Characterization of Chemical Components and Optical Properties of Toluene Secondary Organic Aerosol in Presence of Ferric Chloride Fine Particles
Abstract
1. Introduction
2. Experiments
2.1. Materials
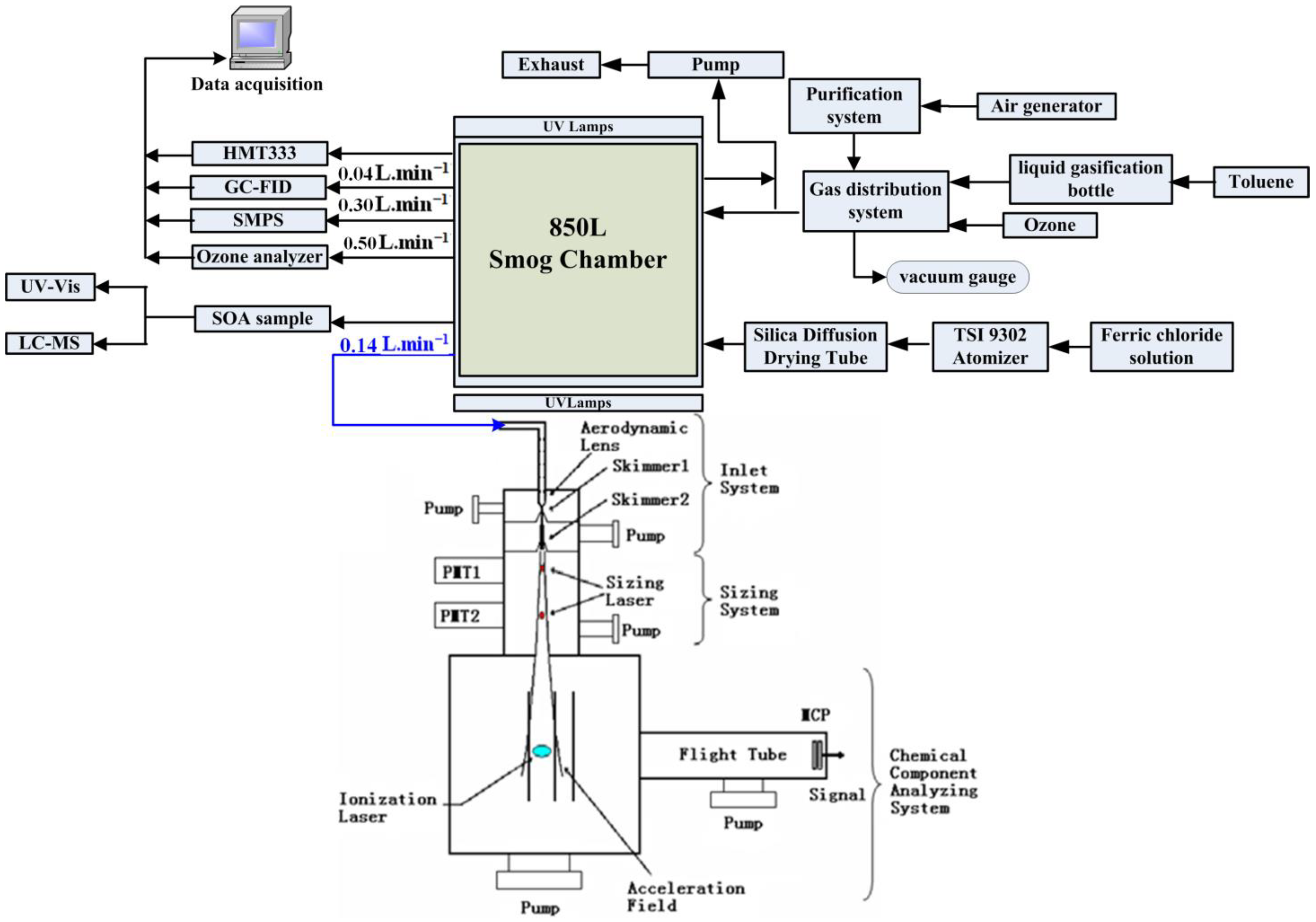
2.2. Smog Chamber Experiments
2.3. Characterization Components of Toluene SOA
2.4. Optical Characterization of Toluene SOA
3. Results
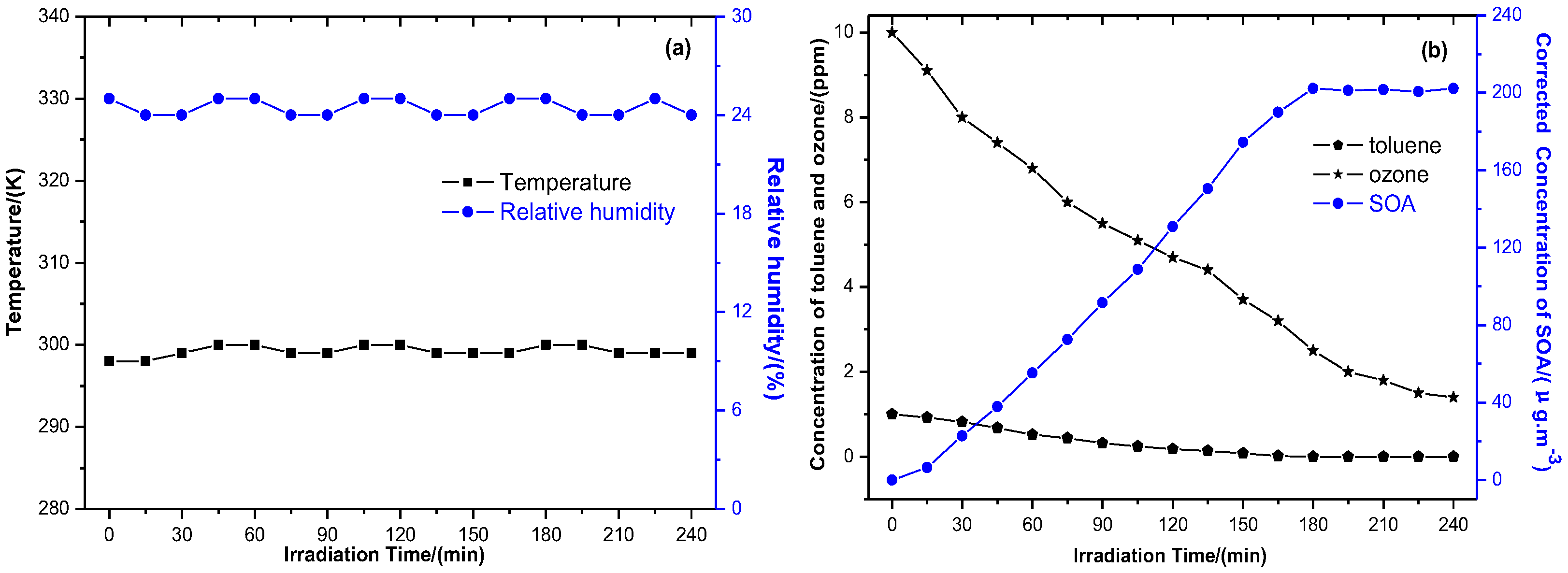
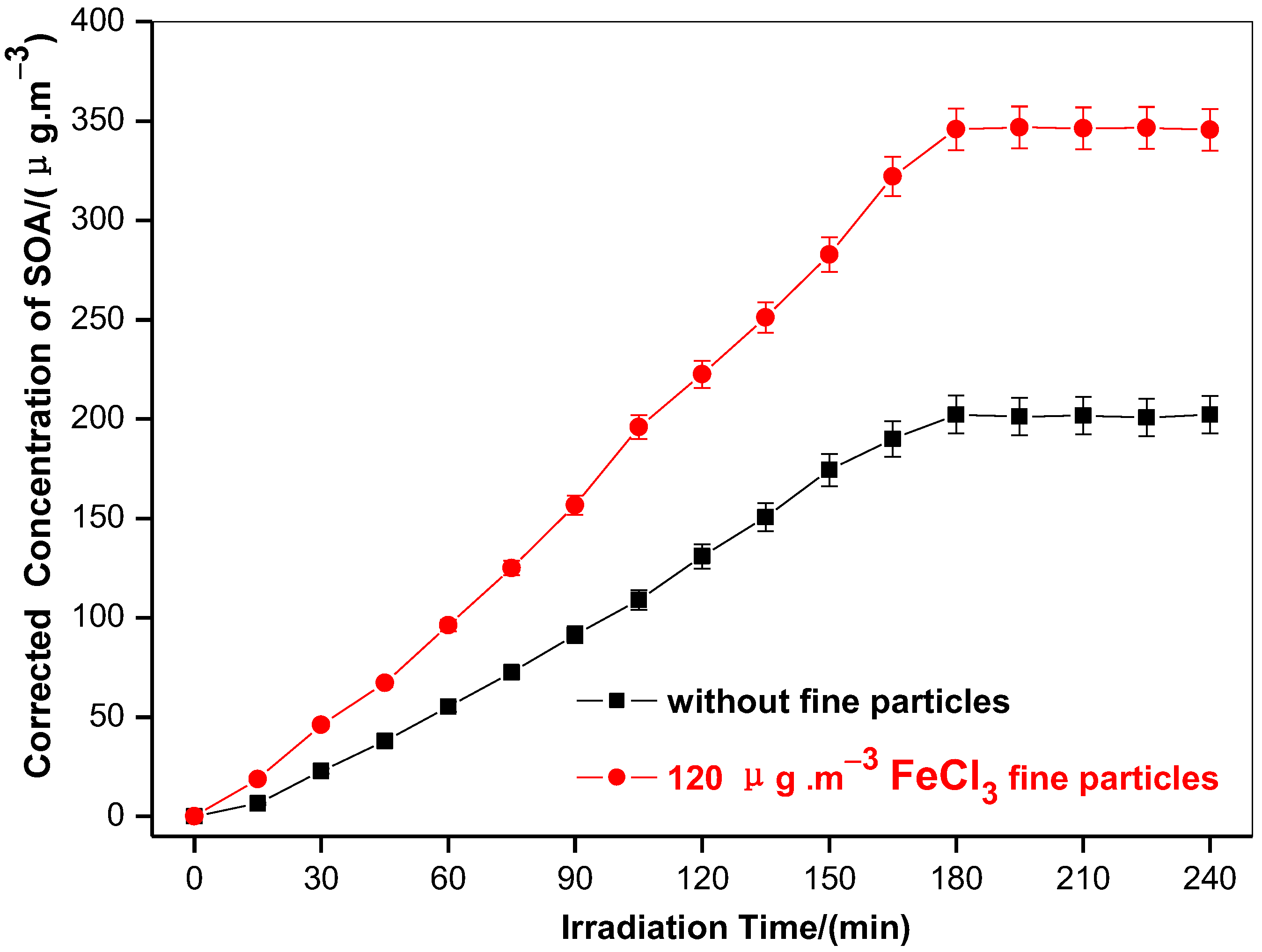
3.1. Generation of Toluene SOA with and without Ferric Chloride Fine Particles
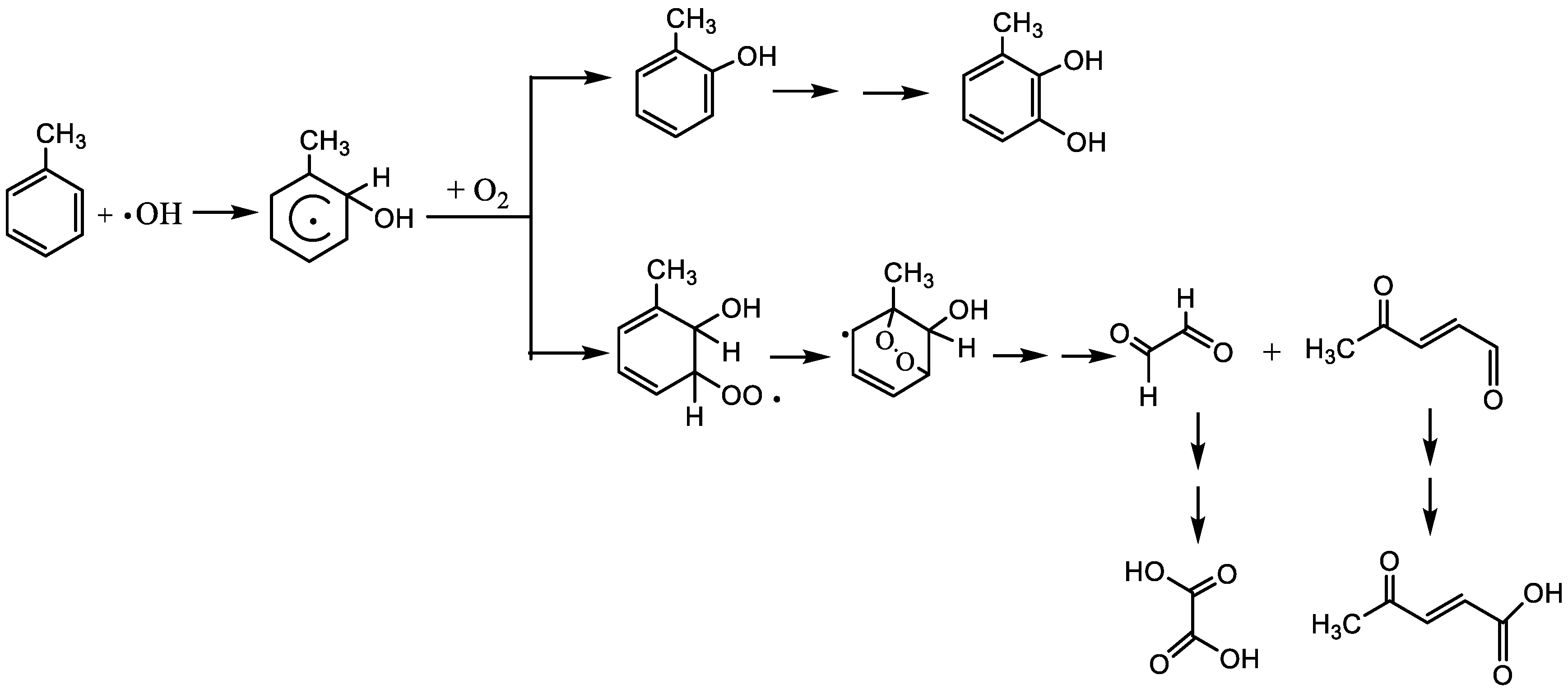
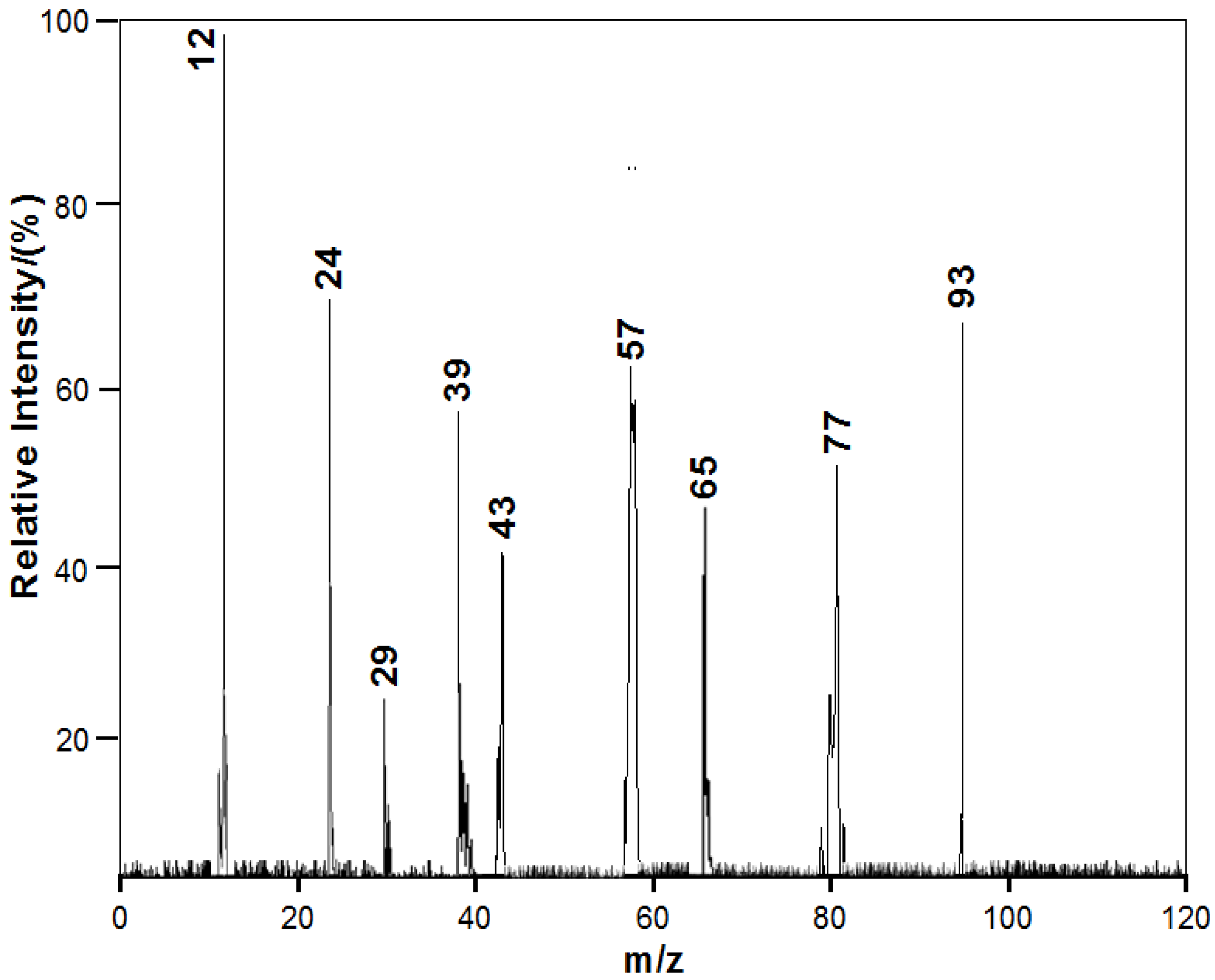
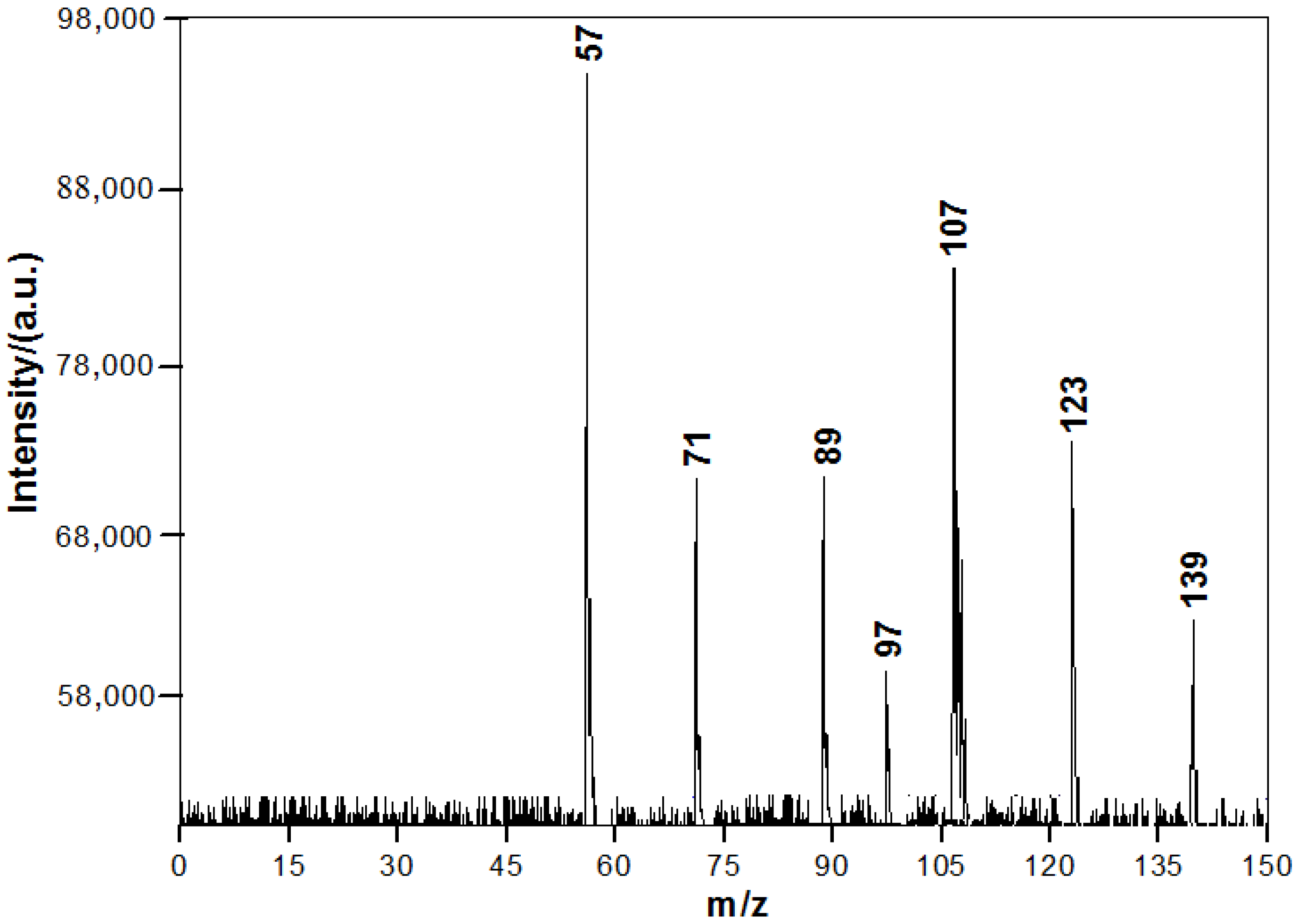
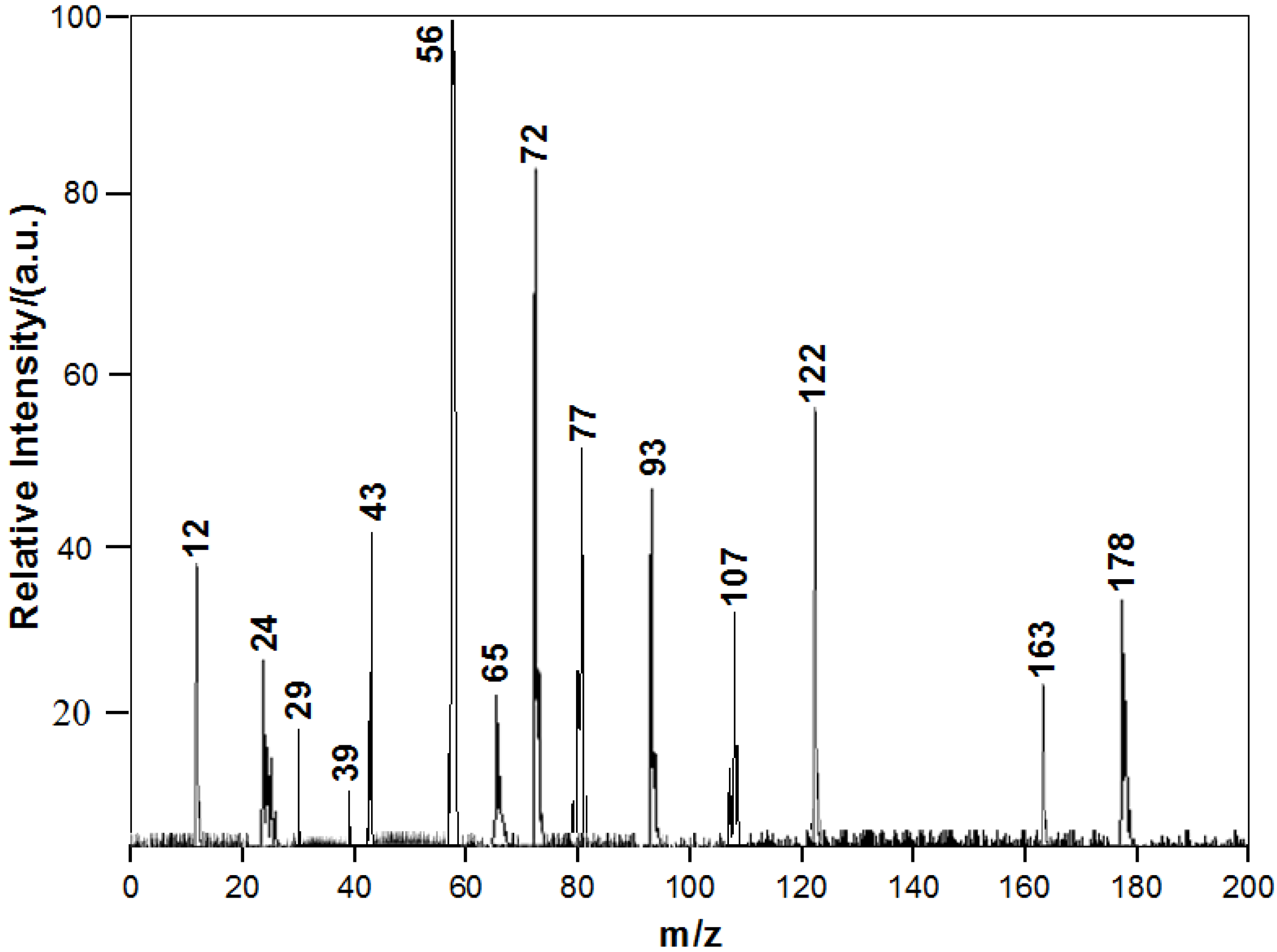
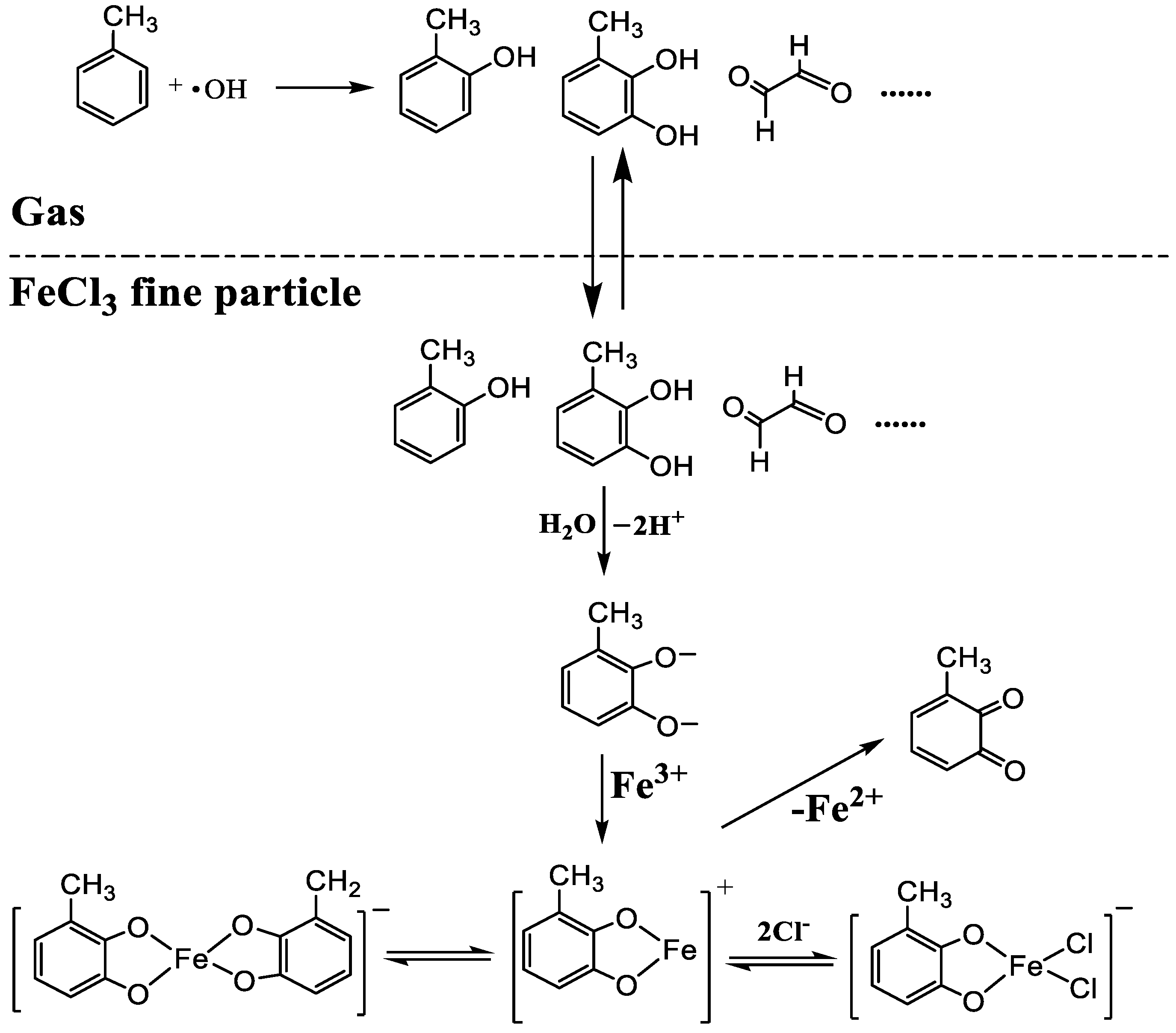
3.2. Chemical Constituents of Toluene SOA with and without Ferric Chloride Fine Particles
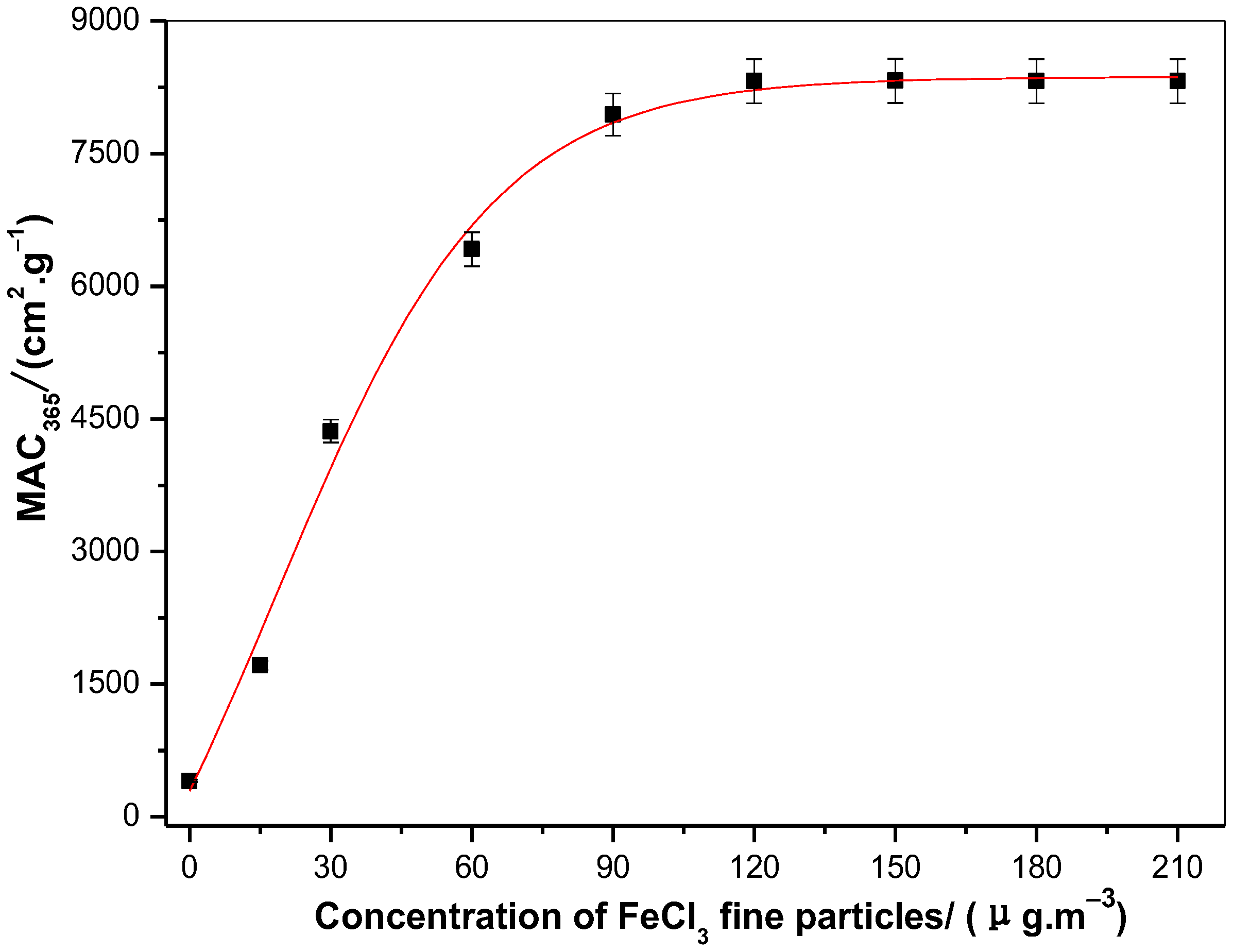
3.3. MAC of Toluene SOA under Different Concentrations of Ferric Chloride Fine Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partha, D.B.; Cassidy-Bushrow, A.E.; Huang, Y.X. Global preterm births attributable to BTEX (benzene, toluene, ethylbenzene, and xylene) exposure. Sci. Total Environ. 2022, 838, 156390. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Feng, X.S. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng.J. 2022, 435, 134825. [Google Scholar]
- Behnami, A.; Jafari, N.; Benis, K.Z.; Fanaei, F.; Abdolahnejad, A. Spatio-temporal variations, ozone and secondary organic aerosol formation potential, and health risk assessment of BTEX compounds in east of Azerbaijan Province, Iran. Urban Clim. 2023, 47, 101360. [Google Scholar] [CrossRef]
- Al-Naiema, I.M.; Offenberg, J.H.; Madler, C.J.; Lewandowski, M.; Stone, E.A. Secondary organic aerosols from aromatic hydrocarbons and their contribution to fine particulate matter in Atlanta, Georgia. Atmos. Environ. 2020, 223, 223. [Google Scholar] [CrossRef]
- Lewis, A.C. The changing face of urban air pollution. Science 2018, 359, 744–745. [Google Scholar] [CrossRef]
- Moise, T.; Flores, J.M.; Rudich, Y. Optical properties of secondary organic aerosols and their changes by chemical processes. Chem. Rev. 2015, 115, 4400–4439. [Google Scholar] [CrossRef]
