An Overview of the Automated and On-Line Systems to Assess the Oxidative Potential of Particulate Matter
Abstract
1. Introduction
1.1. Particulate Matter and Induced Oxidative Stress
1.2. OP of PM as Target for Rapid Environmental Evaluation
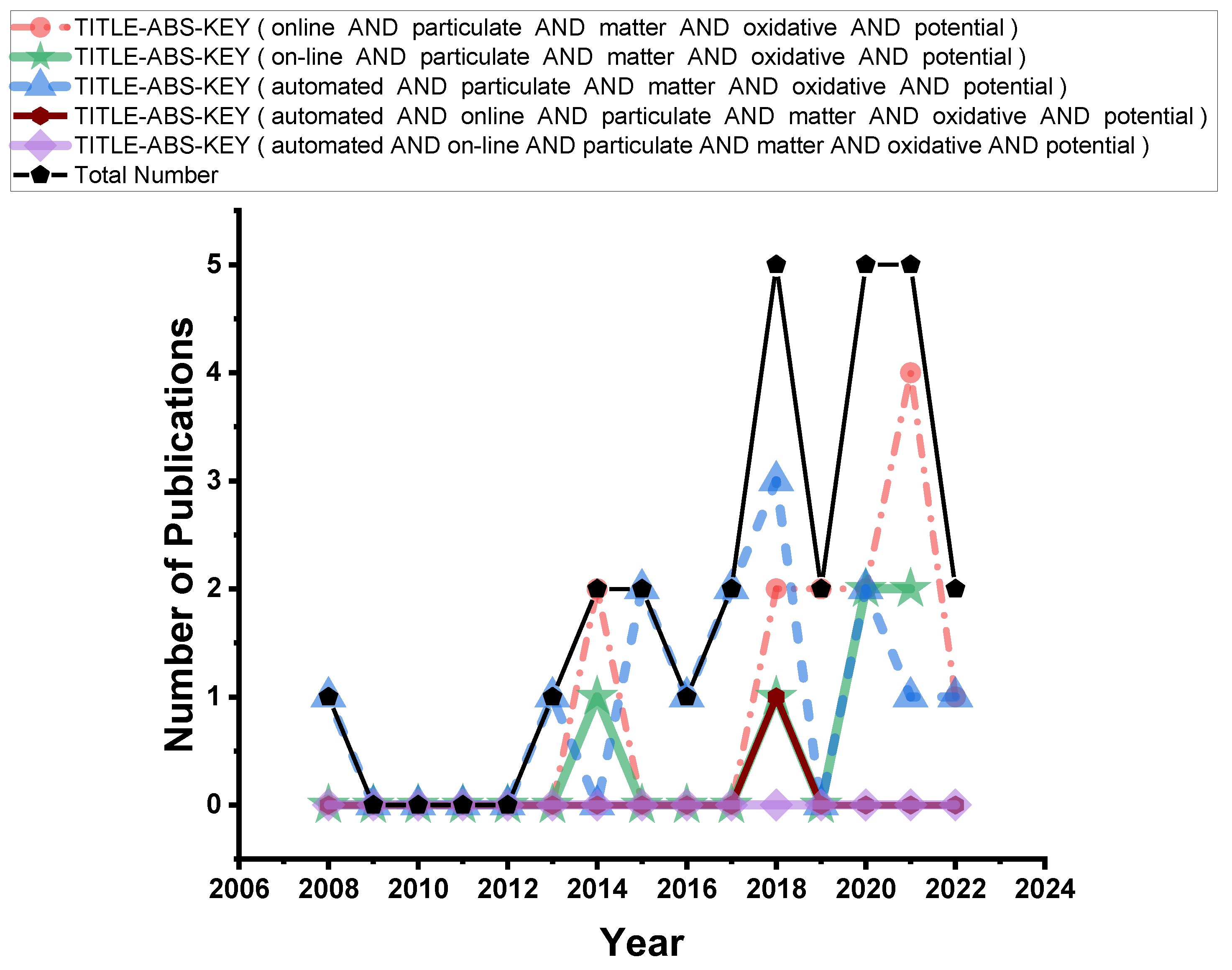
1.3. Literature Overview of Online Methods
2. Automated and OnLine Analysis Systems Based on DCFH
2.1. DCFH Method to Assess OP
2.2. On-Field Methods for PM Analysis
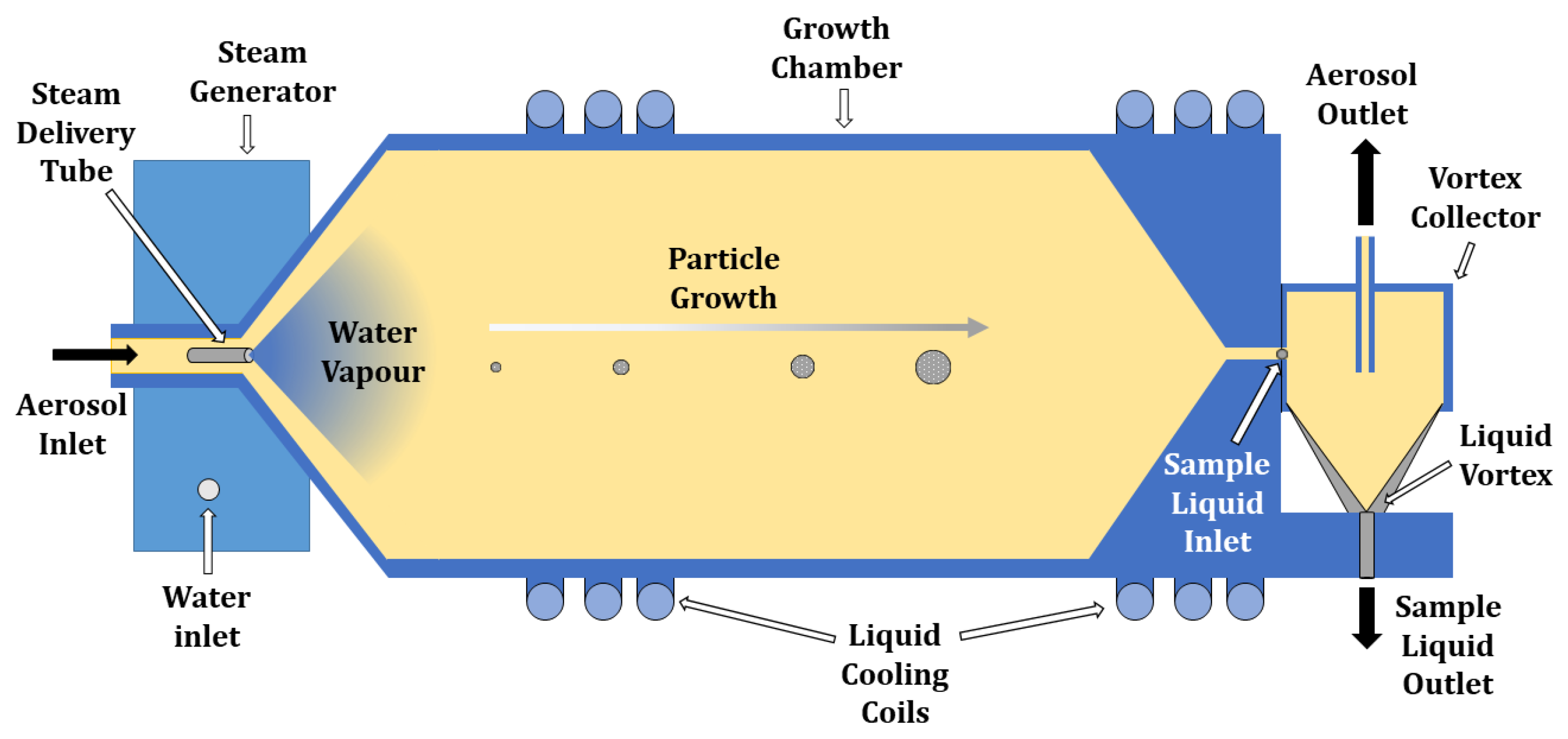
3. The Instrument Particle into Nitroxide Quencher PINQ
4. Automated and OnLine Analysis System Based on Ascorbic Acid
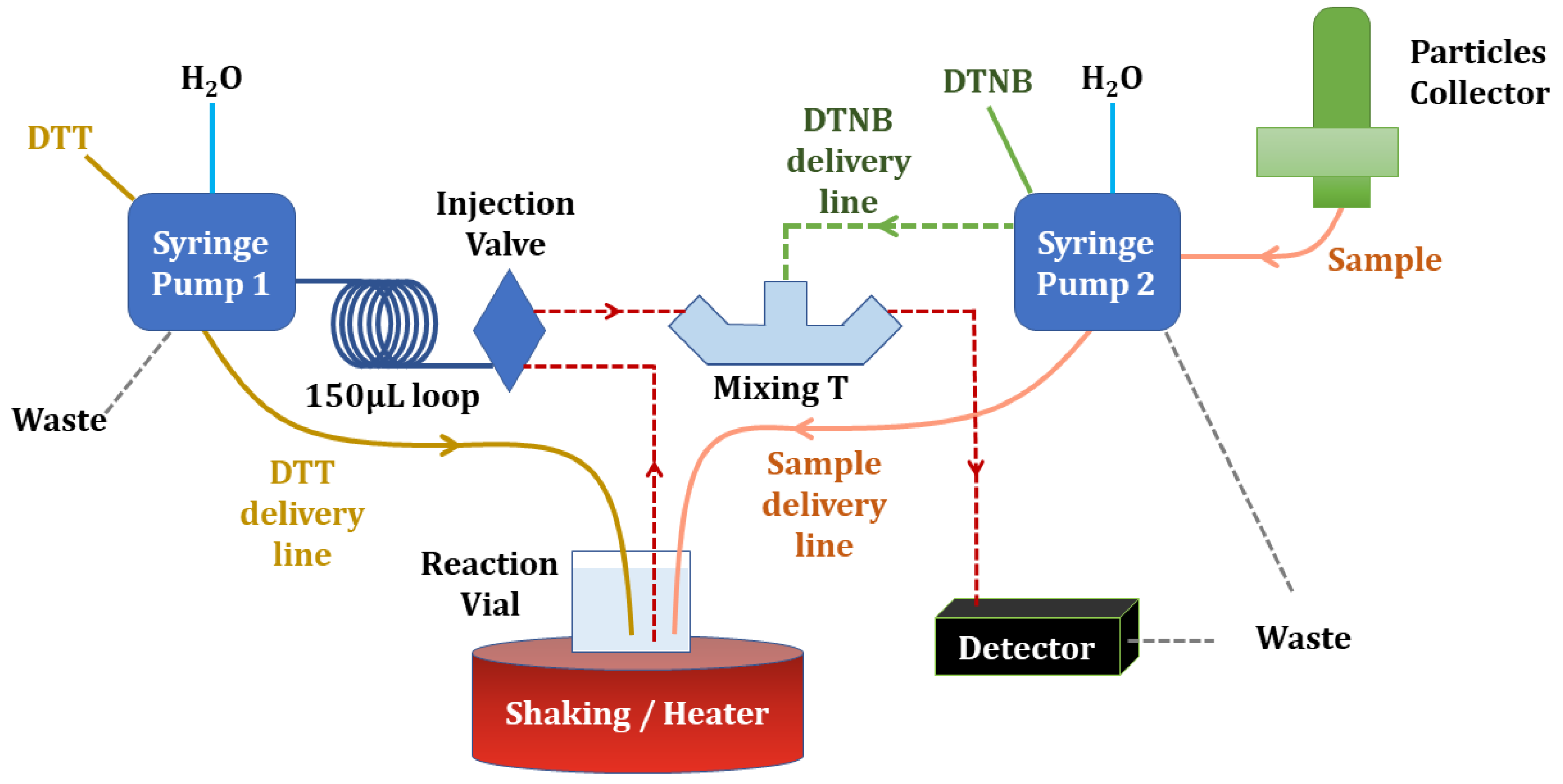
5. Automated and Online Analysis Systems Based on DTT Assay
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemmar, A.; Raza, H.; Subramaniyan, D.; Yasin, J.; John, A.; Ali, B.H.; Kazzam, E.E. Short-Term Systemic Effects of Nose-Only Cigarette Smoke Exposure in Mice: Role of Oxidative Stress. Cell Physiol. Biochem. 2013, 31, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The Contribution of Outdoor Air Pollution Sources to Premature Mortality on a Global Scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Guascito, M.R.; Giordano, M.E.; Caricato, R.; de Bartolomeo, A.R.; Romano, M.P.; Conte, M.; Dinoi, A.; Contini, D. Oxidative Potential, Cytotoxicity, and Intracellular Oxidative Stress Generating Capacity of PM10: A Case Study in South of Italy. Atmosphere 2021, 12, 464. [Google Scholar] [CrossRef]
- Gauderman, W.J.; McConnell, R.; Gilliland, F.; London, S.; Thomas, D.; Avol, E.; Vora, H.; Berhane, K.; Rappaport, E.B.; Lurmann, F.; et al. Association between Air Pollution and Lung Function Growth in Southern California Children. Am. J. Respir. Crit. Care Med. 2000, 162, 1383–1390. [Google Scholar] [CrossRef]
- de Kok, T.M.; Hogervorst, J.G.; Briedé, J.J.; van Herwijnen, M.H.; Maas, L.M.; Moonen, E.J.; Driece, H.A.; Kleinjans, J.C. Genotoxicity and Physicochemical Characteristics of Traffic-Related Ambient Particulate Matter. Environ. Mol. Mutagen. 2005, 46, 71–80. [Google Scholar] [CrossRef]
- Verma, V.; Pakbin, P.; Cheung, K.L.; Cho, A.K.; Schauer, J.J.; Shafer, M.M.; Kleinman, M.T.; Sioutas, C. Physicochemical and Oxidative Characteristics of Semi-Volatile Components of Quasi-Ultrafine Particles in an Urban Atmosphere. Atmos. Environ. 2011, 45, 1025–1033. [Google Scholar] [CrossRef]
