Climate Change Facilitates the Potentially Suitable Habitats of the Invasive Crop Insect Ectomyelois ceratoniae (Zeller)
Abstract
1. Introduction
2. Materials and Methods
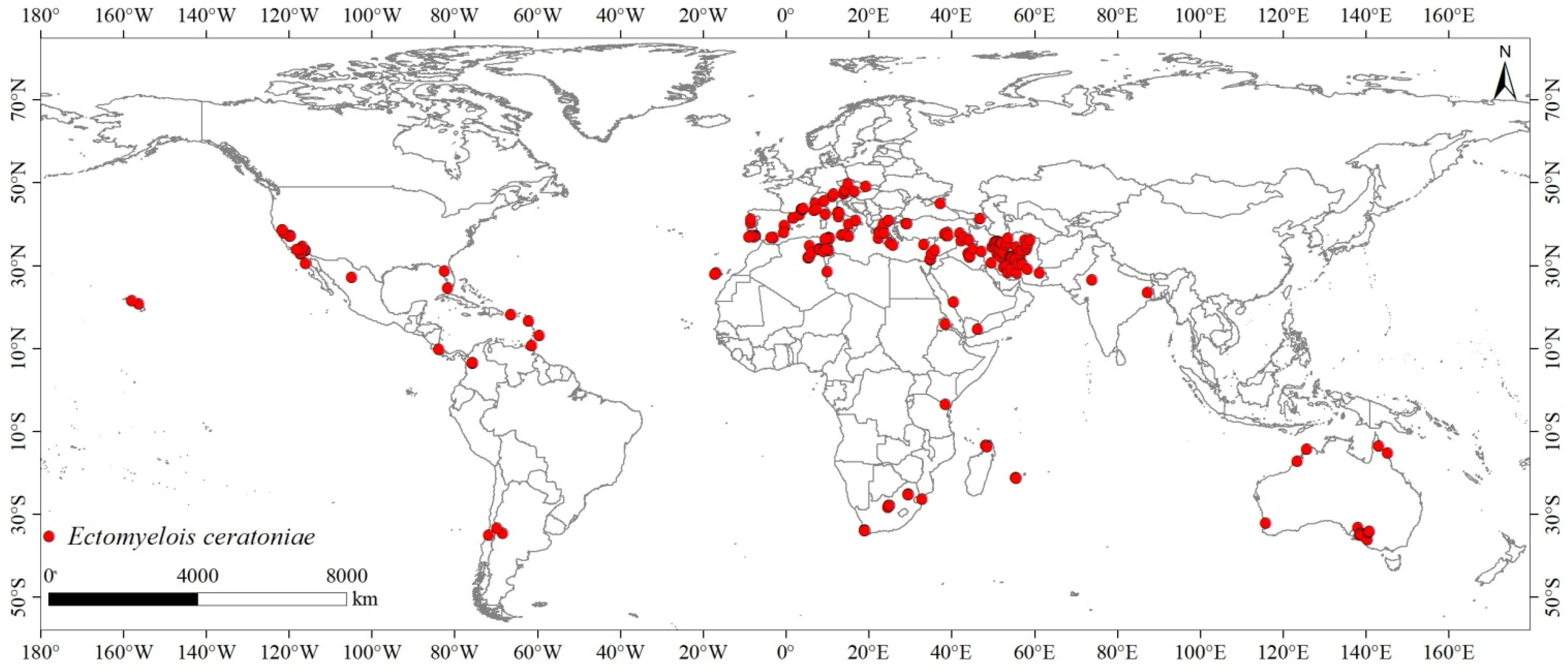
2.1. Distribution Records of Ectomyelois ceratoniae
2.2. Environmental Variables
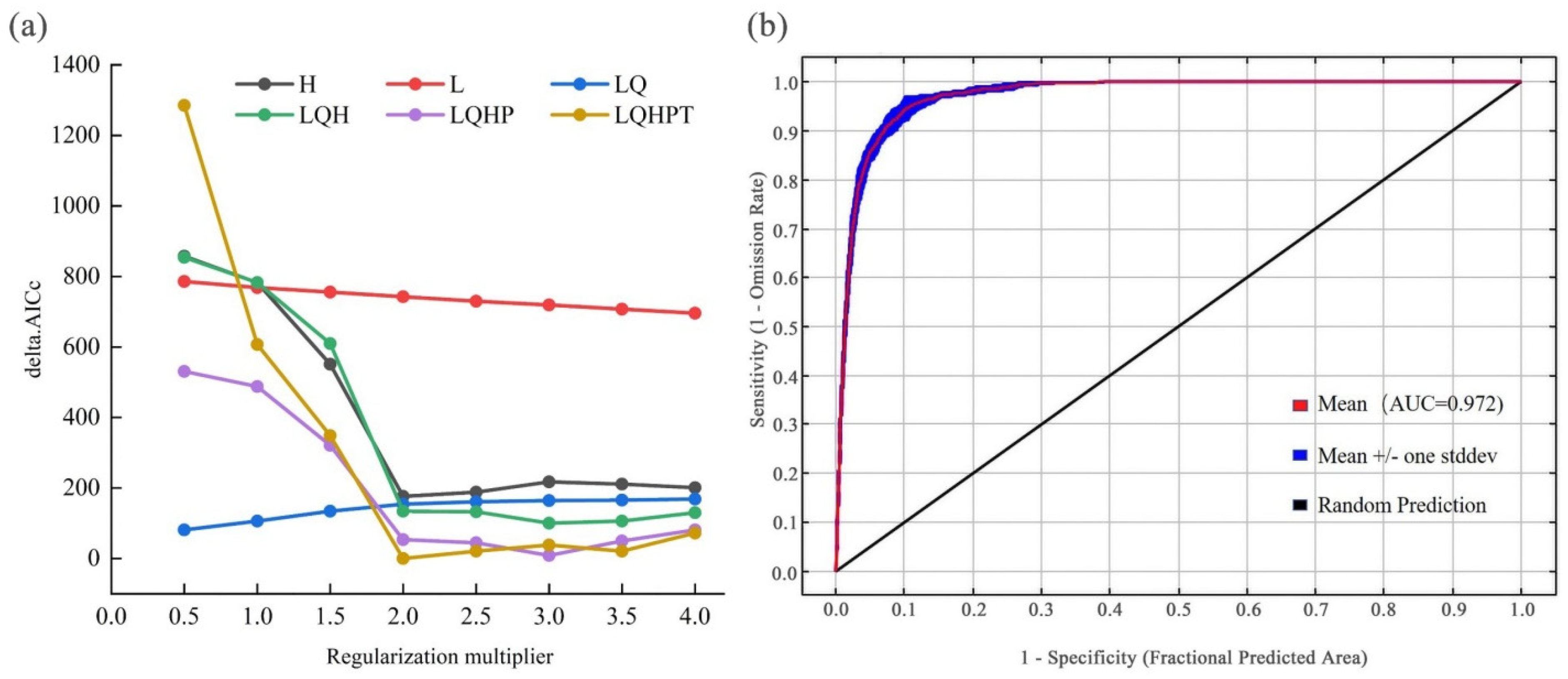
2.3. Model Calibration, Construction, and Evaluation
2.4. Delineation of Potentially Suitable Habitats (PSH)
3. Results
3.1. Model Performance
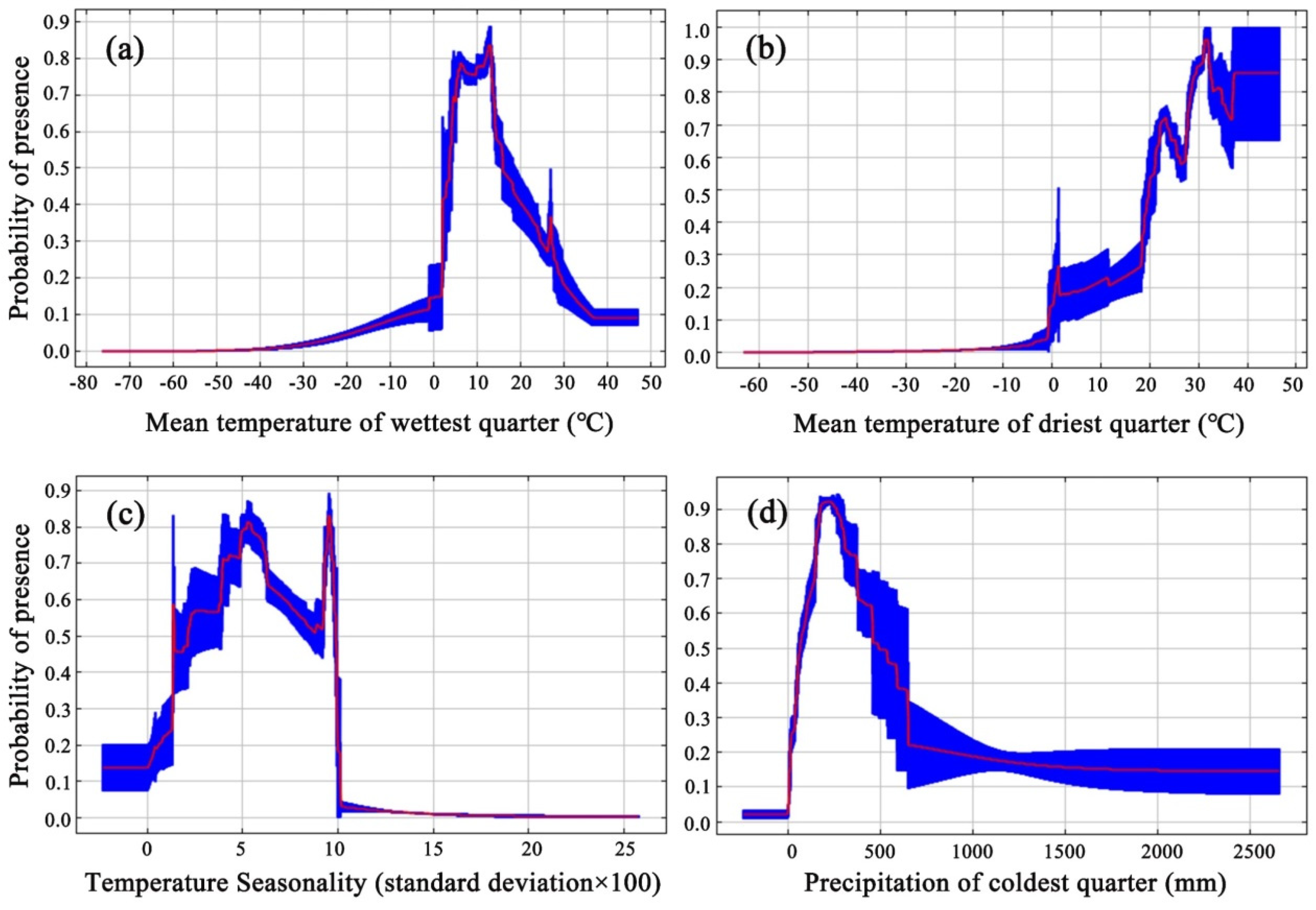
3.2. Significant Environmental Variables (EVs)
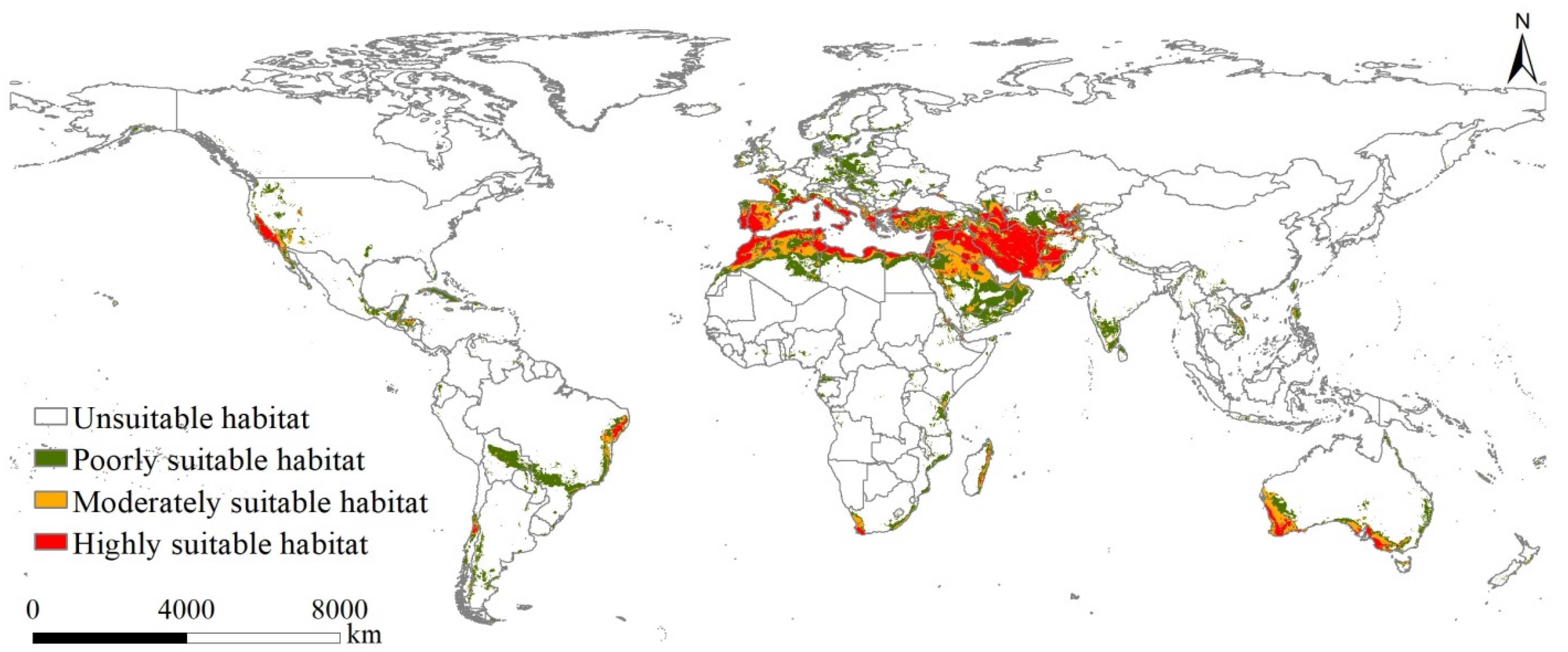
3.3. Potentially Suitable Habitats under Current Climatic Scenarios
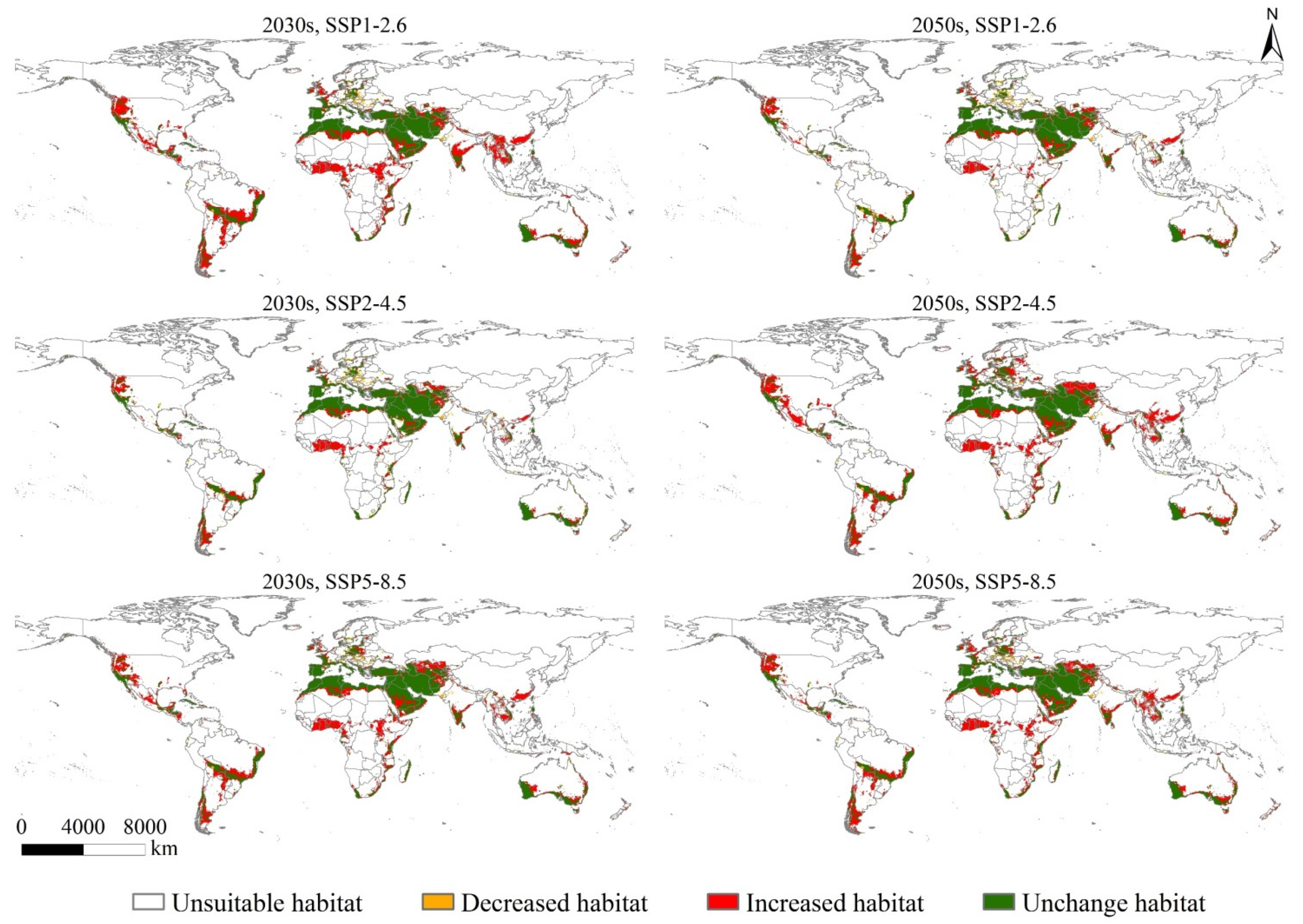
3.4. Potentially Suitable Habitats (PSH) under Future Climatic Conditions
3.5. Potentially Suitable Habitats (PSH) Change
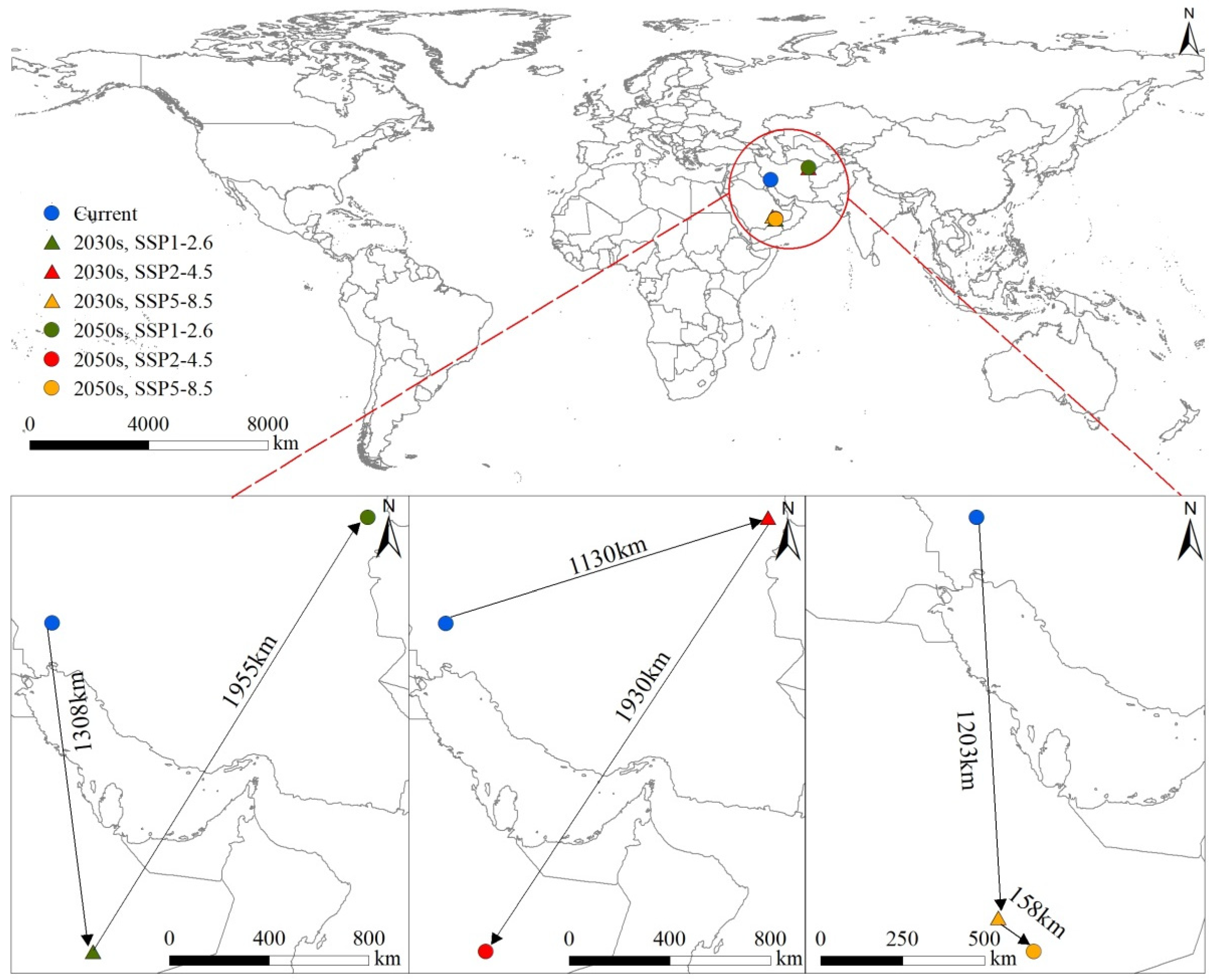
3.6. Center Transfer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gentili, R.; Schaffner, U.; Martinoli, A.; Citterio, S. Invasive alien species and biodiversity: Impacts and management. Biodiversity 2021, 22, 1–3. [Google Scholar] [CrossRef]
- Kenis, M.; Auger-Rozenberg, M.-A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Klem, C.C.; Zaspel, J. Pest Injury Guilds, Lepidoptera, and Placing Fruit-Piercing Moths in Context: A Review. Ann. Entomol. Soc. Am. 2019, 112, 421–432. [Google Scholar] [CrossRef]
- Njuguna, E.; Nethononda, P.; Maredia, K.; Mbabazi, R.; Kachapulula, P.; Rowe, A.; Ndolo, D. Experiences and Perspectives on Spodoptera frugiperda (Lepidoptera: Noctuidae) Management in Sub-Saharan Africa. J. Integr. Pest Manag. 2021, 12, 7. