Abstract
The high isoprene emission capacity of palm species can decrease regional air quality and enhance the greenhouse effect when land is converted to palm plantations. Propagation of low-emitting individuals can be a strategy for reducing isoprene emission from palms. However, the identification of low-emitting individuals requires large-scale sampling. Thus, we aimed to develop a rapid method in which the isoprene emission rate of leaf segments is observed. We examined the temperature response and effect of incubation length on the isoprene emission rate of palm leaf and found that leaf temperatures at 25 to 30 °C and an incubation length of 40 min are suitable parameters. To further examine the validity of the method, we applied both the enclosure method and this method to the same sections of leaves. High coefficient of determinations (0.993 and 0.982) between the results of the two methods were detected regardless of seasonal temperature. This result proves that the method is capable of measuring the isoprene emission rate under any growth conditions if the incubation temperature is controlled. By using a water bath tank and a tested light source, we can simply implement a unified environmental control of multiple samples at once, which achieves a higher time efficiency than conventional enclosure measurements.
1. Introduction
Isoprene (2-methyl-1,3-butadiene, C5H8) is the most abundant volatile organic compound (VOC) emitted from biogenic sources. It is estimated to constitute approximately 400–700 Tg of annual carbon and to account for 50–70% of total terrestrial biogenic VOC (BVOC) emissions [1,2]. These values are equivalent to those of global methane emissions (410–660 Tg of carbon per year) and are five times larger than global anthropogenic emissions of non-methane VOCs [3,4].
Isoprene can affect the oxidative capacity of the atmosphere and, thus, its chemical balance [5,6]. For instance, the oxidation of isoprene in the presence of nitrogen oxides (NOx ≡ NO + NO2) dominates ozone production in the troposphere [7]. High ground-level ozone concentrations were observed in areas with the interaction of isoprene emission from oil palm plantation and agroindustrial exhaust [8], semi-urban regions with a mixture of biogenic and anthropogenic emissions [9], and cities with rich vegetation sources [10,11]. Ground-level ozone can consequently reduce net ecosystem productivity and, thus, may impact the terrestrial carbon sink [12,13]. Depending on the concentrations of isoprene and NOx, 1–6% of the total mass of isoprene can also be converted to secondary organic aerosols [14,15]. Additionally, isoprene, which is highly reactive, can potentially compete with methane for radicals and consequently alter its lifetime [16,17,18]. Thus, the monitoring of isoprene emission from plants provides critical information for the management and control of these negative effects.
Simulation is the most commonly used method of monitoring isoprene dynamics. The Model of Emissions of Gases and Aerosols from Nature (MEGAN) version 2.1, a widely used simulation model of global-scale BVOC emissions [1], uses the framework of Guenther et al. [19] to estimate isoprene emissions from plants. It calculates the rate by adjusting the isoprene emission rate recorded at or standardized to a photosynthetic photon flux density (PPFD) of 1000 μmol m−2 s−1 and a leaf temperature of 30 °C (i.e., the basal isoprene emission factor) to reflect instantaneous changes in factors such as leaf temperature and light intensity [1]. The basal isoprene emission factor is usually conducted independently by species or group. To obtain the basal isoprene emission factors for a species, a branch/leaf enclosure method is typically recommended [20]. Several studies have used portable gas exchange measuring systems (e.g., LI-6400, Li-Cor Inc., Lincoln, NE, USA) with an external VOC trap and air pump for isoprene emission measurement with accurate leaf-scale temperature, light control, and better mobility (e.g., [21,22,23,24,25]). However, this method is limited by its short reach (less than 3 m between the platform and branch); in addition, a raised platform is required for plants with high canopy heights. Moreover, the equilibration time of gas exchange in the cuvette/chamber limits the number of leaves sampled. The reason is that, empirically, CO2 exchange does not become stable in the cuvette for at least 3 min, and the time required is even longer when the light intensity or temperature is adjusted [26,27,28,29]. Moreover, a previous study recorded a period of more than 30 min for the leaves of a shrub (Edgeworthia chrysantha) to reach the stabilized isoprene emission rate in a cuvette [21].
Palms (the Arecaceae family) are monocotyledonous flowering plants with 181 genera and 2600 species [30]; they are distributed in tropical and subtropical areas worldwide [31]. Most species of Arecaceae have tree-like woody trunks which develop by the growth of extremely wide primary stems, progressive lignification, and thickening of the cell wall with age [32]. Tropical plants tend to have a high isoprene emission capacity because isoprene can protect plants from thermal damage [33,34,35]. As tall plants living in tropical and subtropical areas, palm trees encounter transient and continuous heat stresses; thus, palm species can be expected to have a high isoprene emission capacity. Previous studies also found high isoprene emission rates in several palm species (e.g., Calamus gracilis, Elaeis guineensis, Salacca secanda, Euterpe precatoria, and Socratea exorrhiza) [36,37,38]. Palm species are widely planted for agricultural purposes [39]. From 2000 to 2020, the area covered by palms has expanded rapidly, for example, from 10.4 to 28.7 million ha for oil palms, from 10.6 to 11.6 million ha for coconut palms, and from 1.0 to 1.2 million ha for date palms [40]. The high isoprene emission capacity can decrease regional air quality and enhance the greenhouse effect when land is converted to palm plantations. The propagation of low-emitting individuals can be a strategy for reducing isoprene emission from palms. However, the identification of low-emitting individuals requires large-scale sampling, which is difficult using the conventional enclosure measurement. To achieve efficient sampling, this study aimed to develop a rapid method in which the isoprene emission rate of leaf segments is observed. The isoprene emission rate of excised leaves is measured after treatment (i.e., incubation under constant light and temperature). The two main goals of this study are (1) to clarify the appropriate process parameters, including incubation period and temperature and (2) to validate this method by comparing its results to those obtained using an enclosure method.
