Abstract
Exogenous nitrogen (N) inputs greatly change the emission and uptake of carbon dioxide (CO2) and methane (CH4) from temperate grassland soils, thereby affecting the carbon (C) budget of regional terrestrial ecosystems. Relevant research focused on natural grassland, but the effects of N fertilization on C exchange fluxes from different forage soils and the driving mechanisms were poorly understood. Here, a three-year N addition experiment was conducted on cultivated grassland planted with alfalfa (Medicago sativa) and bromegrass (Bromus inermis) in Inner Mongolia. The fluxes of soil-atmospheric CO2 and CH4; the content of the total dissolved N (TDN); the dissolved organic N (DON); the dissolved organic C (DOC); NH4+–N and NO3−–N in soil; enzyme activity; and auxiliary variables (soil temperature and moisture) were simultaneously measured. The results showed that N fertilization (>75 kg N ha−1 year−1) caused more serious soil acidification for alfalfa planting than for brome planting. N fertilization stimulated P-acquiring hydrolase (AP) in soil for growing Bromus inermis but did not affect C- and N-acquiring hydrolases (AG, BG, CBH, BX, LAP, and NAG). The oxidase activities (PHO and PER) of soil for planting Bromus inermis were higher than soil for planting Medicago sativa, regardless of N, whether fertilization was applied or not. Forage species and N fertilization did not affect soil CO2 flux, whereas a high rate of N fertilization (150 kg N ha−1 year−1) significantly inhibited CH4 uptake in soil for planting Medicago sativa. A synergistic effect between CO2 emission and CH4 uptake in soil was found over the short term. Our findings highlight that forage species affect soil enzyme activity in response to N fertilization. Soil enzyme activity may be an important regulatory factor for C exchange from temperate artificial grassland soil in response to N fertilization.
1. Introduction
Nitrogen (N) is one of the dominant drivers for the net primary productivity of terrestrial ecosystems [1]. N fertilization has the potential to increase aboveground biomass through altering its allocation of photosynthate [2]. It can also reduce plant species’ richness, especially populations of nitrogen-sensitive plants such as forbs Plantago Lanceolata, Campanula rotundifolia and Euohrasia officinalis [3]. Moreover, it improves litter decomposition and increases fine-root growth [4,5]. Most importantly, an increased soil N availability mitigates the negative atmospheric effects of increased CO2, thereby enhancing the carbon (C) sequestration capacity of terrestrial ecosystems [6,7]. In terrestrial ecosystems, the content of C in soil is much higher than in vegetation [8]. The main pathway of C emissions from terrestrial ecosystems is the release of CO2 into the atmosphere [9]. In the soil, CO2 is produced depending on the oxidation of organic substrates by plant roots’ respiration and heterotrophic microorganisms, whereas CH4 production results from microorganisms decomposing organic materials in anaerobic conditions [10]. CH4 is reported as the second most radioactive trace gas after CO2, increasing by 0.3% year−1 [11]. Aerobic soils were defined as the only biological atmospheric CH4 sinks varying from 20 to 45 Tg year−1 globally [12]. Furthermore, temperate grasslands are also non-ignorable sinks for atmospheric CH4 [13].
Temperate grasslands account for 60–70% of the global agricultural land representing significant C pools and non-ignorable sinks for atmospheric CH4 [14]. Grassland soils contain at least 200–300 Pg C on a global scale [13,15,16]. A lot of C accumulates under grass in grassland ecosystems covering 10% of the planet’s total ecosystems [17]. Grassland ecosystems are sensitive to N fertilization, which directly affects CO2 emission and CH4 uptake [18,19,20]. Several reviews revealed that inorganic N addition hinders CH4 uptake and promotes CO2 release compared with natural grasslands [21,22]. It was reported that CO2 efflux and CH4 uptake were unaffected by increased N-inputs from unmanaged grasslands during the growing season [23]. Research on an alpine meadow has shown that N fertilization tended to decrease CH4 uptake and to reduce CO2 emission [24]. However, in an alpine grassland, N deposition was observed to increase CH4 uptake and CO2 emission [25]. In a cultivated grassland ecosystem, Kammann et al. [26] found no inhibitory effect on CH4 oxidation and CO2 emission, whatever the amount of annual N fertilization. However, Peng et al. [9] observed that N fertilization promoted soil CO2 emission regardless of N fertilization concentration. The conclusions were contradictory. Therefore, knowledge of soil C dynamics with its controlling factors following N input in grassland ecosystems may be essential to evaluate greenhouse gas emissions and increase grasslands productivity. In China, grasslands make up 40% of the whole land area [27], mainly in Inner Mongolia. This region has experienced a 12-fold increase in livestock from 1947 to 2006 (Bureau of Statistics of Inner Mongolia, 2007), causing such serious deterioration of grasslands that people have to plant palatability forages and apply fertilizer to enhance grass yield. In the Hulunber region, legumes (Medicago sativa) and non-legumes (Bromus inermis) are planted to produce high quality forages. However, there is currently little information on the effects of managed forages on CO2 emissions and CH4 uptakes under N fertilizer. Generally, the evaluation of soil C fluxes depends on many factors. Soil C efflux is determined in part by decomposition, which could increase or decrease as N is added [28,29]. These divergent trends may be explained by the opposite reactions of different extracellular microbial enzymes which degrade soil organic matter (SOM) and plant litter [30]. Soil microbial enzyme activities act as a rate-limiting step in catalyzing the decomposition of organic matter, becoming indicators to reflect the effect of N deposition in many field experiments [31]. Soil extracellular enzymes generally include hydrolases and oxidases. A typical group of hydrolases including β-1,4-glucosidase (BG), β-1,4-xylosidase (BX), cellobiohydrolase (CBH) and α-1,4-glucosidase (AG) are used to decompose polysaccharides. The enzymes related to microbial N acquisition conclude β-N-acetyl-glucosaminidase (NAG) and leucine amino peptidase (LAP). The enzyme related to microbial P acquisition is acidic phosphatase (AP). Phenol oxidase (PHO) and peroxidase (PEO) are the two most commonly detected oxidases. Studies of two meta-analyses demonstrated that C acquisition and P acquisition enzymes were stimulated as N was added [32,33]. However, no effects of N addition on cellobiohydrolase activity or a neutral to a positive effect on soil C, N, and P acquisition enzymes were observed in natural grassland ecosystems [34,35,36]. Some studies also showed that added N in grassland ecosystems suppressed or stimulated oxidase activities, depending on study sites [28,30]. In a cultivated grassland ecosystem, the activities of hydrolytic enzymes responded significantly to N addition, watering and their combinations [37]. The activities of hydrolases (phosphatase and polysaccharide-degrading enzymes) increased obviously in response to the amount of nitrate added [38]. In the context of short-term N addition (three years), the above-mentioned response rules of enzymes to N addition in managed grassland are still unknown, as is is its influence on carbon fluxes.
