Investigation of the Gas-Phase Reaction of Nopinone with OH Radicals: Experimental and Theoretical Study
Abstract
:1. Introduction
2. Methodology
2.1. Experimental
2.2. Computational Details
3. Results and Discussion
3.1. Experimental Kinetic Study
3.2. Theoretical Study
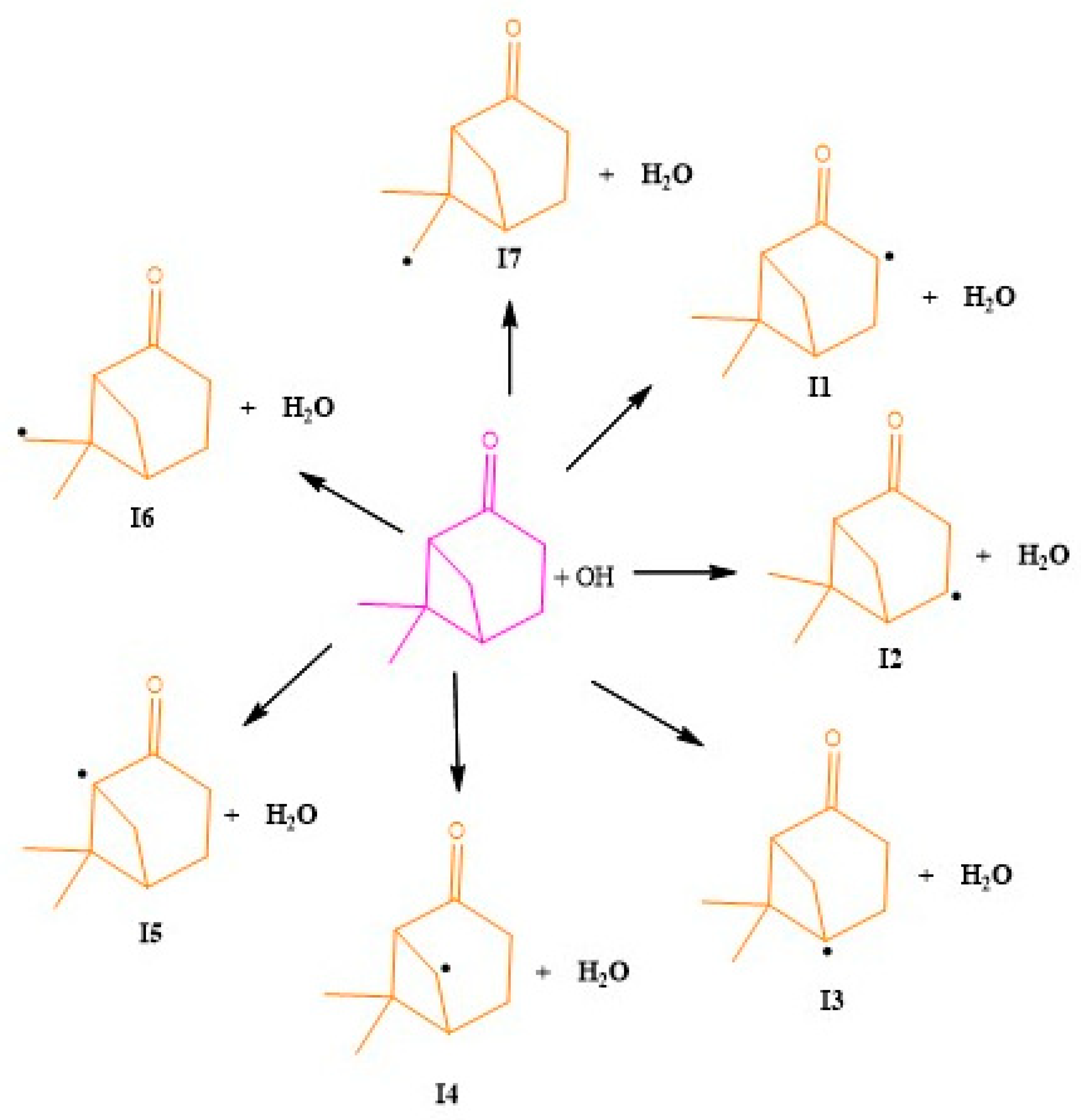
- Initial H-atom abstraction from the -CH2 group (Pathways 1, 2 and 4):
- Initial H-atom abstraction from the -CH group (Pathways 3 and 5):
- Initial H-atom abstraction from the -CH3 group (Pathways 6 and 7):
- Secondary reaction mechanism of nopinone with OH radicals:
- Kinetics of Nopinone with OH radicals:
4. Atmospheric Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, R.; Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Hoffmann, T.; Odum, J.R.; Bowman, F.; Collins, D.; Klockow, D.; Flagan, R.C.; Seinfeld, H.J. Formation of Organic Aerosols from the Oxidation of Biogenic Hydrocarbons. J. Atmos. Chem. 1997, 26, 189–222. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Hamilton, J.F.; Lewis, A.C.; Carey, T.J.; Wenger, J.C.; Garcia, E.B.i.; Muñoz, A. Reactive oxidation products promote secondary organic aerosol formation from green leaf volatiles. Atmos. Chem. Phys. 2009, 9, 3815–3823. [Google Scholar] [CrossRef]
- Grossmann, D.; Moortgat, G.K.; Kibler, M.; Schlomski, S.; Bächmann, K.; Alicke, B.; Geyer, A.; Platt, U.; Hammer, M.-U.; Vogel, B.; et al. Hydrogen peroxide, organic peroxides, carbonyl compounds, and organic acids measured at Pabstthum during BERLIOZ. J. Geophys. Res. Atmos. 2003, 108, 001096. [Google Scholar] [CrossRef]
