Characteristics and Sources of Water-Soluble Inorganic Ions in PM2.5 in Urban Nanjing, China
Abstract
1. Introduction
2. Materials and Methods
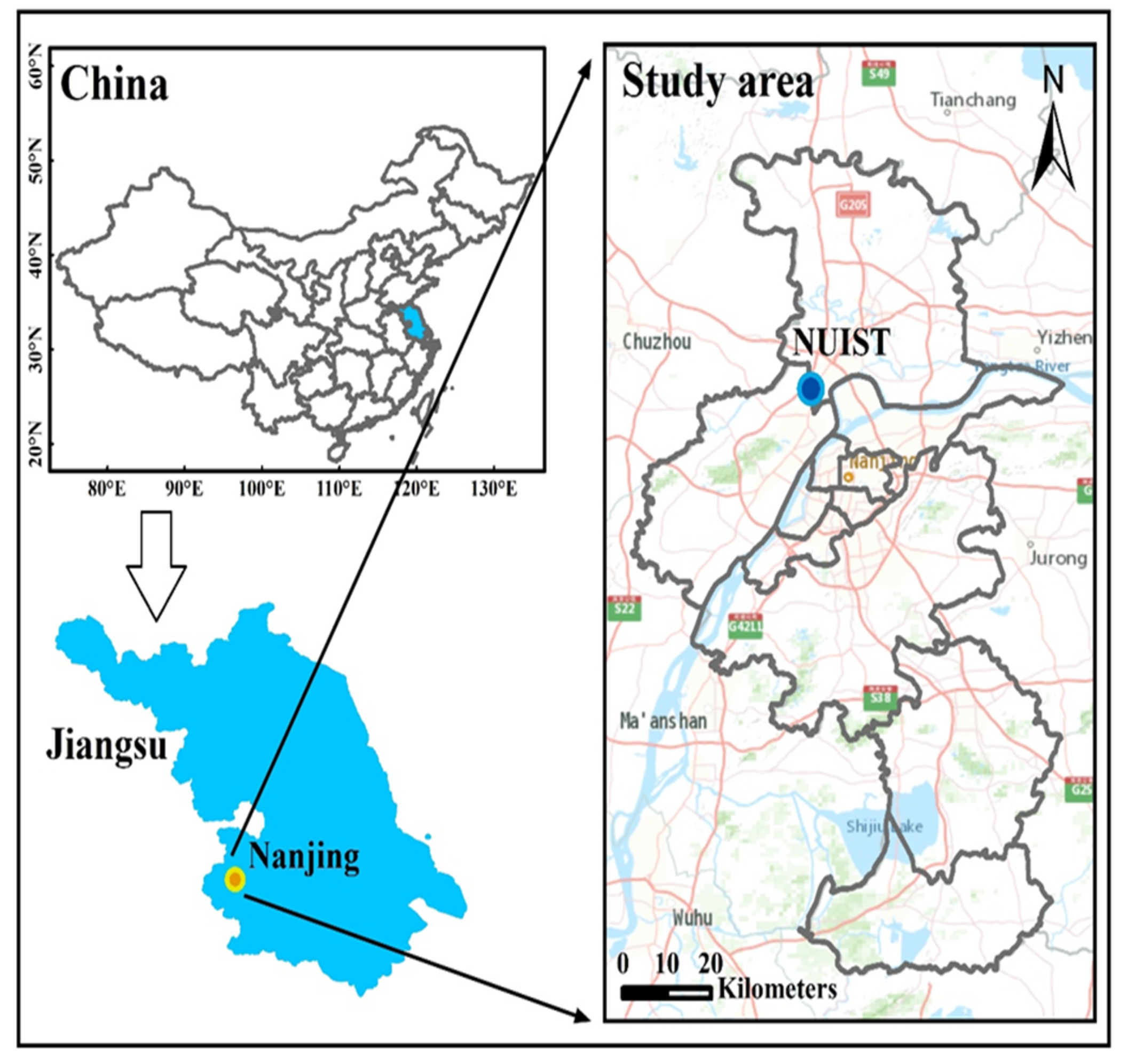
2.1. Site Description and Instrumentation
2.2. Positive Matrix Factorization Model
2.3. Potential Source Contribution Function Analysis
3. Results and Discussion
3.1. General Patterns of WSIIs in PM2.5
3.2. Variability of SNA
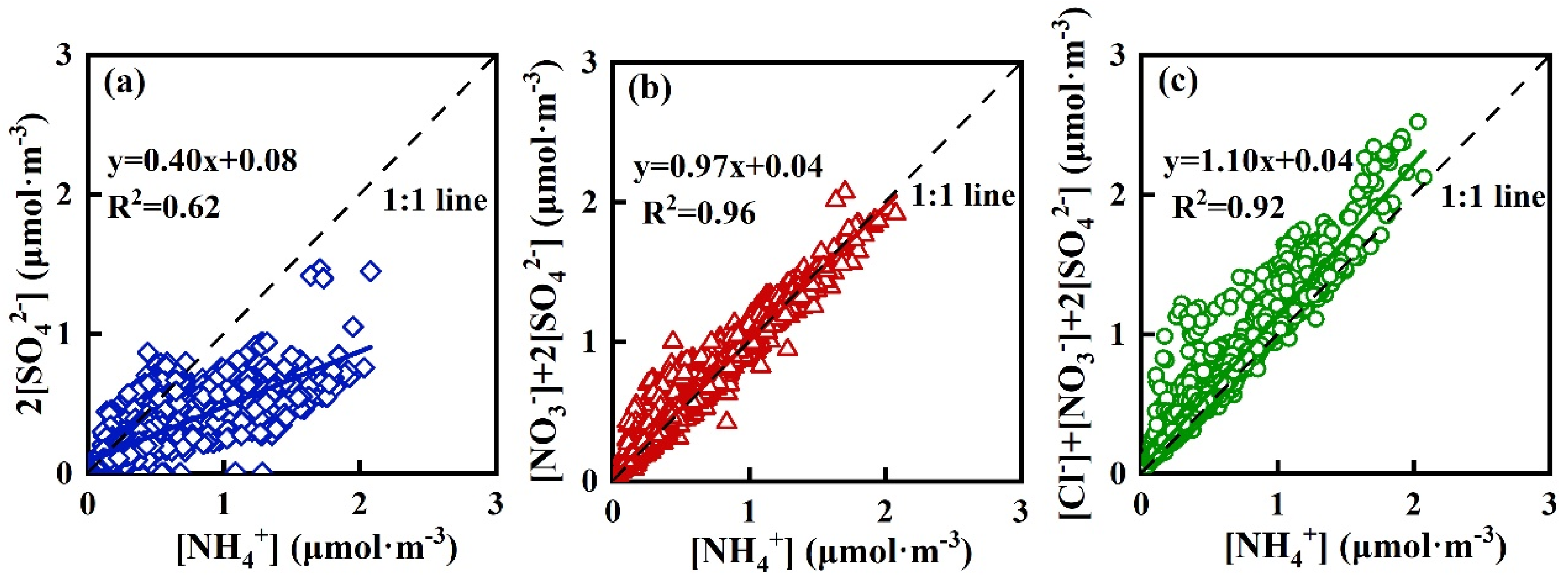
3.3. Aerosol Acidity and Chemical Forms of WSIIs
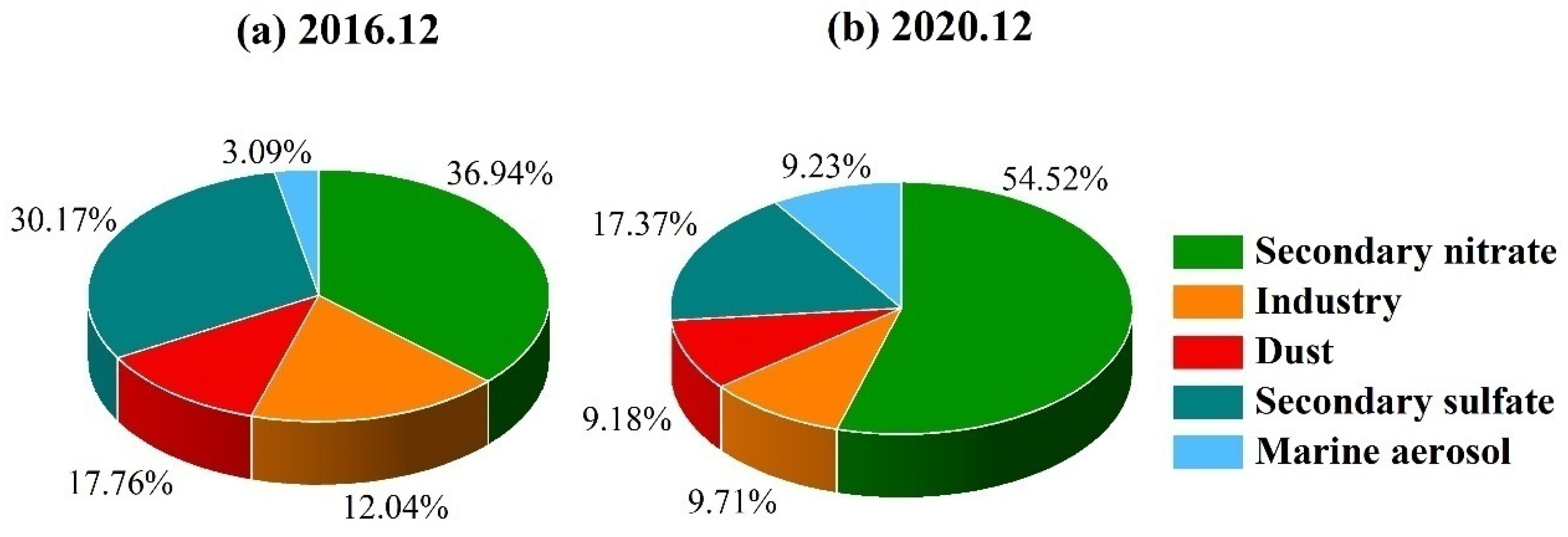
3.4. Source Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, L.P.; Zhang, L.; Cheng, I.; Aherne, J.; Wentworth, G.R. Impacts and effects indicators of atmospheric deposition of major pollutants to various ecosystems—A Review. Aerosol Air Qual. Res. 2018, 18, 1953–1992. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Lv, W.; Liu, B.; Aikawa, M.; Wang, J. Characteristics and risk assessments of heavy metals in fine and coarse particles in an industrial area of central China. Ecotox. Environ. Saf. 2019, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Y.; Hu, B.; Wen, T.; Xin, J.; Li, X.; Wang, Y. Size-resolved aerosol water-soluble ions during the summer and winter seasons in Beijing: Formation mechanisms of secondary inorganic aerosols. Chemosphere 2017, 183, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Quan, J.; Tie, X.; Li, X.; Liu, Q.; Gao, Y.; Zhao, D. Effects of meteorology and secondary particle formation on visibility during heavy haze events in Beijing, China. Sci. Total Environ. 2015, 502, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Gao, J.; Cao, G.; Ouyang, F. Chemical composition and source identification of PM2.5 in the suburb of Shenzhen, China. Atmos. Res. 2013, 122, 391–400. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Guo, L.; Zhang, Y.; Zhang, G.; Wang, X. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Gao, X.; Xue, L.; Wang, X.; Wang, Z.; Gao, J.; Zhang, Q.; Wang, W. Continuous observations of water-soluble ions in PM2.5 at Mount Tai (1534 m a.s.l.) in central-eastern China. J. Atmos. Chem. 2010, 64, 107–127. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, P.; Su, J.; Dong, Q.; Du, X.; Zhang, Y. Aerosol pH and its driving factors in Beijing. Atmos. Chem. Phys. 2019, 19, 7939–7954. [Google Scholar] [CrossRef]
- Su, J.; Zhao, P.; Ding, J.; Du, X.; Dou, Y. Insights into measurements of water-soluble ions in PM2.5 and their gaseous precursors in Beijing. J. Environ. Sci. 2021, 102, 123–137. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, R.; Yu, Y.; Yan, Y.; Liu, Y.; Di, Y.; Wu, D.; Zhang, W. Size-resolved aerosol water-soluble ions at a regional background station of Beijing, Tianjin, and Hebei, North China. J. Environ. Sci. 2017, 55, 146–156. [Google Scholar] [CrossRef]