The purpose of this study is to assess the response of enzyme activity as a functional characteristics of soil microbial community to N fertilization. An additional aim is to evaluate the effect of different forages on soil C fluxes under N fertilization, especially on CO2 emissions and CH4 uptakes. Specifically, we arranged field experiments to assess the C dynamic effects in Inner Mongolia, China. Except for gas fluxes, soil properties and various N contents, the activities of major enzymes which affect microorganisms and substrates were also measured. We hypothesized that (1) N fertilization would increase soil C (CO2 and CH4) fluxes, and (2) soil C fluxes variations may be caused by the changes in enzyme activities under N fertilization.
2. Materials and Methods
2.1. Study Site and Experimental Design
The study was conducted at the Hulunber Grassland Ecosystem Observation and Research Station (49°19′ N, 119°56′ E), located in the northeast of Inner Mongolia, China (Figure 1). The region has a semi-arid continental climate. The mean annual precipitation is 320 mm, and 80% of rainfall is between June and September [20]. The average monthly temperature varies from −48.5 °C in January to 36.2 °C in July with a mean annual temperature of −1.5 °C [20]. The frostless season is about 90 to 110 days. The land use type in the last 30 years has been farmland and the crops grown are mainly oilseed rape (Brassica campestris). Since 2010, oilseed rape has been gradually replaced by bromegrass (Bromus inermis) and alfalfa (Medicago sativa), respectively. The soil is classical chernozem (FAO classification). Topsoil (0–10 cm) total C content is 48.38 g kg−1, total N is 4.36 g kg−1, total P is 0.61 g kg−1, and pH is 7.35 [37].
Figure 1.
Location of the study area.
The N fertilization experiment was conducted using a random block design. The seeding amount of the Medicago sativa and Bromus inermis grasslands was 15.0 kg/ha. Nitrogen fertilizer concentration was based on 0 times, 5 times and 10 times of forage seeding amount. Two forages (Medicago sativa and Bromus inermis) were applied at three rates of urea: 0, 75, and 150 kg N ha−1 year−1. The experiment consists of six treatments—specifically, Medicago sativa soil was fertilized at three levels of N: MN0, MN75 and MN150. Bromus inermis soil was treated at three levels: BN0, BN75 and BN150. Each treatment had four replicates. Each plot had an area of 70 m2 (7 m × 10 m), and a 3-m buffer was set between two adjacent plots. In the reviving stage and heading stage (usually mid-May to early June), solid urea fertilizers (analytical reagent, N concentration of 46%) were applied in two equal parts to the N addition plot. The N addition experiment started in May 2016. Soil samples of 0–10 cm were collected from May to September 2019.
2.2. Measurement of Soil C Fluxes and Auxiliary Variables
Opaque static chambers and gas chromatography techniques were used to measure soil CO2 and CH4 fluxes. The concentrations of CO2 and CH4 of gas samples were measured using a gas chromatograph (Agilent 7890A, Santa Clara, CA, USA). The CO2 and CH4 fluxes at each plot were calculated using a linear fitting approach. During the growing season (from May to September) of 2019, soil CO2 and CH4 fluxes were measured three times a month. During the gas sampling, soil temperature and moisture in 0–10 cm surface layer were monitored using portable temperature probes (JM624 digital thermometer, Living-Jinming Ltd., Shenzhen, China) and a moisture probe meter (TDR 100, Spectrum, Aurora, IL, USA), respectively.
2.3. Measurement of Soil Dissolved C and N Concentrations and pH
After gas sampling, the soil samples at a depth of 0–10 cm were collected to determine the dissolved C and N concentration and pH. All soil samples were immediately passed through a 2-mm sieve and stored at 4 °C for further analysis. Soils were extracted with 2 M KCl solutions (soil:water = 1:10), and the extracts were determined with the concentrations of NH4+-N, NO3−–N, and the total dissolved nitrogen (TDN) on an automatic N analyzer (AA3, SEAL, Norderstedt, Germany). The dissolved organic N (DON) was approximately calculated from the difference between TDN and mineral N (NH4+–N + NO3−–N). Further soil subsamples were extracted with deionized water (soil:water = 1:10) and the supernatant was filtered using 0.45 μm polytetrafluoroethylene filters. The filtrates were determined on a TOC analyzer (Liqui TOCII, Elementar, Hanau, Germany). The soil pH was determined in a 1:2.5 soil: water suspension using a standard pH meter (Mettler Toledo, Urdorf, Switzerland).
2.4. Measurement of Soil Enzyme Activities
The activities of nine enzymes involved in soil C, N, and P cycles were measured using the microplate fluorescence method [35]. The activities of seven hydrolases including β-1,4-glucosidase (BG); β-1,4-xylosidase (BX); β-D-cellobiohydrolase (CBH); α-1,4-glucosidase (AG); β-N-acetylglucosaminidase (NAG); acid phosphatase (AP); and leucine amino peptidase (LAP) were determined using a fluorimetric microplate assay. The activities of two oxidases including phenol oxidase (PHO) and peroxidase (PER) were measured by the absorbance of samples. Specific details refer to the method described by Liu et al. (2020) [39]. Plates were scanned at an excitation of 365 nm and an emission of 450 nm on the microplate reader (SynergyH4, BioTek, Winooski, VT, USA). Absorbance was read at 450 nm during the oxidase measurement [40].
2.5. Statistical Analyses
All data were presented as mean ± standard error (SE). The Shapiro-Wilk and Leven tests were used to test data normality and variance homogeneity before analysis. A repeated measure analysis of variance (RANOVA) combined with a Duncan test was used to evaluate the difference in soil C fluxes and related soil variables among treatments with different N addition rates. The independent samples t-test was developed to test the difference of multiple variables between forage species. Pearson correlations were adopted to examine abiotic factors and biotic factors affecting soil C fluxes. Linear regression models using ordinary least squares (OLS) were used to predict the coupling relationships between the response ratio (RR) of soil C fluxes and the response ratio of enzyme activities. All the above analyses were performed using an SPSS software package (version 25.0, Stanford, CA, USA), and a correlation heatmap was drawn using the corrplot package of R (version 3.6.1, Beijing, China).
