Vertical Divergence Characteristics of Dissolved Inorganic Carbon and Influencing Factors in a Karst Deep-Water Reservoir, Southwest China
Abstract
1. Introduction
2. Materials and Methods
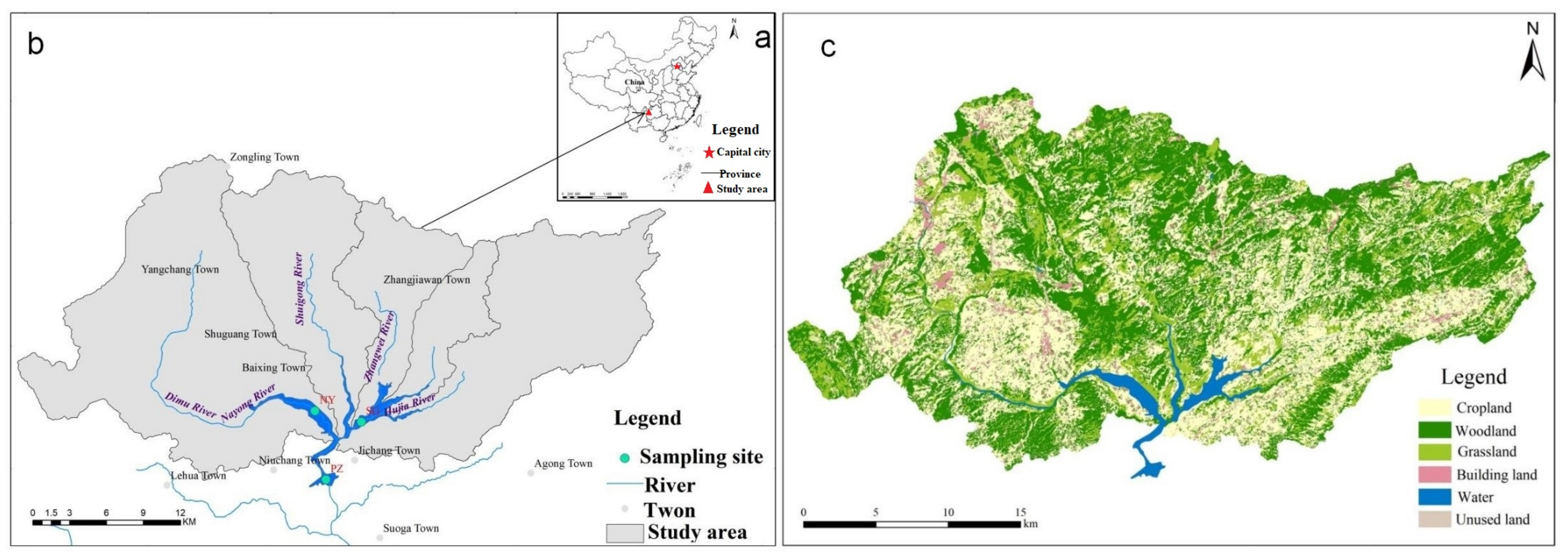
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Data Processing
3. Results
3.1. Characteristics of Twater, DO Changes, and Reservoir Thermal Stratification Patterns
3.2. Distribution Characteristics of DIC and Vertical Variation of δ13CDIC
3.3. Characteristics of SIc and pCO2Variation in the Pingzhai Reservoir
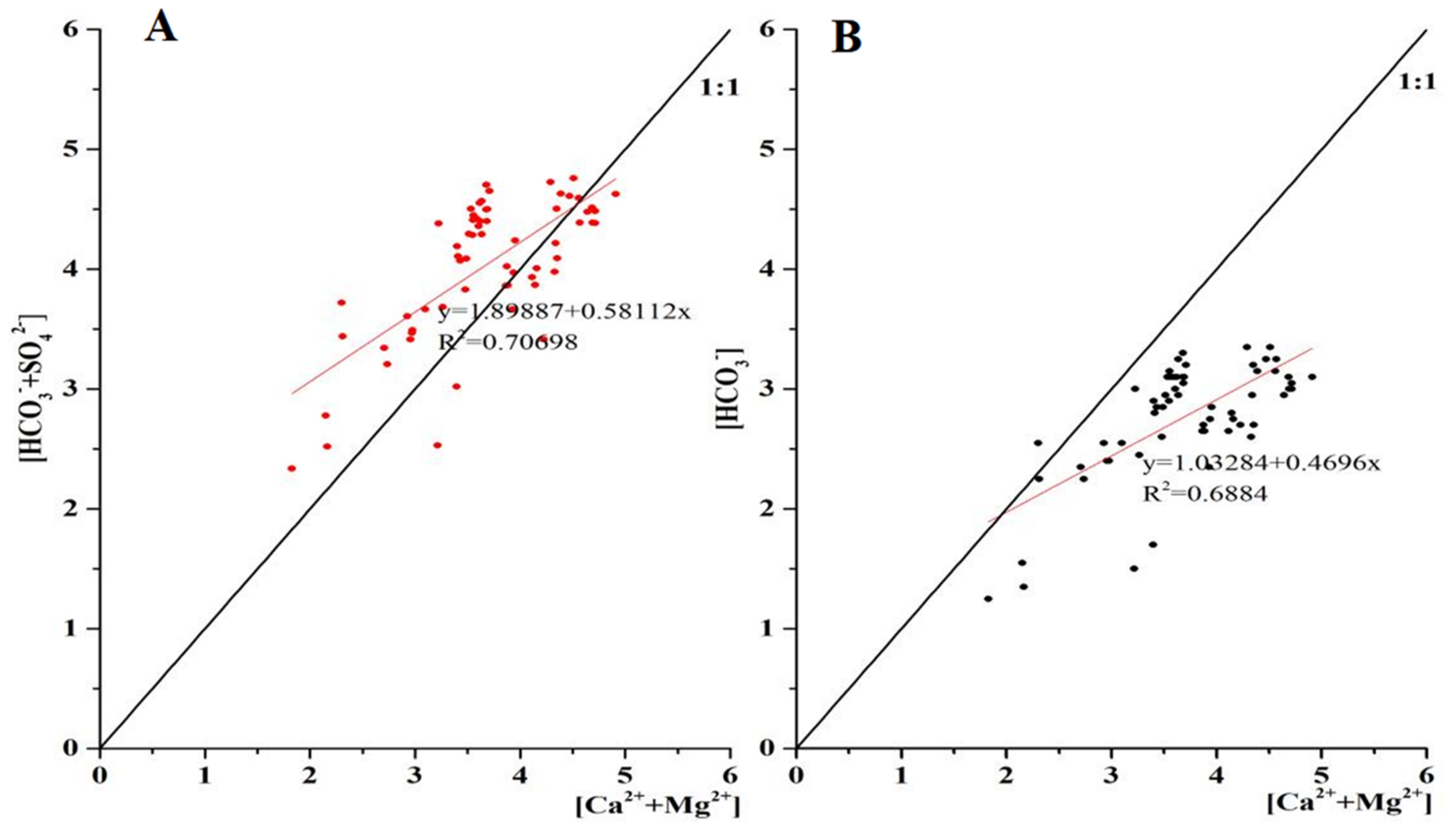
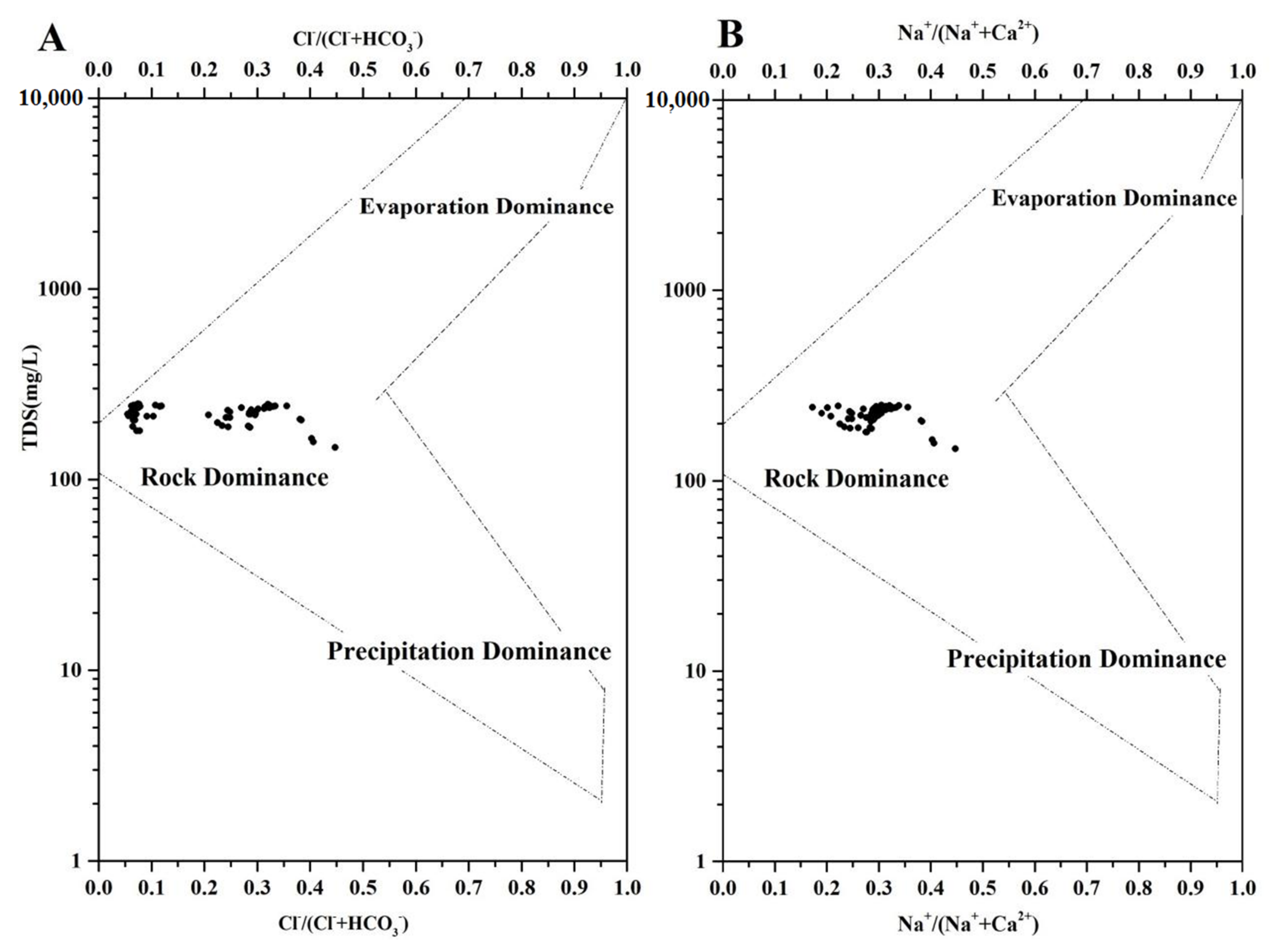
3.4. DIC Sources in Pingzhai Reservoir
4. Discussion
4.1. Mechanism of Subthermocline Formation and Its Influence on the Vertical Distribution of DIC
4.2. Source Influences the Vertical Difference in DIC Concentration
4.3. Impact of Human Activities on DIC Concentration in the Reservoir
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, H.J.; Bouillon, S.; Andre, L.; Cardinal, D. The effects of weathering variability and anthropogenic pressures upon silicon cycling in an intertropical watershed (Tana River, Kenya). Chem. Geol. 2012, 308, 18–25. [Google Scholar] [CrossRef]
- Dong, N.P.; Yu, Z.B.; Gu, H.H.; Yang, C.G.; Yang, M.X.; Wei, J.H.; Wang, H.; Arnault, J.; Laux, P.; Kunstmann, H. Climate-induced hydrological impact mitigated by a high-density reservoir network in the Poyang Lake Basin. J. Hydrol. 2019, 579, 124148. [Google Scholar] [CrossRef]
- Xie, J.Q.; You, J.J.; Ma, Z.Z.; Deng, X.Y.; Lin, P.F.; Gao, J.J.; Klemes, J.J. Methodology for including reservoir regulation in water scarcity evaluation. J. Clean. Prod. 2022, 365, 132657. [Google Scholar] [CrossRef]
- Doretto, A.; Piano, E.; Larson, C.E. The River Continuum Concept: Lessons from the past and perspectives for the future. Can. J. Fish. Aquat. Sci. 2020, 77, 1853–1864. [Google Scholar] [CrossRef]
- Carey, J.C.; Fulweiler, R.W. Human activities directly alter water-shed dissolved silica fluxes. Biogeochemistry 2012, 111, 125–138. [Google Scholar] [CrossRef]
- Haojun, D.; Zhen, T.; Quanzhou, G.; Ling, Y.; Yong, F.; Yinhua, L. Research Advance of Changing Biogenic Substance Cycling in River Systems by Damming. Adv. Earth Sci. 2018, 33, 1237–1247. [Google Scholar]
- Vorosmarty, C.J.; Sharma, K.P.; Fekete, B.M.; Copeland, A.H.; Holden, J.; Marble, J.; Lough, J.A. The storage and aging of continentalrunoff in large reservoir systems of the world. Ambio 1997, 26, 210–219. [Google Scholar]