- Hoek, G.; Brunekreef, B.; Goldbohm, S.; Fischer, P.; van den Brandt, P.A. Association between Mortality and Indicators of Traffic-Related Air Pollution in the Netherlands: A Cohort Study. Lancet 2002, 360, 1203–1209. [Google Scholar] [CrossRef]
- Naidja, L.; Ali-Khodja, H.; Khardi, S. Sources and Levels of Particulate Matter in North African and Sub-Saharan Cities: A Literature Review. Environ. Sci. Pollut. Res. 2018, 25, 12303–12328. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A Review on the Human Health Impact of Airborne Particulate Matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary Health Effects of Air Pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef]
- Ichoku, C.; Andreae, M.O.; Andreae, T.W.; Meixner, F.X.; Schebeske, G.; Formenti, P.; Maenhaut, W.; Cafmeyer, J.; Ptasinski, J.; Karnieli, A.; et al. Interrelationships between Aerosol Characteristics and Light Scattering during Late Winter in an Eastern Mediterranean Arid Environment. J. Geophys. Res. Atmos. 1999, 104, 24371–24393. [Google Scholar] [CrossRef]
- Ghelfi, E.; Rhoden, C.R.; Wellenius, G.A.; Lawrence, J.; Gonzalez-Flecha, B. Cardiac Oxidative Stress and Electrophysiological Changes in Rats Exposed to Concentrated Ambient Particles Are Mediated by TRP-Dependent Pulmonary Reflexes. Toxicol. Sci. 2008, 102, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hatzis, C.; Godleski, J.J.; González-Flecha, B.; Wolfson, J.M.; Koutrakis, P. Ambient Particulate Matter Exhibits Direct Inhibitory Effects on Oxidative Stress Enzymes. Environ. Sci. Technol. 2006, 40, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Kim, S.; Wang, M.; Froines, J.; Sioutas, C.; Nel, A. Use of a Stratified Oxidative Stress Model to Study the Biological Effects of Ambient Concentrated and Diesel Exhaust Particulate Matter. Inhal. Toxicol. 2002, 14, 459–486. [Google Scholar] [CrossRef]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of Proteomics to Demonstrate a Hierarchical Oxidative Stress Response to Diesel Exhaust Particle Chemicals in a Macrophage Cell Line. J. Biol. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef]