- Meng, C.C.; Wang, L.T.; Zhang, F.F.; Wei, Z.; Ma, S.M.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, L.; Huang, Z.; Zhang, H.; He, M.; Dai, X.; Zheng, J.; Xiao, H. Seasonal variation and size distributions of water-soluble inorganic ions and carbonaceous aerosols at a coastal site in Ningbo, China. Sci. Total Environ. 2018, 639, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.S.; Xu, M.X.; Snape, C.; He, J.; Behera, S.N.; Xu, H.H.; Ji, D.S.; Wang, C.J.; Yu, H.; Xiao, H. Temporal and spatial variation in major ion chemistry and source identification of secondary inorganic aerosols in Northern Zhejiang Province, China. Chemosphere 2017, 179, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xing, Z.; Deng, J.; Du, K. Characterizing and sourcing ambient PM2.5 over key emission regions in China I: Water-soluble ions and carbonaceous fractions. Atmos. Environ. 2016, 135, 20–30. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, Q.; Hu, J.; Zhang, H. Spatial and temporal variations of six criteria air pollutants in 31 provincial capital cities in China during 2013–2014. Environ. Int. 2014, 73, 413–422. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.Z.; Ho, S.S.H.; Xu, J.; Wu, W.-S.; Wan, C.H.; Wang, X.; Wang, X.; Wang, L. The chemical composition of inorganic and carbonaceous materials in PM2.5 in Nanjing, China. Atmos. Environ. 2005, 39, 3735–3749. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Yu, Y.; Gao, S.; Feng, J.; Gao, S.; Wang, L. Chemical characterization of water-soluble components of PM10 and PM2.5 atmospheric aerosols in five locations of Nanjing, China. Atmos. Environ. 2003, 37, 2893–2902. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Ji, G.; Ying, R.; Shan, Y.; Lin, Y. Seasonal variations and source apportionment of water-soluble inorganic ions in PM2.5 in Nanjing, a megacity in southeastern China. J. Atmos. Chem. 2019, 76, 73–88. [Google Scholar] [CrossRef]
- Wang, G.; Huang, L.; Gao, S.; Gao, S.; Wang, L. Characterization of water-soluble species of PM10 and PM2.5 aerosols in urban area in Nanjing, China. Atmos. Environ. 2002, 36, 1299–1307. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Shen, L.; Xu, H.; An, J.; Xue, G.; Cao, J. Water-soluble ions in atmospheric aerosols measured in five sites in the Yangtze River Delta, China: Size-fractionated, seasonal variations and sources. Atmos. Environ. 2015, 123, 370–379. [Google Scholar] [CrossRef]