- Fang, Z.; Li, C.L.; He, Q.F.; Czech, H.; Gröger, T.; Zeng, J.Q.; Fang, H.; Xiao, S.X.; Pardo, M.; Hartner, E.; et al. Secondary organic aerosols produced from photochemical oxidation of secondarily evaporated biomass burning organic gases: Chemical composition, toxicity, optical properties, and climate effect. Environ. Int. 2021, 157, 106801. [Google Scholar] [CrossRef]
- Qi, X.; Zhu, S.P.; Zhu, C.Z.; Hu, J.; Lou, S.R.; Xu, L.; Dong, J.G.; Cheng, P. Smog chamber study of the effects of NOx and NH3 on the formation of secondary organic aerosols and optical properties from photo-oxidation of toluene. Sci. Total Environ. 2020, 727, 138632. [Google Scholar] [CrossRef]
- Babar, Z.B.; Park, J.H.; Lim, H.J. Influence of NH3 on secondary organic aerosols from the ozonolysis and photooxidation of α-pinene in a flow reactor. Atmos. Environ. 2017, 164, 71–84. [Google Scholar] [CrossRef]
- Liu, S.J.; Huang, D.D.; Wang, Y.Q.; Zhang, S.; Liu, X.D.; Wu, C.; Du, W.; Wang, G.H. Synergetic effects of NH3 and NOx on the production and optical absorption of secondary organic aerosol formation from toluene photooxidation. Atmos. Chem. Phys. 2021, 21, 17759–17773. [Google Scholar] [CrossRef]
- Chowdhury, P.H.; He, Q.F.; Male, T.L.; Brune, W.H.; Rudich, Y.; Pardo, M. Exposure of lung epithelial cells to photochemically aged secondary organic aerosol shows increased toxic effects. Environ. Sci. Tech. Let. 2018, 5, 424–430. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.J.; Lee, H.Y.; Son, J.M.; Lim, H.B.; Kim, H.W.; Shin, H.J.; Ji, Y.L.; Choi, S.D. Characteristics of volatile organic compounds in the metropolitan city of Seoul, South Korea: Diurnal variation, source identification, secondary formation of organic aerosol, and health risk. Sci. Total Environ. 2022, 838, 156344. [Google Scholar] [CrossRef]
- Guan, Q.Y.; Li, F.C.; Yang, L.Q.; Zhao, R.; Yang, Y.Y.; Luo, H.P. Spatial-temporal variations and mineral dust fractions in particulate matter mass concentrations in an urban area of northwestern China. J. Environ. Manag. 2018, 222, 95–103. [Google Scholar] [CrossRef]
- Deguillaume, L.; Leriche, M.; Desboeufs, K.; Mailhot, G.; George, C.; Chaumerliac, N. Transition metals in atmospheric liquid phases: Sources, reactivity, and sensitive parameters. Chem. Rev. 2005, 105, 3388–3431. [Google Scholar] [CrossRef]
