Controlled Laboratory Generation of Atmospheric Black Carbon Using Laser Excitation-Based Soot Generator: From Basic Principles to Application Perspectives: A Review
Abstract
:1. Introduction
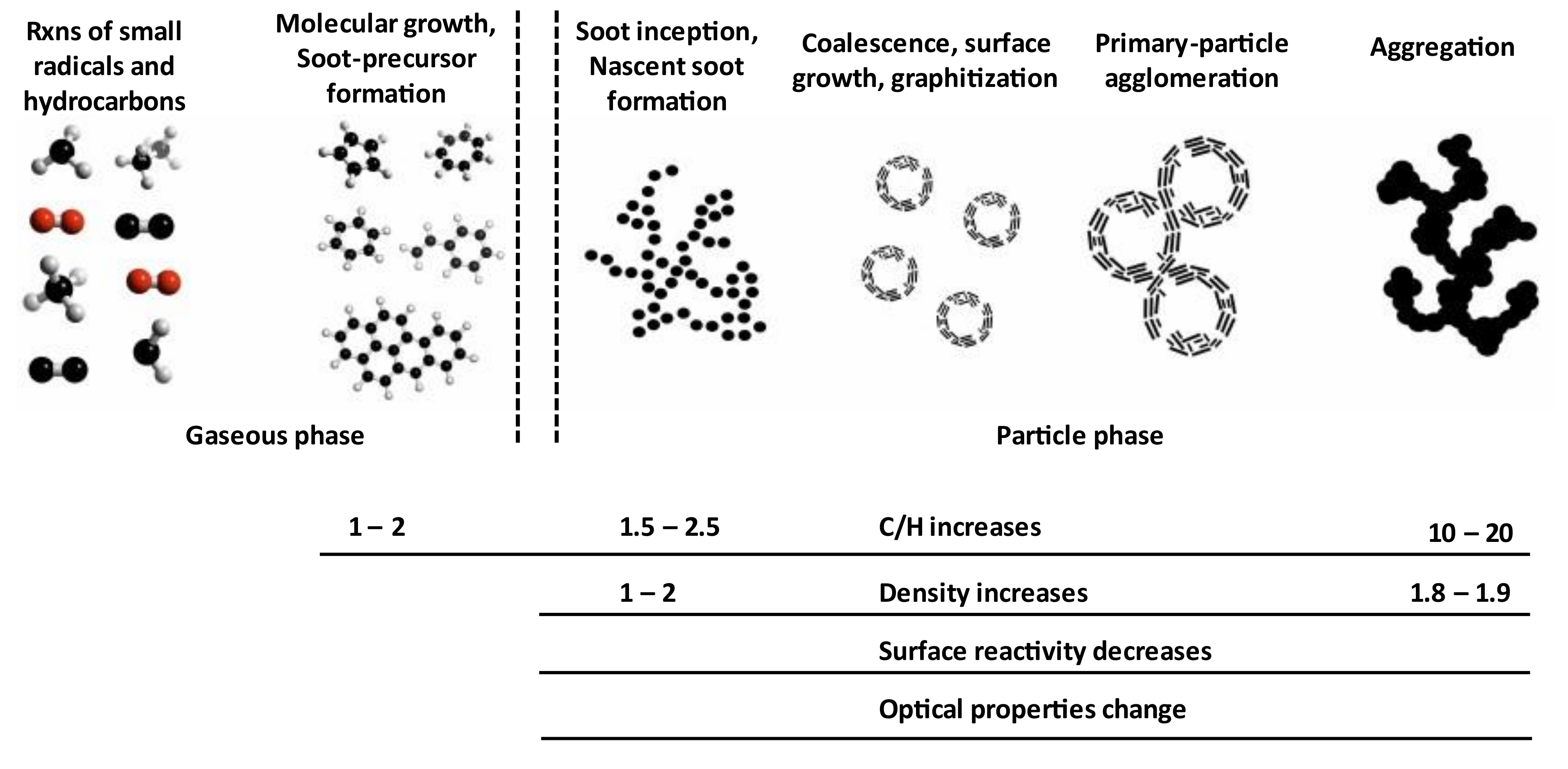
2. Soot Formation Using Different Excitation Mechanisms and Precursor Types
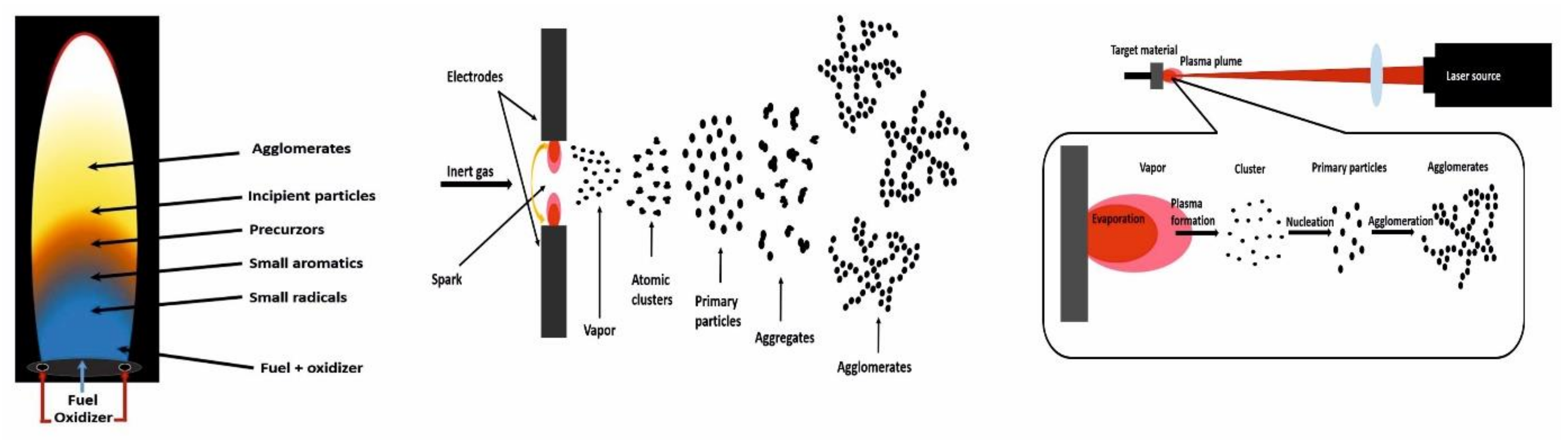
2.1. Flames
2.2. Spark Discharges
2.3. Laser Ablation
3. Experimental Set-Ups for Soot Generation with Different Mechanisms
3.1. Flames
3.2. Spark Discharges
3.3. Laser Ablation
4. Characteristic Performance of Soot Particles Using Different Excitations
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lohmann, U.; Friebel, F.; Kanji, Z.A.; Mahrt, F.; Mensah, A.A.; Neubauer, D. Future Warming Exacerbated by Aged-Soot Effect on Cloud Formation. Nat. Geosci. 2020, 13, 674–680. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; Deangelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Liu, P.; Kaplan, J.O.; Mickley, L.J.; Li, Y.; Chellman, N.J.; Arienzo, M.M.; Kodros, J.K.; Pierce, J.R.; Sigl, M.; Freitag, J.; et al. Improved Estimates of Preindustrial Biomass Burning Reduce the Magnitude of Aerosol Climate Forcing in the Southern Hemisphere. Sci. Adv. 2021, 7, eabc1379. [Google Scholar] [CrossRef]
- Costa, D.L. Historical Highlights of Air Pollution Toxicology. Toxicol. Sci. 2018, 164, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, W.; Yu, W. A Progress Review of Practical Soot Modelling Development in Diesel Engine Combustion. J. Traffic Transp. Eng. Engl. Ed. 2020, 7, 269–281. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A., III; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Martin, J.W.; Salamanca, M.; Kraft, M. Soot Inception: Carbonaceous Nanoparticle Formation in Flames. Prog. Energy Combust. Sci. 2022, 88, 100956. [Google Scholar] [CrossRef]
- Ajtai, T.; Kiss-Albert, G.; Utry, N.; Tóth, Á.; Hoffer, A.; Szabó, G.; Bozóki, Z. Diurnal Variation of Aethalometer Correction Factors and Optical Absorption Assessment of Nucleation Events Using Multi-Wavelength Photoacoustic Spectroscopy. J. Environ. Sci. China 2019, 83, 96–109. [Google Scholar] [CrossRef]
- Petzold, A.; Ogren, J.A.; Fiebig, M.; Laj, P.; Li, S.M.; Baltensperger, U.; Holzer-Popp, T.; Kinne, S.; Pappalardo, G.; Sugimoto, N.; et al. Recommendations for Reporting Black Carbon Measurements. Atmos. Chem. Phys. 2013, 13, 8365–8379. [Google Scholar] [CrossRef]
- Andreae, M.O.; Gelencsér, A. Black Carbon or Brown Carbon? The Nature of Light-Absorbing Carbonaceous Aerosols. Atmos. Chem. Phys. 2006, 6, 3131–3148. [Google Scholar] [CrossRef] [Green Version]
- Török, S.; Malmborg, V.B.; Simonsson, J.; Eriksson, A.; Martinsson, J.; Mannazhi, M.; Pagels, J.; Bengtsson, P.E. Investigation of the Absorption Ångström Exponent and Its Relation to Physicochemical Properties for Mini-CAST Soot. Aerosol Sci. Technol. 2018, 52, 757–767. [Google Scholar] [CrossRef]