- Samake, A.; Uzu, G.; Martins, J.M.F.; Calas, A.; Vince, E.; Parat, S.; Jaffrezo, J.L. The Unexpected Role of Bioaerosols in the Oxidative Potential of PM. Sci. Rep. 2017, 7, 10978. [Google Scholar] [CrossRef]
- Prahalad, A.K.; Soukup, J.M.; Inmon, J.; Willis, R.; Ghio, A.J.; Becker, S.; Gallagher, J.E. Ambient Air Particles: Effects on Cellular Oxidant Radical Generation in Relation to Particulate Elemental Chemistry. Toxicol. Appl. Pharm. 1999, 158, 81–91. [Google Scholar] [CrossRef]
- Clarke, R.W.; Catalano, P.J.; Koutrakis, P.; Murthy, G.G.K.; Sioutas, C.; Paulauskis, J.; Coull, B.; Ferguson, S.; Godleski, J.J. Urban air particulate inhalation alters pulmonary function and induces pulmonary inflammation in a rodent model of chronic bronchitis. Inhal. Toxicol. 2008, 11, 637–656. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D.; Bagchi, M. Toxicity of trace elements in tobacco smoke. Inhal. Toxicol. 2008, 9, 867–890. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid Redox Cycling as a Mechanism for Sustained Free Radical Generation by Inhaled Airborne Particulate Matter. Free Radic. Biol. Med. 2001, 31, 1132–1138. [Google Scholar] [CrossRef]
- Jovanovic, M.V.; Savic, J.Z.; Salimi, F.; Stevanovic, S.; Brown, R.A.; Jovasevic-Stojanovic, M.; Manojlovic, D.; Bartonova, A.; Bottle, S.; Ristovski, Z.D. Measurements of Oxidative Potential of Particulate Matter at Belgrade Tunnel; Comparison of BPEAnit, DTT and DCFH Assays. Int. J. Environ. Res. Public Health 2019, 16, 4906. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Kelly, F.; Künzli, N.; Schins, R.P.F.; Donaldson, K. Oxidant Generation by Particulate Matter: From Biologically Effective Dose to a Promising, Novel Metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Daellenbach, K.R.; Uzu, G.; Jiang, J.; Cassagnes, L.E.; Leni, Z.; Vlachou, A.; Stefenelli, G.; Canonaco, F.; Weber, S.; Segers, A.; et al. Sources of Particulate-Matter Air Pollution and Its Oxidative Potential in Europe. Nature 2020, 587, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox Activity of Airborne Particulate Matter at Different Sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chirizzi, D.; Cesari, D.; Guascito, M.R.; Dinoi, A.; Giotta, L.; Donateo, A.; Contini, D. Influence of Saharan Dust Outbreaks and Carbon Content on Oxidative Potential of Water-Soluble Fractions of PM2.5 and PM10. Atmos. Environ. 