Particle Size Matters: Distribution, Source, and Seasonality Characteristics of Airborne and Pathogenic Bacteria in Wastewater Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the WWTPs and Sampling Sites
2.2. Bioaerosols and Wastewater Collection
2.3. DNA Extraction
2.4. Amplicon Sequencing and Bioinformatic Analysis
2.5. Statistical Analysis
3. Results and Discussion
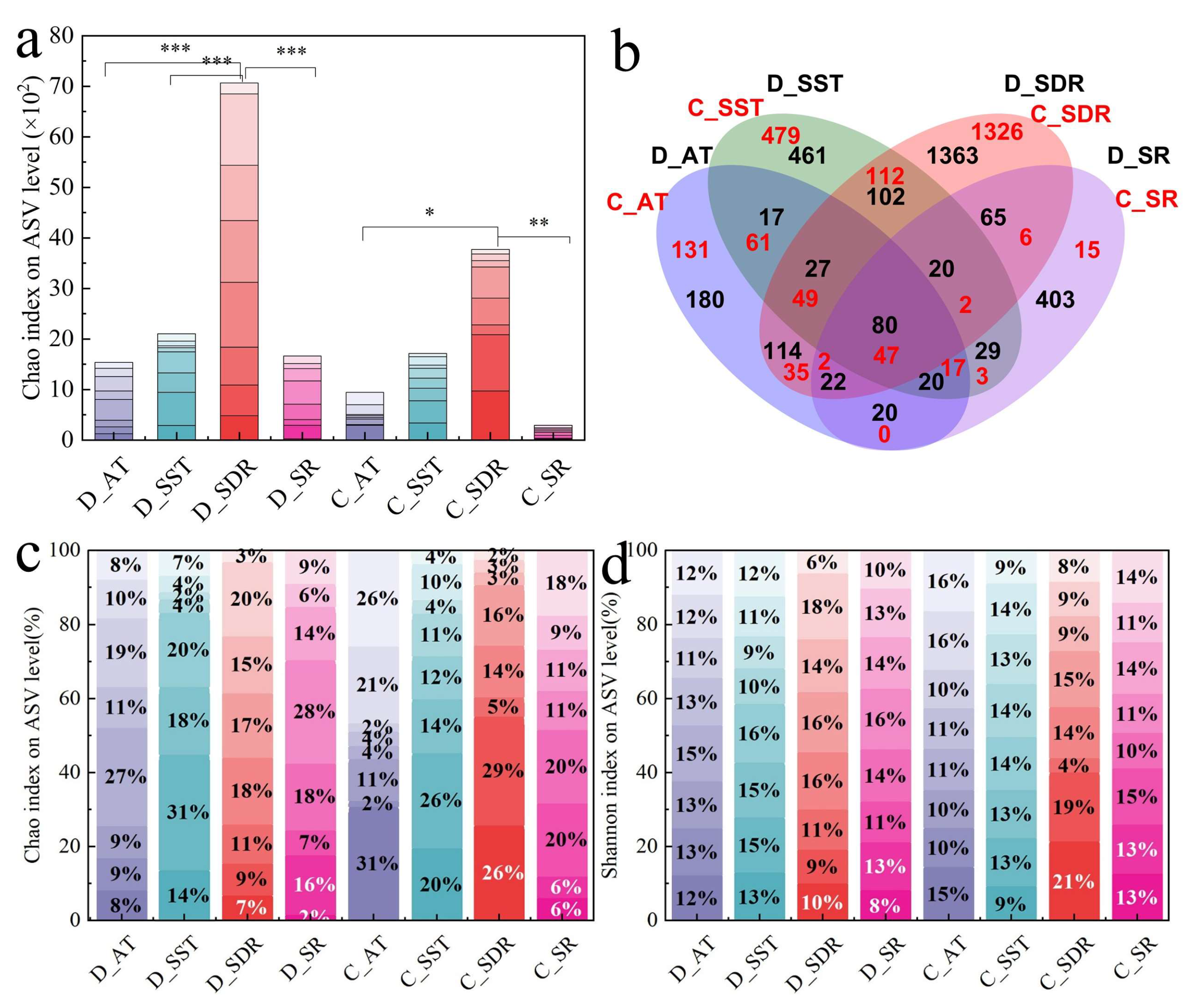
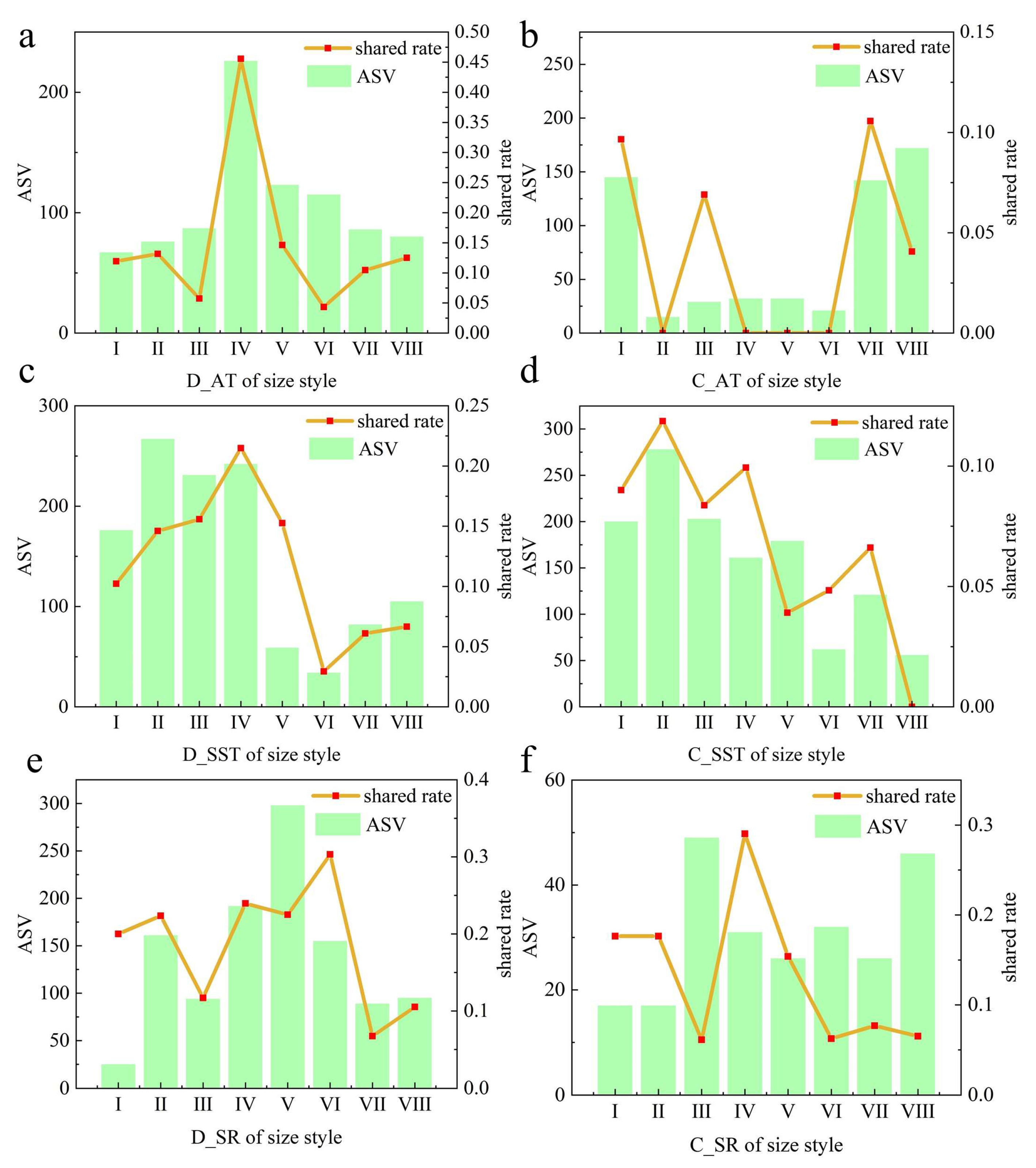
3.1. Emission Levels and Size Distributions of Bacteria Aerosols
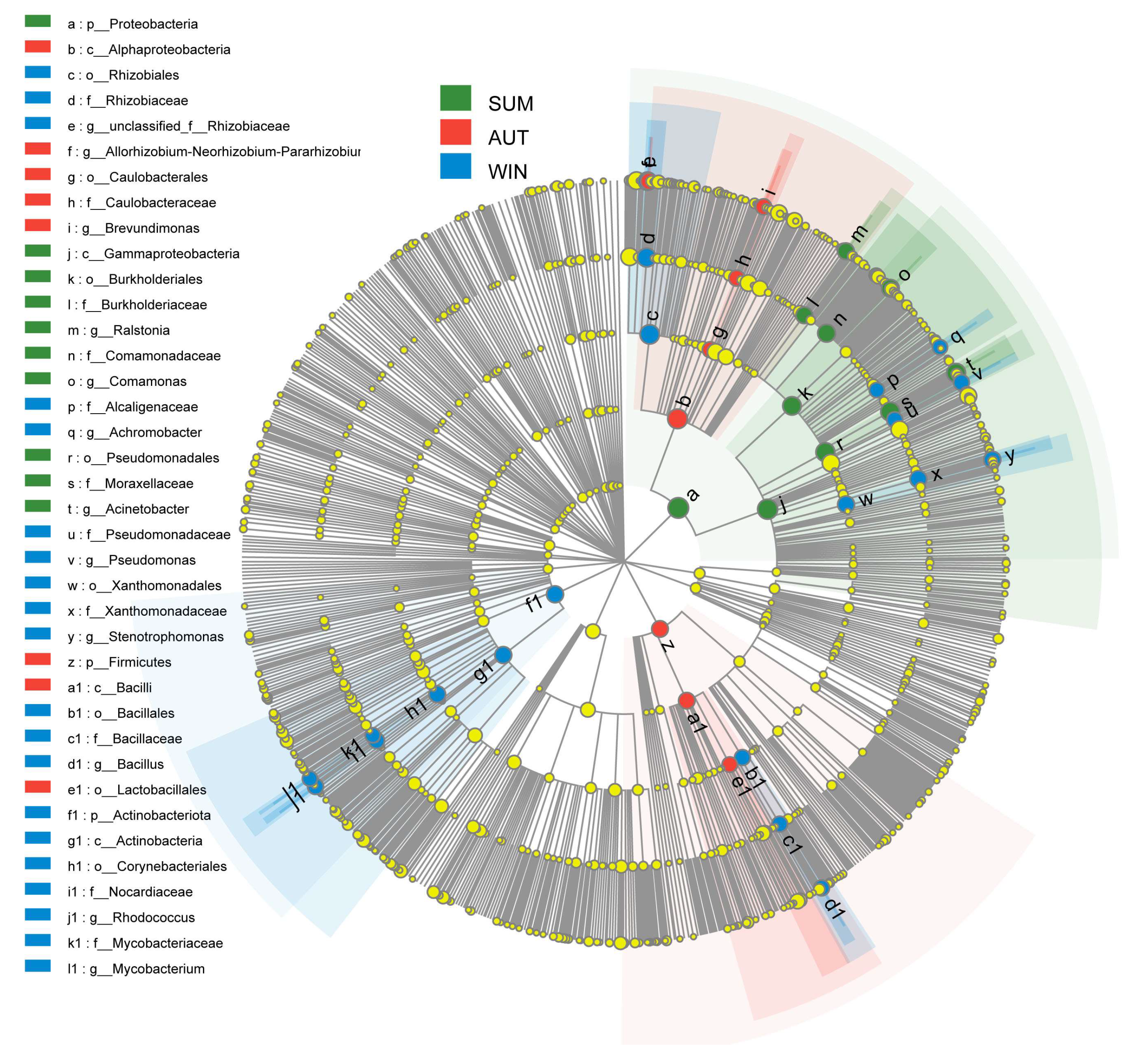
3.2. Bacterial Aerosols Community Composition
3.3. Contribution of Water Sources to Bacterial Aerosols
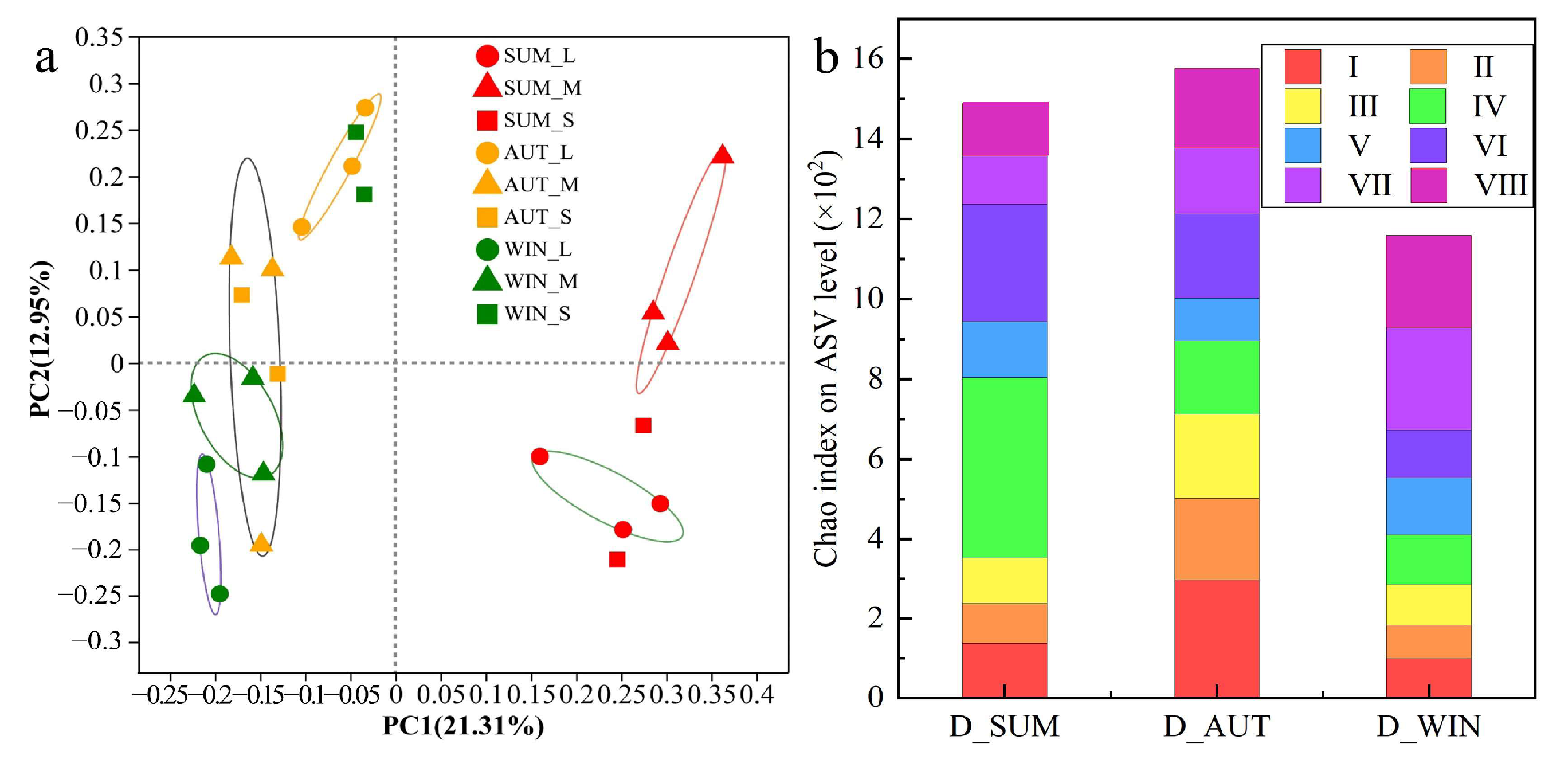
3.4. Seasonal Effects of Bacterial Aerosols on Aeration Tank
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Yao, M.; Hwang, J.; Wang, C. Bioaerosol: A Key Vessel between Environment and Health. Front. Environ. Sci. Eng. 2021, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E.; Pawlak, M. Health Risks Associated with Occupational Exposure to Biological Air Pollutants Occurring during the Processing of Biomass for Energy Purposes: A Case Study. Energies 2021, 14, 2086. [Google Scholar] [CrossRef]
- Schlosser, O. Bioaerosols and health: Current knowledge and gaps in the field of waste management. Detritus 2019, 5, 111–125. [Google Scholar] [CrossRef]
- Adhikari, A.; Reponen, T.; Grinshpun, S.A.; Martuzevicius, D.; LeMasters, G. Correlation of ambient inhalable bioaerosols with particulate matter and ozone: A two-year study. Environ. Pollut. 2006, 140, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E. The Dose of Fungal Aerosol Inhaled by Workers in a Waste-Sorting Plant in Poland: A Case Study. Int. J. Environ. Res. Public Health 2020, 17, 177. [Google Scholar] [CrossRef]