- Zhan, Y.; Xie, M.; Gao, D.; Wang, T.; Zhang, M.; An, F. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 during a wintertime particle pollution episode in Nanjing, China. Atmos. Res. 2021, 262, 105769. [Google Scholar] [CrossRef]
- Fu, Q.; Zhuang, G.; Wang, J.; Xu, C.; Huang, K.; Li, J.; Hou, B.; Lu, T.; Streets, D.G. Mechanism of formation of the heaviest pollution episode ever recorded in the Yangtze River Delta, China. Atmos. Environ. 2008, 42, 2023–2036. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.G.; Yang, M.; Li, F.; Wang, J.; Sun, Y.; Wang, C.; Wu, H.; Qian, X. Chemical characterization and source apportionment of PM2.5 aerosols in a megacity of Southeast China. Atmos. Res. 2016, 181, 288–299. [Google Scholar] [CrossRef]
- Liu, A.; Wang, H.; Cui, Y.; Shen, L.; Yin, Y.; Wu, Z.; Guo, S.; Shi, S.; Chen, K.; Zhu, B. Characteristics of Aerosol during a Severe Haze-Fog Episode in the Yangtze River Delta: Particle Size Distribution, Chemical Composition, and Optical Properties. Atmosphere 2020, 11, 56. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Shen, L.; Kang, H. Size distributions of aerosol and water-soluble ions in Nanjing during a crop residual burning event. J. Environ. Sci. 2012, 24, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, H.L.; Yin, Y.; Zhu, B.; Xia, L.; Zhang, Z.F.; Chen, K. Measurement of ambient aerosols by single particle mass spectrometry in the Yangtze River Delta, China: Seasonal variations, mixing state and meteorological effects. Atmos. Res. 2018, 213, 562–575. [Google Scholar] [CrossRef]
- Chen, X.; Walker, J.T.; Geron, C. Chromatography related performance of the Monitor for AeRosols and GAses in ambient air (MARGA): Laboratory and field-based evaluation. Atmos. Meas. Tech. 2017, 10, 3893–3908. [Google Scholar] [CrossRef]
- Wen, L.; Chen, J.; Yang, L.; Wang, X.; Caihong, X.; Sui, X.; Yao, L.; Zhu, Y.; Zhang, J.; Zhu, T. Enhanced formation of fine particulate nitrate at a rural site on the North China Plain in summer: The important roles of ammonia and ozone. Atmos. Environ. 2015, 101, 294–302. [Google Scholar] [CrossRef]
- Li, L.; Lai, W.; Pu, J.; Mo, H.; Dai, D.; Wu, G.; Deng, S. Polar organic tracers in PM2.5 aerosols from an inland background area in Southwest China: Correlations between secondary organic aerosol tracers and source apportionment. J. Environ. Sci. 2018, 69, 281–293. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Cao, J.; Zhong, L.; Chen, D.; Yang, Y.; Chen, D.; Chen, L.; Zhang, Z.; Wu, Y. Source apportionment of PM2.5 at urban and suburban areas of the Pearl River Delta region, south China—With emphasis on ship emissions. Sci. Total Environ. 2017, 574, 1559–1570. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, H.; Yi, A.; Zhang, Z.; Zheng, N.; Fang, X.; Xiao, H. Characterization and source analysis of water–soluble ions in PM2.5 at a background site in Central China. Atmos. Res. 2020, 239, 104881. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhao, Q.; Zhang, T.; Schauer, J.J. Increases in the formation of water soluble organic nitrogen during Asian dust storm episodes. Atmos. Res. 2021, 253, 105486. [Google Scholar]
- Zhang, Y.; Sheesley, R.J.; Schauer, J.J.; Lewandowski, M.; Jaoui, M.; Offenberg, J.H.; Kleindienst, T.E.; Edney, E.O. Source apportionment of primary and secondary organic aerosols using positive matrix factorization (PMF) of molecular markers. Atmos. Environ. 2009, 43, 5567–5574. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Chen, N.; Yan, Y.; Liu, D.; Zhu, B.; Xu, K.; Cao, W.; Ding, Q.; Lan, B. Significant changes in the chemical compositions and sources of PM2.5 in Wuhan since the city lockdown as COVID-19. Sci. Total Environ. 2020, 739, 140000. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hu, J.; Xu, B.; Hu, X.; Sun, P.; Han, W.; Gu, Z.; Yu, X.; Wu, M. Characteristics and seasonal variation of organic matter in PM2.5 at a regional background site of the Yangtze River Delta region, China. Atmos. Environ. 2015, 123, 288–297. [Google Scholar] [CrossRef]
- Polissar, A.V.; Hopke, P.K.; Paatero, P.; Kaufmann, Y.J.; Hall, D.K.; Bodhaine, B.A.; Dutton, E.G.; Harris, J.M. The aerosol at Barrow, Alaska: Long-term trends and source locations. Atmos. Environ. 1999, 33, 2441–2458. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, M.; Liu, W.; Mao, Y.; Hu, J.; Tian, Q.; Chen, Z.; Hu, T.; Xing, X.; Qi, S. Characteristics and source apportionment of water-soluble inorganic ions in PM2.5 during a wintertime haze event in Huanggang, central China. Atmos. Pollut. Res. 2021, 12, 111–123. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Sun, Y.; Ji, D.; Wang, Y. Long-range transport and regional sources of PM2.5 in Beijing based on long-term observations from 2005 to 2010. Atmos. Res. 2015, 157, 37–48. [Google Scholar] [CrossRef]
- Huang, T.; Chen, J.; Zhao, W.; Cheng, J.; Cheng, S. Seasonal variations and correlation analysis of water-soluble inorganic ions in PM2.5 in Wuhan, 2013. Atmosphere 2016, 7, 49. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, Y.C.; Amesho, K.T.T.; Shangdiar, S.; Chou, F.C.; Cheng, P.C. Chemical characterization of PM2.5 emissions and atmospheric metallic element concentrations in PM2.5 emitted from mobile source gasoline-fueled vehicles. Sci. Total Environ. 2020, 739, 139942. [Google Scholar] [CrossRef]