- Tang, M.J.; Cziczo, D.J.; Grassian, V.H. Interactions of water with mineral dust aerosol: Water adsorption, hygroscopicity, cloud condensation, and ice nucleation. Chem. Rev. 2016, 116, 4205–4259. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, H.B. Microscopic morphology and seasonal variation of health effect arising from heavy metals in PM2.5 and PM10: One-year measurement in a densely populated area of urban Beijing. Atmos. Res. 2018, 212, 213–226. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef]
- Tang, M.J.; Huang, X.; Lu, K.D.; Ge, M.F.; Li, Y.J.; Cheng, P.; Zhu, T.; Ding, A.J.; Zhang, Y.H.; Gligorovski, S.; et al. Heterogeneous reactions of mineral dust aerosol: Implications for tropospheric oxidation capacity. Atmos. Chem. Phys. 2017, 17, 11727–11777. [Google Scholar] [CrossRef]
- Babar, Z.B.; Park, J.H.; Kang, J.; Lim, H.J. Characterization of a smog chamber for studying formation and physicochemical properties of secondary organic aerosol. Aerosol Air Qual. Res. 2016, 16, 3102–3113. [Google Scholar] [CrossRef]
- Shao, Y.Q.; Wang, Y.; Du, M.; Voliotis, A.; Alfarra, M.R.; O’Meara, S.P.; Turner, S.F.; McFiggans, G. Characterization of the Manchester aerosol chamber facility. Atmos. Meas. Tech. 2022, 15, 539–559. [Google Scholar] [CrossRef]
- Chu, B.W.; Chen, T.Z.; Liu, Y.C.; Ma, Q.X.; Mu, Y.J.; Wang, Y.H.; Ma, J.Z.; Zhang, P.; Liu, J.; Liu, C.S.; et al. Application of smog chambers in atmospheric process studies. Natl. Sci. Rev. 2022, 9, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.W.; Hao, J.M.; Takekawa, H.; Li, J.H.; Wang, K.; Jiang, J.K. The remarkable effect of FeSO4 seed aerosols on secondary organic aerosol formation from photooxidation of α-pinene/NOx and toluene/ NOx. Atmos. Environ. 2012, 55, 26–34. [Google Scholar] [CrossRef]
- Chu, B.W.; Liu, Y.C.; Li, J.H.; Takekawa, H.; Liggio, J.; Li, S.M.; Jiang, J.K.; Hao, J.M.; He, H. Decreasing effect and mechanism of FeSO4 seed particles on secondary organic aerosol in α-pinene photooxidation. Environ. Pollut. 2014, 193, 88–93. [Google Scholar] [CrossRef]