- Madsen, A.M.; Alwan, T.; Ørberg, A.; Uhrbrand, K.; Jørgensen, M.B. Waste Workers’ Exposure to Airborne Fungal and Bacterial Species in the Truck Cab and During Waste Collection. Ann. Occup. Hyg. 2016, 60, 651–668. [Google Scholar] [CrossRef]
- Han, Y.; Yang, T.; Xu, G.; Li, L.; Liu, J. Characteristics and interactions of bioaerosol microorganisms from wastewater treatment plants. J. Hazard. Mater. 2020, 391, 122256. [Google Scholar] [CrossRef]
- Kataki, S.; Patowary, R.; Chatterjee, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Kamboj, D.V. Bioaerosolization and pathogen transmission in wastewater treatment plants: Microbial composition, emission rate, factors affecting and control measures. Chemosphere 2022, 287, 132180. [Google Scholar] [CrossRef]
- Singh, N.K.; Sanghvi, G.; Yadav, M.; Padhiyar, H.; Thanki, A. A state-of-the-art review on WWTP associated bioaerosols: Microbial diversity, potential emission stages, dispersion factors, and control strategies. J. Hazard. Mater. 2021, 410, 124686. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Bi, X.; Cheng, L.; Zheng, X.; Wang, X.; Zhou, X. Submicron aerosols share potential pathogens and antibiotic resistomes with wastewater or sludge. Sci. Total Environ. 2022, 821, 153521. [Google Scholar] [CrossRef]
- Espigares, E.; Bueno, A.; Espigares, M.; Gálvez, R. Isolation of Salmonella serotypes in wastewater and effluent: Effect of treatment and potential risk. Int. J. Hyg. Environ. Health 2006, 209, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, L.; Pietronave, S.; Rinaldi, M.; Martinotti, M.G. Site-related airborne biological hazard and seasonal variations in two wastewater treatment plants. Water Res. 2006, 40, 1985–1994. [Google Scholar] [CrossRef]
