Numerical Simulation of Aspergillus Niger Spore Deposition in Nasal Cavities of a Population in Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Objects
2.2. Medical Imaging
2.3. Three-Dimensional (3D) Reconstruction and CFD Simulation
2.4. Statistical Methods
3. Results
3.1. Model Validation
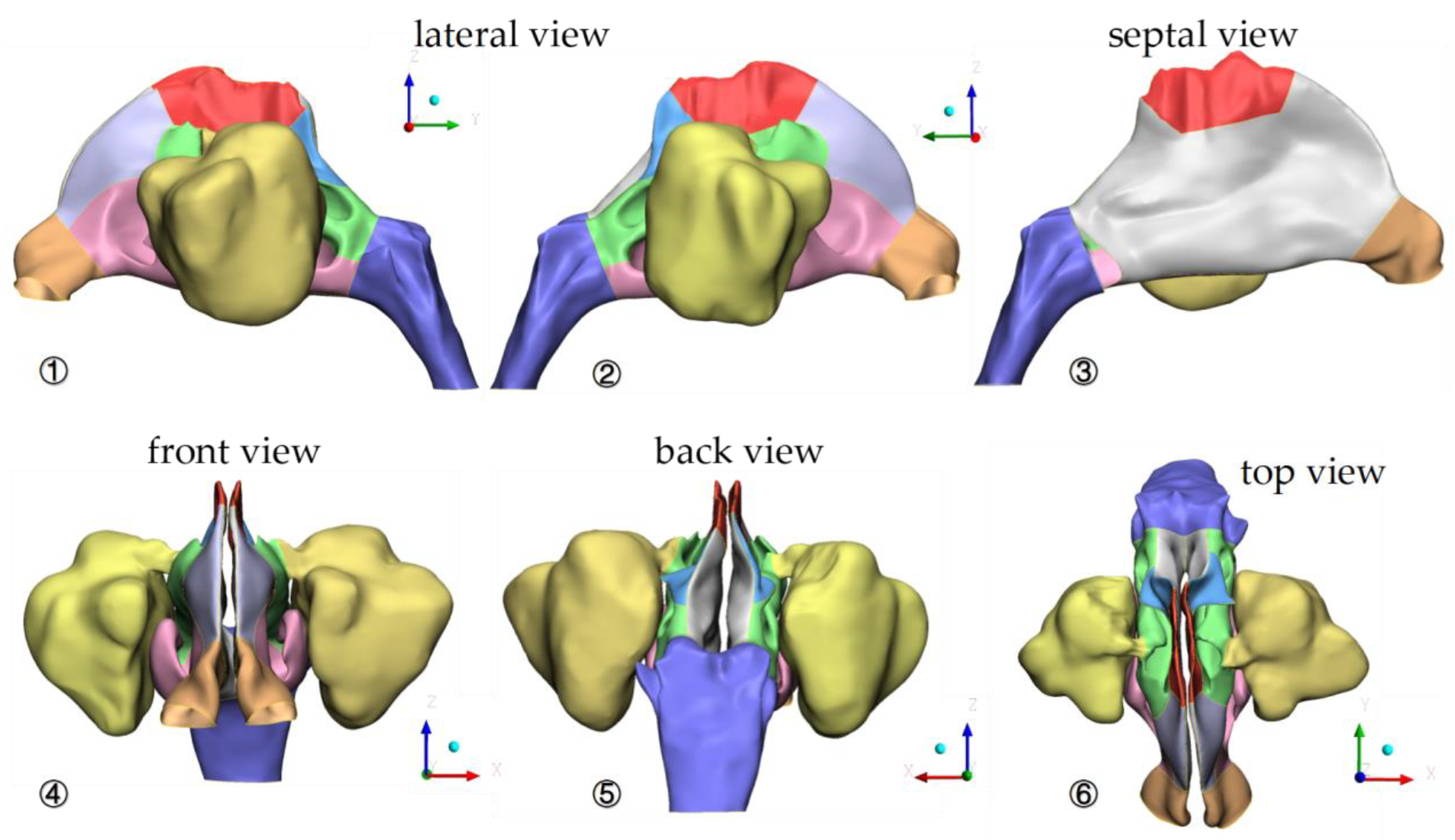
3.2. Geometric Characteristics of Nasal Cavity Model
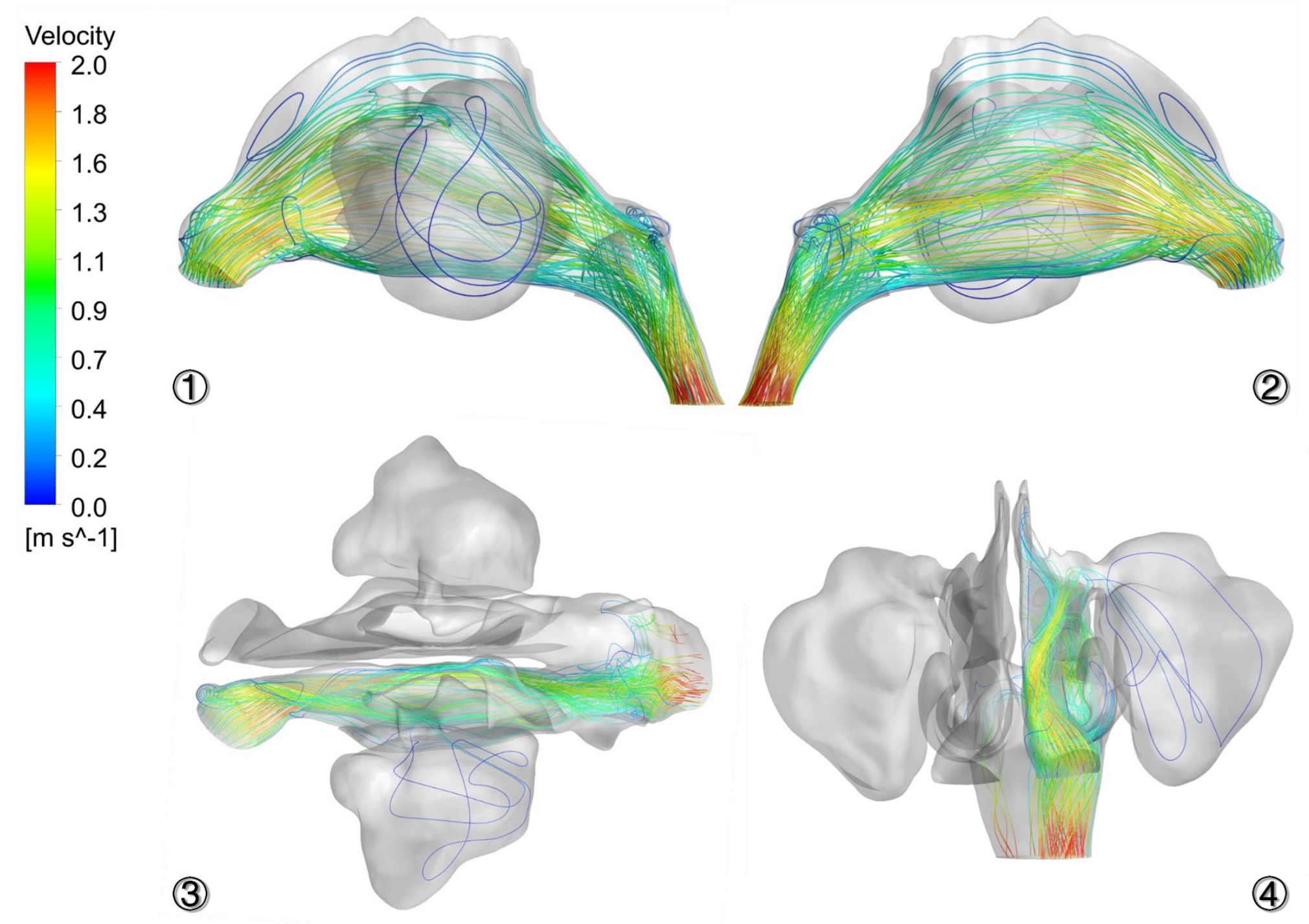
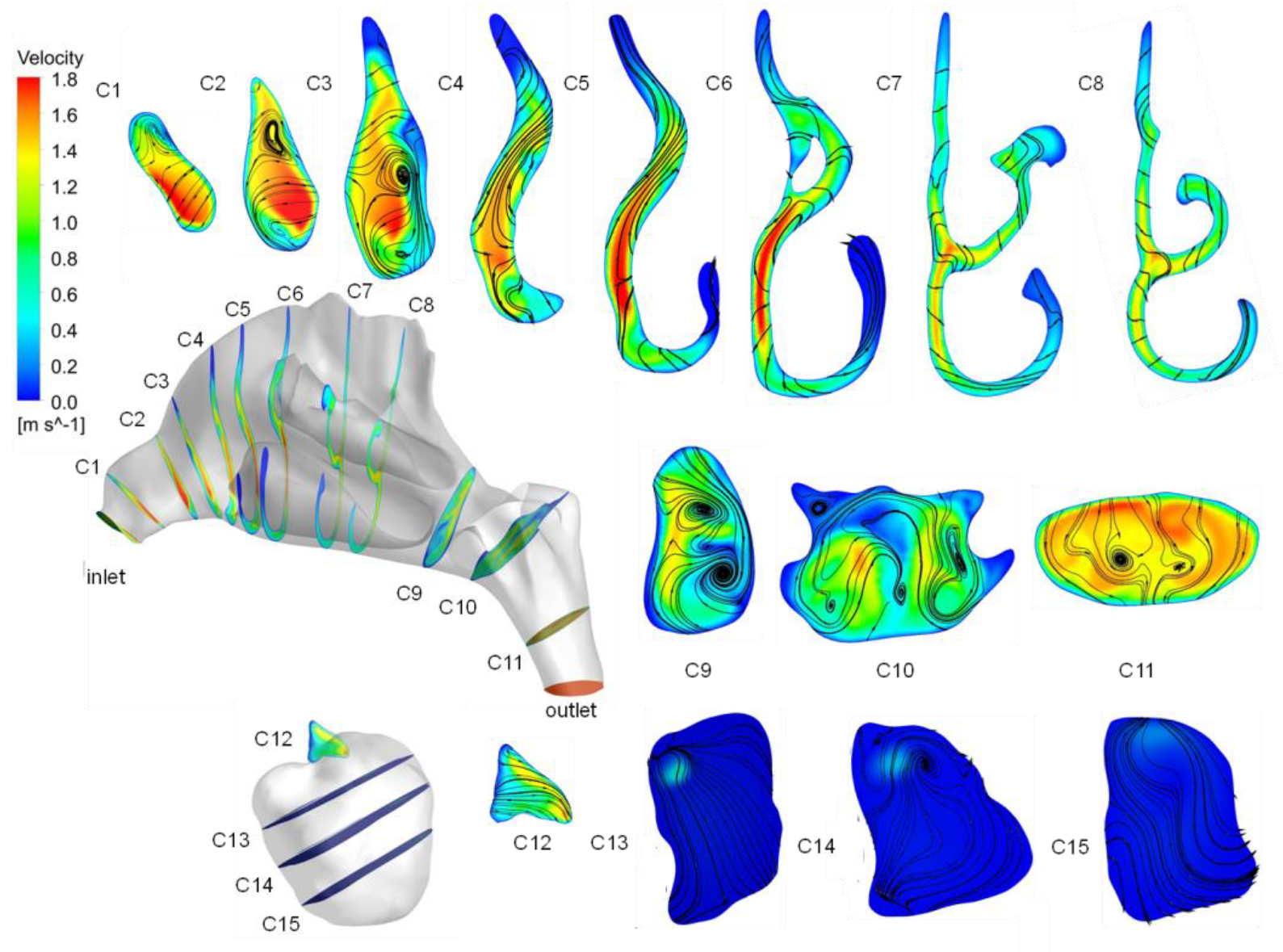
3.3. Airflow Characteristics in Nasal Cavity
3.4. Spore-Deposition Distribution and Particle Trajectories in Nasal Cavity
3.5. Effects of Variables
3.5.1. Different Airflow
3.5.2. Different Body Positions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bush, R.K.; Portnoy, J.M.; Saxon, A.; Terr, A.I.; Wood, R.A. The medical effects of mold exposure. J. Allergy Clin. Immunol. 2006, 117, 326–333. [Google Scholar] [CrossRef]
- Dykewicz, M.S.; Rodrigues, J.M.; Slavin, R.G. Allergic fungal rhinosinusitis. J. Allergy Clin. Immunol. 2018, 142, 341–351. [Google Scholar] [CrossRef]
- Sharpe, R.A.; Bearman, N.; Thornton, C.R.; Husk, K.; Osborne, N.J. Indoor fungal diversity and asthma: A meta-analysis and systematic review of risk factors. J. Allergy Clin. Immunol. 2015, 135, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M.; Akgül, H.; Tosunoglu, A. Temporal variations in fungal spores in Mardin city atmosphere, upper Mesopotamia, SE-Turkey. Grana 2022, 61, 67–80. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Homburger, H.A.; Frigas, E.; Gaffey, T.A.; Roberts, G.D. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin. Proc. 1999, 74, 877–884. [Google Scholar] [CrossRef]