- Humborg, C.; Conley, D.J.; Rahm, L.; Wulff, F.; Cociasu, A.; Ittekkot, V. Silicon retention in river basins: Far-reaching effects on biogeochemistry and aquatic food webs in coastal marine environments. AMBIO J. Hum. Environ. 2000, 29, 45–50. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Thornton, P. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Sience Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 46–570. [Google Scholar]
- Stocker, T.; Plattner, G.K.; Dahe, Q. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate ChangeWG1AR5; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2013; pp. 465–570. [Google Scholar]
- Temino-Boes, R.; Romero, I.; Paches, M.; Martinez-Guijarro, R.; Romero-Lopez, R. Anthropogenic impact on nitrification dynamics in coastal waters of the Mediterranean sea. Mar. Pollut. Bull. 2019, 145, 14–22. [Google Scholar] [CrossRef]
- Ivlev, A.A. Global redox cycle of biospheric carbon: Interaction of photosynthesis and earth crust processes. BioSystems 2015, 137, 1–11. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion and the global carbon budget. Environ. Int. 2003, 29, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.L.; Corman, J.R.; Havig, J.R. Carbon and nitrogen recycling during cyanoHABs in dreissenid-invaded and non-invaded US midwestern lakes and reservoirs. Hydrobiologia 2020, 847, 939–965. [Google Scholar] [CrossRef]
- Tan, D.B.; Luo, T.F.; Zhao, D.Z.; Li, C. Carbon dioxide emissions from the Geheyan Reservoir over the Qingjiang River Basin, China. Ecohydrol. Hydrobiol. 2019, 19, 499–514. [Google Scholar] [CrossRef]
- Fuhrmann, B.C.; Beutel, M.W.; O’Day, P.A.; Tran, C.; Funk, A.; Brower, S.; Seelos, M. Effects of mercury, organic carbon, and microbial inhibition on methylmercury cycling at the profundal sediment-water interface of a sulfate-rich hypereutrophic reservoir. Environ. Pollut. 2021, 268, 115853. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lang, Y.; Liu, C.Q.; Qin, Y.; Yu, N.; Wang, B. Flux of organic carbon burial and carbon emission from a large reservoir: Implications for the cleanliness assessment of hydropower. Sci. Bull. 2019, 64, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Abril, G.; Richard, S.; Guerin, F. In situ measurements of dissolved gases 440 (CO2 and CH4) in a wide range of concentrations in a tropical reservoir using an equilibrator. Sci. Total Environ. 2006, 354, 246–251. [Google Scholar] [CrossRef]