- Tian, M.; Wang, H.; Chen, Y.; Zhang, L.; Shi, G.; Liu, Y.; Yu, J.; Zhai, C.; Wang, J.; Yang, F. Highly time-resolved characterization of water-soluble inorganic ions in PM2.5 in a humid and acidic mega city in Sichuan Basin, China. Sci. Total Environ. 2017, 580, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ding, F.; Mu, Y.; Xie, M.; Wang, Q.G. High time-resolved PM2.5 composition and sources at an urban site in Yangtze River Delta, China after the implementation of the APPCAP. Chemosphere 2020, 261, 127746. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tong, Y.; Wei, Y.; Wang, T.; Li, J.; Yang, W.; Fan, S. The analysis of chemistry composition of fine-mode particles in Nanjing. Trans. Atmos. Sci. 2009, 32, 723–733. (In Chinese) [Google Scholar]
- Zhang, Y.; Ren, L.; Sun, J.; Zhu, Z.; Chen, Y. Features of water-soluble components pollution of atmospheric fine particles in Nanjing. Environ. Monitor. China 2013, 29, 25–27. (In Chinese) [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Zheng, J.; Wang, Z.; Zhou, Y. Characterization apportionment of carbonaceous aerosol and water-soluble ions in PM2.5 at suburban Nanjing. Sci. Tech. Eng. 2015, 15, 91–99. (In Chinese) [Google Scholar]
- Wang, H.; An, J.; Cheng, M.; Shen, L.; Zhu, B.; Li, Y.; Wang, Y.; Duan, Q.; Sullivan, A.; Xia, L. One year online measurements of water-soluble ions at the industrially polluted town of Nanjing, China: Sources, seasonal and diurnal variations. Chemosphere 2016, 148, 526–536. [Google Scholar] [CrossRef]
- Du, W.; Hong, Y.; Xiao, H.; Zhang, Y.; Chen, Y.; Xu, L.; Chen, J.; Deng, J. Chemical characterization and source apportionment of PM2.5 during spring and winter in the Yangtze River Delta, China. Aerosol Air Qual. Res. 2017, 17, 2165–2180. [Google Scholar] [CrossRef]
- Li, S.W.; Chang, M.H.; Li, H.M.; Cui, X.Y.; Ma, L.Q. Chemical compositions and source apportionment of PM2.5 during clear and hazy days: Seasonal changes and impacts of Youth Olympic Games. Chemosphere 2020, 256, 1127163. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, Q.; Chen, S.; Zhu, B.; Zhang, Y.; Yu, J.; Guo, Z. Study on pollution behavior and sulfate formation during the typical haze event in Nanjing with water soluble inorganic ions and sulfur isotopes. Atmos. Res. 2019, 217, 198–207. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhang, Y.L.; Fan, M.Y.; Bao, M.Y. Heterogeneous formation of particulate nitrate under ammonium-rich regimes during the high-PM2.5 events in Nanjing, China. Atmos. Chem. Phys. 2020, 20, 3999–4011. [Google Scholar] [CrossRef]