3. Results
3.1. Soil General Properties
Soil temperature and moisture in the 0–10 cm layer exhibited significant seasonal variations (p < 0.001, Figure 2a,b). There were no significant differences in soil temperature among the treatments (Table 1). In the growing season, the average content of soil moisture in zero N addition treatments varied from 7.01 to 39.47% with an average of 25.52% and 26.61% for BN0 and MN0, respectively (Figure 2b, Table 1). Moisture contents were significantly higher in soils for cultivating bromegrass than in soils for cultivating alfalfa under an N fertilization rate of 75 kg N ha−1 year−1 (Table 1, p < 0.001).
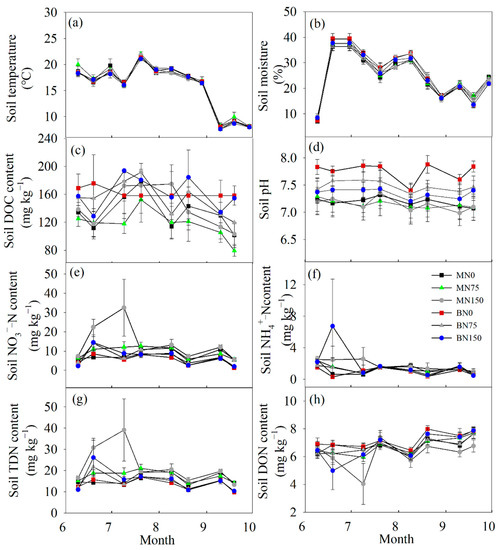
Figure 2.
Seasonal variations of soil temperature (a), moisture (b), DOC (c), pH (d), NO3−–N (e), NH4+–N (f), TDN (g) and DON (h) in the 0–10 cm layer under six treatments. Data were shown by mean ± SE (n = 6).

Table 1.
Soil properties under N addition treatments in different pastures.
There was no consistent temporal variation in the soil DOC concentration in each treatment (Figure 2c). Soil DOC concentration was significantly lower in the MN75 treatment than in the MN0 and BN75 treatments (Table 1, p < 0.001). The soil pH at each plot was generally stable, but N fertilization at a rate of 75 kg N ha−1 year−1 and 150 kg N ha−1 year−1 led to a decline of 0.35 units and 0.52 units in soil pH for cultivating alfalfa rather than bromegrass, respectively (Figure 2d, Table 1). Further, the soil pH of the BN75 treatment was significantly lower than that of the MN75 treatment (Table 1, p < 0.01).
Except for individual measurements, the concentrations of soil dissolved N (NH4+−N, NO3−−N, TDN, and DON) in each treatment did not vary significantly over time (Figure 2e–h). Forage species and N application rate did not affect the soil NH4+–N concentration, but a high rate of N fertilization significantly increased the concentrations of NO3−–N and TDN in alfalfa soil by 33.77% (Table 1, p < 0.001) and 23.05% (Table 1, p < 0.01), respectively. Moreover, under the same rate of N fertilization, NO3−–N and TDN accumulations were more significant in alfalfa soil than in bromegrass soil (Table 1). Further, N fertilization with a rate of 150 kg N ha−1 year−1 tended to decrease the DON concentration in bromegrass soil (Table 1, p = 0.05).
3.2. Soil Enzyme Activity
Except for CBH, the activities of the remaining six hydrolases (BG, NAG, AP, BX, AG, and LAP) and two oxidases (PHO and PER) exhibited significant seasonal variations (Figure 3, Table 2). In the soils for cultivating bromegrass, the low and high values of BG, NAG, AP, and BX activities appeared in mid-July and mid-August, respectively (Figure 3a–d). However, the high values of the two soil oxidase activities (PHO and PER) appeared in mid-August (Figure 3h,i). The two oxidase activities were significantly higher in alfalfa soil than in brome soil, regardless of whether N fertilizer was applied or not (Figure 4h,i). Nitrogen fertilization and its interaction with forage species only significantly affected soil LAP activity (Table 2). Under a high rate of N fertilization (150 kg N ha−1 year−1), the LAP activity in bromegrass soil was higher than that of alfalfa soil (Figure 4g). N75 and N150 treatments significantly increased the AP activities in bromegrass soils by 30.90% and 48.99%, respectively (Figure 4c).
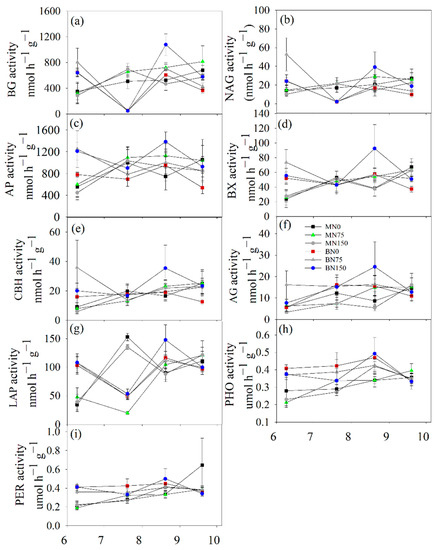
Figure 3.
Seasonal variations of hydrolases (BG (a), NAG (b), AP (c), BX (d), CBH (e), AG (f) and LAP (g)) and oxidases (PHO (h) and PER (i)). Data were shown by mean ± SE (n = 6).

Table 2.
Repeated measure analysis of variance of soil extracellular enzyme activity and soil C fluxes.
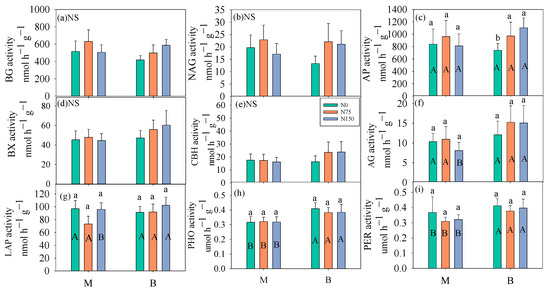
Figure 4.