2017, 163, 1–8. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Shahpoury, P.; Zhang, Z.W.; Arangio, A.; Celo, V.; Dabek-Zlotorzynska, E.; Harner, T.; Nenes, A. The Influence of Chemical Composition, Aerosol Acidity, and Metal Dissolution on the Oxidative Potential of Fine Particulate Matter and Redox Potential of the Lung Lining Fluid. Environ. Int. 2021, 148. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bacco, D.; Trentini, A.; Russo, M. Effect of Filter Extraction Solvents on the Measurement of the Oxidative Potential of Airborne PM2.5. Environ. Sci. Pollut. Res. Int. 2021, 28, 29551–29563. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Casari, L.; Demaria, G.; Russo, M. Indoor Air Quality in Domestic Environments during Periods Close to Italian COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 4060. [Google Scholar] [CrossRef]
- Venkatachari, P.; Hopke, P.K. Development and Laboratory Testing of an Automated Monitor for the Measurement of Atmospheric Particle-Bound Reactive Oxygen Species (ROS). Aerosol Sci. Technol. 2008, 42, 629–635. [Google Scholar] [CrossRef]
- Puthussery, J.V.; Singh, A.; Rai, P.; Bhattu, D.; Kumar, V.; Vats, P.; Furger, M.; Rastogi, N.; Slowik, J.G.; Ganguly, D.; et al. Real-Time Measurements of PM2.5Oxidative Potential Using a Dithiothreitol Assay in Delhi, India. Environ. Sci. Technol. Lett. 2020, 7, 504–510. [Google Scholar] [CrossRef]
- Hopke, P.K.; Wang, Y.; Sun, L.; Chalupa, D.C.; Utell, M.J. Laboratory and Field Testing of an Automated Atmospheric Particle-Bound Reactive Oxygen Species Sampling-Analysis System. J. Toxicol. 2011, 2011, 419476. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Guo, H.; King, L.E.; Edgerton, E.S.; Weber, R.J. A Semi-Automated System for Quantifying the Oxidative Potential of Ambient Particles in Aqueous Extracts Using the Dithiothreitol (DTT) Assay: Results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE). Atmos. Meas. Tech. 2015, 8, 471–482. [Google Scholar] [CrossRef]
- Gao, D.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J. A Method for Measuring Total Aerosol Oxidative Potential (OP) with the Dithiothreitol (DTT) Assay and Comparisons between an Urban and Roadside Site of Water-Soluble and Total OP. Atmos. Meas. Tech. 2017, 10, 2821–2835. [Google Scholar] [CrossRef]