- Ess, M.N.; Vasilatou, K. Characterization of a New MiniCAST with Diffusion Flame and Premixed Flame Options: Generation of Particles with High EC Content in the Size Range 30 Nm to 200 Nm. Aerosol Sci. Technol. 2019, 53, 29–44. [Google Scholar] [CrossRef]
- Mason, Y.C.; Schoonraad, G.L.; Orasche, J.; Bisig, C.; Jakobi, G.; Zimmermann, R.; Forbes, P.B.C. Comparative Sampling of Gas Phase Volatile and Semi-Volatile Organic Fuel Emissions from a Combustion Aerosol Standard System. Environ. Technol. Innov. 2020, 19, 100945. [Google Scholar] [CrossRef]
- Hagen, F.P.; Rinkenburger, A.; Günther, J.; Bockhorn, H.; Niessner, R.; Suntz, R.; Loukou, A.; Trimis, D.; Haisch, C. Spark Discharge-Generated Soot: Varying Nanostructure and Reactivity against Oxidation with Molecular Oxygen by Synthesis Conditions. J. Aerosol Sci. 2020, 143, 105530. [Google Scholar] [CrossRef]
- Bischof, O.F.; Weber, P.; Bundke, U.; Petzold, A.; Kiendler-Scharr, A. Characterization of the Miniaturized Inverted Flame Burner as a Combustion Source to Generate a Nanoparticle Calibration Aerosol. Emiss. Control. Sci. Technol. 2020, 6, 37–46. [Google Scholar] [CrossRef]
- Haller, T.; Rentenberger, C.; Meyer, J.C.; Felgitsch, L.; Grothe, H.; Hitzenberger, R. Structural Changes of CAST Soot during a Thermal-Optical Measurement Protocol. Atmos. Meas. Tech. 2019, 12, 3503–3519. [Google Scholar] [CrossRef]
- Ajtai, T.; Utry, N.; Pintér, M.; Kiss-Albert, G.; Puskás, R.; Tápai, C.; Kecskeméti, G.; Smausz, T.; Hopp, B.; Bozóki, Z.; et al. Microphysical Properties of Carbonaceous Aerosol Particles Generated by Laser Ablation of a Graphite Target. Atmos. Meas. Tech. 2015, 8, 1207–1215. [Google Scholar] [CrossRef]
- Preining, O.D.E.J.; der, W.Ö.A. (Eds.) History of Aerosol Science: Proceedings of Symposium on the History of Aerosol Science Held in Vienna; Österreichische Akademie der Wissenschaften: Vienna, Austria, 2000. [Google Scholar]
- Faccinetto, A.; Irimiea, C.; Minutolo, P.; Commodo, M.; D’anna, A.; Nuns, N.; Carpentier, Y.; Pirim, C.; Desgroux, P.; Focsa, C.; et al. Evidence on the formation of dimers of polycyclic aromatic hydrocarbons in a laminar diffusion flame. Commun. Chem. 2020, 3, 112. [Google Scholar] [CrossRef]
- Sabbah, H.; Commodo, M.; Picca, F.; De Falco, G.; Minutolo, P.; D’Anna, A.; Joblin, C. Molecular content of nascent soot: Family characterization using two-step laser desorption laser ionization mass spectrometry. Proc. Combust. Inst. 2021, 38, 1241–1248. [Google Scholar] [CrossRef]
- Michelsen, H.A. Effects of maturity and temperature on soot density and specific heat. Proc. Combust. Inst. 2021, 38, 1197–1205. [Google Scholar] [CrossRef]