- Calogirou, A.; Jensen, N.R.; Nielsen, C.J.; Kotzias, D.; Hjorth, J. Gas-Phase Reactions of Nopinone, 3-Isopropenyl-6-oxo-heptanal, and 5-Methyl-5-vinyltetrahydrofuran-2-ol with OH, NO3, and Ozone. Environ. Sci. Technol. 1999, 33, 453–460. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M. Atmospheric chemistry of the monoterpene reaction products nopinone, camphenilone, and 4-acetyl-1-methylcyclohexene. J. Atmos. Chem. 1990, 16, 337–348. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Izumi, K.; Fukuyama, T.; Akimoto, H.; Washida, N. Reactions of OH with α-pinene and β-pinene in air: Estimate of global CO production from the atmospheric oxidation of terpenes. J. Geophys. Res. Atmos. 1991, 96, 947–958. [Google Scholar] [CrossRef]
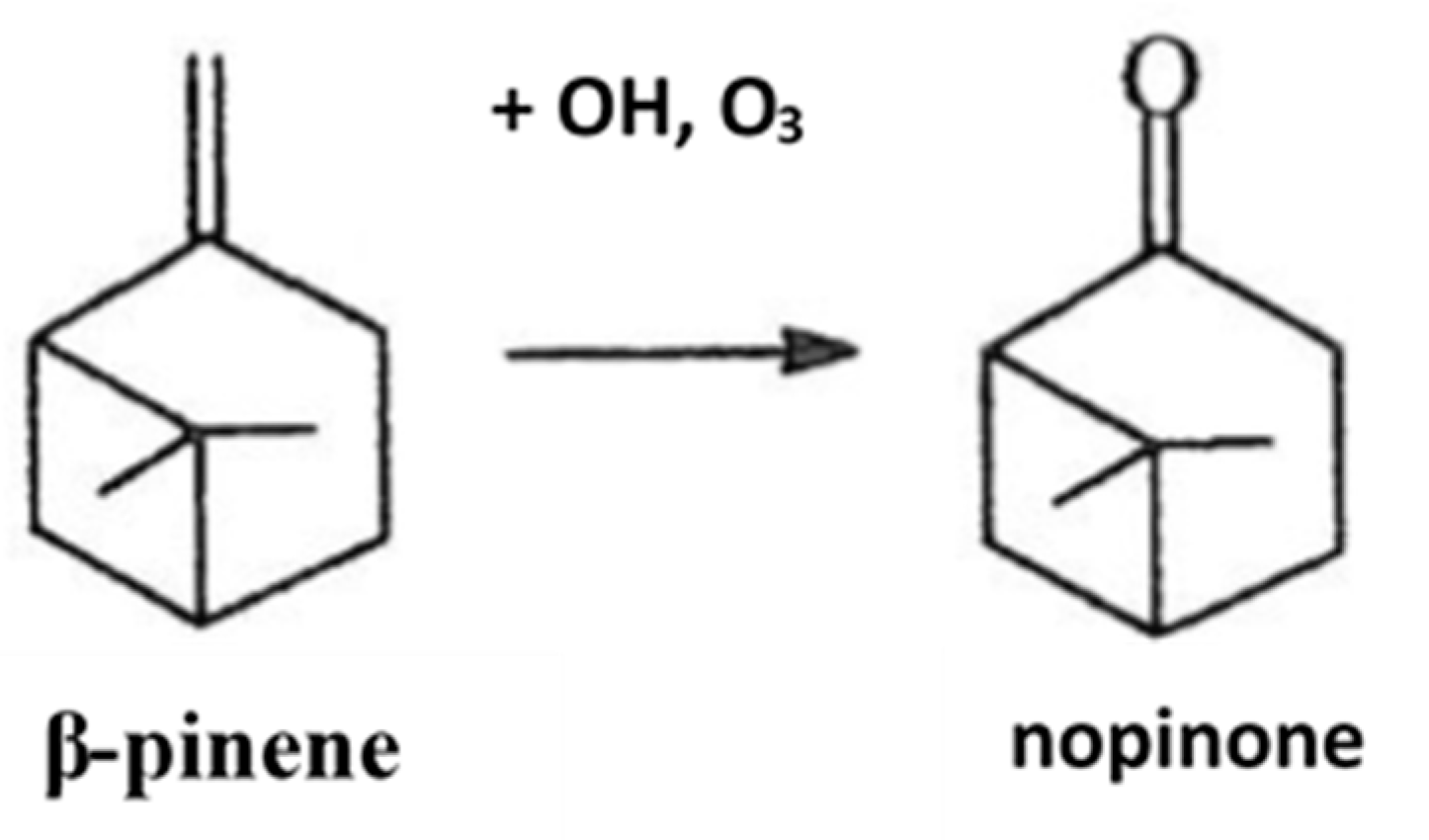
- Stolle, A. Synthesis of Nopinone from β-Pinene—A Journey Revisiting Methods for Oxidative Cleavage of C=C Bonds in Terpenoid Chemistry. Eur. J. Org. Chem. 2013, 2013, 2265–2278. [Google Scholar] [CrossRef]
- Lewis, P.J.; Bennett, K.A.; Harvey, J.N. A computational study of the atmospheric oxidation of nopinone. Phys. Chem. Chem. Phys. 2005, 7, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Dib, G.E.; Aazaad, B.; Lakshmipathi, S.; Laversin, H.; Roth, E.; Chakir, A. An experimental and theoretical study on the kinetics of the reaction between 4-hydroxy-3-hexanone CH3CH2C(O)CH(OH)CH2CH3 and OH radicals. Int. J. Chem. Kinet. 2018, 50, 556–567. [Google Scholar] [CrossRef]
- Priya, A.M.; Lakshmipathi, S.; Chakir, A.; El Dib, G. First Experimental and Theoretical Kinetic Study of the Reaction of 4-Hydroxy-4-methyl 2-pentanone as a Function of Temperature. Int. J. Chem. Kinet. 2016, 48, 584–600. [Google Scholar] [CrossRef]
- Bouzidi, H.; Aslan, L.; El Dib, G.; Coddeville, P.; Fittschen, C.; Tomas, A. Investigation of the Gas-Phase Photolysis and Temperature-Dependent OH Reaction Kinetics of 4-Hydroxy-2-butanone. Environ. Sci. Technol. 2015, 549, 12178–12186. [Google Scholar] [CrossRef]