Average of BG (a), NAG (b), AP (c), BX (d), CBH (e), AG (f), LAP (g), PHO (h) and PER (i) activity under six treatments. Data were shown by mean ± SE (n = 6). Different small letters represented significant differences among different N levels under the same forage species, different capital letters showed significant differences between the two forages at the same N level. All statistical differences were at 0.05 level. NS indicated no difference under six treatments.
3.3. Soil C Fluxes
The soil CO2 flux at each experimental treatment showed a significant single-peak seasonal variation and the high value generally appeared from mid-July to early August (Figure 5a, Table 2, p < 0.001). Soil CO2 fluxes ranged from 4.71 to 138.73 mg C m−2 h−1 in the unfertilized treatment with an average of 53.55 mg C m−2 h−1 (Figure 5b). Neither forage nor N fertilization rate significantly changed soil CO2 flux (Figure 5b).
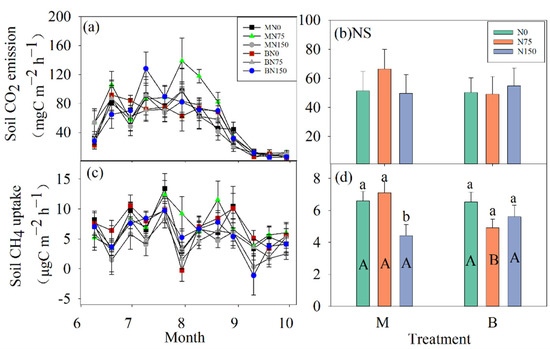
Figure 5.
Seasonal variations and mean values of soil CO2 emission (a,b) and CH4 (c,d) uptake under six treatments. The difference of nitrogen fertilization at the same forage species was shown by small letters. The difference of forage species at the same N level was expressed by capital letters. All statistical differences were at 0.05 level. NS indicated no difference under six treatments.
The temperate artificial grassland acted as a sink for atmospheric CH4. The CH4 uptake flux from different treatment soils also exhibited significant seasonal fluctuations (Figure 5c, Table 2, p < 0.001). During the monitoring period, the soil CH4 uptake flux in the unfertilized treatment averaged between 0.11 and 13.42 μg C m−2 h−1 (Figure 5c). The high rate of N fertilization (150 kg N ha−1 year−1) significantly decreased soil CH4 uptake in alfalfa fields by 32.86% (Figure 5d, p < 0.01). However, N fertilization did not affect the CH4 uptake in bromegrass fields (Figure 5d). Further, under the same fertilization rate (75 kg N ha−1 year−1), the soil CH4 uptake was significantly lower in bromegrass fields than in alfalfa fields (Figure 5d, p < 0.01).
3.4. Relationships between Soil C Fluxes and Soil Properties
The soil CO2 flux was significantly positively correlated with soil temperature and moisture, but significantly negatively correlated with soil NH4+−N and DON contents (Figure 6). The soil CH4 uptake flux was positively correlated with the topsoil temperature, whereas it was negatively correlated with the soil moisture and DON contents (Figure 6). Except for AG, the activities of other hydrolytic enzymes were significantly negatively correlated with the soil pH (Figure 6). The soil temperature was significantly positively correlated with the activities of four hydrolases (BX, CBH, AG, LAP) and two oxidases (PHO and PER), whereas the soil moisture was positively correlated with the activities of NAG, AG, and PHO (Figure 6). Further, the soil DON content was negatively correlated with the activities of AP, BX, CBH, and AG (Figure 6).
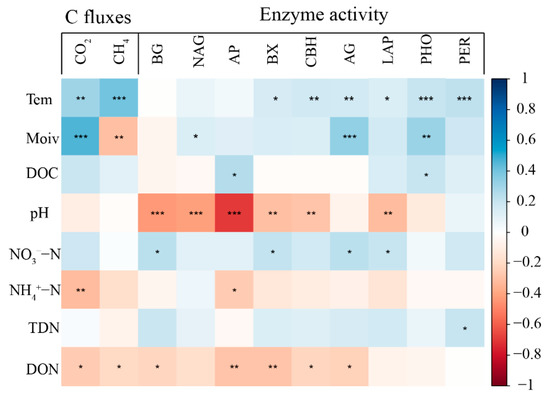
Figure 6.
Soil general properties, enzyme activities and soil C fluxes related heatmap. Each color block corresponds to coefficient r of Spearman correlation ranging from −1 to 1, r < 0 and r > 0 mean negative correlation and positive correlation, respectively. The *, ** and *** represent significant p value < 0.05, < 0.01 and < 0.001, respectively.
Under N addition, the response ratio (RR) of soil CO2 emission flux was positively correlated with the response ratio (RR) of soil PHO activity, and the relationship between them could be well fitted by a linear equation (Figure 7a). However, a negative linear relationship between the response ratio (RR) of soil CH4 uptake flux and the response ratio (RR) of soil NAG activity was found (Figure 7b). In addition, putting all the experimental treatments together, the relationship between soil CO2 emission flux and soil CH4 uptake flux was well fitted using an exponential growth equation (Figure 8).
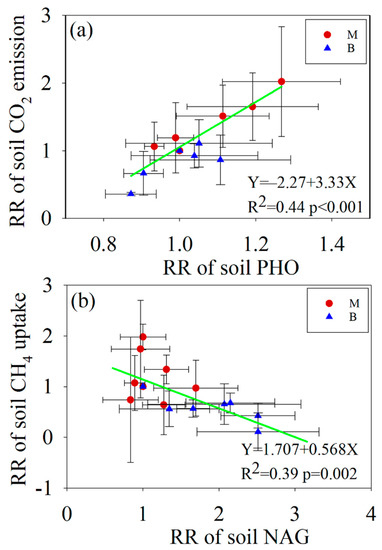
Figure 7.
(a) the relationships between RR of enzyme activities and RR of soil C fluxes. The bivariate relationship between RR of soil PHO and RR of soil CO2 emission; (b) the bivariate relationship between RR of soil NAG and RR of soil CH4 uptake.
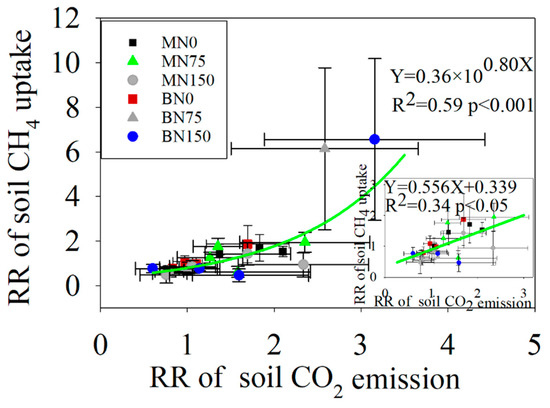
Figure 8.