- Sun, P.; Nie, W.; Wang, T.; Chi, X.; Huang, X.; Xu, Z.; Zhu, C.; Wang, L.; Qi, X.; Zhang, Q. Impact of air transport and secondary formation on haze pollution in the Yangtze River Delta: In situ online observations in Shanghai and Nanjing. Atmos. Environ. 2020, 225, 117350. [Google Scholar] [CrossRef]
- Yang, L.; Shang, Y.; Hannigan, M.P.; Zhu, R.; Wang, Q.G.; Qin, C.; Xie, M. Collocated speciation of PM2.5 using tandem quartz filters in northern nanjing, China: Sampling artifacts and measurement uncertainty. Atmos. Environ 2021, 246, 118066. [Google Scholar] [CrossRef]
- Qiu, C.; Gong, H.; Yu, X.; Ding, C.; Hou, S.; Zhang, R.; Hou, X. Seasonal characteristics and sources apportionment of water-soluble ions in PM2.5 of Nanjing Jiangbei New Area. Acta Sci. Circumstantiae 2021, 41, 1718–1726. (In Chinese) [Google Scholar] [CrossRef]
- Qiao, B.; Chen, Y.; Tian, M.; Wang, H.; Yang, F.; Shi, G.; Zhang, L.; Peng, C.; Luo, Q.; Ding, S. Characterization of water soluble inorganic ions and their evolution processes during PM2.5 pollution episodes in a small city in southwest China. Sci. Total Environ. 2019, 650, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Y.; Kong, S.; Wen, B.; Chen, K.; Yuan, L.; Li, Q. Altitudinal effect to the size distribution of water soluble inorganic ions in PM at Huangshan, China. Atmos. Environ. 2014, 98, 242–252. [Google Scholar] [CrossRef]
- Agarwal, A.; Satsangi, A.; Lakhani, A.; Kumari, K.M. Seasonal and spatial variability of secondary inorganic aerosols in PM2.5 at Agra: Source apportionment through receptor models. Chemosphere 2020, 242, 125132. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Yu, G.; Qin, X.; Ren, J.; Qin, D. Characteristics of water soluble ionic species in fine particles from a high altitude site on the northern boundary of Tibetan Plateau: Mixture of mineral dust and anthropogenic aerosol. Atmos. Res. 2014, 143, 43–56. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, Z.; Nie, T.; Wang, K.; Nie, L.; Pan, T.; Wu, X.; Tian, H.; Zhong, L.; Li, J. Trends of multiple air pollutants emissions from residential coal combustion in Beijing and its implication on improving air quality for control measures. Atmos. Environ. 2016, 142, 303–312. [Google Scholar] [CrossRef]