- El Dib, G.; Sleiman, C.; Canosa, A.; Travers, D.; Courbe, J.; Sawaya, T.; Mokbel, I.; Chakir, A. First Experimental Determination of the Absolute Gas-Phase Rate Coefficient for the Reaction of OH with 4-Hydroxy-2-Butanone (4H2B) at 294 K by Vapor Pressure Measurements of 4H2B. J. Phys. Chem. A 2013, 117, 117–125. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Xie, H.-B.; Li, C.; He, N.; Wang, C.; Zhang, S.; Chen, J. Atmospheric Chemical Reactions of Monoethanolamine Initiated by OH Radical: Mechanistic and Kinetic Study. Environ. Sci. Technol. 2014, 48, 1700–1706. [Google Scholar] [CrossRef]
- Arathala, P.; Musah, R.A. Computational Study Investigating the Atmospheric Oxidation Mechanism and Kinetics of Dipropyl Thiosulfinate Initiated by OH Radicals and the Fate of Propanethiyl Radical. J. Phys. Chem. A 2020, 124, 8292–8304. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal. Coord. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Generalized Transition State Theory. Bond Energy-Bond Order Method for Canonical Variational Calculations with Application to Hydrogen Atom Transfer Reactions. J. Am. Chem. Soc. 1979, 101, 4534–4548. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Criterion of minimum state density in the transition state theory of bimolecular reactions. J. Chem. Phys. 1979, 70, 1593–1598. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G.; Grev, R.S.; Magnuson, A.W. Improved treatment of threshold contributions in variational transition-state theory. J. Phys. Chem. 1980, 84, 1730–1748. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, S.; Lynch, B.; Corchado, J.; Chuang, Y.; Fast, P.; Hu, W.; Liu, Y.; Lynch, G.; Nguyen, K. Polyrate; Version 2010-a; University of Minnesota: Minneapolis, MN, USA, 2010. [Google Scholar]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Priya, A.M.; Lakshmipathi, S. DFT study on abstraction reaction mechanism of oh radical with 2-methoxyphenol. J. Phys. Org. Chem. 2017, 30, e3713. [Google Scholar] [CrossRef]