- Chu, B.W.; Liggio, J.; Liu, Y.C.; He, H.; Takekawa, H.; Li, S.M.; Hao, J.M. Influence of metal-mediated aerosol-phase oxidation on secondary organic aerosol formation from the ozonolysis and OH-oxidation of α-pinene. Sci. Rep. 2016, 7, 40311. [Google Scholar] [CrossRef] [PubMed]
- Scheinhardt, S.; Müller, K.; Spindler, G.; Herrmann, H. Complexation of trace metals in size-segregated aerosol particles at nine sites in Germany. Atmos. Environ. 2013, 74, 102–109. [Google Scholar] [CrossRef]
- Furukawa, T.; Takahashi, Y. Oxalate metal complexes in aerosol particles: Implications for the hygroscopicity of oxalate-containing particles. Atmos. Chem. Phys. 2011, 11, 4289–4301. [Google Scholar] [CrossRef]
- Singh, D.K.; Gupta, T. Role of transition metals with water soluble organic carbon in the formation of secondary organic aerosol and metallo-organics in PM1 sampled during post monsoon and pre-winter time. J. Aerosol Sci. 2016, 94, 56–69. [Google Scholar] [CrossRef]
- Singh, D.K.; Gupta, T. Role of ammonium ion and transition metals in the formation of secondary organic aerosol and metallo-organic complex within fog processed ambient deliquescent submicron particles collected in central part of Indo-Gangetic Plain. Chemosphere 2017, 181, 725–737. [Google Scholar] [CrossRef]
- Moretti, S.; Smets, W.; Hofman, J.; Mubiana, K.V.; Oerlemans, E.; Vandenheuvel, D.; Samson, R.; Blust, R.; Lebeer, S. Human inflammatory response of endotoxin affected by particulate matter-bound transition metals. Environ. Pollut. 2019, 244, 118–126. [Google Scholar] [CrossRef]
- Kodikara, M.S.; Stranger, R.; Humphrey, M.G. Computational studies of the nonlinear optical properties of organometallic complexes. Coordin. Chem. Rev. 2019, 375, 389–409. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A. Aging of atmospheric aerosols and the role of iron in catalyzing brown carbon formation. Environ. Sci. Atmos. 2021, 1, 297–345. [Google Scholar] [CrossRef]
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Hao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 2015, 15, 10127–10147. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Shao, L.Y.; Jones, T.; Feng, X.L.; Ge, S.Y.; Yang, C.X.; Cao, Y.X.; BéruBé, K.; Zhang, D.Z. Atmospheric iron particles in PM2.5 from a subway station, Beijing, China. Atmos. Environ. 2022, 283, 119175. [Google Scholar] [CrossRef]
