral aerosols. J. Aerosol Sci. 2010, 41, 944–952. [Google Scholar] [CrossRef]
- Rovelli, G.; Miles, R.E.H.; Reid, J.P.; Clegg, S.L. Accurate Measurements of Aerosol Hygroscopic Growth over a Wide Range in Relative Humidity. J. Phys. Chem. A 2016, 120, 4376–4388. [Google Scholar] [CrossRef]
- Gregson, F.K.A.; Robinson, J.F.; Miles, R.E.H.; Royall, C.P.; Reid, J.P. Drying Kinetics of Salt Solution Droplets: Water Evaporation Rates and Crystallization. J. Phys. Chem. B 2019, 123, 266–276. [Google Scholar] [CrossRef]
- Cai, C.; Miles, R.E.; Cotterell, M.I.; Marsh, A.; Rovelli, G.; Rickards, A.M.; Zhang, Y.H.; Reid, J.P. Comparison of Methods for Predicting the Compositional Dependence of the Density and Refractive Index of Organic-Aqueous Aerosols. J. Phys. Chem. A 2016, 120, 6604–6617. [Google Scholar] [CrossRef]
- Davies, J.F.; Haddrell, A.E.; Reid, J.P. Time-Resolved Measurements of the Evaporation of Volatile Components from Single Aerosol Droplets. Aerosol Sci. Technol. 2012, 46, 666–677. [Google Scholar] [CrossRef]
- Kulmala, M.; Vesala, T.; Wagner, P.E. An analytical expression for the rate of binary condensational particle growth. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1993, 441, 589–605. [Google Scholar] [CrossRef]
- Davies, J.F.; Haddrell, A.E.; Rickards, A.M.; Reid, J.P. Simultaneous analysis of the equilibrium hygroscopicity and water transport kinetics of liquid aerosol. Anal. Chem. 2013, 85, 5819–5826. [Google Scholar] [CrossRef] [PubMed]
- Kreidenweis, S.M.; Koehler, K.; DeMott, P.J.; Prenni, A.J.; Carrico, C.; Ervens, B. Water activity and activation diameters from hygroscopicity data-Part I: Theory and application to inorganic salts. Atmos. Chem. Phys. 2005, 5, 1357–1370. [Google Scholar] [CrossRef]
- Baldelli, A.; Boraey, M.A.; Nobes, D.S.; Vehring, R. Analysis of the Particle Formation Process of Structured Microparticles. Mol. Pharm. 2015, 12, 2562–2573. [Google Scholar] [CrossRef] [PubMed]
- Nenninger, A.; Mastroianni, G.; Robson, A.; Lenn, T.; Xue, Q.; Leake, M.C.; Mullineaux, C.W. Independent mobility of proteins and lipids in the plasma membrane of Escherichia coli. Mol. Microbiol. 2014, 92, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Clegg, S.L.; Brimblecombe, P.; Wexler, A.S. Thermodynamic Model of the System H+−NH4+−Na+−SO42−−NO3−−Cl−−H2O at 298.15 K. J. Phys. Chem. A 1998, 102, 2155–2171. [Google Scholar] [CrossRef]
- Fernandez, M.O.; Thomas, R.J.; Garton, N.J.; Hudson, A.; Haddrell, A.; Reid, J.P. Assessing the airborne survival of bacteria in populations of aerosol droplets with a novel technology. J. R. Soc. Interface 2019, 16, 20180779. [Google Scholar] [CrossRef]
- Keune, K.; van Loon, A.; Boon, J.J. SEM backscattered-electron images of paint cross sections as information source for the presence of the lead white pigment and lead-related degradation and migration phenomena in oil paintings. Microsc. Microanal. 2011, 17, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.W.; Tian, J.; Haddrell, A.E.; Oswin, H.P.; Neal, E.; Hardy, D.A.; Otero-Fernandez, M.; Mann, J.F.S.; Cogan, T.A.; Finn, A.; et al. Mucin Transiently Sustains Coronavirus Infectivity through Heterogenous Changes in Phase Morphology of Evaporating Aerosol. Viruses 2022, 14, 1856. [Google Scholar] [CrossRef]
- Bourouiba, L.; Dehandschoewercker, E.; Bush, J.W. Violent expiratory events: On coughing and sneezing. J. Fluid Mech. 2014, 745, 537–563. [Google Scholar] [CrossRef]
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. Jama 2020, 323, 1837–1838. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gil, J.; Keough, K.M.W. Interfacial properties of surfactant proteins. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1998, 1408, 203–217. [Google Scholar] [CrossRef]
- Lighthart, B.; Shaffer, B.T. Increased Airborne Bacterial Survival as a Function of Particle Content and Size. Aerosol Sci. Technol. 1997, 27, 439–446. [Google Scholar] [CrossRef]
- Lou, M.; Liu, S.; Gu, C.; Hu, H.; Tang, Z.; Zhang, Y.; Xu, C.; Li, F. The bioaerosols emitted from toilet and wastewater treatment plant: A literature review. Environ. Sci. Pollut. Res. Int. 2021, 28, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Nicas, M.; Nazaroff, W.W.; Hubbard, A. Toward understanding the risk of secondary airborne infection: Emission of respirable pathogens. J. Occup. Environ. Hyg. 2005, 2, 143–154. [Google Scholar] [CrossRef]
- Johnson, G.R.; Morawska, L.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Chao, C.Y.H.; Wan, M.P.; Li, Y.; Xie, X.; Katoshevski, D.; et al. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011, 42, 839–851. [Google Scholar] [CrossRef]
- de Mik, G.; de Groot, I. The germicidal effect of the open air in different parts of The Netherlands. J. Hyg. 1977, 78, 175–187. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.S. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, O.W.; Chotirmall, S.H. Mucus, Microbiomes and Pulmonary Disease. Biomedicines 2021, 9, 675. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and molecular biology of airway mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar] [CrossRef]
