Resolving the Loss of Intermediate-Size Speech Aerosols in Funnel-Guided Particle Counting Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subject
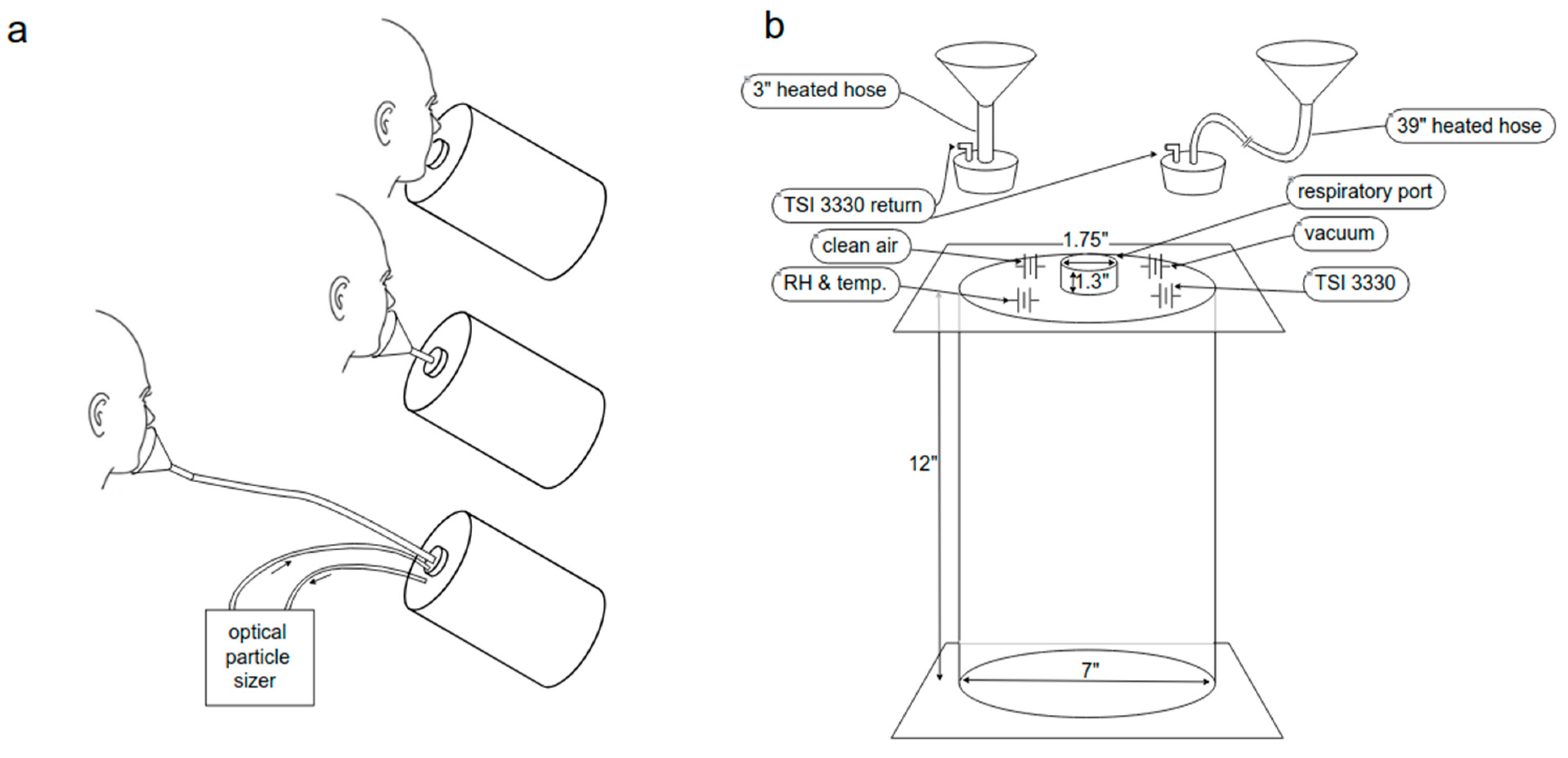
2.2. Experimental Setup
2.3. Expiratory Activities
2.4. Uncertainty in Aerosol Counts
2.5. Aerosol Detection Methods
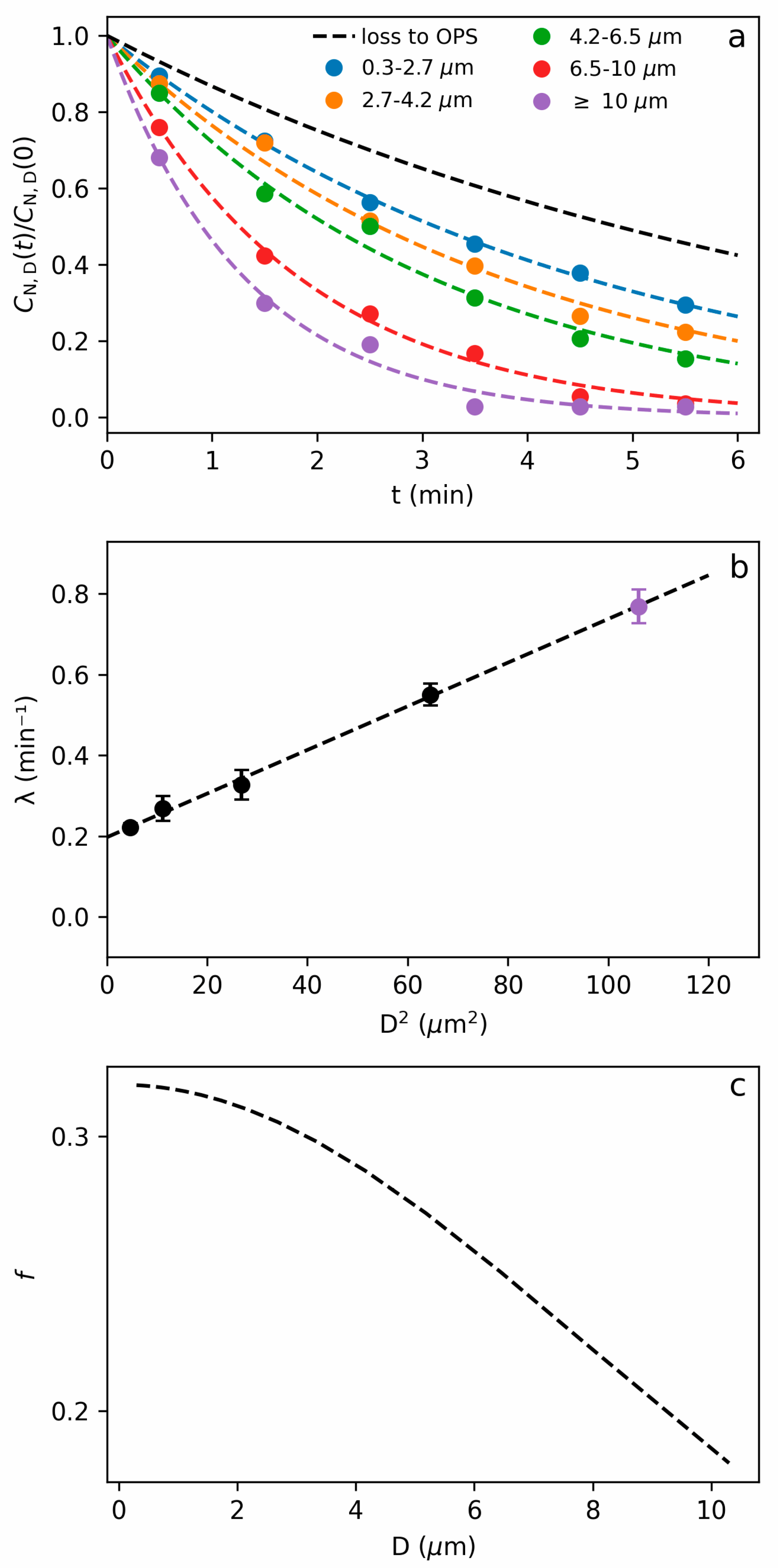
2.6. Aerosol Sedimentation Velocity
3. Results
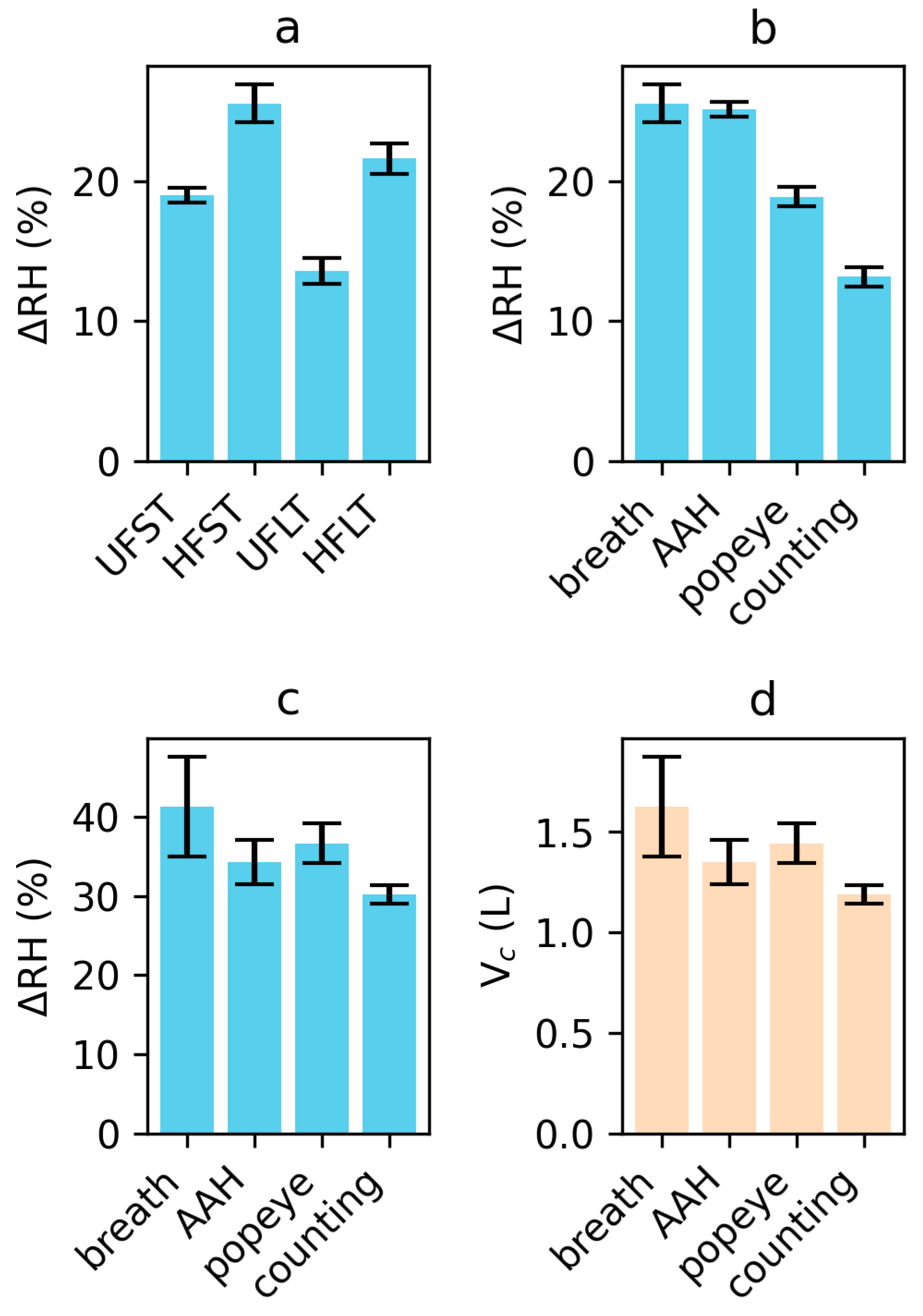
3.1. Condensation during Transport
3.2. Sedimentation during Measurement
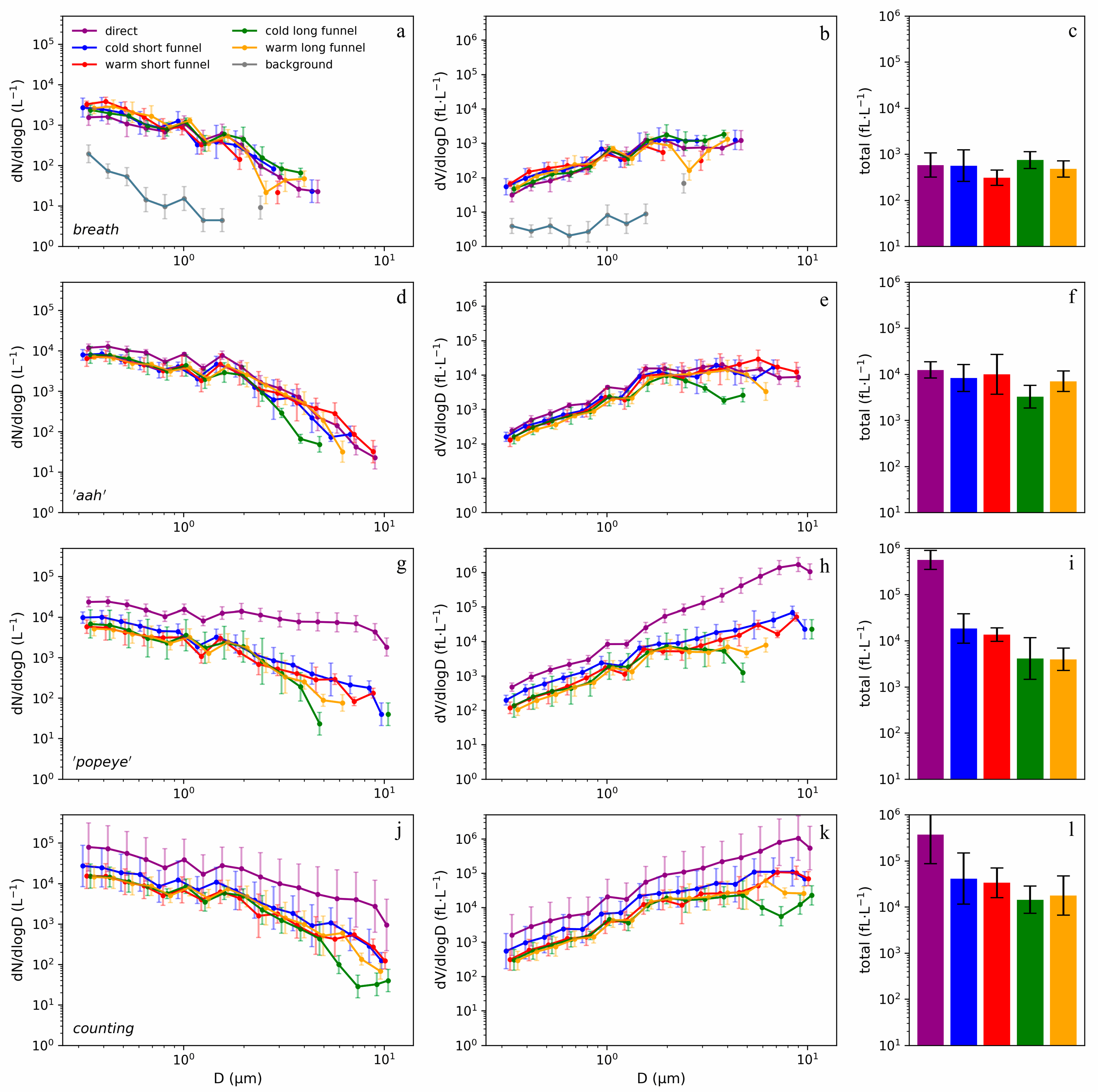
3.3. Breathing
3.4. Vowel ‘aah’
3.5. Plosives
3.6. Counting
4. Discussion
4.1. Impact on Disease Transmission
4.2. Impact on Ventilation Requirements
4.3. Limitations
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | absolute humidity |
| AHb | absolute humidity of exhaled breath |
| AHc | absolute humidity of air in chamber |
| αOPS | chamber ventilation rate by the OPS |
| αwall | rate of particle losses due to non-gravitational deposition chamber walls |
| APS | aerodynamic particle sizer |
| CN,D(t) | particle number concentration at time t |
| D | particle diameter |
| ID | inner diameter |
| ΔRH | increase in chamber relative humidity |
| f | fraction of chamber aerosol sampled |
| fL | femtoliter |
| H | chamber height |
| HEPA | high efficiency particulate air [filter] |
| λ | total rate of particle depletion in chamber |
| N | number of virions |
| N0 | infectious dose number of virions |
| OPS | optical particle sizer |
| P(N) | probability of infection upon inhaling N virions |
| PCR | polymerase chain reaction |
| ρ | particle mass density |
| RH | relative humidity |
| RHc | relative humidity in detection chamber |
| RNA | ribonucleic acid |
| s | second |
| t | Time |
| T | duration of chamber sampling by OPS |