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, G.; Onkara Naik, S.; Rami Reddy, P.V. The Shoot and Fruit Borer, Conogethes punctiferalis (Guenee): An Important Pest of Tropical and Subtropical Fruit Crops. In The Black Spotted, Yellow Borer, Conogethes Punctiferalis Guenée and Allied Species; Chakravarthy, A.K., Ed.; Springer: Singapore, 2018; pp. 165–191. [Google Scholar]
- Ahmad, R.; Khuroo, A.A.; Charles, B.; Hamid, M.; Rashid, I.; Aravind, N.A. Global distribution modelling, invasion risk assessment and niche dynamics of Leucanthemum vulgare (Ox-eye Daisy) under climate change. Sci. Rep. 2019, 9, 11395. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-López, M.A. Ectomyelois Ceratoniae (Carob Moth); CABI Compendium; CABI International: Wallingford, UK, 2022; p. 11. [Google Scholar] [CrossRef]
- Abedi, Z.; Golizadeh, A.; Soufbaf, M.; Hassanpour, M.; Jafari-Nodoushan, A.; Akhavan, H.-R. Relationship between Performance of Carob Moth, Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) and Phytochemical Metabolites in Various Pomegranate Cultivars. Front. Physiol. 2019, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C. American moths of the subfamily Phycitinae. Bulletin 1956, 207, 581. [Google Scholar]
- Abedi, Z.; Golizadeh, A.; Hassanpour, M.; Soufbaf, M. Performance of Habrobracon hebetor (Say) (Hymenoptera: Braconidae) parasitizing Ectomyelois ceratoniae (Zeller) (Lepidoptera: Pyralidae) on different pomegranate cultivars. Int. J. Trop. Insect Sci. 2021, 41, 95–105. [Google Scholar] [CrossRef]
- Mehrnejad, M. The current status of pistachio pests in Iran. Cah. Options Méditerranéennes 2001, 56, 315–322. [Google Scholar]