- Liu, C.; Singh, A.V.; Saggese, C.; Tang, Q.; Chen, D.; Wan, K.; Vinciguerra, M.; Comodo, M.; De Falco, G.; Minutolo, P.; et al. Flame-formed carbon nanoparticles exhibit quantum dot behaviors. Proc. Natl. Acad. Sci. USA 2019, 116, 12692–12697. [Google Scholar] [CrossRef] [PubMed]
- Constantine, M.M.; Richard, A.D. Comparison of soot growth and oxidation in smoking and non–smoking ethylene diffusion flames. Combust. Sci. Technol. 1989, 66, 1–16. [Google Scholar] [CrossRef]
- Helsper, C.; Mölter, W.; Löffler, F.; Wadenpohl, C.; Kaufmann, S.; Wenninger, G. Investigations of a New Aerosol Generator for the Production of Carbon Aggregate Particles. Atmos. Environ. Part A Gen. Top. 1993, 27, 1271–1275. [Google Scholar] [CrossRef]
- Baumgardner, D.; Popovicheva, O.; Allan, J.; Bernardoni, V.; Cao, J.; Cavalli, F.; Cozic, J.; Diapouli, E.; Eleftheriadis, K.; Genberg, P.J.; et al. Soot Reference Materials for Instrument Calibration and Intercomparisons: A Workshop Summary with Recommendations. Atmos. Meas. Tech. 2012, 5, 1869–1887. [Google Scholar] [CrossRef]
- Kohut, A.; Ludvigsson, L.; Meuller, B.O.; Deppert, K.; Messing, M.E.; Galbács, G.; Geretovszky, Z. From Plasma to Nanoparticles: Optical and Particle Emission of a Spark Discharge Generator. Nanotechnology 2017, 28, 475603. [Google Scholar] [CrossRef]
- Feng, J.; Huang, L.; Ludvigsson, L.; Messing, M.E.; Maisser, A.; Biskos, G.; Schmidt-Ott, A. General Approach to the Evolution of Singlet Nanoparticles from a Rapidly Quenched Point Source. J. Phys. Chem. C 2016, 120, 621–630. [Google Scholar] [CrossRef]
- Hakjoon, K.; Jinho, K.; Youngjoo, C.; Hyenchul, O.; Jungbum, C.; Sangsoo, K. Generation of Model Diesel Particles by Spark Discharge and Hydrocarbon Condensation. J. Mech. Sci. Technol. 2006, 20, 1972–1979. [Google Scholar] [CrossRef]
- Roth, C.; Ferron, G.A.; Karg, E.; Lentner, B.; Schumann, G.; Takenaka, S.; Heyder, J. Generation of Ultrafine Particles by Spark Discharging. Aerosol Sci. Technol. 2010, 38, 228–235. [Google Scholar] [CrossRef]
- Itina, T.E.; Voloshko, A. Nanoparticle Formation by Laser Ablation in Air and by Spark Discharges at Atmospheric Pressure. Appl. Phys. B Lasers Opt. 2013, 113, 473–478. [Google Scholar] [CrossRef]
- Garrison, B.J.; Itina, T.E.; Zhigilei, L.V. Limit of Overheating and the Threshold Behavior in Laser Ablation. Phys. Rev. E 2003, 68, 041501. [Google Scholar] [CrossRef] [Green Version]
- Vidal, F.; Johnston, T.W.; Laville, S.; Barthélemy, O.; Chaker, M.; le Drogoff, B.; Margot, J.; Sabsabi, M. Critical-Point Phase Separation in Laser Ablation of Conductors. Phys. Rev. Lett. 2001, 86, 2573. [Google Scholar] [CrossRef] [PubMed]
- Raizer, Y.P. Gas Discharge Physics; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar] [CrossRef]