| v | particle gravitational velocity |
| Vb | exhaled breath volume |
| Vc | chamber volume |
References
- Wang Chia, C.; Prather Kimberly, A.; Sznitman, J.; Jimenez Jose, L.; Lakdawala Seema, S.; Tufekci, Z.; Marr Linsey, C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Stadnytskyi, V.; Anfinrud, P.; Bax, A. Breathing, speaking, coughing or sneezing: What drives transmission of SARS-CoV-2? J. Intern. Med. 2021, 290, 1010–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Zhang, A.L.; Wang, Y.; Molina, M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857. [Google Scholar] [CrossRef]
- Duguid, J.P. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J. Hyg. 1946, 44, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef]
- Gandhi, M.; Yokoe, D.S.; Havlir, D.V. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control COVID-19. N. Engl. J. Med. 2020, 382, 2158–2160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.R.; Morawska, L. The Mechanism of Breath Aerosol Formation. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Piralla, A.; Pariani, E.; Rovida, F.; Campanini, G.; Muzzi, A.; Emmi, V.; Iotti, G.A.; Pesenti, A.; Conaldi, P.G.; Zanetti, A.; et al. Segregation of Virulent Influenza A(H1N1) Variants in the Lower Respiratory Tract of Critically Ill Patients during the 2010–2011 Seasonal Epidemic. PLoS ONE 2011, 6, e28332. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, G.; Morawska, L.; Stabile, L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: Prospective and retrospective applications. Environ. Int. 2020, 145, 10. [Google Scholar] [CrossRef]
- Miller, S.L.; Nazaroff, W.W.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Marr, L.C.; Morawska, L.; Noakes, C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021, 31, 314–323. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef] [PubMed]