- Phyoe, W.W.; Wang, F. A review of carbon sink or source effect on artificial reservoirs. Int. J. Environ. Sci. Technol. 2019, 16, 2161–2174. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Liu, C.Q.; Wang, F.; Peng, X.; Liu, X.L. Carbon biogeochemical cycle is enhanced by damming in a karst river. Sci. Total Environ. 2018, 616, 1181–1189. [Google Scholar] [CrossRef]
- Biswas, H.; Mukhopadhyay, S.K.; De, T.K.; Sen, S.; Jana, T.K. Biogenic controls on the air-water carbon dioxide exchange in the Sundarban mangrove environment, northeast coast of Bay of Bengal, India. Limnol. Oceanogr. 2004, 49, 95–101. [Google Scholar] [CrossRef]
- Hammer, K.J.; Kragh, T.; Sand-Jensen, K. Inorganic carbon promotes photosynthesis, growth, and maximum biomass of phytoplankton in eutrophic water bodies. Freshw. Biol. 2019, 64, 1956–1970. [Google Scholar] [CrossRef]
- Lash, G.G. Significance of stable carbon isotope trends in carbonate concretions formed in association with anaerobic oxidation of methane (AOM), Middle and Upper Devonian shale succession, western New York State, USA. Mar. Pet. Geol. 2018, 91, 470–479. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.; Chung, J. Reduction in carbon dioxide and production of methane by biological reaction in the electronics industry. Int. J. Hydrogen Energy 2013, 38, 3488–3496. [Google Scholar] [CrossRef]
- Chuang, P.C.; Yang, T.F.; Wallmann, K.; Matsumoto, R.; Hu, C.Y.; Chen, H.W.; Dale, A.W. Carbon isotope exchange during Anaerobic Oxidation of Methane (AOM) in sediments of the northeastern South China Sea. Geochim. Cosmochim. Acta 2019, 246, 138–155. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, S.B.; Liu, D.F.; Chen, W.Z.; Wang, Y.C.; Chen, X.Y.; Duan, Y.J. Fluxes of greenhouse gases from Xiangxi River in summer and their influencing factors. Environ. Sci. 2012, 33, 1471–1475. [Google Scholar]
- Chanudet, V.; Descloux, S.; Harby, A.; Sundt, H.; Hansen, B.H.; Brakstad, O.; Serça, D.; Guerin, F. Gross CO2 and CH4 emissions from the Nam Ngum and Nam Leuk sub-tropical reservoirs in Lao PDR. Sci. Total Environ. 2011, 409, 5382–5391. [Google Scholar] [CrossRef]
- Wang, W.F.; Li, S.L.; Zhong, J.; Maberly, S.C.; Li, C.; Wang, F.S.; Xiao, H.Y.; Liu, C.Q. Climatic and anthropogenic regulation of carbon transport and transformation in a karst river-reservoir system. Sci. Total Environ. 2020, 707, 135628. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Li, S.L.; Zhong, J.; Wang, L.C.; Yang, H.; Xiao, H.Y.; Liu, C.Q. CO2 emissions from karst cascade hydropower reservoirs: Mechanisms and reservoir effect. Environ. Res. Lett. 2021, 16, 044013. [Google Scholar] [CrossRef]