- Berg, K.E.; Clark, K.M.; Li, X.; Carter, E.M.; Volckens, J.; Henry, C.S. High-Throughput, Semi-Automated Dithiothreitol (DTT) Assays for Oxidative Potential of Fine Particulate Matter. Atmos. Environ. 2020, 222, 117132. [Google Scholar] [CrossRef]
- Sameenoi, Y.; Koehler, K.; Shapiro, J.; Boonsong, K.; Sun, Y.; Collett, J.; Volckens, J.; Henry, C.S. Microfluidic Electrochemical Sensor for On-Line Monitoring of Aerosol Oxidative Activity. J. Am. Chem. Soc. 2012, 134, 10562–10568. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Kreisberg, N.; Hering, S. An Online Monitor of the Oxidative Capacity of Aerosols (o-MOCA). Atmos. Meas. Tech. 2017, 10, 633–644. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Zhang, Y.; Zeng, L.; Dong, H.; Huo, P.; Fang, D.; Schauer, J.J. Development of an Automated Sampling-Analysis System for Simultaneous Measurement of Reactive Oxygen Species (ROS) in Gas and Particle Phases: GAC-ROS. Atmos. Environ. 2016, 134, 18–26. [Google Scholar] [CrossRef]
- Wragg, F.P.H.; Fuller, S.J.; Freshwater, R.; Green, D.C.; Kelly, F.J.; Kalberer, M. An Automated Online Instrument to Quantify Aerosol-Bound Reactive Oxygen Species (ROS) for Ambient Measurement and Health-Relevant Aerosol Studies. Atmos. Meas. Tech. 2016, 9, 4891–4900. [Google Scholar] [CrossRef]
- Puthussery, J.V.; Zhang, C.; Verma, V. Development and Field Testing of an Online Instrument for Measuring the Real-Time Oxidative Potential of Ambient Particulate Matter Based on Dithiothreitol Assay. Atmos. Meas. Tech. 2018, 11, 5767–5780. [Google Scholar] [CrossRef]
- Zhou, J.; Bruns, E.A.; Zotter, P.; Stefenelli, G.; Prévôt, A.S.H.; Baltensperger, U.; El-Haddad, I.; Dommen, J. Development, Characterization and First Deployment of an Improved Online Reactive Oxygen Species Analyzer. Atmos. Meas. Tech. 2018, 11, 65–80. [Google Scholar] [CrossRef]
- Brown, A.R.; Stevanovic, S.; Bottle, S.; Ristovski, D.Z. An Instrument for the Rapid Quantification of PM-Bound ROS: The Particle into Nitroxide Quencher (PINQ). Atmos. Meas. Tech. 2019, 12, 2387–2401. [Google Scholar] [CrossRef]