- Pourfattah, F.; Wang, L.P.; Deng, W.; Ma, Y.F.; Hu, L.; Yang, B. Challenges in simulating and modeling the airborne virus transmission: A state-of-the-art review. Phys. Fluids 2021, 33, 101302. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, G.; Thiede, B.; Hejazi, B.; Schlenczek, O.; Bodenschatz, E. An upper bound on one-to-one exposure to infectious human respiratory particles. Proc. Natl. Acad. Sci. USA 2021, 118, e2110117118. [Google Scholar] [CrossRef] [PubMed]
- Prentiss, M.; Chu, A.; Berggren, K.K. Finding the infectious dose for COVID-19 by applying an airborne-transmission model to superspreader events. PLoS ONE 2022, 17, e0265816. [Google Scholar] [CrossRef]
- Mikszewski, A.; Stabile, L.; Buonanno, G.; Morawska, L. The airborne contagiousness of respiratory viruses: A comparative analysis and implications for mitigation. Geosci. Front. 2022, 13, 101285. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Kasibhatla, P.; Mukherjee, A.; Pan, W.L.; Morrison, G.; Mishra, S.; Murty, V.K. Analysis of overdispersion in airborne transmission of COVID-19. Phys. Fluids 2022, 34, 051914. [Google Scholar] [CrossRef]
- Wells, W.F. Airborne Contagion and Air Hygiene; Harvard University Press: Cambridge, UK, 1955. [Google Scholar]
- To, G.N.S.; Chao, C.Y.H. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air 2010, 20, 2–16. [Google Scholar]
- Archer, J.; McCarthy, L.P.; Symons, H.E.; Watson, N.A.; Orton, C.M.; Browne, W.J.; Harrison, J.; Moseley, B.; Philip, K.E.J.; Calder, J.D.; et al. Comparing aerosol number and mass exhalation rates from children and adults during breathing, speaking and singing. Interface Focus 2022, 12, 20210078. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Bar-On, Y.M.; Gleizer, S.; Bernshtein, B.; Flamholz, A.; Phillips, R.; Milo, R. The total number and mass of SARS-CoV-2 virions. Proc. Natl. Acad. Sci. USA 2021, 118, e2024815118. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Bhopal, R.; Nguyen Tien, H. Review of infective dose, routes of transmission and outcome of COVID-19 caused by the SARS-COV-2: Comparison with other respiratory viruses. Epidemiol. Infect. 2021, 149, e96. [Google Scholar] [CrossRef]
- Killingley, B.; Mann, A.J.; Kalinova, M.; Boyers, A.; Goonawardane, N.; Zhou, J.; Lindsell, K.; Hare, S.S.; Brown, J.; Frise, R.; et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 2022, 28, 1031–1041. [Google Scholar] [CrossRef]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 11875–11877. [Google Scholar] [CrossRef]
- Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.Y.H.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Effect of voicing and articulation manner on aerosol particle emission during human speech. PLoS ONE 2020, 15, e0227699. [Google Scholar] [CrossRef] [PubMed]
- Gregson, F.K.A.; Watson, N.A.; Orton, C.M.; Haddrell, A.E.; McCarthy, L.P.; Finnie, T.J.R.; Gent, N.; Donaldson, G.C.; Shah, P.L.; Calder, J.D.; et al. Comparing aerosol concentrations and particle size distributions generated by singing, speaking and breathing. Aerosol Sci. Technol. 2021, 55, 681–691. [Google Scholar] [CrossRef]
- Caracci, E.; Stabile, L.; Ferro, A.R.; Morawska, L.; Buonanno, G. Respiratory particle emission rates from children during speaking. Sci. Rep. 2023, 13, 18294. [Google Scholar] [CrossRef]
- Alsved, M.; Matamis, A.; Bohlin, R.; Richter, M.; Bengtsson, P.E.; Fraenkel, C.J.; Medstrand, P.; Londahl, J. Exhaled respiratory particles during singing and talking. Aerosol Sci. Technol. 2020, 54, 1245–1248. [Google Scholar] [CrossRef]