- Kong, S.; Wen, B.; Chen, K.; Yin, Y.; Li, L.; Li, Q.; Yuan, L.; Li, X.; Sun, X. Ion chemistry for atmospheric size-segregated aerosol and depositions at an offshore site of Yangtze River Delta region, China. Atmos. Res. 2014, 147–148, 205–226. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Qian, W.B. Comprehensive characterization of PM2.5 aerosols in Singapore. J. Geophys. Res. 2003, 108, 4523. [Google Scholar] [CrossRef]
- Nair, P.R.; Parameswaran, K.; Abraham, A.; Jacob, S. Wind-dependence of sea-salt and non-sea-salt aerosols over the oceanic environment. J. Atmos. Sol. Terr. Phy. 2005, 67, 884–898. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Wang, S.; Hao, J.; Zhang, H.; He, C. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Y.; Nielsen, C.P. Benefits of China’s efforts in gaseous pollutant control indicated by the bottom-up emissions and satellite observations 2000–2014. Atmos. Environ. 2016, 136, 43–53. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Xu, Y.; Chen, J. Characterization of water-soluble inorganic ions in size-segregated aerosols in coastal city, Xiamen. Atmos. Res. 2011, 99, 546–562. [Google Scholar] [CrossRef]
- Pant, P.; Harrison, R.M. Critical review of receptor modelling for particulate matter: A case study of India. Atmos. Environ. 2012, 49, 1–12. [Google Scholar] [CrossRef]
- Deng, X.L.; Shi, C.E.; Wu, B.W.; Yang, Y.J.; Jin, Q.; Wang, H.L.; Zhu, S.; Yu, C. Characteristics of the water-soluble components of aerosol particles in Hefei, China. J. Environ. Sci. 2016, 42, 32–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Zhang, X.; Huang, K.; Xu, C.; Tang, A.; Chen, J.; An, Z. The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai. Atmos. Environ. 2006, 40, 2935–2952. [Google Scholar] [CrossRef]
- Manktelow, P.T.; Carslaw, K.S.; Mann, G.W.; Spracklen, D.V. The impact of dust on sulfate aerosol, CN and CCN during an East Asian dust storm. Atmos. Chem. Phys. 2010, 10, 365–382. [Google Scholar] [CrossRef]
- Deng, C.; Tian, S.; Li, Z.; Li, K. Spatiotemporal characteristics of PM2.5 and ozone concentrations in Chinese urban clusters. Chemosphere 2022, 295, 133813. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Yi, H.; Tang, X.; Yu, Q.; Zhao, S.; Gao, F.; Zhou, Y.; Zhong, T.; Wang, Y. Trends in air pollutant emissions from the sintering process of the iron and steel industry in the Fenwei Plain and surrounding regions in China, 2014–2017. Chemosphere 2022, 291, 132917. [Google Scholar] [CrossRef]
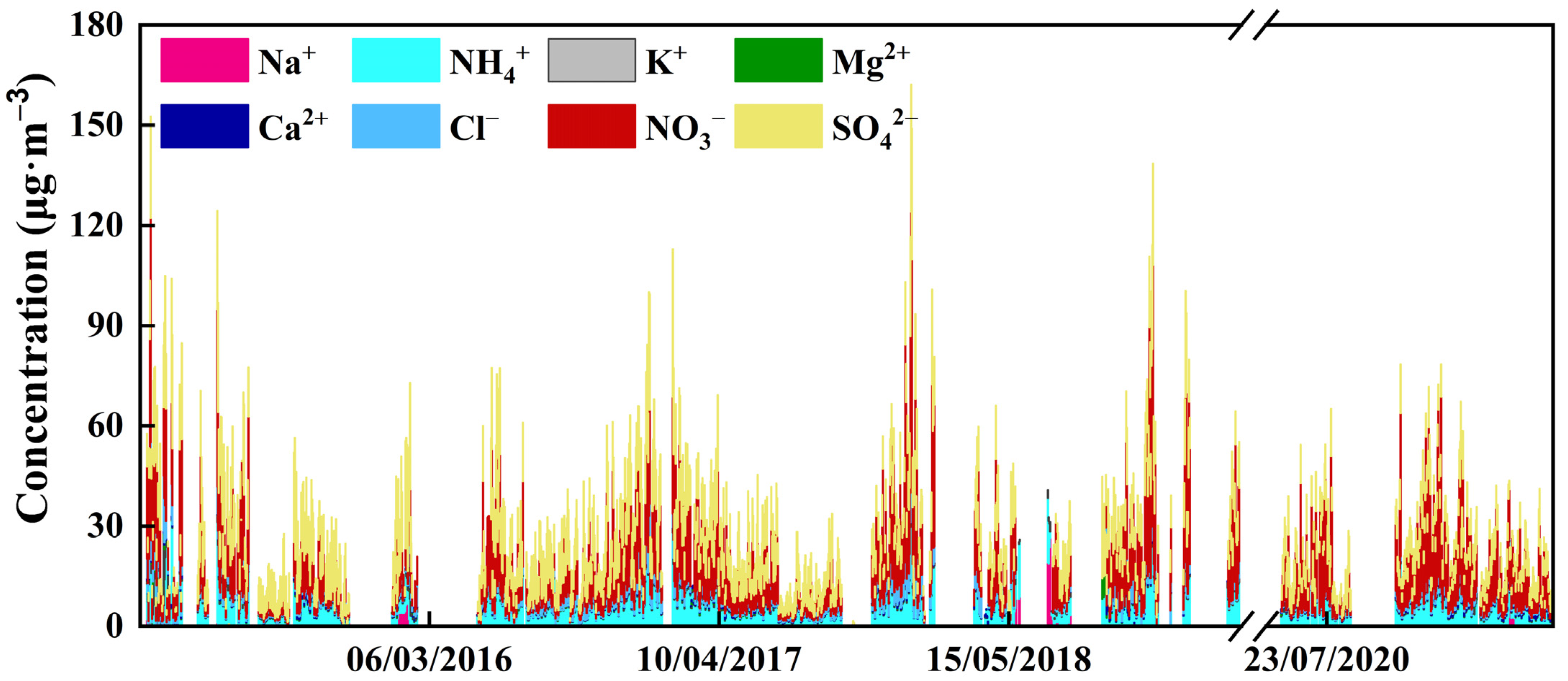
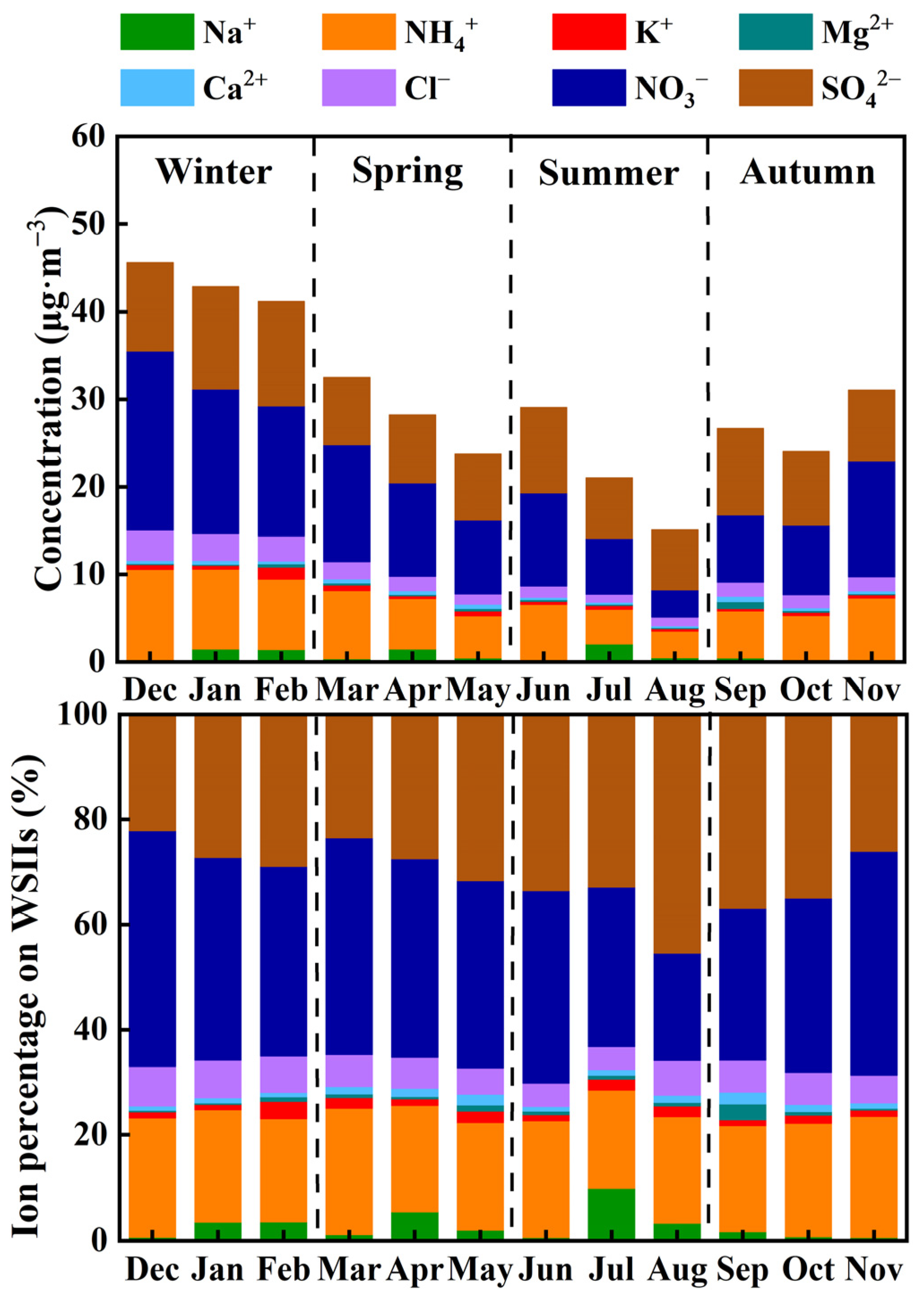

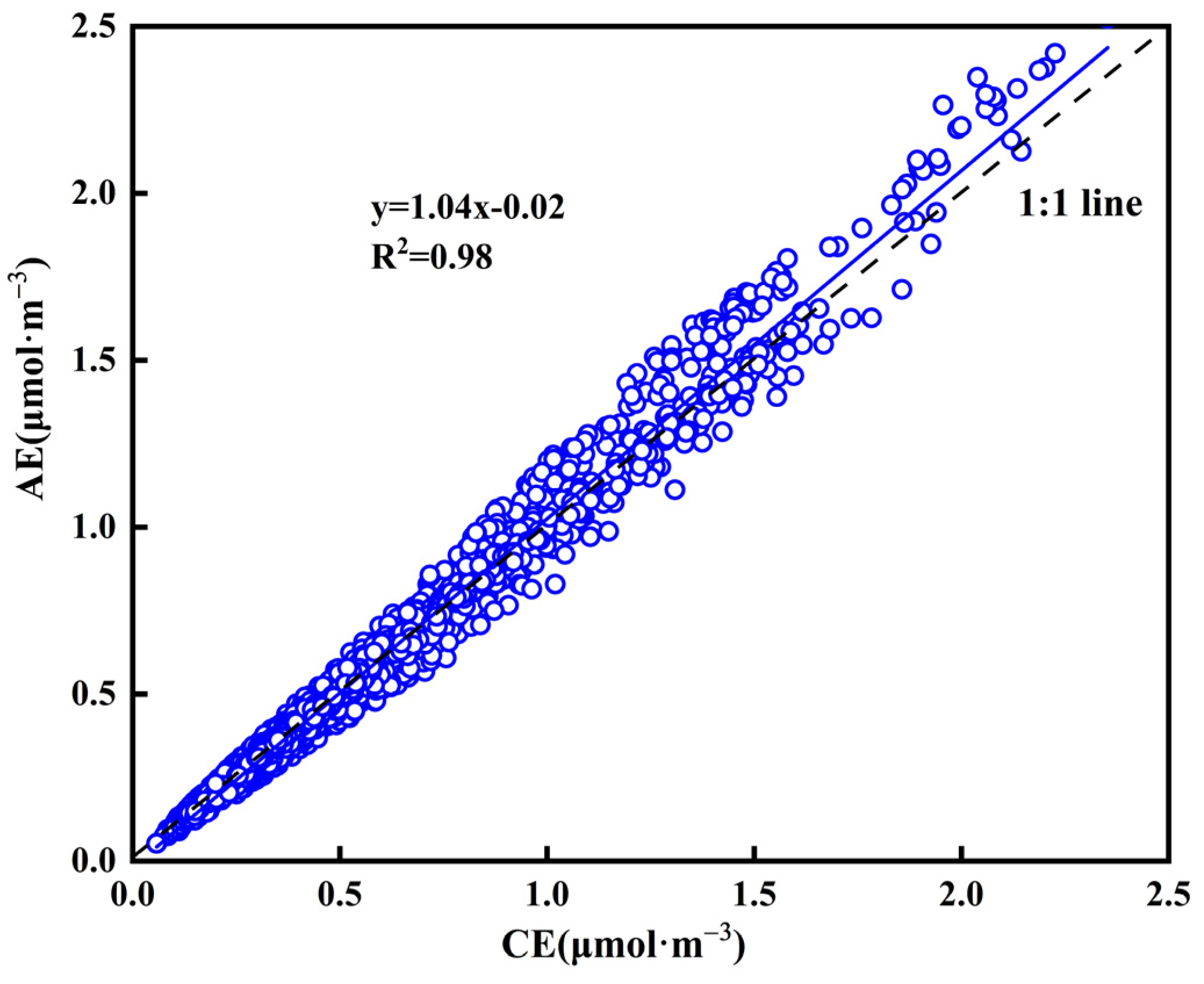


| Maximum | Minimum | Median | Mean | Standard Deviation | |
|---|---|---|---|---|---|