The relationship between RR of soil CO2 emissions and RR of soil CH4 uptakes.
4. Discussion
4.1. The Response of Soil Main Properties to Each Treatment
In our study, NO3−−N accumulated and pH decreased in alfalfa soil at the conditions of medium (75 kg N ha−1 year−1) and high (150 kg N ha−1 year−1) nitrogen, indicating that nitrification was enhanced, soil leaching was strengthened, and soil acidification was accelerated. This agrees with findings from other studies in perennial grasslands [41]. Similarly, In the alpine meadow, a study concluded that N addition dramatically leads to soil acidification [42]. A global meta-analysis also suggested that N addition increased soil NO3− concentration, soil inorganic N leaching and nitrification by 429%, 461% and 154%, respectively [43]. In our study, no significant difference in NH4+−N concentration at any treatment might be explained by the following aspects. First, the soil in our study area was characteristic by N-limited [41], creating a strong ability to fix exogenous NH4+−N. The applied NH4+−N was rapidly immobilized by SOM and absorbed by negatively charged soil organic matter and clay minerals [44]. On the other hand, NH4+−N is easily volatile, especially in alkaline soil [45]. The DON content decreased in bromegrass soil as N was added. Previous studies showed that dissolved organic matter is mainly composed of microbial biomass and metabolites that are continuously decomposed or produced by soil microorganisms [46,47]. The potential of the proteolytic enzymes produced by soil microorganisms during the ammonification process of DON is higher in semi-arid ecosystems than other ecosystems such as boreal ecosystems, mainly because the optimal pH of protease activity (neutral to alkaline) is similar to that of semi-arid soils [48,49]. Proteolytic fungi rich in these soils when added a high concentration of N may promote soil organic matter depolymerization [18,47]. Furthermore, a meta-analysis of independent N fertilization experiments demonstrated that plants in semi-arid ecosystems were N-limited [50]. Plants from these ecosystems have been shown to obtain organic N such as amino acids through root uptake [51,52]. High concentrations of N are easily absorbed by plants.
4.2. The Response of Soil C Fluxes to N Fertilization
CO2 fluxes did not respond to short-term N fertilization in the managed grassland, which contradicts our first hypothesis. Generally, root/rhizosphere respiration and microbial respiration together constitute the soil CO2 fluxes. The response of soil autotrophic respiration and heterotrophic respiration to N fertilization is inconsistent which results in different responses of soil CO2 fluxes to N fertilization [44]. For temperate N-limited grasslands, fine root yield and turnover were increased by N fertilization [1], whereas microbial heterotrophic respiration and soil microbial biomass was decreased [53]. Thus, the trade-off between promoting root autotrophic respiration and inhibiting microbial heterotrophic respiration determines the diverse effects of N fertilization on CO2 fluxes. CH4 uptakes were inhibited in alfalfa soil at a high N input which is also inconsistent with our first hypothesis. The possible principles that decreased soil CH4 uptakes by N fertilization mainly include chemical inhibition and reduced physical diffusion. The chemical inhibition mechanism is that increased nitrate concentration and nitrification could directly inhibit methanotrophs activities [54,55]. The intermediate products (NH2(OH), NO2−) in nitrification could decrease methanotrophs activities effectively [56]. Furthermore, under N fertilization, nitrate accumulation leads to the soil pH decrease, which affects the community composition of methanotrophs [57]. Additionally, soil acidification results in an increase in Al3+ concentration which has a toxic effect on methanotrophs in the soil solution [58,59]. Concerning physical diffusion, the maximum consumption rate of soil CH4 occurs in the oxidized soil layer. When litters on the oxidized soil thickened in growing seasons, ethylene and monoterpenes accumulated, resulting in a decrease in the level of atmospheric CH4 entering the soil [56,60]. In addition, although the reacted substrates for methanogens increased as N inputs, the short-term CH4 production was partly offset by the oxidation of soil CH4, leading to a decrease in net CH4 absorption [61,62].
Further, the C fluxes in the two forage soils were not significantly different. C acquisition hydrolases (AG, BG, CBH and BX) did not differ between forage species. Moreover, no significant difference in fine root biomass between the two forages may also lead to no difference in root autotrophic respiration, which ultimately leads to no difference in soil CO2 emissions between the two forage species [20]. It is worth noting that alfalfa is a typical N-fixing plant. Alfalfa can fix N in the atmosphere through biological N fixation [63], thereby providing additional available N and increasing NH4+ accumulation near the plant roots. It was shown that aggregated NH4+ inhibits methanotroph activity, resulting in decreased CH4 uptake [64]. However, suppressed CH4 fluxes were not found in our experiment. The effect of N fixation likely takes a long time to manifest, depending on pasture species [65]. Another potential explanation is that alfalfa cropped in N fertilized soil becomes lazy, reducing N fixation, whereas in an N depleted ecosystem legumes are inclined to fix N efficiently [66].
4.3. The Response of Soil C Fluxes to Enzyme Activities
Many studies showed that N addition had a positive effect on C-acquisition (especially BG) hydrolase and P-acquisition (AP) hydrolase [33,67,68], suggesting that N addition may stimulate microbes to need C and P, thereby producing enzymes to hydrolyze labile SOM promoting soil C emission. On the other hand, neither C acquisition hydrolases nor soil CO2 emission was significantly different as N was added which may be related to the shorter N addition time (three years). N-targeting hydrolytic enzymes NAG was found to negatively respond to soil CH4 uptake in our study, which was partially consistent with our second hypothesis. NAG are mainly derived from cellulose degradation. The general substrates of NAG are chitin and peptidoglycan. Adding N to the soil not only provides the necessary foundation for the construction of enzymes, but also may increase the demand for C by microorganisms. Many studies usually use NAG activity to characterize soil chitinase activity and N uptake [69,70]. Increased N may stimulate an increase in chemoautotrophic ammonia-oxidizing nitrifiers. The nitrifiers compete with methanotrophs for the same substrates, thereby inhibiting methane oxidation [71].