- Braham, M. Insect larvae associated with dropped pomegranate fruits in an organic orchard in Tunisia. J. Entomol. Nematol. 2015, 7, 5–10. [Google Scholar] [CrossRef]
- Perring, T.M.; El-Shafie, H.A.F.; Wakil, W. Carob Moth. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Wakil, W., Romeno Faleiro, J., Miller, T.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 109–167. [Google Scholar]
- Al-Izzi, M.A.J.; Al-Maliky, S.K.; Younis, M.A.; Jabbo, N.F. Bionomics of Ectomyelois ceratoniae (Lepidoptera: Pyralidae) on Pomegranates in Iraq. Environ. Entomol. 1985, 14, 149–153. [Google Scholar] [CrossRef]
- Warner, R.L.; Barnes, M.M.; Laird, E.F. Chemical Control of a Carob Moth, Ectomyelois ceratoniae (Lepidoptera: Pyralidae), and Various Nitidulid Beetles (Coleoptera) on ‘Deglet Noor’ Dates in California. J. Econ. Entomol. 1990, 83, 2357–2361. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Goldansaz, S.H.; Fotoukkiaii, S.M.; Menken, S.B.J.; Groot, A.T. Seasonal pattern of infestation by the carob moth Ectomyelois ceratoniae in pomegranate cultivars. Crop Prot. 2017, 102, 19–24. [Google Scholar] [CrossRef]
- Warner, R.L.; Barnes, M.M.; Laird, E.F. Reduction of Insect Infestation and Fungal Infection by Cultural Practice in Date Gardens. Environ. Entomol. 1990, 19, 1618–1623. [Google Scholar] [CrossRef]
- Vetter, R.S.; Tatevossian, S.; Baker, T. Reproductive behavior of the female carob moth, (Lepidoptera: Pyralidae). Pan-Pac. Entomol. 1997, 73, 28–35. [Google Scholar]
- Solis, M. Key to Selected Pyraloidea (Lepidoptera) Larvae Intercepted at U. S. Ports of Entry: Revision of Pyraloidea in “Keys to Some Frequently Intercepted Lepidopterous Larvae” by Weisman 1986 (Updated 2006). Proc. Entomol. Soc. Wash. 2006, 101. [Google Scholar]