- Kohut, A.; Galbács, G.; Márton, Z.; Geretovszky, Z. Characterization of a Copper Spark Discharge Plasma in Argon Atmosphere Used for Nanoparticle Generation. Plasma Sources Sci. Technol. 2017, 26, 045001. [Google Scholar] [CrossRef]
- Pintér, M.; Ajtai, T.; Kiss-Albert, G.; Utry, N.; Kiss, D.; Smausz, T.; Kohut, A.; Hopp, B.; Galbács, G.; Kukovecz, Á.; et al. Thermo-Optical Properties of Residential Coals and Combustion Aerosols. Atmos. Environ. 2018, 178, 118–128. [Google Scholar] [CrossRef]
- Utry, N.; Ajtai, T.; Pintér, M.; Bozóki, Z.; Szabó, G. Wavelength-Dependent Optical Absorption Properties of Artificial and Atmospheric Aerosol Measured by a Multi-Wavelength Photoacoustic Spectrometer. Int. J. Thermophys. 2014, 35, 2246–2258. [Google Scholar] [CrossRef]
- Ajtai, T.; Filep, Á.; Kecskeméti, G.; Hopp, B.; Bozóki, Z.; Szabó, G.; Ajtai, T.; Filep, Á.; Kecskeméti, G.; Szabó, G.; et al. Wavelength Dependent Mass-Specific Optical Absorption Coefficients of Laser Generated Coal Aerosols Determined from Multi-Wavelength Photoacoustic Measurements. Appl. Phys. A 2010, 103, 1165–1172. [Google Scholar] [CrossRef]
- Bockhorn, H. (Ed.) Soot Formation in Combustion; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Bockhorn, H.; D’Anna, A.; Sarofim, A.F.; Wang, H. (Eds.) Combustion Generated Fine Carbonaceous Particles; Universitätsverlag Karlsruhe: Karlsruhe, Germany, 2009. [Google Scholar]
- Xi, J.; Yang, G.; Cai, J.; Gu, Z. A Review of Recent Research Results on Soot: The Formation of a Kind of Carbon-Based Material in Flames. Front. Mater. 2021, 8, 179. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.H. Soot formation in laminar counterflow flames. Prog. Energy Combust. Sci. 2019, 74, 152–238. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Shi, D.; Huang, Y.; Zhou, L. Study of soot formations in co-flow laminar diffusion flames of n-heptane and oxygenated aromatic biofuels from atmospheric condition to 2.3 bar. Fuel 2021, 297, 120753. [Google Scholar] [CrossRef]
- Sun, Z.; Dally, B.; Alwahabi, Z.; Nathan, G. The effect of oxygen concentration in the co-flow of laminar ethylene diffusion flames. Combust. Flame 2020, 211, 96–111. [Google Scholar] [CrossRef]
- Horvath, H.; Gangl, M. A Low-Voltage Spark Generator for Production of Carbon Particles. J. Aerosol Sci. 2003, 34, 1581–1588. [Google Scholar] [CrossRef]
- Feng, J.; Hontañón, E.; Blanes, M.; Meyer, J.; Guo, X.; Santos, L.; Paltrinieri, L.; Ramlawi, N.; Smet, L.C.P.M.D.; Nirschl, H.; et al. Scalable and Environmentally Benign Process for Smart Textile Nanofinishing. ACS Appl. Mater. Interfaces 2016, 8, 14756–14765. [Google Scholar] [CrossRef] [PubMed]
- Schnaiter, M.; Horvath, H.; Möhler, O.; Naumann, K.H.; Saathoff, H.; Schöck, O.W. UV-VIS-NIR Spectral Optical Properties of Soot and Soot-Containing Aerosols. J. Aerosol Sci. 2003, 34, 1421–1444. [Google Scholar] [CrossRef]