- Priya, M.; Senthilkumar, L. Degradation of methyl salicylate through Cl initiated atmospheric oxidation—A theoretical study. RSC Adv. 2014, 4, 23464–23475. [Google Scholar] [CrossRef]
- Kurylo, M.J.; Orkin, V.L. Determination of Atmospheric Lifetimes via the Measurement of OH Radical Kinetics. Chem. Rev. 2003, 103, 5049–5076. [Google Scholar] [CrossRef] [PubMed]
- Arathala, P.; Musah, R.A. Thermochemistry and Kinetics of the Atmospheric Oxidation Reactions of Propanesulfinyl Chloride Initiated by OH Radicals: A Computational Approach. J. Phys. Chem. A 2022, 126, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F., Jr.; Kerr, J.A.; Rossi, M.J.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement VI. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1997, 26, 1329–1499. [Google Scholar] [CrossRef]


 Theoretical values (this work) in the range of 278–350 K;
Theoretical values (this work) in the range of 278–350 K;  Experimental rate constant values (this work);
Experimental rate constant values (this work);  Experimental rate constant obtained by Calogirou et al. (1999) [7];
Experimental rate constant obtained by Calogirou et al. (1999) [7];  Experimental rate constant obtained by Atkinson et al. (1993) [8].
Experimental rate constant obtained by Atkinson et al. (1993) [8].
 Theoretical values (this work) in the range of 278–350 K;
Theoretical values (this work) in the range of 278–350 K;  Experimental rate constant values (this work);
Experimental rate constant values (this work);  Experimental rate constant obtained by Calogirou et al. (1999) [7];
Experimental rate constant obtained by Calogirou et al. (1999) [7];  Experimental rate constant obtained by Atkinson et al. (1993) [8].