- Huang, M.Q.; Zhang, W.J.; Wang, Z.Y.; Fang, L.; Kong, R.H.; Shan, X.B.; Liu, F.Y.; Sheng, L.S. Mass spectrometry study of OH-initiated photooxidation of toluene. Chin. J. Chem. Phys. 2011, 24, 672–678. [Google Scholar] [CrossRef]
- Huang, M.Q.; Hao, L.Q.; Gu, X.J.; Hu, C.J.; Zhao, W.X.; Wang, Z.Y.; Fang, L.; Zhang, W.J. Effects of inorganic seed aerosols on the growth and chemical composition of secondary organic aerosol formed from OH-initiated oxidation of toluene. J. Atmos. Chem. 2013, 70, 151–164. [Google Scholar] [CrossRef]
- Lu, T.T.; Huang, M.Q.; Zhao, W.X.; Hu, C.J.; Gu, X.J.; Zhang, W.J. Influence of ammonium sulfate seed particle on optics and compositions of toluene derived organic aerosol in photochemistry. Atmosphere 2020, 11, 961. [Google Scholar] [CrossRef]
- Huang, M.Q.; Hao, L.Q.; Cai, S.Y.; Gu, X.J.; Zhang, W.X.; Hu, C.J.; Wang, Z.Y.; Fang, L.; Zhang, W.J. Effects of inorganic seed aerosols on the particulate products of aged 1,3,5-trimethylbenzene secondary organic aerosol. Atmos. Environ. 2017, 152, 490–502. [Google Scholar] [CrossRef]
- Assaf, E.; Fittschen, C. Cross section of OH radical overtone transition near 7028 cm–1 and measurement of the rate constant of the reaction of OH with HO2 radicals. J. Phys. Chem. A 2016, 120, 7051–7059. [Google Scholar] [CrossRef]
- Carlton, A.G.; Turpin, B.; Altieri, K.E.; Seitzinger, S.; Reff, A.; Lim, H.-J.; Ervens, B. Atmospheric oxalic acid and SOA production from glyoxal: Results of aqueous photooxidation experiments. Atmos. Environ. 2007, 41, 7588–7602. [Google Scholar] [CrossRef]
- Updyke, K.M.; Nguyen, T.B.; Nizkorodov, S.A. Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors. Atmos. Environ. 2012, 63, 22–31. [Google Scholar] [CrossRef]
- Powelson, M.H.; Espelien, B.M.; Hawkins, L.N.; Galloway, M.M.; De Haan, D.O. Brown carbon formation by aqueous-phase carbonyl compound reactions with amines and ammonium sulfate. Environ. Sci. Technol. 2014, 48, 985–993. [Google Scholar] [CrossRef]
- Lin, X.; Huang, M.Q.; Lu, T.T.; Zhao, W.X.; Hu, C.J.; Gu, X.J.; Zhang, W.J. Characterization of imidazole compounds in aqueous secondary organic aerosol generated from evaporation of droplets containing pyruvaldehyde and inorganic ammonium. Atmosphere 2022, 13, 970. [Google Scholar] [CrossRef]