For oxidases, PHO activity exhibited a positive response to soil CO2 emission in our study. Oxidases and hydrolases are often distributed on separate axes with multidimensional scaling analyses and principal component methods [69,72]. This dichotomy suggests that the controls on microbial enzyme expression and environmental enzyme turnover differ for these two broad classes of enzymes. In general, PHO is characterized by a low substrate specificity and catalyzes the macromolecular aromatic compounds into available substrates. Owing to its regulatory role in the decomposition of organic matter and acting as an ‘enzymic latch’ to store C, the importance of PHO in global carbon storages has been paid more and more attention [73]. In peatland, decreased PHO activity is mainly caused by the low pH, temperature and oxygen availability, leading to the accumulation of phenolic and decreased hydrolase activity. Following this, the decomposition of SOM is slowed down. Finally, C may be stored in peatlands because of a reduced DOC release and low microbial activity. However, this phenomenon of storing C is not applicable to other ecosystems with a high temperature neutral pH and high nutrients. For example, Luo and Gu [74] conducted a field experiment in a subtropical mangrove ecosystem and found that the ‘enzyme latch’ did not work in the condition of high PHO activity. The PHO activity was always high in temperate grasslands which might promote C loss rather than C storage. In our study, it may be possible to use PHO activity as an indicator for evaluating CO2 emission in temperate grasslands.
With the development of microbiology technology, in addition to enzyme activity representing microbial functional attributes, a growing number of studies has emphasized the significance of microbial community composition in driving soil C emissions [75,76]. Future studies could focus on microbial community composition, diversity and microbial function based on high-throughput sequencing technology to explain the variation of soil C fluxes under N addition.
5. Conclusions
This study set out to explore whether soils grown with alfalfa and bromegrass under the condition of N fertilization could affect soil CO2 emissions and CH4 uptakes. N fertilization in alfalfa soil increases soil acidification. Forage species have a greater effect on soil oxidase activity than hydrolase activity. PHO may be an indicator for evaluating soil CO2 emissions from temperate managed grasslands. CH4 uptake was inhibited in alfalfa soils which indirectly indicates an increase in greenhouse gas emissions (N2O increased, unpublished data). In view of the results of soil acidification and the inhibited CH4 uptake of legume soils under N fertilizer management, from the perspective of ecological effects, managed grassland can be mainly planted with bromegrass.
Author Contributions
Conceptualization, Y.Y., H.F. and S.C.; methodology, Y.Y., H.F., S.C.; software, Y.Y.; formal analysis, Y.Y., L.X., S.C., H.F., M.L., Y.G., Y.L. and Y.Z.; investigation, H.F.; resources, H.F. and S.C.; data curation, Y.Y., H.F. and S.C.; writing—original draft preparation, Y.Y.; writing—review and editing, Y.Y., S.C. and H.F.; visualization, Y.Y.; supervision, H.F. and S.C.; project administration, H.F.; funding acquisition, H.F. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 41977041, 41907036, 31770558); the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (No. 2019QZKK1003); the National Key R&D Program of China (Nos. 2017YFA0604802, 2017YFA0604804, 2016YFC0500603, 2016YFC0503603); the CAS Strategic Priority Program (Nos. XDA200204020, XDA23060401); and the “Thousand Talents Plan” Project of High-End Innovative Talents of Qinghai Province (No. TTPPHEITQP—2019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Allen, R.B.; Clinton, P.W. The influence of N addition on nutrient content, leaf carbon isotope ratio, and productivity in a Nothofagus forest during stand development. Can. J. For. Res. 2004, 34, 2037–2048. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dise, N.B.; Mountford, J.O.; Gowing, D.J. Impact of nitrogen deposition on the species richness of grasslands. Science 2004, 303, 1876–1879. [Google Scholar] [CrossRef]
- Friend, A.L.; Eide, M.R.; Hinckley, T.M. Nitrogen stress alters root proliferation in Douglas-fir seedlings. Can. J. For. Res. 1990, 20, 1524–1529. [Google Scholar] [CrossRef]
- Aerts, R.; de Caluwe, H. Nitrogen deposition effects on carbon dioxide and methane emissions from temperate peatland soils. Oikos 1999, 84, 44–54. [Google Scholar] [CrossRef]
- Sutton, M.A.; Simpson, D.; Levy, P.E.; Smith, R.I.; Reis, S.; Van Oijen, M.; De Vries, W. Uncertainties in the relationship between atmospheric nitrogen deposition and forest carbon sequestration. Glob. Chang. Biol. 2008, 14, 2057–2063. [Google Scholar] [CrossRef]
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Yang, J.; Banger, K.; Huntzinger, D.N.; Schwalm, C.R.; Michalak, A.M.; Cook, R.; Ciais, P.; Hayes, D. Global patterns and controls of soil organic carbon dynamics as simulated by multiple terrestrial biosphere models: Current status and future directions. Glob. Biogeochem. Cycles 2015, 29, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Dong, Y.; Qi, Y.; Xiao, S.; He, Y.; Ma, T. Effects of nitrogen fertilization on soil respiration in temperate grassland in Inner Mongolia, China. Environ. Earth Sci. 2011, 62, 1163–1171. [Google Scholar] [CrossRef]
- Yamulki, S.; Jarvis, S. Short-term effects of tillage and compaction on nitrous oxide, nitric oxide, nitrogen dioxide, methane and carbon dioxide fluxes from grassland. Biol. Fertil. Soils 2002, 36, 224–231. [Google Scholar] [CrossRef]
- Khalil, M.; Rasmussen, R. Atmospheric methane: Trends over the last 10,000 years. Atmos. Environ. 1987, 21, 2445–2452. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Chen, W.; Wolf, B.; Zheng, X.; Yao, Z.; BUTTERBACH-BAHL, K.; Brueggemann, N.; Liu, C.; Han, S.; Han, X. Annual methane uptake by temperate semiarid steppes as regulated by stocking rates, aboveground plant biomass and topsoil air permeability. Glob. Chang. Biol. 2011, 17, 2803–2816. [Google Scholar] [CrossRef]