- Wang, B.-X.; Zhu, L.; Ma, G.; Najar-Rodriguez, A.; Zhang, J.-P.; Zhang, F.; Avila, G.A.; Ma, C.-S. Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios. Biology 2023, 12, 435. [Google Scholar] [CrossRef]
- Zhu, H.; Kumar, S.; Neven, L.G. Codling Moth (Lepidoptera: Tortricidae) Establishment in China: Stages of Invasion and Potential Future Distribution. J. Insect. Sci. 2017, 17, 85. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B.; Khojastehpour, M.; Golzarian, M.R. Carob moth, Ectomyelois ceratoniae, detection in pomegranate using visible/near infrared spectroscopy. Comput. Electron. Agric. 2016, 129, 9–14. [Google Scholar] [CrossRef]
- Abid, I.; Laghfiri, M.; Bouamri, R.; Aleya, L.; Bourioug, M. Integrated pest management (IPM) for Ectomyelois ceratoniae on date palm. Curr. Opin. Environ. Sci. Health 2021, 19, 100219. [Google Scholar] [CrossRef]
- Ranjbar, M.; Zibaee, A.; Sendi, J.J. Purification and characterization of a digestive lipase in the midgut of Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae). Front. Life Sci. 2015, 8, 64–70. [Google Scholar] [CrossRef]
- Alotaibi, S.S.; Darwish, H.; Zaynab, M.; Alharthi, S.; Alghamdi, A.; Al-Barty, A.; Asif, M.; Wahdan, R.H.; Baazeem, A.; Noureldeen, A. Isolation, Identification, and Biocontrol Potential of Entomopathogenic Nematodes and Associated Bacteria against Virachola livia (Lepidoptera: Lycaenidae) and Ectomyelois ceratoniae (Lepidoptera: Pyralidae). Biology 2022, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Morland, G. The morphology and ecology of the Carob moth (Ectomyelois ceratoniae) (Zeller) in citrus