- Pu, J.; Li, J.; Zhang, T.; Martin, J.B.; Yuan, D. Varying thermal structure controls the dynamics of CO2 emissions from a subtropical reservoir, south China. Water Res. 2020, 178, 115831. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, J.; Li, C.; Yi, Y.; Chen, S.; Chen, L.; Lang, Y.; Li, S. The influence of cascade reservoir construction on water chemistry distribution in Karst area. J. Lake Sci. 2020, 32, 713–725. [Google Scholar]
- Barros, N.; Cole, J.J.; Tranvik, L.J.; Prairie, Y.T.; Bastviken, D.; Huszar, V.L.; Roland, F. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nat. Geosci. 2011, 4, 593–596. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Huang, X.F.; Zhou, Y.C. Spatial heterogeneity of soil organic carbon in a karst region under different land use patterns. Ecosphere 2020, 11, e03077. [Google Scholar] [CrossRef]
- Cao, M.; Zhou, Z.; Zhang, J.; Zhang, S.; Yin, C.; Yan, L. Study on the physicochemical characteristics and environmental indication of dolomite karst cave water in the Shuanghe Cave, Guizhou Province. Geochimica 2017, 46, 87–97. [Google Scholar]
- Liang, J.; Yi, Y.; Li, X.; Yuan, Y.; Zhai, Y. Detecting changes in water level caused by climate, land cover and dam construction in interconnected riverlake systems. Sci. Total Environ. 2021, 788, 147692. [Google Scholar] [CrossRef] [PubMed]
- Invers, O.; Zimmerman, R.C.; Alberte, R.S.; Pérez, M.; Romero, J. Inorganic carbon sources for seagrass photosynthesis: An experimental evaluation of bicarbonate use in species inhabiting temperate waters. J. Exp. Mar. Biol. Ecol. 2001, 265, 203–217. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef]
- Myrbo, A.; Shapley, M.D. Seasonal water-column dynamics of dissolved inorganic carbon stable isotopic compositions (δ13CDIC) in small hardwater lakes in Minnesota and Montana. Geochim. Cosmochim. Acta 2006, 70, 2699–2714. [Google Scholar] [CrossRef]
- Liu, X. Study on Geochemical Characteristics of Dissolved Inorganic Carbon in Lakes of Karst Plateau Area: A Case Study in Pingzhai Reservoir; Guizhou Normal University: Guiyang, China, 2021. [Google Scholar] [CrossRef]
- Chun, H.Z.Y.; Cheng, Z.; Xiaoyu, G.; Chen, W.; Deng, J. Reservoir storage estimation of karst underground reservoir associated with Pingzhai Reservoir in South tributary of Wu River. Resour. Environ. Eng. 2021, 35, 478–483. [Google Scholar]
- Beck, M.B. Mathematical Modeling of Water Quality; IIASA Working Paper; IIASA: Laxenburg, Austria, 1978. [Google Scholar]