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Chemistry of atmospheric brown carbon. Chem. Rev. 2015, 115, 4335–4382. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, E.N.; Andersson, A.; Han, J.; Lee, M.; Gustafsson, Ö. Sources and light absorption of water-soluble organic carbon aerosols in the outflow from northern China. Atmos. Chem. Phys. 2014, 14, 1413–1422. [Google Scholar] [CrossRef]
- Liu, J.M.; Scheuer, E.; Dibb, J.; Ziemba, L.D.; Thornhill, K.L.; Anderson, B.E.; Wisthaler, A.; Mikoviny, T.; Devi, J.J.; Bergin, M.; et al. Brown carbon in the continental troposphere. Geophys. Res. Lett. 2014, 41, 2191–2195. [Google Scholar] [CrossRef]
- Liu, J.; Bergin, M.; Guo, H.; King, L.; Kotra, N.; Edgerton, E.; Weber, R.J. Size-resolved measurements of brown carbon in water and methanol extracts and estimates of their contribution to ambient fine-particle light absorption. Atmos. Chem. Phys. 2013, 13, 12389–12404. [Google Scholar] [CrossRef]
- Shetty, N.J.; Pandey, A.; Baker, S.; Hao, W.M.; Chakrabarty, R.K. Measuring light absorption by freshly emitted organic aerosols: Optical artifacts in traditional solvent-extraction- based methods. Atmos. Chem. Phys. 2019, 19, 8817–8830. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, X.D.; Wang, Y.Q.; Zhang, S.; Wu, C.; Du, W.; Wang, G.H. Effect of NOx and RH on the secondary organic aerosol formation from toluene photooxidation. J. Environ. Sci. China 2022, 114, 1–9. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhao, J.Y.; Wang, Y.; Seinfeld, J.H.; Zhang, R.Y. Multigeneration production of secondary organic aerosol from toluene photooxidation. Environ. Sci. Technol. 2021, 55, 8592–8603. [Google Scholar] [CrossRef]
- White, S.J.; Jamie, I.M.; Angove, D.E. Chemical characterisation of semi-volatile and aerosol compounds from the photooxidation of toluene and NOx. Atmos. Environ. 2014, 83, 237–244. [Google Scholar] [CrossRef]
- Dong, Z.K.; Tang, R.Y.; Liu, H.F.; Zhang, Q.Z.; Zong, W.S.; Cheng, J.M.; Shi, X.L. The formation mechanism of highly oxygenated organic molecules produced by toluene in the urban atmosphere. Atmos. Environ. 2023, 295, 119555. [Google Scholar] [CrossRef]