- Han, Y.; Yang, T.; Yan, X.; Li, L.; Liu, J. Effect of aeration mode on aerosol characteristics from the same wastewater treatment plant. Water Res. 2020, 170, 115324. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, K.; Yang, T.; Zhang, M.; Li, L. Bioaerosols emission and exposure risk of a wastewater treatment plant with A2O treatment process. Ecotoxicol. Environ. Saf. 2019, 169, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bruni, E.; Simonetti, G.; Bovone, B.; Casagrande, C.; Castellani, F.; Riccardi, C.; Pomata, D.; Di Filippo, P.; Federici, E.; Buiarelli, F.; et al. Evaluation of bioaerosol bacterial components of a wastewater treatment plant through an integrate approach and in vivo assessment. Int. J. Environ. Res. Public Health 2020, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Szyłak-Szydłowski, M.; Kulig, A.; Miaśkiewicz-Pęska, E. Seasonal changes in the concentrations of airborne bacteria emitted from a large wastewater treatment plant. Int. Biodeterior. Biodegrad. 2016, 115, 11–16. [Google Scholar] [CrossRef]
- Han, Y.; Li, L.; Wang, Y.; Ma, J.; Li, P.; Han, C.; Liu, J. Composition, dispersion, and health risks of bioaerosols in wastewater treatment plants: A review. Front. Environ. Sci. Eng. 2020, 15, 38. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Li, L.; Xu, G.; Liu, J.; Yang, K. Bacterial population and chemicals in bioaerosols from indoor environment: Sludge dewatering houses in nine municipal wastewater treatment plants. Sci. Total Environ. 2018, 618, 469–478. [Google Scholar] [CrossRef]
- Malakootian, M.; Radhakrishna, N.; Mazandarany, M.P.; Hossaini, H. Bacterial-aerosol emission from wastewater treatment plant. Desalin. Water Treat. 2013, 51, 4478–4488. [Google Scholar] [CrossRef]