- Li, J.; Pu, J.; Zhang, T. Transport and transformation of dissolved inorganic carbon in a subtropical groundwater-fed reservoir, south China. Water Res. 2022, 209, 117905. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, P. Preliminary study on thermocline in Lake Fuxian. Trans. Oceanol. Limnol. 1982, 4, 1–9. [Google Scholar]
- Das, A.; Krishnaswami, S.; Bhattacharya, S.K. Carbon isotope ratio of dissolved inorganic carbon (DIC) in rivers draining the Deccan Traps, India: Sources of DIC and their magnitudes. Earth Planet. Sci. Lett. 2005, 236, 419–429. [Google Scholar] [CrossRef]
- Helie, J.F.; Hillaire-Marcel, C.; Rondeau, B. Seasonal changes in the sources and fluxes of dissolved inorganic carbon through the St. Lawrence River—Isotopic and chemical constraint. Chem. Geol. 2002, 186, 117–138. [Google Scholar] [CrossRef]
- Liu, C.Q.; Jiang, Y.K.; Tao, F.X.; Lang, Y.C.; Li, S.L. Chemical weathering of carbonate rocks by sulfuric acid and carbon cycling in Southwest China. Geochimica 2008, 37, 404–414. [Google Scholar]
- Cheng, T.Y.; Zhou, T.; Qin, Y.; Phoye, W.; Wang, F.S. Characters and sources of dissolved inorganic carbon isotope in channel reservoir: A case of Wanan Reservoir. Chin. J. Ecol. 2018, 37, 661–666. [Google Scholar]
- Yang, X.Y.; Li, Y.J.; Wang, B.L.; Xiao, J.; Yang, M.L.; Liu, C.Q. Effect of hydraulic load on thermal stratification in karst cascade hydropower reservoirs, Southwest China. J. Hydrol. Reg. Stud. 2020, 32, 100748. [Google Scholar] [CrossRef]
- Belolipetskii, V.M.; Genova, S.N. Simplified Mathematical Model of the Hydrothermal Regime of the Krasnoyarsk Reservoir. Therm. Sci. 2019, 23, S455–S462. [Google Scholar] [CrossRef]
- Qiu, X. Study on Water Quality Evolution Law and Eutrophication of Stratified Reservoirs in North China; Xi’an University of Architecture and Technology: Xi’an, China, 2016. [Google Scholar]
- Varol, M.; Li, S.Y. Biotic and abiotic controls on CO2 partial pressure and CO2 emission in the Tigris River, Turkey. Chem. Geol. 2017, 449, 182–193. [Google Scholar] [CrossRef]
- Herczeg, A.L. A stable carbon isotope study of dissolved inorganic carbon cycling in a softwater lake. Biogeochemistry 1987, 4, 231–263. [Google Scholar] [CrossRef]
- Reiss, R.S.; Lemmin, U.; Cimatoribus, A.A.; Barry, D.A. Wintertime Coastal Upwelling in Lake Geneva: An Efficient Transport Process for Deepwater Renewal in a Large, Deep Lake. J. Geophys. Res.-Ocean. 2020, 125, e2020JC016095. [Google Scholar] [CrossRef]