- Bahl, P.; de Silva, C.; Bhattacharjee, S.; Stone, H.; Doolan, C.; Chughtai, A.A.; MacIntyre, C.R. Droplets and Aerosols generated by singing and the risk of COVID-19 for choirs. Clin. Infect. Dis. 2020, 72, e639–e641. [Google Scholar] [CrossRef]
- Schlenczek, O.; Thiede, B.; Turco, L.; Stieger, K.; Kosub, J.M.; Muller, R.; Scheithauer, S.; Bodenschatz, E.; Bagheri, G. Experimental measurement of respiratory particles dispersed by wind instruments and analysis of the associated risk of infection transmission. J. Aerosol Sci. 2023, 167, 106070. [Google Scholar] [CrossRef]
- Roth, A.; Frantz, D.; Stiti, M.; Berrocal, E. High-speed scattered-light imaging for sizing respiratory droplets. J. Aerosol Sci. 2023, 174, 106257. [Google Scholar] [CrossRef]
- Hu, N.; Yuan, F.; Gram, A.; Yao, R.; Sadrizadeh, S. Review of experimental measurements on particle size distribution and airflow behaviors during human respiration. Build. Environ. 2024, 247, 110994. [Google Scholar] [CrossRef]
- Roth, A.; Stiti, M.; Frantz, D.; Corber, A.; Berrocal, E. Exhaled aerosols and saliva droplets measured in time and 3D space: Quantification of pathogens flow rate applied to SARS-CoV-2. Nat Sci. 2023, 3, e20230007. [Google Scholar] [CrossRef]
- Shen, Y.; Courtney, J.M.; Anfinrud, P.; Bax, A. Hybrid measurement of respiratory aerosol reveals a dominant coarse fraction resulting from speech that remains airborne for minutes. Proc. Natl. Acad. Sci. USA 2022, 119, e2203086119. [Google Scholar] [CrossRef]
- Netz, R. Mechanisms of airborne infection via evaporating and sedimenting droplets produced by speaking. J. Phys. Chem. B 2020, 124, 7093–7101. [Google Scholar] [CrossRef]