orchards of the Western Cape, South Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Leyequien, E.; Verrelst, J.; Slot, M.; Schaepman-Strub, G.; Heitkonig, I.; Skidmore, A. Capturing the fugitive: Applying remote sensing to terrestrial animal distribution and diversity. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 1–20. [Google Scholar] [CrossRef]
- Lodge, D.M.; Simonin, P.W.; Burgiel, S.W.; Keller, R.P.; Bossenbroek, J.M.; Jerde, C.L.; Kramer, A.M.; Rutherford, E.S.; Barnes, M.A.; Wittmann, M.E.; et al. Risk Analysis and Bioeconomics of Invasive Species to Inform Policy and Management. Annu. Rev. Environ. Resour. 2016, 41, 453–488. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Gillison, A.N.; Winter, J. Domain: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Busby, J.R. Bioclim—A bioclimate analysis and prediction system. Plant Prot. Q. 1991, 61, 8–9. [Google Scholar]
- Jie, L.; Yang, J.-B.; Li, W. Potential distribution analysis of an invasive alien species Parapediasia teterrella (Lepidoptera, Crambidae) in East Asia. J. Asia-Pac. Entomol. 2020, 23, 219–223. [Google Scholar] [CrossRef]
- San Blas, G.; Obholz, G.; Dias, F.; Specht, A.; Casagrande, M.; Mielke, O. Global Potential Distribution of the South American Cutworm Pest Agrotis robusta (Lepidoptera: Noctuidae). Neotrop. Entomol. 2022, 51, 198. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Fotheringham, A.; Oshan, T. Geographically weighted regression and multicollinearity: Dispelling the myth. J. Geogr. Syst. 2016, 18, 303–329. [Google Scholar] [CrossRef]