- You, R.; Radney, J.G.; Zachariah, M.R.; Zangmeister, C.D. Measured Wavelength-Dependent Absorption Enhancement of Internally Mixed Black Carbon with Absorbing and Nonabsorbing Materials. Environ. Sci. Technol. 2016, 50, 7982–7990. [Google Scholar] [CrossRef]
- Horender, S.; Auderset, K.; Vasilatou, K. Facility for Calibration of Optical and Condensation Particle Counters Based on a Turbulent Aerosol Mixing Tube and a Reference Optical Particle Counter. Rev. Sci. Instrum. 2019, 90, 075111. [Google Scholar] [CrossRef] [PubMed]
- Moosmüller, H.; Chakrabarty, R.K.; Arnott, W.P. Aerosol Light Absorption and Its Measurement: A Review. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 844–878. [Google Scholar] [CrossRef]
- Bescond, A.; Yon, J.; Ouf, F.X.; Rozé, C.; Coppalle, A.; Parent, P.; Ferry, D.; Laffon, C. Soot Optical Properties Determined by Analyzing Extinction Spectra in the Visible Near-UV: Toward an Optical Speciation According to Constituents and Structure. J. Aerosol Sci. 2016, 101, 118–132. [Google Scholar] [CrossRef]
- Lefevre, G.; Yon, J.; Liu, F.; Coppalle, A. Spectrally Resolved Light Extinction Enhancement of Coated Soot Particles. Atmos. Environ. 2018, 186, 89–101. [Google Scholar] [CrossRef]
- Malmborg, V.; Eriksson, A.; Gren, L.; Török, S.; Shamun, S.; Novakovic, M.; Zhang, Y.; Kook, S.; Tunér, M.; Bengtsson, P.E.; et al. Characteristics of BrC and BC Emissions from Controlled Diffusion Flame and Diesel Engine Combustion. Aerosol Sci. Technol. 2021, 55, 769–784. [Google Scholar] [CrossRef]
- Ess, M.N.; Bertò, M.; Irwin, M.; Modini, R.L.; Gysel-Beer, M.; Vasilatou, K. Optical and Morphological Properties of Soot Particles Generated by the MiniCAST 5201 BC Generator. Aerosol Sci. Technol. 2021, 55, 828–847. [Google Scholar] [CrossRef]
- Byeon, J.H.; Park, J.H.; Yoon, K.Y.; Ko, B.J.; Ji, J.H.; Hwang, J. Removal of Volatile Organic Compounds by Spark Generated Carbon Aerosol Particles. Carbon 2006, 44, 2106–2108. [Google Scholar] [CrossRef]
- Chae, S.; Lee, D.; Kim, M.C.; Kim, D.S.; Choi, M. Wire-in-Hole-Type Spark Discharge Generator for Long-Time Consistent Generation of Unagglomerated Nanoparticles. Aerosol Sci. Technol. 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; de Luca, O.; Rudolf, P.; Zhang, B.; Sabia, P.; de Joannon, M. Easy tuning of nanotexture and N doping of carbonaceous particles produced by spark discharge. Carbon Trends 2021, 5, 100134. [Google Scholar] [CrossRef]
- Burtscher, H. Application of a Spark Discharge Generator for Production of Combustion-Like Aerosols. In Spark Ablation; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2020; pp. 421–445. [Google Scholar] [CrossRef]