- Reiss, R.S.; Lemmin, U.; Barry, D.A. Wind-Induced Hypolimnetic Upwelling Between the Multi-Depth Basins of Lake Geneva During Winter: An Overlooked Deepwater Renewal Mechanism? J. Geophys. Res.-Ocean. 2022, 127, e2021JC018023. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Z.H.; Luo, W.; Ma, T.; Wang, Y.X. Multiple isotope geochemistry and hydrochemical monitoring of karst water in a rapidly urbanized region. J. Contam. Hydrol. 2018, 218, 44–58. [Google Scholar] [CrossRef]
- Rao, W.B.; Zheng, F.W.; Tan, H.B.; Yong, B.; Jin, K.; Wang, S.; Zhang, W.B.; Chen, T.Q.; Wang, Y.N. Major ion chemistry of a representative river in South-central China: Runoff effects and controlling mechanisms. J. Hazard. Mater. 2019, 378, 120755. [Google Scholar] [CrossRef]
- Peng, Y.M.; Xiao, Q.; Xue, H.L.; Lv, M.H.; Lin, G.Q.; Zhao, H.J.; Wu, P.Y.; Guo, Y.L. Comparison of groundwater hydrogeochemistry of karst areas in northern and southern China with emphasis on their performance in karst development. Carbonates Evaporites 2022, 37, 76. [Google Scholar]
- Huang, Q.; Xiaoqun, Q.I.N.; Ruirui, C.H.E.N.G. Research progress of sulfuric acid rain participating in the dissolution of carbonate rocks. Carsologica Sin. 2019, 38, 149–156. [Google Scholar]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering, Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]



| Ca2+ | K+ | Mg2+ | Na+ | Cl− | NO3− | SO42− | DIC | pH | δ13CDIC | DO | Chl-a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | |||||||||||
| K+ | 0.573 ** | 1 | ||||||||||
| Mg2+ | 0.470 ** | 0.932 ** | 1 | |||||||||
| Na+ | 0.288 * | 0.810 ** | 0.845 ** | 1 | ||||||||
| Cl- | 0.209 | 0.488 ** | 0.523 ** | 0.684 ** | 1 | |||||||
| NO3- | 0.576 ** | 0.271 | 0.165 | −0.133 | 0.098 | 1 | ||||||
| SO42- | 0.408 ** | 0.538 ** | 0.607 ** | 0.586 ** | 0.894 ** | 0.362 ** | 1 | |||||
| DIC | 0.363 ** | 0.120 | 0.175 | 0.038 | 0.171 | 0.336 * | 0.365 ** | 1 | ||||
| pH | −0.227 | 0.036 | 0.149 | 0.244 | 0.002 | −0.363 ** | −0.065 | −0.425 ** | 1 | |||
| δ13CDIC | −0.684 ** | −0.436 ** | −0.325 * | −0.046 | −0.074 | −0.684 ** | −0.267 | −0.272 | 0.463 ** | 1 | ||
| DO | −0.091 | 0.054 | 0.095 | 0.077 | −0.172 | −0.096 | −0.166 | −0.310 * | 0.863 ** | 0.214 | 1 | |
| Chl-a | 0.151 | −0.063 | −0.058 | −0.185 | 0.003 | 0.211 | 0.092 | 0.489 ** | −0.152 | −0.231 | 0.045 | 1 |
| Surface Layer | 10 m Depth | 20 m Depth | 30 m Depth | 40 m Depth | 50 m Depth | 60 m Depth | |
|---|---|---|---|---|---|---|---|
| Ca2+ | 0.885 ** | 0.273 | 0.4 | 0.706 * | 0.188 | 0.371 | 0.491 |
| Mg2+ | 0.627 | 0.078 | 0.633 | 0.832 ** | −0.58 | −0.381 | 0.253 |
| K+ | 0.545 | −0.035 | 0.293 | 0.691 * | −0.654 | −0.455 | 0.229 |
| Na+ | 0.411 | 0.099 | 0.680 * | 0.836 ** | −0.593 | −0.428 | 0.256 |