- GBIF.org (26 August 2022) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.73j6ct (accessed on 26 August 2022).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Zhao, L.; Cui, S. Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model. Insects 2023, 14, 182. [Google Scholar] [CrossRef]
- Ning, Y.; Lei, J.R.; Song, X.Q.; Han, S.M.; Zhong, Y.F. Modeling the potential suitable habitat of Impatiens hainanensis, a limestone-endemic plant. Chin. J. Plant Ecol. 2018, 42, 946–954. [Google Scholar] [CrossRef]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Ben-David, A. About the relationship between ROC curves and Cohen’s kappa. Eng. Appl. Artif. Intell. 2008, 21, 874–882. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Altamiranda-Saavedra, M.; Amat, E.; Gómez-P, L.-M. Influence of montane altitudinal ranges on species distribution models; evidence in Andean blow flies. PeerJ 2020, 8, e10370. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Lei, X.; Chen, J.; Zhu, Z.; Guo, X.; Liu, P.; Jiang, X. How to locate urban–rural transit hubs from the viewpoint of county integration? Phys. A Stat. Mech. Its Appl. 2022, 606, 128148. [Google Scholar] [CrossRef]
- Huang, X.F. The Study of Landuse Change and Ecological Suitability in Pudong New Area; East China Normal University: Shanghai, China, 2009. [Google Scholar]
- Frank, S.D. Review of the direct and indirect effects of warming and drought on scale insect pests of forest systems. For. Int. J. For. Res. 2021, 94, 167–180. [Google Scholar] [CrossRef]
- Cox, P.D. The influence of temperature and humidity on the life-cycle of Ectomyelois ceratoniae (Zeller) (Lepidoptera: Phycitidae). J. Stored Prod. Res. 1976, 12, 111–117. [Google Scholar] [CrossRef]