- King, M. Viscoelastic properties of airway mucus. Fed. Proc. 1980, 39, 3080–3085. [Google Scholar] [PubMed]
- Small, P.; Blankenhorn, D.; Welty, D.; Zinser, E.; Slonczewski, J.L. Acid and base resistance in Escherichia coli and Shigella flexneri: Role of rpoS and growth pH. J. Bacteriol. 1994, 176, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Brmblecombe, P.; Clegg, S.L. The henry’s law constants of pyruvic and methacrylic acids. Environ. Technol. 1992, 13, 587–593. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Z.; Tong, W.; Ding, Y.; Liu, B.; Shi, Y.; Wang, J.; Sun, S.; Liu, M.; Wang, Y.; et al. An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat. Commun. 2020, 11, 1496. [Google Scholar] [CrossRef]
- Handley, B.A.; Webster, A.J.F. Some factors affecting the airborne survival of bacteria outdoors. J. Appl. Bacteriol. 1995, 79, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.F.; Damon, E.B.; Ferry, R.M. Studies of the loss of viability of bacterial aerosols: III. Factors affecting death rates of certain non-pathogens. J. Hyg. 1958, 56, 389–403. [Google Scholar] [CrossRef]
- Chan, W.L.; Chung, W.T.; Ng, T.W. Airborne Survival of Escherichia coli under Different Culture Conditions in Synthetic Wastewater. Int. J. Environ. Res. Public Health 2019, 16, 4745. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.D.; Stewart, I.W.; Reading, A.R.; Futter, S.J. Effect of aerosolisation, growth phase and residence time in spray and collection fluids on the culturability of cells and spores. J. Aerosol Sci. 1996, 27, 803–820. [Google Scholar] [CrossRef]
- Jenkins, D.E.; Chaisson, S.A.; Matin, A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J. Bacteriol. 1990, 172, 2779–2781. [Google Scholar] [CrossRef]
- Navarro Llorens, J.M.; Tormo, A.; Martínez-García, E. Stationary phase in gram-negative bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef]
- Lacour, S.; Landini, P. SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: Function of sigmaS-dependent genes and identification of their promoter sequences. J. Bacteriol. 2004, 186, 7186–7195. [Google Scholar] [CrossRef] [PubMed]
- Battesti, A.; Majdalani, N.; Gottesman, S. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011, 65, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Barer, M.R.; Harwood, C.R. Bacterial viability and culturability. Adv. Microb. Physiol. 1999, 41, 93–137. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Grinshpun, S.A.; Willeke, K.; Macher, J.M.; Donnelly, J.; Clark, S.; Juozaitis, A. Factors Affecting Microbiological Colony Count Accuracy for Bioaerosol Sampling and Analysis. Am. Ind. Hyg. Assoc. J. 1995, 56, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Girolamini, L.; Brattich, E.; Marino, F.; Pascale, M.R.; Mazzotta, M.; Spiteri, S.; Derelitto, C.; Tositti, L.; Cristino, S. Cooling towers influence in an urban environment: A predictive model to control and prevent Legionella risk and Legionellosis events. Build. Environ. 2023, 228, 109891. [Google Scholar] [CrossRef]
- Kataki, S.; Patowary, R.; Chatterjee, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Kamboj, D.V. Bioaerosolization and pathogen transmission in wastewater treatment plants: Microbial composition, emission rate, factors affecting and control measures. Chemosphere 2022, 287, 132180. [Google Scholar] [CrossRef]
- Knowlton, S.D.; Boles, C.L.; Perencevich, E.N.; Diekema, D.J.; Nonnenmann, M.W.; Program, C.D.C.E. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrob. Resist. Infect. Control 2018, 7, 16. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero-Fernandez, M.; Thomas, R.J.; Oswin, H.; Alexander, R.; Haddrell, A.; Reid, J.P. Inactivation Mechanisms of Escherichia coli in Simulants of Respiratory and Environmental Aerosol Droplets. Atmosphere 2024, 15, 511. https://doi.org/10.3390/atmos15040511
Otero-Fernandez M, Thomas RJ, Oswin H, Alexander R, Haddrell A, Reid JP. Inactivation Mechanisms of Escherichia coli in Simulants of Respiratory and Environmental Aerosol Droplets. Atmosphere. 2024; 15(4):511. https://doi.org/10.3390/atmos15040511
Chicago/Turabian StyleOtero-Fernandez, Mara, Richard J. Thomas, Henry Oswin, Robert Alexander, Allen Haddrell, and Jonathan P. Reid. 2024. "Inactivation Mechanisms of Escherichia coli in Simulants of Respiratory and Environmental Aerosol Droplets" Atmosphere 15, no. 4: 511. https://doi.org/10.3390/atmos15040511
APA StyleOtero-Fernandez, M., Thomas, R. J., Oswin, H., Alexander, R., Haddrell, A., & Reid, J. P. (2024). Inactivation Mechanisms of Escherichia coli in Simulants of Respiratory and Environmental Aerosol Droplets. Atmosphere, 15(4), 511. https://doi.org/10.3390/atmos15040511