- Buzina, W.; Braun, H.; Freudenschuss, K.; Lackner, A.; Habermann, W.; Stammberger, H. Fungal biodiversity—As found in nasal mucus. Med. Mycol. 2003, 41, 149–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharpe, R.; Thornton, C.R.; Osborne, N.J. Modifiable factors governing indoor fungal diversity and risk of asthma. Clin. Exp. Allergy 2014, 44, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M. The Effects of Fungus Spores on Asthma. J. Bacteriol. Mycol. Open Access 2017, 5, 00163. [Google Scholar]
- Martinez-Bracero, M.; Markey, E.; Clancy, J.H.; McGillicuddy, E.J.; Sewell, G.; O’Connor, D.J. Airborne Fungal Spore Review, New Advances and Automatisation. Atmosphere 2022, 13, 308. [Google Scholar] [CrossRef]
- de Shazo, R.D.; Chapin, K.; Swain, R.E. Fungal sinusitis. N. Engl. J. Med. 1997, 337, 254–259. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Bassiouni, A.; Tanjararak, K.; Vreugde, S.; Wormald, P.J.; Psaltis, A.J. Role of fungi in chronic rhinosinusitis through ITS sequencing. Laryngoscope 2018, 128, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Inthavong, K.; Wen, J.; Tu, J.Y.; Xue, C.L. Comparison of micron- and nanoparticle deposition patterns in a realistic human nasal cavity. Respir. Physiol. Neurobiol. 2009, 166, 142–151. [Google Scholar] [CrossRef]
- Rozsasi, A.; Leiacker, R.; Rettinger, G.; Lindemann, J.; Keck, T. Impact of resection of the turbinates and the lateral nasal wall on particle deposition. Laryngoscope 2004, 114, 646–651. [Google Scholar] [CrossRef]
- Le Guellec, S.; Ehrmann, S.; Vecellio, L. In vitro-in vivo correlation of intranasal drug deposition. Adv. Drug Deliv. Rev. 2021, 170, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shang, Y.; Inthavong, K.; Tong, Z.; Sun, B.; Zhu, K.; Yu, A.; Zheng, G. Computational investigation of dust mite allergens in a realistic human nasal cavity. Inhal. Toxicol. 2019, 31, 224–235. [Google Scholar] [CrossRef]