- Ben-Amor, R.; Dhouibi, M.H.; Aguayo, E. Hot water treatments combined with cold storage as a tool for Ectomyelois ceratoniae mortality and maintenance of Deglet Noor palm date quality. Postharvest Biol. Technol. 2016, 112, 247–255. [Google Scholar] [CrossRef]
- Ahmadi, B.; Moharramipour, S.; Sinclair, B.J. Overwintering biology of the carob moth Apomyelois ceratoniae (Lepidoptera: Pyralidae). Int. J. Pest Manag. 2016, 62, 69–74. [Google Scholar] [CrossRef]
- Baradevanal, G.; Chander, S.; Jayanthi, P.D.K.; Singh, H.S.; Reddy, D.S. Assessing the risk of mango quarantine pest Deanolis sublimbalis Snellen under different climate change scenarios. J. Plant Dis. Prot. 2021, 128, 853–863. [Google Scholar] [CrossRef]
- Mellado-Negrete, A.; Peña-Vázquez, G.I.; Urías-Orona, V.; De La Garza, A.L. Polyphenol Bioaccessibility and Antioxidant Activity of Pomegranate (Punica granatum) Peel Supplementation in Diet-Induced Obese Rats. J. Med. Food 2023, 26, 570–579. [Google Scholar] [CrossRef]
- Park, J.-J.; Perring, T. Development of a Binomial Sampling Plan for the Carob Moth (Lepidoptera: Pyralidae), a Pest of California Dates. J. Econ. Entomol. 2010, 103, 1474–1482. [Google Scholar] [CrossRef]
- Hached, W.; Romdhane, S.; Sahraoui, H.; Lebdi, K. Control trials against Ectomyelois ceratoniae Zeller 1881 (Lepidoptera: Pyralidae) under controlled conditions and in citrus orchard. J. New Sci. Agric. Biotechnol. 2018, 49, 2961–2970. [Google Scholar]
- Mnif, I.; Elleuch, M.; Châabouni, S.E.; Ghribi, D.J.C.P. Bacillus subtilis SPB1 biosurfactant: Production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae. Crop Prot. 2013, 50, 66–72. [Google Scholar] [CrossRef]


| Variable | Description | Contribution (%) |