- Jeong, B.; Lee, J. Effective Density and Light Absorption Cross Section of Black Carbon Generated in a Spark Discharger. J. Aerosol Sci. 2017, 107, 55–64. [Google Scholar] [CrossRef]
- An, P.; Sun, W.; Li, G.; Tan, M.; Lai, C.; Chen, S. Characteristics of Particle Size Distributions About Emissions in A Common-Rail Diesel Engine with Biodiesel Blends. Procedia Environ. Sci. 2011, 11, 1371–1378. [Google Scholar] [CrossRef]
- Ess, M.N.; Bladt, H.; Mühlbauer, W.; Seher, S.I.; Zöllner, C.; Lorenz, S.; Brüggemann, D.; Nieken, U.; Ivleva, N.P.; Niessner, R. Reactivity and Structure of Soot Generated at Varying Biofuel Content and Engine Operating Parameters. Combust. Flame 2016, 163, 157–169. [Google Scholar] [CrossRef]
- Caroca, J.C.; Millo, F.; Vezza, D.; Vlachos, T.; de Filippo, A.; Bensaid, S.; Russo, N.; Fino, D. Detailed Investigation on Soot Particle Size Distribution during DPF Regeneration, Using Standard and Bio-Diesel Fuels. Ind. Eng. Chem. Res. 2011, 50, 2650–2658. [Google Scholar] [CrossRef]
- Kittelson, D.B. Engines and Nanoparticles: A Review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Lu, T.; Huang, Z.; Cheung, C.S.; Ma, J. Size Distribution of EC, OC and Particle-Phase PAHs Emissions from a Diesel Engine Fueled with Three Fuels. Sci. Total Environ. 2012, 438, 33–41. [Google Scholar] [CrossRef]
- Abegglen, M.; Brem, B.T.; Ellenrieder, M.; Durdina, L.; Rindlisbacher, T.; Wang, J.; Lohmann, U.; Sierau, B. Chemical Characterization of Freshly Emitted Particulate Matter from Aircraft Exhaust Using Single Particle Mass Spectrometry. Atmos. Environ. 2016, 134, 181–197. [Google Scholar] [CrossRef]
- Lobo, P.; Durdina, L.; Smallwood, G.J.; Rindlisbacher, T.; Siegerist, F.; Black, E.A.; Yu, Z.; Mensah, A.A.; Hagen, D.E.; Miake-Lye, R.C.; et al. Measurement of Aircraft Engine Non-Volatile PM Emissions: Results of the Aviation-Particle Regulatory Instrumentation Demonstration Experiment (A-PRIDE) 4 Campaign. Aerosol Sci. Technol. 2015, 49, 472–484. [Google Scholar] [CrossRef]
- Popovitcheva, O.B.; Persiantseva, N.M.; Trukhin, M.E.; Rulev, G.B.; Shonija, N.K.; Buriko, Y.Y.; Starik, A.M.; Demirdjian, B.; Ferry, D.; Suzanne, J. Experimental Characterization of Aircraft Combustor Soot: Microstructure, Surface Area, Porosity and Water Adsorption. Phys. Chem. Chem. Phys. 2000, 2, 4421–4426. [Google Scholar] [CrossRef]
- Durdina, L.; Lobo, P.; Trueblood, M.B.; Black, E.A.; Achterberg, S.; Hagen, D.E.; Brem, B.T.; Wang, J. Response of Real-Time Black Carbon Mass Instruments to Mini-CAST Soot. Aerosol Sci. Technol. 2016, 50, 906–918. [Google Scholar] [CrossRef]