| Cl− | 0.138 | −0.079 | 0.366 | 0.888 ** | −0.26 | −0.333 | −0.325 |
| NO3− | 0.915 ** | −0.172 | −0.279 | −0.14 | 0.317 | −0.286 | −0.215 |
| SO42− | 0.299 | −0.035 | 0.534 | 0.837 ** | 0.089 | −0.373 | −0.191 |
| Twater | −0.989 ** | −0.539 | −0.597 | −0.903 ** | −0.760 * | 0.01 | −0.005 |
| DO | −0.671 * | 0.211 | 0.445 | −0.291 | −0.551 | −0.432 | −0.344 |
| pH | −0.780 * | 0.063 | 0.506 | 0.417 | 0.555 | −0.187 | 0.073 |
| chl-a | −0.662 | 0.105 | 0.17 | −0.581 | −0.447 | 0.289 | −0.518 |
| Humidity | −0.328 | - | - | - | - | - | - |
| Wind speed | 0.048 | - | - | - | - | - | - |
| Air temperature | −0.811 ** | - | - | - | - | - | - |
| Barometric pressure | 0.499 | - | - | - | - | - | - |
| Atmospheric CO2 concentration | 0.879 ** | - | - | - | - | - | - |
| Surface Layer | 10 m Depth | 20 m Depth | 30 m Depth | 40 m Depth | 50 m Depth | 60 m Depth | |
|---|---|---|---|---|---|---|---|
| Ca2+ | 0.373 | 0.468 | 0.519 | 0.705 * | 0.464 | 0.268 | 0.921 ** |
| Mg2+ | 0.51 | 0.043 | 0.826 ** | 0.875 ** | 0.628 | 0.832 ** | 0.878 ** |
| K+ | 0.645 | 0.009 | 0.532 | 0.778* | 0.541 | 0.799 ** | 0.830 ** |
| Na+ | 0.733 * | 0.204 | 0.895 ** | 0.902 ** | 0.599 | 0.764 * | 0.824 ** |
| Cl− | 0.594 | −0.406 | 0.245 | 0.869 ** | −0.085 | 0.525 | −0.603 |
| NO3− | 0.258 | −0.505 | −0.467 | 0.082 | −0.157 | 0.613 | −0.224 |
| SO42− | 0.578 | −0.377 | 0.307 | 0.832 ** | 0.409 | 0.307 | −0.374 |
| Twater | 0.014 | −0.889 ** | −0.911 ** | −0.694 * | −0.618 | 0.192 | −0.253 |
| DO | 0.681 * | 0.792 * | 0.889 ** | 0.461 | −0.06 | 0.116 | −0.181 |
| EC | 0.105 | 0.006 | 0.489 | 0.802 ** | 0.268 | −0.161 | 0.172 |
| pH | 0.585 | 0.897 ** | 0.924 ** | 0.933 ** | 0.804 ** | 0.175 | 0.877 ** |
| chl-a | −0.204 | −0.585 | −0.136 | −0.682 * | −0.695 * | 0.1 | −0.712 * |
| Humidity | −0.533 | - | - | - | - | - | - |
| Wind speed | −0.263 | - | - | - | - | - | - |
| Air temperature | 0.347 | - | - | - | - | - | - |
| Barometric pressure | −0.596 | - | - | - | - | - | - |
| Atmospheric CO2 concentration | −0.271 | - | - | - | - | - | - |
| Surface Layer | 10 m Depth | 20 m Depth | 30 m Depth | 40 m Depth | 50 m Depth | 60 m Depth | |
|---|---|---|---|---|---|---|---|
| Ca2+ | 0.547 | 0.017 | −0.198 | −0.269 | −0.146 | −0.338 | −0.505 |
| Mg2+ | 0.125 | 0.285 | −0.508 | −0.546 | −0.368 | 0.047 | −0.646 |
| K+ | −0.002 | 0.239 | −0.305 | −0.393 | −0.241 | 0.109 | −0.587 |
| Na+ | −0.119 | 0.147 | −0.625 | −0.622 | −0.358 | 0.174 | −0.541 |
| Cl− | −0.353 | 0.3 | 0.037 | −0.526 | −0.083 | 0.153 | 0.525 |