- Bagheri, G.; Schlenczek, O.; Turco, L.; Thiede, B.; Stieger, K.; Kosub, J.M.; Clauberg, S.; Pohlker, M.L.; Pohlker, C.; Molacek, J.; et al. Size, concentration, and origin of human exhaled particles and their dependence on human factors with implications on infection transmission. J. Aerosol Sci. 2023, 168, 106102. [Google Scholar] [CrossRef]
- Volckens, J.; Peters, T.M. Counting and particle transmission efficiency of the aerodynamic particle sizer. J. Aerosol Sci. 2005, 36, 1400–1408. [Google Scholar] [CrossRef]
- Abkarian, M.; Stone, H.A. Stretching and break-up of saliva filaments during speech: A route for pathogen aerosolization and its potential mitigation. Phys. Rev. Fluids 2020, 5, 102301. [Google Scholar] [CrossRef]
- Oswin, H.P.; Haddrell, A.E.; Otero-Fernandez, M.; Mann, J.F.S.; Cogan, T.A.; Hilditch, T.G.; Tian, J.; Hardy, D.A.; Hill, D.J.; Finn, A.; et al. The dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc. Natl. Acad. Sci. USA 2022, 119, e2200109119. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Marr, L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface 2018, 15, 20170939. [Google Scholar] [CrossRef]
- Corner, J.; Pendlebury, E.D. The coagulation and deposition of a stirred aerosol. Proc. Phys. Soc. Lond. B 1951, 64, 645–654. [Google Scholar] [CrossRef]
- Bake, B.; Larsson, P.; Ljungkvist, G.; Ljungstrom, E.; Olin, A.C. Exhaled particles and small airways. Respir. Res. 2019, 20, 8. [Google Scholar] [CrossRef]
- Pöhlker, M.L.; Pöhlker, C.; Krüger, O.O.; Förster, J.D.; Berkemeier, T.; Elbert, W.; Fröhlich-Nowoisky, J.; Pöschl, U.; Bagheri, G.; Bodenschatz, E.; et al. Respiratory aerosols and droplets in the transmission of infectious diseases. Rev. Mod. Phys. 2023, 95, 045001. [Google Scholar] [CrossRef]