- Oyama, Y.; Furukawa, K.; Chikahisa, L.; Hatakeyama, Y. Effect of N,N-Diethyldithiocarbamate on Ionomycin-Induced Increase in Oxidation of Cellular 2′,7′-Dichlorofluorescin in Dissociated Cerebellar Neurons. Brain Res. 1994, 660, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, R.; Schwiers, E.; Ames, B.N. Detection of Picomole Levels of Hydroperoxides Using a Fluorescent Dichlorofluorescein Assay. Anal. Biochem. 1983, 134, 111–116. [Google Scholar] [CrossRef]
- Antonini, J.M.; Clarke, R.W.; Krishna Murthy, G.G.; Sreekanthan, P.; Jenkins, N.; Eagar, T.W.; Brain, J.D. Freshly Generated Stainless Steel Welding Fume Induces Greater Lung Inflammation in Rats as Compared to Aged Fume. Toxicol. Lett. 1998, 98, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.F.; Wang, C.S. Experimental Determination of Reactive Oxygen Species in Taipei Aerosols. J. Aerosol Sci. 2001, 32, 1201–1211. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a Fluorescent Probe for Reactive Oxygen Species Measurement: Forty Years of Application and Controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- King, L.E.; Weber, R.J. Development and Testing of an Online Method to Measure Ambient Fine Particulate Reactive Oxygen Species (ROS) Based on the 2′,7′-Dichlorofluorescin (DCFH) Assay. Atmos. Meas. Tech. 2013, 6, 1647–1658. [Google Scholar] [CrossRef]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Baeza Squiban, A. Oxidative Potential of Particulate Matter 2.5 as Predictive Indicator of Cellular Stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative Potential of Particulate Matter Collected at Sites with Different Source Characteristics. Sci. Total Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 Oxidative Potential: Importance of Chemical Species and Comparison of Two Spectrophotometric Cell-Free Assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.J.; Utinger, B.; Lienhard, D.M.; Paulson, S.E.; Shen, J.; Griffiths, P.T.; Stell, A.C.; Kalberer, M. Development of a Physiologically Relevant Online Chemical Assay to Quantify Aerosol Oxidative Potential. Anal. Chem. 2019, 91, 13088–13095. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.A.; Shapiro, J.; Sameenoi, Y.; Henry, C.; Volckens, J. Laboratory Evaluation of a Microfluidic Electrochemical Sensor for Aerosol Oxidative Load. Aerosol Sci. Technol. 2014, 48, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Sameenoi, Y.; Panymeesamer, P.; Supalakorn, N.; Koehler, K.; Chailapakul, O.; Henry, C.S.; Volckens, J. Microfluidic Paper-Based Analytical Device for Aerosol Oxidative Activity. Environ. Sci. Technol. 