- Niazi, S.; Hassanvand, M.S.; Mahvi, A.H.; Nabizadeh, R.; Alimohammadi, M.; Nabavi, S.; Faridi, S.; Dehghani, A.; Hoseini, M.; Moradi-Joo, M.; et al. Assessment of bioaerosol contamination (bacteria and fungi) in the largest urban wastewater treatment plant in the Middle East. Environ. Sci. Pollut. Res. Int. 2015, 22, 16014–16021. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Han, Y.; Liu, J.; Yang, K. Intestinal bacteria in bioaerosols and factors affecting their survival in two oxidation ditch process municipal wastewater treatment plants located in different regions. Ecotoxicol. Environ. Saf. 2018, 154, 162–170. [Google Scholar] [CrossRef]
- Yang, K.; Li, L.; Wang, Y.; Xue, S.; Han, Y.; Liu, J. Airborne bacteria in a wastewater treatment plant: Emission characterization, source analysis and health risk assessment. Water Res. 2019, 149, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Han, Y.; Liu, J.; Li, L. Aerosols from a wastewater treatment plant using oxidation ditch process: Characteristics, source apportionment, and exposure risks. Environ. Pollut. 2019, 250, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Zhang, H.F.; Qiu, X.H.; Zhang, Y.; Wang, H. Dispersion and Risk Assessment of Bacterial Aerosols Emitted from Rotating-Brush Aerator during Summer in a Wastewater Treatment Plant of Xi’an, China. Aerosol Air Qual. Res. 2013, 13, 1807–1814. [Google Scholar] [CrossRef]
- Yamamoto, N.; Bibby, K.; Qian, J.; Hospodsky, D.; Rismani-Yazdi, H.; Nazaroff, W.W.; Peccia, J. Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J. 2012, 6, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Nel, A. Air Pollution-Related Illness: Effects of Particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef]