- Morawska, L.; Buonanno, G.; Mikszewski, A.; Stabile, L. The physics of respiratory particle generation, fate in the air, and inhalation. Nat. Rev. Phys. 2022, 4, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Gralton, J.; Tovey, E.; McLaws, M.L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–442. [Google Scholar] [CrossRef]
- Bax, A.; Shen, Y.; Kakeshpour, T.; Fennelly, K.P. Snoring may transmit infectious aerosols from the upper to the lower respiratory tract. Med. Hypotheses 2022, 168, 110966. [Google Scholar] [CrossRef]
- Holmgren, H.; Bake, B.; Olin, A.-C.; Ljungström, E. Relation Between Humidity and Size of Exhaled Particles. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Schumm, B.; Heiber, M.; Grätz, F.; Stabile, L.; Buonanno, G.; Schönfelder, M.; Hain, R.; Kähler, C.J.; Wackerhage, H. Respiratory aerosol particle emission and simulated infection risk is greater during indoor endurance than resistance exercise. Proc. Natl. Acad. Sci. USA 2023, 120, e2220882120. [Google Scholar] [CrossRef]
- Mutsch, B.; Heiber, M.; Grätz, F.; Hain, R.; Schönfelder, M.; Kaps, S.; Schranner, D.; Kähler, C.J.; Wackerhage, H. Aerosol particle emission increases exponentially above moderate exercise intensity resulting in superemission during maximal exercise. Proc. Natl. Acad. Sci. USA 2022, 119, e2202521119. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Rojas, A.L.P.; Kropff, E.; Bahnfleth, W.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Li, Y.; Loomans, M.G.L.C.; Marr, L.C.; et al. Practical Indicators for Risk of Airborne Transmission in Shared Indoor Environments and Their Application to COVID-19 Outbreaks. Environ. Sci. Technol. 