- Ya, Z.; Luyao, Z.; Fen, H.; Jingbin, Z.; Miao, L.; Bin, S.; Kang, Z.; Guoxi, Z.; Zhenbo, T. Computational investigation of Artemisia pollen deposition in realistic nasal cavities of residents in northwest China. Chin. J. Otorhinolaryngol. Head Neck Surg. 2019, 54, 741–747. [Google Scholar]
- Tian, Z.F.; Inthavong, K.; Tu, J.Y. Deposition of inhaled wood dust in the nasal cavity. Inhal. Toxicol. 2007, 19, 1155–1165. [Google Scholar] [CrossRef]
- Inthavong, K.; Wen, J.; Tian, Z.; Tu, J. Numerical study of fibre deposition in a human nasal cavity. J. Aerosol Sci. 2008, 39, 253–265. [Google Scholar] [CrossRef]
- Kabilan, S.; Suffield, S.R.; Recknagle, K.P.; Jacob, R.E.; Einstein, D.R.; Kuprat, A.P.; Carson, J.P.; Colby, S.M.; Saunders, J.H.; Hines, S.A.; et al. Computational fluid dynamics modeling of Bacillus anthracis spore deposition in rabbit and human respiratory airways. J. Aerosol Sci. 2016, 99, 64–77. [Google Scholar] [CrossRef]
- Tian, L.; Inthavong, K.; Lidén, G.; Shang, Y.; Tu, J. Transport and Deposition of Welding Fume Agglomerates in a Realistic Human Nasal Airway. Ann. Occup. Hyg. 2016, 60, 731–747. [Google Scholar] [CrossRef]
- Inthavong, K. From indoor exposure to inhaled particle deposition: A multiphase journey of inhaled particles. Experi-Ment. Comput. Multiph. Flow 2020, 2, 59–78. [Google Scholar] [CrossRef]
- Kiakojuri, K.; Armaki, M.T.; Rajabnia, R.; Pournajaf, A.; Karami, M.; Khademian, A.; Omran, S.M. Outer Ear Infections in Iran: A Review. Open Access Maced. J. Med. Sci. 2019, 7, 1233–1240. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; van Veluw, G.J.; Wang, F.; Müller, W.H.; Dijksterhuis, J.; Wösten, H.A. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef]
- Shang, Y.D.; Inthavong, K.; Tu, J.Y. Detailed micro-particle deposition patterns in the human nasal cavity influenced by the breathing zone. Comput. Fluids 2015, 114, 141–150. [Google Scholar] [CrossRef]