2013, 47, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, J.V.; Dave, J.; Shukla, A.; Gaddamidi, S.; Singh, A.; Vats, P.; Salana, S.; Ganguly, D.; Rastogi, N.; Tripathi, S.N.; et al. Effect of Biomass Burning, Diwali Fireworks, and Polluted Fog Events on the Oxidative Potential of Fine Ambient Particulate Matter in Delhi, India. Environ. Sci. Technol. 2022, 56, 14605–14616. [Google Scholar] [CrossRef]
- Romano, M.P.; Lionetto, M.G.; Mangone, A.; de Bartolomeo, A.R.; Giordano, M.E.; Contini, D.; Guascito, M.R. Development and Characterization of a Gold Nanoparticles Glassy Carbon Modified Electrode for Dithiotreitol (DTT) Detection Suitable to Be Applied for Determination of Atmospheric Particulate Oxidative Potential. Anal. Chim. Acta 2022, 1206, 339556. [Google Scholar] [CrossRef]

| Quantitation Method | On-Field Application | Validation on Real Samples | Automated | Online | Dynamic Range | Linear Range | Collector Flow | Flow during Analysis | LoD | Link |
|---|---|---|---|---|---|---|---|---|---|---|
| fluorescence | No | N.A. | Yes | Yes | 50 to 800 nM | 0.051 to 0.8µM | 14–25 L/min | N.A. | 81.1 nM | [30] |
| fluorescence | Yes | Yes | Yes | Yes | N.A. | 0.1–0.4 µM H2O2 | 16.7 L/min | N.A. | N.A. | [32] |
| fluorescence | Yes | Yes | Yes | Yes | N.A. | 0.01–0.2 µM | 20 L/min | 0.4 mL/min | 0.28 nM H2O2 equivalents | [48] |
| fluorescence | Yes | Yes | Yes | Yes | 4–2000 nmol [H2O2] equivalents/m3 air | 0.025–1.0 μM (calibration with H2O2) | 5 L/min | N.A. | 3.85 nmol [H2O2] equivalents/m3 air | [39] |
| fluorescence | Yes | Yes | Yes | Yes | N.A. | 0.1–0.4 µM (calibration with H2O2) | 16.7 L/min | N.A. | gas-phase ROS: 0.16 nmol H2O2/m3; particle-phase ROS: 0.12 nmol H2O2/m3 | [38] |
| fluorescence | Yes | Yes | Partially | Yes | N.A. | 0–0.15 µM | 1.7 L/min | 0.3 mL/min | 1.3 nmol/L (offline); 2 nmol/m3 (online) | [41] |
| Quantitation Method | On-Field Application | Validation on Real Samples | Automated | Online | Linear Range | Collector Flow | Flow during Analysis | LoD | Link |
|---|---|---|---|---|---|---|---|---|---|
| Electrochemical | No | Yes | Yes | Yes | 10–100 μM | 12.5 L/min | N.A. | 2.49 ± 0.20 μM | [36] |