2022, 56, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Lam-Hine, T.; McCurdy, S.A.; Santora, L.; Duncan, L.; Corbett-Detig, R.; Kapusinszky, B.; Willis, M. Outbreak Associated with SARS-CoV-2 B.1.617.2 (Delta) Variant in an Elementary School—Marin County, California, May–June 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wang, J. Quantitative modeling of the impact of facemasks and associated leakage on the airborne transmission of SARS-CoV-2. Sci. Rep. 2021, 11, 19403. [Google Scholar] [CrossRef] [PubMed]
- Majra, D.; Benson, J.; Pitts, J.; Stebbing, J. SARS-CoV-2 (COVID-19) superspreader events. J. Infect. 2021, 82, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Loudon, R.G.; Roberts, R.M. Droplet expulsion from respiratory tract. Am. Rev. Resp. Dis. 1967, 95, 435–442. [Google Scholar] [PubMed]
- Wells, W.F.; Wells, M.W.; Mudd, S. Infection of Air Bacteriologic and Epidemiologic Factors. Am. J. Public Health 1939, 29, 863–880. [Google Scholar] [CrossRef]
- Anfinrud, P.; Stadnytskyi, V.; Bax, C.E.; Bax, A. Visualizing Speech-Generated Oral Fluid Droplets with Laser Light Scattering. N. Engl. J. Med. 2020, 382, 2061–2063. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakeshpour, T.; Bax, A. Resolving the Loss of Intermediate-Size Speech Aerosols in Funnel-Guided Particle Counting Measurements. Atmosphere 2024, 15, 570. https://doi.org/10.3390/atmos15050570
Kakeshpour T, Bax A. Resolving the Loss of Intermediate-Size Speech Aerosols in Funnel-Guided Particle Counting Measurements. Atmosphere. 2024; 15(5):570. https://doi.org/10.3390/atmos15050570
Chicago/Turabian StyleKakeshpour, Tayeb, and Adriaan Bax. 2024. "Resolving the Loss of Intermediate-Size Speech Aerosols in Funnel-Guided Particle Counting Measurements" Atmosphere 15, no. 5: 570. https://doi.org/10.3390/atmos15050570
APA StyleKakeshpour, T., & Bax, A. (2024). Resolving the Loss of Intermediate-Size Speech Aerosols in Funnel-Guided Particle Counting Measurements. Atmosphere, 15(5), 570. https://doi.org/10.3390/atmos15050570