| NO3− | 0.566 | 0.337 | 0.618 | 0.165 | −0.099 | 0.117 | 0.246 |
| SO42− | −0.225 | 0.357 | 0.207 | −0.443 | −0.418 | −0.424 | 0.433 |
| Twater | −0.803 ** | 0.567 | 0.816 ** | 0.509 | 0.898 ** | 0.692 * | 0.505 |
| DO | −0.894 ** | −0.791 * | −0.928 ** | −0.659 | 0.056 | 0.391 | 0.035 |
| EC | 0.614 | 0.383 | 0.017 | −0.412 | −0.432 | −0.472 | 0.042 |
| pH | −0.962 ** | −0.906 ** | −0.909 ** | −0.972 ** | −0.987 ** | −0.981 ** | −0.962 ** |
| chl-a | −0.556 | 0.394 | 0.44 | 0.704* | 0.905 ** | 0.793 * | 0.534 |
| Humidity | −0.059 | - | - | - | - | - | - |
| Wind speed | 0.294 | - | - | - | - | - | - |
| Air temperature | −0.851 ** | - | - | - | - | - | - |
| Barometric pressure | 0.796 * | - | - | - | - | - | - |
| Atmospheric CO2 concentration | 0.867 ** | - | - | - | - | - | - |
| DIC (mg/L) | SO42− (mg/L) | NO3− (mg/L) | |
|---|---|---|---|
| Sources of carbonate rock | −0.548 ** | −0.335 | −0.492 ** |
| Sources of soil organic matter | 0.548 ** | 0.335 | 0.492 ** |
| Sources of air | −0.548 | −0.335 | −0.492 |
| Cl−/Na+ | SO42−/Na+ | NO3−/Na+ | |
|---|---|---|---|
| Min. value | 0.164 | 2.116 | 0.276 |
| Max. value | 0.782 | 7.001 | 1.743 |
| Mean value | 0.491 | 4.320 | 0.870 |
| Standard deviation | 0.098 | 0.858 | 0.276 |
| Coefficient of variation | 0.200 | 0.199 | 0.317 |
| Critical Value of significant (t-test) | 0.516 | 4.536 | 0.940 |
| Critical value of highly significant (t-test) | 0.525 | 4.607 | 0.963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wang, C.; Li, Y.; Zhang, Y.; Kong, J. Vertical Divergence Characteristics of Dissolved Inorganic Carbon and Influencing Factors in a Karst Deep-Water Reservoir, Southwest China. Atmosphere 2023, 14, 1111. https://doi.org/10.3390/atmos14071111
Zhou Z, Wang C, Li Y, Zhang Y, Kong J. Vertical Divergence Characteristics of Dissolved Inorganic Carbon and Influencing Factors in a Karst Deep-Water Reservoir, Southwest China. Atmosphere. 2023; 14(7):1111. https://doi.org/10.3390/atmos14071111
Chicago/Turabian StyleZhou, Zhongfa, Cui Wang, Yongliu Li, Yongrong Zhang, and Jie Kong. 2023. "Vertical Divergence Characteristics of Dissolved Inorganic Carbon and Influencing Factors in a Karst Deep-Water Reservoir, Southwest China" Atmosphere 14, no. 7: 1111. https://doi.org/10.3390/atmos14071111
APA StyleZhou, Z., Wang, C., Li, Y., Zhang, Y., & Kong, J. (2023). Vertical Divergence Characteristics of Dissolved Inorganic Carbon and Influencing Factors in a Karst Deep-Water Reservoir, Southwest China. Atmosphere, 14(7), 1111. https://doi.org/10.3390/atmos14071111