- Cheng, M.D.; Corporan, E.; Dewitt, M.J.; Landgraf, B. Emissions of Volatile Particulate Components from Turboshaft Engines Operated with JP-8 and Fischer-Tropsch Fuels. Aerosol Air Qual. Res. 2009, 9, 237–256. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; You, R.; Lunny, E.M.; Jacobson, A.E.; Okumura, M.; Zachariah, M.R.; Radney, J.G. Measured In-Situ Mass Absorption Spectra for Nine Forms of Highly-Absorbing Carbonaceous Aerosol. Carbon 2018, 136, 85–93. [Google Scholar] [CrossRef]
- Drinovec, L.; Gregoric, A.; Zotter, P.; Wolf, R.; Anne Bruns, E.; Bruns, E.A.; Prevot, A.S.H.; Favez, O.; Sciare, J.; Arnold, I.J.; et al. The Filter-Loading Effect by Ambient Aerosols in Filter Absorption Photometers Depends on the Coating of the Sampled Particles. Atmos. Meas. Tech. 2017, 10, 1043–1059. [Google Scholar] [CrossRef]
- Zhang, G.; Peng, L.; Lian, X.; Lin, Q.; Bi, X.; Chen, D.; Li, M.; Li, L.; Wang, X.; Sheng, G. An Improved Absorption Ångström Exponent (AAE)-Based Method for Evaluating the Contribution of Light Absorption from Brown Carbon with a High-Time Resolution. Aerosol Air Qual. Res. 2019, 19, 15–24. [Google Scholar] [CrossRef]
- Costabile, F.; Alas, H.; Aufderheide, M.; Avino, P.; Amato, F.; Argentini, S.; Barnaba, F.; Berico, M.; Bernardoni, V.; Biondi, R.; et al. First Results of the “Carbonaceous Aerosol in Rome and Environs (CARE)” Experiment: Beyond Current Standards for PM10. Atmosphere 2017, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.H.; Ziemba, L.D.; Dutcher, D.; Beyersdorf, A.J.; Chan, K.; Crumeyrolle, S.; Raymond, T.M.; Thornhill, K.L.; Winstead, E.L.; Anderson, B.E. Mapping the Operation of the Miniature Combustion Aerosol Standard (Mini-CAST) Soot Generator. Aerosol Sci. Technol. 2014, 48, 467–479. [Google Scholar] [CrossRef]
- Kuznetsov, B.v.; Rakhmanova, T.A.; Popovicheva, O.B.; Shonija, N.K. Water Adsorption and Energetic Properties of Spark Discharge Soot: Specific Features of Hydrophilicity. J. Aerosol Sci. 2003, 34, 1465–1479. [Google Scholar] [CrossRef]
- Palásti, D.J.; Metzinger, A.; Ajtai, T.; Bozóki, Z.; Hopp, B.; Kovács-Széles, E.; Galbács, G. Qualitative Discrimination of Coal Aerosols by Using the Statistical Evaluation of Laser-Induced Breakdown Spectroscopy Data. Spectrochim. Acta Part B At. Spectrosc. 2019, 153, 34–41. [Google Scholar] [CrossRef]
- Tumolva, L.; Park, J.Y.; Kim, J.S.; Miller, A.L.; Chow, J.C.; Watson, J.G.; Park, K. Morphological and Elemental Classification of Freshly Emitted Soot Particles and Atmospheric Ultrafine Particles Using the TEM/EDS. Aerosol Sci. Technol. 2010, 44, 202–215. [Google Scholar] [CrossRef]
- Utry, N.; Ajtai, T.; Filep, Á.; Pintér, M.; Török, Z.; Bozóki, Z.; Szabó, G. Correlations between Absorption Angström Exponent (AAE) of Wintertime Ambient Urban Aerosol and Its Physical and Chemical Properties. Atmos. Environ. 2014, 91, 52–59. [Google Scholar] [CrossRef]