- Visser, S.; Slowik, J.G.; Furger, M.; Young, D.E.; Williams, P.I.; Detournay, J.F.; Halios, C.H.; Fleming, Z.L.; Baltensperger, U.; Prévôt, A.S. Kerb and urban increment of highly time-resolved trace elements in PM10, PM2.5 and PM1.0 winter aerosol in london during clearflo 2012. Atmos. Chem. Phys. 2015, 15, 2367–2386. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, P.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Optimized DNA extraction and metagenomic sequencing of airborne microbial communities. Nat. Protoc. 2015, 10, 768–779. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.; Bier, R.; Li, Z.; Langenheder, S.; Gorokhova, E.; Sobek, A. Association between Aquatic Micropollutant Dissipation and River Sediment Bacterial Communities. Environ. Sci. Technol. 2020, 54, 14380–14392. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Liu, J.; Zang, N.; Liu, X.; Tian, H.; Kang, X. Bioaerosols dispersed from a typical wastewater treatment plant with a membrane bioreactor: Emission characteristics, source analysis and health risk. Process Saf. Environ. Prot. 2022, 160, 976–987. [Google Scholar] [CrossRef]
- Humbal, C.; Gautam, S.; Trivedi, U. A review on recent progress in observations, and health effects of bioaerosols. Environ. Int. 2018, 118, 189–193. [Google Scholar] [CrossRef]
- Uhrbrand, K.; Schultz, A.C.; Koivisto, A.J.; Nielsen, U.; Madsen, A.M. Assessment of airborne bacteria and noroviruses in air emission from a new highly-advanced hospital wastewater treatment plant. Water Res. 2017, 112, 110–119. [Google Scholar] [CrossRef]
- Gao, M.; Jia, R.; Qiu, T.; Han, M.; Wang, X. Size-related bacterial diversity and tetracycline resistance gene abundance in the air of concentrated poultry feeding operations. Environ. Pollut. 2017, 220, 1342–1348. [Google Scholar] [CrossRef]