- Rogger, J.; Hörtnagl, L.; Buchmann, N.; Eugster, W. Carbon dioxide fluxes of a mountain grassland: Drivers, anomalies and annual budgets. Agric. For. Meteorol. 2022, 314, 108801. [Google Scholar] [CrossRef]
- Poeplau, C.; Zopf, D.; Greiner, B.; Geerts, R.; Korvaar, H.; Thumm, U.; Don, A.; Heidkamp, A.; Flessa, H. Why does mineral fertilization increase soil carbon stocks in temperate grasslands? Agric. Ecosyst. Environ. 2018, 265, 144–155. [Google Scholar] [CrossRef]
- Scurlock, J.; Hall, D. The global carbon sink: A grassland perspective. Glob. Chang. Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Soussana, J.F.; Loiseau, P.; Vuichard, N.; Ceschia, E.; Balesdent, J.; Chevallier, T.; Arrouays, D. Carbon cycling and sequestration opportunities in temperate grasslands. Soil Use Manag. 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Collins, S.L.; Sinsabaugh, R.L.; Crenshaw, C.; Green, L.; Porras-Alfaro, A.; Stursova, M.; Zeglin, L.H. Pulse dynamics and microbial processes in aridland ecosystems. J. Ecol. 2008, 96, 413–420. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, L.; Li, L.; Li, A.; Wu, M.; Wan, S. Diversity-dependent stability under mowing and nutrient addition: Evidence from a 7-year grassland experiment. Ecol. Lett. 2012, 15, 619–626. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Tang, X.; Xin, X.; Ye, L.; Yang, G.; Tang, H.; Lv, S.; Xu, D.; Zhang, Z. Managed grassland alters soil N dynamics and N2O emissions in temperate steppe. J. Environ. Sci. 2018, 66, 20–30. [Google Scholar] [CrossRef]
- Jones, S.K.; Helfter, C.; Anderson, M.; Coyle, M.; Campbell, C.; Famulari, D.; Marco, C.D.; Dijk, N.v.; Tang, Y.S.; Topp, C.F. The nitrogen, carbon and greenhouse gas budget of a grazed, cut and fertilised temperate grassland. Biogeosciences 2017, 14, 2069–2088. [Google Scholar] [CrossRef]
- Jones, S.; Rees, R.; Skiba, U.; Ball, B. Greenhouse gas emissions from a managed grassland. Glob. Planet. Chang. 2005, 47, 201–211. [Google Scholar] [CrossRef]
- Ambus, P.; Robertson, G. The effect of increased N deposition on nitrous oxide, methane and carbon dioxide fluxes from unmanaged forest and grassland communities in Michigan. Biogeochemistry 2006, 79, 315–337. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, G.; Fang, H.; Cao, G.; Li, Y. Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China. Atmos. Environ. 2010, 44, 2920–2926. [Google Scholar] [CrossRef]
- Li, K.; Gong, Y.; Song, W.; He, G.; Hu, Y.; Tian, C.; Liu, X. Responses of CH4, CO2 and N2O fluxes to increasing nitrogen deposition in alpine grassland of the Tianshan Mountains. Chemosphere 2012, 88, 140–143. [Google Scholar] [CrossRef]
- Kammann, C.; Grünhage, L.; Jäger, H.-J.; Wachinger, G. Methane fluxes from differentially managed grassland study plots: The important role of CH4 oxidation in grassland with a high potential for CH4 production. Environ. Pollut. 2001, 115, 261–273. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hu, H.-W.; Han, H.-Y.; Du, Y.; Wan, S.-Q.; Xu, Z.-W.; Chen, B.-D. Abundance and community structure of ammonia-oxidizing Archaea and Bacteria in response to fertilization and mowing in a temperate steppe in Inner Mongolia. FEMS Microbiol. Ecol. 2014, 89, 67–79. [Google Scholar] [CrossRef]
- Carreiro, M.; Sinsabaugh, R.; Repert, D.; Parkhurst, D. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915–917. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Stursova, M.; Sinsabaugh, R.L.; Collins, S.L. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 2007, 154, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, T.; Guo, R.; Cao, H.; Shi, L.; Guo, J.; Sun, W. Response of soil enzyme activity to warming and nitrogen addition in a meadow steppe. Soil Res. 2015, 53, 242–252. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Gao, Y.; Xing, F.; Sun, S.; Zhang, T.; Zhu, X.; Chen, C.; Li, Z. Responses of soil extracellular enzyme activities and microbial community properties to interaction between nitrogen addition and increased precipitation in a semi-arid grassland ecosystem. Sci. Total Environ. 2020, 703, 134691. [Google Scholar] [CrossRef]
- Xu, M.; Xu, L.; Fang, H.; Cheng, S.; Yu, G.; Yang, Y.; Lu, M. Alteration in enzymatic stoichiometry controls the response of soil organic carbon dynamic to nitrogen and water addition in temperate cultivated grassland. Eur. J. Soil Biol. 2020, 101, 103248. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.A.; Campany, C.E.; Classen, A.T. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Liu, W.; Tian, R.; Peng, Z.; Yang, S.; Liu, X.; Yang, Y.; Zhang, W.; Liu, L. Nonlinear responses of the Vmax and Km of hydrolytic and polyphenol oxidative enzymes to nitrogen enrichment. Soil Biol. Biochem. 2020, 141, 107656. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob. Biogeochem. Cycles 2007, 21, GB4017. [Google Scholar] [CrossRef]
- Chen, S.; Hao, T.; Goulding, K.; Misselbrook, T.; Liu, X. Impact of 13-years of nitrogen addition on nitrous oxide and methane fluxes and ecosystem respiration in a temperate grassland. Environ. Pollut. 2019, 252, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cheng, S.; Yu, G.; Cooch, J.; Wang, Y.; Xu, M.; Li, L.; Dang, X.; Li, Y. Low-level nitrogen deposition significantly inhibits methane uptake from an alpine meadow soil on the Qinghai–Tibetan Plateau. Geoderma 2014, 213, 444–452. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Fang, H.; Yu, G.; Xu, X.; Xu, M.; Wang, L.; Li, X.; Si, G.; Geng, J. Contrasting effects of ammonium and nitrate inputs on soil CO2 emission in a subtropical coniferous plantation of southern China. Biol. Fertil. Soils 2015, 51, 815–825. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, W.; Yang, H.; Yang, H.; Chen, G.; Luo, Y.; Fang, H.; Li, S. Effects of nitrogen addition on soil inorganic N content and soil N mineralization of a cold-temperate coniferous forest in Great Xing’an Mountains. Acta Ecol. Sin. 2015, 35, 130–136. [Google Scholar] [CrossRef]