- Ajtai, T.; Utry, N.; Pintér, M.; Major, B.; Bozóki, Z.; Szabó, G. A Method for Segregating the Optical Absorption Properties and the Mass Concentration of Winter Time Urban Aerosol. Atmos. Environ. 2015, 122, 313–320. [Google Scholar] [CrossRef]

| Property | miniCAST BC Soot | Diesel Soot | Aircaft Soot | Atmospheric Measurement | Spark Discharge GFG1000 | Laser Ablation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diffusion Flame | Premixed Flame | |||||||||
| C/O < 0.25 | 0.25 < C/O < 0.31 | C/O < 0.31 | C/O < 0.3 | C/O < 0.3 | ||||||
| GMD [nm] | ≤60–180 | 180–210 | ≤40–160 | ≤30–180 | ≤40–180 | 5–20, 30–150 | 10–50 | 175.56 | ||
| Number concentration [#/cm3] | 2 × 105–3 × 107 | 1 × 107–3 × 107 | 1 × 107–5 × 107 | 2 × 106–3 × 107 | 7 × 106–4 × 107 | 105–109–106/108 | ∼1013–1015 #/kg fuel | 6 × 106 | 107 | 107 |
| Mass concentration [mg/m3] | 1–60 eBC (880 nm) | 40–180 eBC (880 nm) | 0.2–130 eBC (880 nm) | 0.1–160 eBC (880 nm) | 0.4–150 eBC (880 nm) | 0.1–103 | 0.5–100 mg/kg fuel 20–200 TC | 2.6–2.9 µg/m3 eBC | ||
| Primary particle diameter [nm] | 15–45 20–30 | 16–27 | 20–50 | na | 5–7 15–36 5–10 | 7–13.7 | ||||
| Fractal dimension [–] | 2.1–2.2 | 1.7–2 | na | 2 | 1.65–2.1 | |||||
| AAE [–] | ≤1.4 ± 0.2 | ≤1.2 ± 0.1 1.25 (1064–266) | 1.1–4.5 | 1.1–1.7 | 1.1–4.5 | 1.1 (450–700 nm) 1.3 1.3 (1064–266) 1.04 (370–950 nm) | – | 2.25 (355––1064 nm) 2.23 (370–950) 2 (1064–266) (Winter season) 1.55 (370–950) 1–1.6 (370–950 nm) 1–2.03 (450–660 nm Aethalometer) | 2.1 1.8 (1064–266 nm) | 1.15 (1064–355 nm) 1.04 (1064–266) |
| EC/TC [%] | <70–95 | <90 | 2–60 | 50–95 | 1–90 | 60–85 | ∼10–70 80–100 | – | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajtai, T.; Kohut, A.; Raffai, P.; Szabó, G.; Bozóki, Z. Controlled Laboratory Generation of Atmospheric Black Carbon Using Laser Excitation-Based Soot Generator: From Basic Principles to Application Perspectives: A Review. Atmosphere 2022, 13, 1366. https://doi.org/10.3390/atmos13091366
Ajtai T, Kohut A, Raffai P, Szabó G, Bozóki Z. Controlled Laboratory Generation of Atmospheric Black Carbon Using Laser Excitation-Based Soot Generator: From Basic Principles to Application Perspectives: A Review. Atmosphere. 2022; 13(9):1366. https://doi.org/10.3390/atmos13091366
Chicago/Turabian StyleAjtai, Tibor, Attila Kohut, Péter Raffai, Gábor Szabó, and Zoltán Bozóki. 2022. "Controlled Laboratory Generation of Atmospheric Black Carbon Using Laser Excitation-Based Soot Generator: From Basic Principles to Application Perspectives: A Review" Atmosphere 13, no. 9: 1366. https://doi.org/10.3390/atmos13091366
APA StyleAjtai, T., Kohut, A., Raffai, P., Szabó, G., & Bozóki, Z. (2022). Controlled Laboratory Generation of Atmospheric Black Carbon Using Laser Excitation-Based Soot Generator: From Basic Principles to Application Perspectives: A Review. Atmosphere, 13(9), 1366. https://doi.org/10.3390/atmos13091366