- Ding, W.; Li, L.; Han, Y.; Liu, J.; Liu, J. Site-related and seasonal variation of bioaerosol emission in an indoor wastewater treatment station: Level, characteristics of particle size, and microbial structure. Aerobiologia 2015, 32, 211–224. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Filipkowska, Z.; Gotkowska-Plachta, A.; Janczukowicz, W.; Dixon, B.; Czułowska, M. Determination of emitted airborne microorganisms from a BIO-PAK wastewater treatment plant. Water Res. 2009, 43, 2841–2851. [Google Scholar] [CrossRef]
- Liu, M.; Nobu, M.K.; Ren, J.; Jin, X.; Hong, G.; Yao, H. Bacterial compositions in inhalable particulate matters from indoor and outdoor wastewater treatment processes. J. Hazard. Mater. 2020, 385, 121515. [Google Scholar] [CrossRef]
- Korzeniewska, E. Emission of bacteria and fungi in the air from wastewater treatment plants—A review. FBS 2011, 3, 393–407. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Han, Y.; Liu, J.; Wang, X.; Yan, X.; Liu, J. Linking aerosol characteristics of size distributions, core potential pathogens and toxic metal(loid)s to wastewater treatment process. Environ. Pollut. 2020, 264, 114741. [Google Scholar] [CrossRef]
- Karra, S.; Katsivela, E. Microorganisms in bioaerosol emissions from wastewater treatment plants during summer at a Mediterranean site. Water Res. 2007, 41, 1355–1365. [Google Scholar] [CrossRef]
- Zhang, M.; Zuo, J.; Yu, X.; Shi, X.; Chen, L.; Li, Z. Quantification of multi-antibiotic resistant opportunistic pathogenic bacteria in bioaerosols in and around a pharmaceutical wastewater treatment plant. J. Environ. Sci. 2018, 72, 53–63. [Google Scholar] [CrossRef]