- Sun, X.; Yu, C.; Liu, Y.; Yu, S.; Zhang, J.; Su, Y. 3D Finite Element Model Reconsturction and Numerical Simulation of Airflow in Human Upper Air-way. Space Med. Med. Eng. 2006, 19, 129–133. [Google Scholar] [CrossRef]
- Hahn, I.; Scherer, P.W.; Mozell, M.M. Velocity profiles measured for airflow through a large-scale model of the human nasal cavity. J. Appl. Physiol. 1993, 75, 2273–2287. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Prasad, A.K.; Wexler, A.S. Detailed flow patterns in the nasal cavity. J. Appl. Physiol. 2000, 89, 323–337. [Google Scholar] [CrossRef]
- Keyhani, K.; Scherer, P.W.; Mozell, M.M. A numerical model of nasal odorant transport for the analysis of human olfaction. J. Theor. Biol. 1997, 186, 279–301. [Google Scholar] [CrossRef]
- van Veluw, G.J.; Teertstra, W.R.; de Bekker, C.; Vinck, A.; van Beek, N.; Muller, W.H.; Arentshorst, M.; van der Mei, H.C.; Ram, A.F.; Dijksterhuis, J.; et al. Heterogeneity in liquid shaken cultures of Aspergillus niger inoculated with melanised conidia or conidia of pigmentation mutants. Stud. Mycol. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Feng, Y.; Kleinstreuer, C. Micron-particle transport, interactions and deposition in triple lung-airway bifurcations using a novel modeling approach. J. Aerosol Sci. 2014, 71, 1–15. [Google Scholar] [CrossRef]
- Silva, D.M.; Batista, L.R.; Rezende, E.F.; Fungaro, M.H.; Sartori, D.; Alves, E. Identification of fungi of the genus Aspergillus section nigri using polyphasic taxonomy. Braz. J. Microbiol. 2011, 42, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Shang, Y.; Inthavong, K.; Tu, J.; Chen, R.; Bai, R.; Wang, D.; Chen, C. From the Cover: Comparative Numerical Modeling of Inhaled Nanoparticle Deposition in Human and Rat Nasal Cavities. Toxicol. Sci. 2016, 152, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Doorly, D.J.; Taylor, D.J.; Schroter, R.C. Mechanics of airflow in the human nasal airways. Respir. Physiol. Neurobiol. 