|---|---|---|
| bio9 | Mean temperature of driest quarter (°C) | 50.2 |
| bio8 | Mean temperature of wettest quarter (°C) | 16.9 |
| bio4 | Temperature seasonality (standard deviation × 100) (°C) | 9.7 |
| bio19 | Precipitation of coldest quarter (mm) | 9.1 |
| bio15 | Precipitation seasonality (coefficient of variation) | 3.6 |
| bio18 | Precipitation of warmest quarter (mm) | 3.6 |
| bio13 | Precipitation of wettest month (mm) | 2.9 |
| bio2 | Mean diurnal range (Mean of monthly (max temp–min temp)) (°C) | 2.3 |
| bio17 | Precipitation of driest quarter (mm) | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yang, M.; Li, M.; Jin, Z.; Yang, N.; Yu, H.; Liu, W. Climate Change Facilitates the Potentially Suitable Habitats of the Invasive Crop Insect Ectomyelois ceratoniae (Zeller). Atmosphere 2024, 15, 119. https://doi.org/10.3390/atmos15010119
Liu C, Yang M, Li M, Jin Z, Yang N, Yu H, Liu W. Climate Change Facilitates the Potentially Suitable Habitats of the Invasive Crop Insect Ectomyelois ceratoniae (Zeller). Atmosphere. 2024; 15(1):119. https://doi.org/10.3390/atmos15010119
Chicago/Turabian StyleLiu, Changqing, Ming Yang, Ming Li, Zhenan Jin, Nianwan Yang, Hao Yu, and Wanxue Liu. 2024. "Climate Change Facilitates the Potentially Suitable Habitats of the Invasive Crop Insect Ectomyelois ceratoniae (Zeller)" Atmosphere 15, no. 1: 119. https://doi.org/10.3390/atmos15010119
APA StyleLiu, C., Yang, M., Li, M., Jin, Z., Yang, N., Yu, H., & Liu, W. (2024). Climate Change Facilitates the Potentially Suitable Habitats of the Invasive Crop Insect Ectomyelois ceratoniae (Zeller). Atmosphere, 15(1), 119. https://doi.org/10.3390/atmos15010119