- Fathi, S.; Hajizadeh, Y.; Nikaeen, M.; Gorbani, M. Assessment of microbial aerosol emissions in an urban wastewater treatment plant operated with activated sludge process. Aerobiologia 2017, 33, 507–515. [Google Scholar] [CrossRef]
- Vítězová, M.; Vítěz, T.; Mlejnková, H.; Lošák, T. Microbial contamination of the air at the wastewater treatment plant. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 60, 233–240. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Ko, H.-J.; Kim, D. Assessment of Airborne Microorganisms in a Swine Wastewater Treatment Plant. Environ. Eng. Res. 2012, 17, 211–216. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Zhang, Z.; Huo, Y.; Wang, X.; Wang, Y.; Wu, J.; Huo, M. Particle Size Matters: Distribution, Source, and Seasonality Characteristics of Airborne and Pathogenic Bacteria in Wastewater Treatment Plants. Atmosphere 2023, 14, 465. https://doi.org/10.3390/atmos14030465
Wan J, Zhang Z, Huo Y, Wang X, Wang Y, Wu J, Huo M. Particle Size Matters: Distribution, Source, and Seasonality Characteristics of Airborne and Pathogenic Bacteria in Wastewater Treatment Plants. Atmosphere. 2023; 14(3):465. https://doi.org/10.3390/atmos14030465
Chicago/Turabian StyleWan, Jianan, Zhiruo Zhang, Yang Huo, Xianze Wang, Yifan Wang, Jinghui Wu, and Mingxin Huo. 2023. "Particle Size Matters: Distribution, Source, and Seasonality Characteristics of Airborne and Pathogenic Bacteria in Wastewater Treatment Plants" Atmosphere 14, no. 3: 465. https://doi.org/10.3390/atmos14030465
APA StyleWan, J., Zhang, Z., Huo, Y., Wang, X., Wang, Y., Wu, J., & Huo, M. (2023). Particle Size Matters: Distribution, Source, and Seasonality Characteristics of Airborne and Pathogenic Bacteria in Wastewater Treatment Plants. Atmosphere, 14(3), 465. https://doi.org/10.3390/atmos14030465