| Electrochemical | No | Yes | semi-automated | Yes | N.A. | 13 L/min | 0.18 mL/min | 2 pmol DTT/min (aerosol concentration of 10 μg/m3) | [53] |
| Absorbance | Yes | Yes | semi-automated | No | 0.3–1.9 nmol/min | 1.13 m3/min | N.A. | 0.31 nmol DTT/min | [33] |
| Absorbance | Yes | Yes | Yes | No | 0.22–1.25 nmol/min (from graph) | 1.13 m3/min | N.A. | Water-soluble metals method = K: 0.03 mg/L; Mn: 0.00007 mg/L; Fe: 0.009 mg/L; Cu: 0.0002 mg/L Total metal method = K: 0.03 mg/L; Mn: 0.0002 mg/L; Fe: 0.02 mg/L; Cu: 0.002 mg/L | [34] |
| Colorimetric | Yes | Yes | No | No | 0–12.5 nmol DTT | 4 L/min | N.A. | 7.92 ± 0.3 ng NQ | [54] |
| Absorbance | Yes | Yes | Yes | Yes | 0.025–0.25 µM | 3.0 L/min | continuous-flow DAD | 0.15 nmol/min | [37] |
| Absorbance | Yes | Yes | Yes | Yes | 0.03–0.23 µM/min | 42 L/min | N.A. | 0.24 nmol/min | [40] |
| Absorbance—Electrochemical | No | Yes | semi-automated | No | 0–100 µM DTT | 16.7 L/min | 0.085 mL/min | UV/vis: 0.150 mAU ⋅ min/μM DTT Electrochemical: 0.218 Ip/μM DTT | [35] |
| Electrochemical | No | Yes | No | Yes | Amperometric (BIA): 0.75–200 µM Amperometric (FIA): 10–500 µM | N.A. | 1.5 mL/min | Amperometric (BIA): 0.75 µM Amperometric (FIA): 1.5 µM | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlino, A.; Romano, M.P.; Lionetto, M.G.; Contini, D.; Guascito, M.R. An Overview of the Automated and On-Line Systems to Assess the Oxidative Potential of Particulate Matter. Atmosphere 2023, 14, 256. https://doi.org/10.3390/atmos14020256
Carlino A, Romano MP, Lionetto MG, Contini D, Guascito MR. An Overview of the Automated and On-Line Systems to Assess the Oxidative Potential of Particulate Matter. Atmosphere. 2023; 14(2):256. https://doi.org/10.3390/atmos14020256
Chicago/Turabian StyleCarlino, Alessandro, Maria Pia Romano, Maria Giulia Lionetto, Daniele Contini, and Maria Rachele Guascito. 2023. "An Overview of the Automated and On-Line Systems to Assess the Oxidative Potential of Particulate Matter" Atmosphere 14, no. 2: 256. https://doi.org/10.3390/atmos14020256
APA StyleCarlino, A., Romano, M. P., Lionetto, M. G., Contini, D., & Guascito, M. R. (2023). An Overview of the Automated and On-Line Systems to Assess the Oxidative Potential of Particulate Matter. Atmosphere, 14(2), 256. https://doi.org/10.3390/atmos14020256