- Malik, A.; Gleixner, G. Importance of microbial soil organic matter processing in dissolved organic carbon production. FEMS Microbiol. Ecol. 2013, 86, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Huygens, D.; Díaz, S.; Urcelay, C.; Boeckx, P. Microbial recycling of dissolved organic matter confines plant nitrogen uptake to inorganic forms in a semi-arid ecosystem. Soil Biol. Biochem. 2016, 101, 142–151. [Google Scholar] [CrossRef]
- Ladd, J. Properties of proteolytic enzymes extracted from soil. Soil Biol. Biochem. 1972, 4, 227–237. [Google Scholar] [CrossRef]
- Hofmockel, K.S.; Fierer, N.; Colman, B.P.; Jackson, R.B. Amino acid abundance and proteolytic potential in North American soils. Oecologia 2010, 163, 1069–1078. [Google Scholar] [CrossRef]
- Yahdjian, L.; Gherardi, L.; Sala, O.E. Nitrogen limitation in arid-subhumid ecosystems: A meta-analysis of fertilization studies. J. Arid Environ. 2011, 75, 675–680. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Wolf, G.; Stock, W.D. Cluster roots of Leucadendron laureolum (Proteaceae) and Lupinus albus (Fabaceae) take up glycine intact: An adaptive strategy to low mineral nitrogen in soils? Ann. Bot. 2005, 96, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Carrillo, Y.; Pendall, E.; Dijkstra, F.A.; Evans, R.D.; Morgan, J.A.; Williams, D.G. Soil microbes compete strongly with plants for soil inorganic and amino acid nitrogen in a semiarid grassland exposed to elevated CO2 and warming. Ecosystems 2015, 18, 867–880. [Google Scholar] [CrossRef]
- Tian, Q.; He, H.; Cheng, W.; Bai, Z.; Wang, Y.; Zhang, X. Factors controlling soil organic carbon stability along a temperate forest altitudinal gradient. Sci. Rep. 2016, 6, 18783. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Lee, S.; Hong, J.-H.; Kang, H. Methane oxidation rates in forest soils and their controlling variables: A review and a case study in Korea. Ecol. Res. 2006, 21, 849–854. [Google Scholar] [CrossRef]
- Fang, H.; Yu, G.; Cheng, S.; Zhu, T.; Wang, Y.; Yan, J.; Wang, M.; Cao, M.; Zhou, M. Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences 2010, 7, 395–407. [Google Scholar] [CrossRef]
- Nyerges, G.; Stein, L.Y. Ammonia cometabolism and product inhibition vary considerably among species of methanotrophic bacteria. FEMS Microbiol. Lett. 2009, 297, 131–136. [Google Scholar] [CrossRef]
- Whalen, S.; Reeburgh, W. Effect of nitrogen fertilization on atmospheric methane oxidation in boreal forest soils. Chemosphere-Glob. Chang. Sci. 2000, 2, 151–155. [Google Scholar] [CrossRef]
- Nanba, K.; King, G.M. Response of atmospheric methane consumption by Maine forest soils to exogenous aluminum salts. Appl. Environ. Microbiol. 2000, 66, 3674–3679. [Google Scholar] [CrossRef][Green Version]
- Tamai, N.; Takenaka, C.; Ishizuka, S. Water-soluble Al inhibits methane oxidation at atmospheric concentration levels in Japanese forest soil. Soil Biol. Biochem. 2007, 39, 1730–1736. [Google Scholar] [CrossRef]
- Xu, X.; Inubushi, K. Effects of N sources and methane concentrations on methane uptake potential of a typical coniferous forest and its adjacent orchard soil. Biol. Fertil. Soils 2004, 40, 215–221. [Google Scholar] [CrossRef]
- Acton, S.; Baggs, E. Interactions between N application rate, CH4 oxidation and N2O production in soil. Biogeochemistry 2011, 103, 15–26. [Google Scholar] [CrossRef]
- Jassal, R.S.; Black, T.A.; Roy, R.; Ethier, G. Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 2011, 162, 182–186. [Google Scholar] [CrossRef]
- Barton, L.; Thamo, T.; Engelbrecht, D.; Biswas, W.K. Does growing grain legumes or applying lime cost effectively lower greenhouse gas emissions from wheat production in a semi-arid climate? J. Clean. Prod. 2014, 83, 194–203. [Google Scholar] [CrossRef]
- Li, J.; Tong, X.-j.; Yu, Q. Methane uptake and oxidation by unsaturated soil. Acta Ecol. Sin 2005, 25, 141–147. [Google Scholar]
- Raji, S.G.; Dörsch, P. Effect of legume intercropping on N2O emissions and CH4 uptake during maize production in the Great Rift Valley, Ethiopia. Biogeosciences 2020, 17, 345–359. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Pineau, S.; Crozat, Y.; Jeuffroy, M.-H. The effect of various dynamics of N availability on winter pea–wheat intercrops: Crop growth, N partitioning and symbiotic N2 fixation. Field Crops Res. 2010, 119, 2–11. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, L.; Fang, H.; Yu, G.; Yang, X.; Li, X.; Si, G.; Geng, J.; He, S.; Yu, G. Nonlinear responses of soil nitrous oxide emission to multi-level nitrogen enrichment in a temperate needle-broadleaved mixed forest in Northeast China. Catena 2016, 147, 556–563. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Saiya-Cork, K.; Sinsabaugh, R.; Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Frenzel, P. Contribution of methanotrophic and nitrifying bacteria to CH4 and NH4+ oxidation in the rhizosphere of rice plants as determined by new methods of discrimination. Appl. Environ. Microbiol. 1999, 65, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Floch, C.; Alarcon-Gutiérrez, E.; Criquet, S. ABTS assay of phenol oxidase activity in soil. J. Microbiol. Methods 2007, 71, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gu, J.-D. Seasonal variability of extracellular enzymes involved in carbon mineralization in sediment of a subtropical mangrove wetland. Geomicrobiol. J. 2015, 32, 68–76. [Google Scholar] [CrossRef]
- Whitaker, J.; Ostle, N.; Nottingham, A.T.; Ccahuana, A.; Salinas, N.; Bardgett, R.D.; Meir, P.; McNamara, N.P. Microbial community composition explains soil respiration responses to changing carbon inputs along an A ndes-to-A mazon elevation gradient. J. Ecol. 2014, 102, 1058–1071. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Delgado-Baquerizo, M.; Wang, J.-T.; Hu, H.-W.; Yang, Z.; He, J.-Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).