2008, 163, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, J.; Dong, H.; Zhao, K. Computational modeling and validation of human nasal airflow under various breathing conditions. J. Biomech. 2017, 64, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM(2.5)-Induced Oxidative Stress and Mitochondrial Damage in the Nasal Mucosa of Rats. Int. J. Environ. Res. Public Health 2017, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Wang, Q.; Kuuspalo, K.; Ruusunen, J.; Hao, L.; Fang, D.; Väisänen, O.; Ruuskanen, A.; Sippula, O.; Happo, M.S.; et al. Day and night variation in chemical composition and toxicological responses of size segregated urban air PM samples in a high air pollution situation. Atmos. Environ. 2015, 120, 427–437. [Google Scholar] [CrossRef]
- Barac, A.; Ong, D.S.Y.; Jovancevic, L.; Peric, A.; Surda, P.; Tomic Spiric, V.; Rubino, S. Fungi-Induced Upper and Lower Respiratory Tract Allergic Diseases: One Entity. Front. Microbiol. 2018, 9, 583. [Google Scholar] [CrossRef]
- Inthavong, K.; Shang, Y.; Del Gaudio, J.M.; Wise, S.K.; Edwards, T.S.; Bradshaw, K.; Wong, E.; Smith, M.; Singh, N. Inhalation and deposition of spherical and pollen particles after middle turbinate resection in a human nasal cavity. Respir. Physiol. Neurobiol. 2021, 294, 103769. [Google Scholar] [CrossRef]
- Kanj, A.; Abdallah, N.; Soubani, A.O. The spectrum of pulmonary aspergillosis. Respir. Med. 2018, 141, 121–131. [Google Scholar] [CrossRef]
- Eccles, R. Anatomy and physiology of the nose and control of nasal airflow. Middlet. Allergy Princ. Pract. 2003, 6, 775–787. [Google Scholar]
- Inthavong, K.; Tu, J.; Ahmadi, G. Computational Modelling of Gas-Particle Flows with Different Particle Morphology in the Human Nasal Cavity. J. Comput. Multiph. Flows 2009, 1, 57–82. [Google Scholar] [CrossRef]