Experimental rate constant obtained by Atkinson et al. (1993) [8].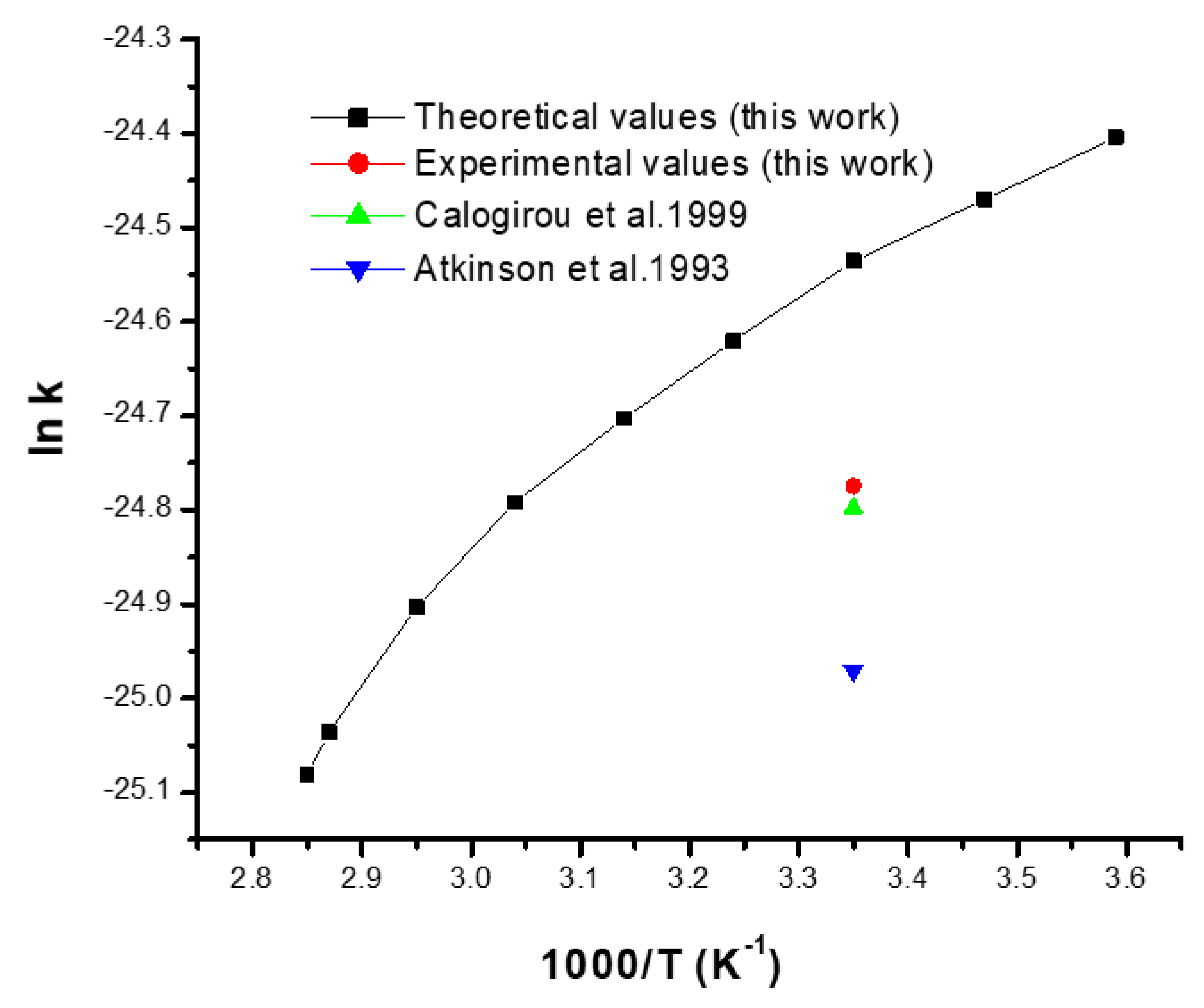
| Pressure /Torr | Linear Velocity /cm s−1 | [OH]0 /1011 radical cm−3 | [nopinone] /1014 molecule cm−3 | k /10−11 cm3 molecule−1 s−1 |
|---|---|---|---|---|
| 7 | 370 | 9 | 0.30–4.35 | 1.75 ± 0.35 |
| 20 | 320 | 11 | 0.44–4.35 | 1.79 ± 0.35 |
| 20 | 320 | 11 | 0.70–4.25 | 1.69 ± 0.35 |
| 80 | 248 | 11 | 0.30–4.65 | 1.73 ± 0.35 |
| 1.74 ± 0.35 a |
| Reaction Pathways | M06-2X/6-311++G(d,p) | |||
|---|---|---|---|---|
| ΔE | ΔH | ΔG | ||
| PATHWAY 1 | R | 0 | 0 | 0 |
| RC1 | −9.41 | −7.48 | 1.30 | |
| TS1 | −0.79 | −2.13 | 7.34 | |
| IC1 | −36.13 | −34.48 | −26.75 | |
| I1 + H2O | −27.16 | −27.90 | −29.11 | |
| PATHWAY 2 | TS2 | 1.37 | 0.06 | 8.44 |
| IC2 | −27.18 | −26.55 | −20.06 | |
| I2 + H2O | −19.26 | −20.62 | −22.34 | |
| PATHWAY 3 | TS3 | 1.12 | 0.02 | 8.10 |
| IC3 | −21.67 | −20.03 | −12.85 | |
| I3 + H2O | −13.59 | −13.92 | −15.32 | |
| PATHWAY 4 | TS4 | 2.26 | 0.71 | 9.38 |
| IC4 | −23.43 | −22.47 | −15.30 | |
| I4 + H2O | −17.84 | −16.53 | −18.11 | |
| PATHWAY 5 | TS5 | −1.23 | −2.45 | 6.81 |
| IC5 | −18.92 | −17.10 | −9.58 | |
| I5 + H2O | −10.61 | −10.93 | −12.38 | |
| PATHWAY 6 | TS6 | 2.64 | 1.24 | 9.97 |
| IC6 | −24.08 | −23.41 | −16.61 | |
| I6 + H2O | −15.80 | −17.08 | −18.53 | |
| PATHWAY 7 | TS7 | −0.95 | −1.75 | 8.32 |
| IC7 | −24.57 | −23.62 | −16.02 | |
| I7 + H2O | −16.13 | −17.34 | −18.95 | |
| Reaction Pathways | M06-2X/6-311++G(d,p) | |||