- Ji, Y.M.; Zhao, J.; Terazono, H.; Terazono, H.; Misawa, K.; Levitt, N.; Li, Y.X.; Lin, Y.; Peng, J.F.; Wang, Y.; et al. Reassessing the atmospheric oxidation mechanism of toluene. Proc. Natl. Acad. Sci. USA 2017, 114, 8169–8174. [Google Scholar] [CrossRef]
- Suh, I.; Zhang, R.; Molina, L.T.; Molina, M.J. Oxidation mechanism of aromatic peroxy and bicyclic radicals from OH-toluene reactions. J. Am. Chem. Soc. 2003, 125, 12655–12665. [Google Scholar] [CrossRef]
- Sato, K.; Takami, A.; Kato, Y.; Seta, T.; Fujitani, Y.; Hikida, T.; Shimono, A.; Imamura, T. AMS and LC/MS analyses of SOA from the photooxidation of benzene and 1,3,5-trimethyl benzene in the presence of NOx: Effects of chemical structure on SOA aging. Atmos. Chem. Phys. 2012, 12, 4667–4682. [Google Scholar] [CrossRef]
- Sato, K.; Takami, A.; Isozaki, T.; Hikida, T.; Shimono, A.; Imamura, T. Mass spectrometric study of secondary organic aerosol formed from the photo-oxidation of aromatic hydrocarbons. Atmos. Environ. 2010, 44, 1080–1087. [Google Scholar] [CrossRef]
- Marković, S.; Tošović, J. Application of time-dependent density functional and natural bond orbital theories to the UV–vis absorption spectra of some phenolic compounds. J. Phys. Chem. A 2015, 119, 9352–9362. [Google Scholar] [CrossRef]
- Arndt, J.; Deboudt, K.; Anderson, A.; Blondel, A.; Eliet, S.; Flament, P.; Fourmentin, M.; Healy, R.M.; Savary, V.; Setyan, A.; et al. Scanning electron microscopy-energy dispersive X-ray spectrometry (SEM-EDX) and aerosol time-of-flight mass spectrometry (ATOFMS) single particle analysis of metallurgy plant emissions. Environ. Pollut. 2016, 210, 9–17. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Mane, M.; Khuroo, M.A.; Peerzada, G.M. Computational survey of ligand properties on iron(III)–iron(II) redox potential: Exploring natural attenuation of nitroaromatic compounds. Monatsh. Chem. 2017, 148, 655–668. [Google Scholar] [CrossRef]
- Albarran, G.; Boggess, W.; Rassolov, V.; Schuler, R.H. Absorption spectrum, mass spectrometric properties, and electronic structure of 1,2-benzoquinone. J. Phys. Chem. A 2010, 114, 7470–7478. [Google Scholar] [CrossRef]