| Na+ | 26.4 | 0.05 | 0.18 | 0.75 | 2.21 |
| NH4+ | 38.0 | 0.10 | 5.01 | 6.26 | 4.85 |
| K+ | 19.7 | 0.09 | 0.28 | 0.50 | 1.09 |
| Mg2+ | 6.2 | 0.06 | 0.09 | 0.18 | 0.48 |
| Ca2+ | 3.6 | 0.09 | 0.25 | 0.34 | 0.29 |
| Cl− | 16.6 | 0.03 | 1.24 | 1.83 | 1.89 |
| NO3− | 75.7 | 0.12 | 8.26 | 10.9 | 9.59 |
| SO42− | 44.3 | 0.06 | 7.30 | 8.72 | 5.94 |
| total | 162.1 | 0.99 | 23.3 | 28.7 | 20.5 |
| Study Period | Method | NO3− | SO42− | NO3−/SO42− | References | Language |
|---|---|---|---|---|---|---|
| February 2001–December 2001 | Offline | 7.5 | 16.3 | 0.46 | [17] | English |
| January 2007–October 2007 | Offline | 9.1 | 28.0 | 0.33 | [43] | Chinese |
| January 2010–December 2010 | Offline | 2.8 | 16.3 | 0.17 | [44] | Chinese |
| August 2012–June 2013 | Offline | 10.3 | 30.8 | 0.33 | [45] | Chinese |
| October 2013–November 2014 | Online | 18.9 | 28.3 | 0.67 | [46] | English |
| December 2014–November 2015 | Offline | 11.8 | 14.9 | 0.79 | [18] | English |
| December 2014–April 2015 | Offline | 16.3 | 16.6 | 0.98 | [47] | English |
| July 2014–May 2015 | Offline | 15.0 | 18.0 | 0.83 | [48] | English |
| December 2015–January 2016 | Offline | 26.5 | 19.0 | 1.39 | [49] | English |
| March 2016–August 2017 | Online | 16.7 | 14.9 | 1.12 | [50] | English |
| January 2017–December 2017 | Online | 12.8 | 9.3 | 1.38 | [42] | English |
| November 2017–June 2018 | Online | 14.2 | 9.1 | 1.56 | [51] | English |
| September 2018–September 2019 | Offline | 12.5 | 9.1 | 1.37 | [52] | English |
| May 2019–October 2019 | Offline | 17.3 | 11.0 | 1.57 | [53] | Chinese |
| February 2015–May 2021 | Online | 10.9 | 8.8 | 1.24 | This work | English |
| NF | Value (μmol·m−3) |
|---|---|
| NH4+ | 0.85 |
| nssK+ | 0.05 |
| nssMg2+ | 0.02 |
| nssCa2+ | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Chen, K.; Xu, G. Characteristics and Sources of Water-Soluble Inorganic Ions in PM2.5 in Urban Nanjing, China. Atmosphere 2023, 14, 135. https://doi.org/10.3390/atmos14010135
Guo Q, Chen K, Xu G. Characteristics and Sources of Water-Soluble Inorganic Ions in PM2.5 in Urban Nanjing, China. Atmosphere. 2023; 14(1):135. https://doi.org/10.3390/atmos14010135
Chicago/Turabian StyleGuo, Qinghao, Kui Chen, and Guojie Xu. 2023. "Characteristics and Sources of Water-Soluble Inorganic Ions in PM2.5 in Urban Nanjing, China" Atmosphere 14, no. 1: 135. https://doi.org/10.3390/atmos14010135
APA StyleGuo, Q., Chen, K., & Xu, G. (2023). Characteristics and Sources of Water-Soluble Inorganic Ions in PM2.5 in Urban Nanjing, China. Atmosphere, 14(1), 135. https://doi.org/10.3390/atmos14010135