|---|---|---|---|---|
| ΔE | ΔH | ΔG | ||
| PATHWAY 8 | I5 + O2 | 0 | 0 | 0 |
| I8 | −43.39 | −40.71 | −35.54 | |
| PATHWAY 9 | I8 + HO2 | 0 | 0 | 0 |
| TS8 | 35.40 | 34.68 | 32.71 | |
| P1 | −77.24 | −75.93 | −76.45 | |
| PATHWAY 10 | I8 + NO | 0 | 0 | 0 |
| I9 | −63.79 | −61.76 | −61.27 | |
| Temp (K) | K CVT/SCT (cm3 molecule−1 s−1) × 10−11 |
|---|---|
| 278 | 2.52 |
| 288 | 2.36 |
| 298 | 2.21 |
| 308 | 2.03 |
| 318 | 1.87 |
| 328 | 1.71 |
| 338 | 1.53 |
| 348 | 1.34 |
| 350 | 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Dib, G.; Mano Priya, A.; Lakshmipathi, S. Investigation of the Gas-Phase Reaction of Nopinone with OH Radicals: Experimental and Theoretical Study. Atmosphere 2022, 13, 1247. https://doi.org/10.3390/atmos13081247
El Dib G, Mano Priya A, Lakshmipathi S. Investigation of the Gas-Phase Reaction of Nopinone with OH Radicals: Experimental and Theoretical Study. Atmosphere. 2022; 13(8):1247. https://doi.org/10.3390/atmos13081247
Chicago/Turabian StyleEl Dib, Gisèle, Angappan Mano Priya, and Senthilkumar Lakshmipathi. 2022. "Investigation of the Gas-Phase Reaction of Nopinone with OH Radicals: Experimental and Theoretical Study" Atmosphere 13, no. 8: 1247. https://doi.org/10.3390/atmos13081247
APA StyleEl Dib, G., Mano Priya, A., & Lakshmipathi, S. (2022). Investigation of the Gas-Phase Reaction of Nopinone with OH Radicals: Experimental and Theoretical Study. Atmosphere, 13(8), 1247. https://doi.org/10.3390/atmos13081247