- Powell, H.K.J.; Taylor, M.C. Interactions of iron(II) and iron(III) with gallic acid and its homologues: A potentiormetric and spectrophotometric study. Aust. J. Chem. 1982, 35, 739–756. [Google Scholar] [CrossRef]
- Slikboer, S.; Grandy, L.; Blair, S.L.; Nizkorodov, S.A.; Smith, R.W.; Al-Abadleh, H.A. Formation of light absorbing soluble secondary organics and insoluble polymeric particles from the dark reaction of catechol and guaiacol with Fe(III). Environ. Sci. Technol. 2015, 49, 7793–7802. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Zhang, J.; Lv, G.C.; George, C.; Herrmann, H.; Fu, H.B.; Li, D.; Zhang, L.W.; Sun, X.M.; Sun, H.; et al. Complexation of Fe(III)/Catechols in atmospheric aqueous phase and the consequent cytotoxicity assessment in human bronchial epithelial cells (BEAS-2B). Ecotoxicol. Environ. Saf. 2020, 202, 110898. [Google Scholar] [CrossRef] [PubMed]
- Dhulipala, S.V.; Bhandari, S.; Hildebrandt Ruiz, L. Formation of oxidized organic compounds from Cl-initiated oxidation of toluene. Atmos. Environ. 2019, 199, 265–273. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Huang, M.; Hu, H.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Characterization of Chemical Components and Optical Properties of Toluene Secondary Organic Aerosol in Presence of Ferric Chloride Fine Particles. Atmosphere 2023, 14, 1075. https://doi.org/10.3390/atmos14071075
Wang W, Huang M, Hu H, Zhao W, Hu C, Gu X, Zhang W. Characterization of Chemical Components and Optical Properties of Toluene Secondary Organic Aerosol in Presence of Ferric Chloride Fine Particles. Atmosphere. 2023; 14(7):1075. https://doi.org/10.3390/atmos14071075
Chicago/Turabian StyleWang, Weichao, Mingqiang Huang, Huimin Hu, Weixiong Zhao, Changjin Hu, Xuejun Gu, and Weijun Zhang. 2023. "Characterization of Chemical Components and Optical Properties of Toluene Secondary Organic Aerosol in Presence of Ferric Chloride Fine Particles" Atmosphere 14, no. 7: 1075. https://doi.org/10.3390/atmos14071075
APA StyleWang, W., Huang, M., Hu, H., Zhao, W., Hu, C., Gu, X., & Zhang, W. (2023). Characterization of Chemical Components and Optical Properties of Toluene Secondary Organic Aerosol in Presence of Ferric Chloride Fine Particles. Atmosphere, 14(7), 1075. https://doi.org/10.3390/atmos14071075