- Shi, H.; Kleinstreuer, C.; Zhang, Z. Modeling of inertial particle transport and deposition in human nasal cavities with wall roughness. J. Aerosol Sci. 2007, 38, 398–419. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Holmes, T.D.; Gao, J.; Guilmette, R.A.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J. Aerosol Med. 2001, 14, 267–280. [Google Scholar] [CrossRef]
- Dong, J.; Ma, J.; Shang, Y.; Inthavong, K.; Qiu, D.; Tu, J.; Frank-Ito, D. Detailed nanoparticle exposure analysis among human nasal cavities with distinct vestibule phenotypes. J. Aerosol Sci. 2018, 121, 54–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, J.; Chen, X.; Lou, M.; Ma, R.; Hu, Z.; Gong, M.; Wang, B.; Tong, Z.; Ren, H.; et al. Numerical Simulation of Aspergillus Niger Spore Deposition in Nasal Cavities of a Population in Northwest China. Atmosphere 2022, 13, 911. https://doi.org/10.3390/atmos13060911
Wang Y, Dong J, Chen X, Lou M, Ma R, Hu Z, Gong M, Wang B, Tong Z, Ren H, et al. Numerical Simulation of Aspergillus Niger Spore Deposition in Nasal Cavities of a Population in Northwest China. Atmosphere. 2022; 13(6):911. https://doi.org/10.3390/atmos13060911
Chicago/Turabian StyleWang, Yusheng, Jingliang Dong, Xiaole Chen, Miao Lou, Ruiping Ma, Zhenzhen Hu, Minjie Gong, Botao Wang, Zhenbo Tong, Hongxian Ren, and et al. 2022. "Numerical Simulation of Aspergillus Niger Spore Deposition in Nasal Cavities of a Population in Northwest China" Atmosphere 13, no. 6: 911. https://doi.org/10.3390/atmos13060911
APA StyleWang, Y., Dong, J., Chen, X., Lou, M., Ma, R., Hu, Z., Gong, M., Wang, B., Tong, Z., Ren, H., Li, C., Zheng, G., & Zhang, Y. (2022). Numerical Simulation of Aspergillus Niger Spore Deposition in Nasal Cavities of a Population in Northwest China. Atmosphere, 13(6), 911. https://doi.org/10.3390/atmos13060911