2. Materials and Methods
2.1. Plant Materials
Three- to five-year old pot saplings of Butia yatay, Washingtonia filifera (three individuals), Phoenix dactylifera ‘Major’, and Phoenix dactylifera ‘Piarom’ were purchased from a local commercial supplier and moved to the campus of the University of Shizuoka, Shizuoka Prefecture, Japan. The trunk heights (vertical length from ground surface to trunk top) of B. yatay, the three W. filifera individuals, P. dactylifera ‘Major’, and P. dactylifera ‘Piarom’ were 0.3, 0.3, 0.2, 0.1, and 0.1 m, respectively. The saplings were watered every fourth day. Phoenix canariensis and Trachycarpus wagnerianus previously planted on the University of Shizuoka campus were also studied. Two individuals of P. canariensis grown separately under different conditions (sunlit/shaded) were used. The trunk heights of the sunlit and shaded P. canariensis and T. wagnerianus were 1.2, 1.4, and 0.6 m, respectively.
After 1 November 2021, the pot saplings were moved to an indoor space. A light intensity of ~250 μmol m−2 s−1 PPFD at the top of the plants was supplied by horticultural fluorescent lamps from 6:00 to 18:00. The air temperature was fixed at ~25 °C.
2.2. Measurement of Temperature Response of Isoprene Emission Rate Using Enclosure Method
To clarify the response of the isoprene emission rate of palm leaves to temperature, B. yatay, W. filifera, P. dactylifera ‘Major’, and P. dactylifera ‘Piarom’ were used for the measurements (B. yatay: 27 October and 11 November 2021; W. filifera: 26 November and 7 December 2021; P. dactylifera ‘Major’: 15 November and 22 November, 2021; and P. dactylifera ‘Piarom’: 12 November and 19 November, 2021). A modified portable photosynthesis measuring system (LI-6400XT, Li-Cor Inc., Lincoln, NE, USA) connected to a proton transfer reaction time-of-flight mass spectrometer (PTR-TOF1000, IONICON Analytik GmbH, Innsbruck, Tyrol, Austria) was used for continuous monitoring of the isoprene emission rate inside the enclosure. The system was assembled using the method described by Tani and Kawawata [22]. The drift tube E/N (where E is the electric field strength, and N is the buffer-gas number density in the drift tube) was kept at 143 Td by maintaining the drift tube voltage, temperature, and pressure at 600 V, 80 °C, and 2.2 mbar, respectively. H3O+ was used as the primary ion. The m/z (where m is the ion’s mass, and z is the charge number of the ion) of ionized isoprene ((C5H8)H+) was 69. It was confirmed that leaves of the palm species exhibited no emission of 2-methyl-3-buten-2-ol. Thus, it was deemed that no overlap on the m/z of 69 by its fragment was produced by hydroxide ion transfer. The system was calibrated with a 10 ppb isoprene standard sample, which was prepared by diluting 1 ppm isoprene gas with pure nitrogen gas (purity > 99.99995%). To direct air emitted from the leaf in the cuvette of the photosynthesis measuring system to the spectrometer, the tube between the cuvette and embedded infrared gas analyzer was replaced with a T-junction of polytetrafluoroethylene (PTFE). This modification added an air pathway that was connected to the sample inlet of the spectrometer. The air inflow rates of the cuvette in the photosynthesis measuring system and the spectrometer were set to 500 μmol s−1 and 0.3 L min−1 (~225 μmol s−1), respectively. A platinum catalyst heated to 400 °C was used to supply VOC-free air to the photosynthesis measuring system. A blank test was performed before the measurement. During the measurement, the entire plant was placed under a 400 W metal halide lamp (D400, Toshiba Lighting & Technology Corporation, Yokosuka, Japan), and the target leaflet was enclosed in the cuvette. The light intensity and CO2 concentration in the cuvette were set to 1000 μmol m−2 s−1 and 400 ppm, respectively. The leaf temperature in the cuvette was sequentially set to 20, 25, 30, and 35 °C to observe the effects of the leaf temperature on the rates of isoprene emission, net photosynthesis, and stomatal conductance (gsw). The isoprene concentration was recorded at 30 s intervals, and the photosynthetic rate was recorded at 1 min intervals.
2.3. Isoprene Emission Rate for Different Incubation Periods
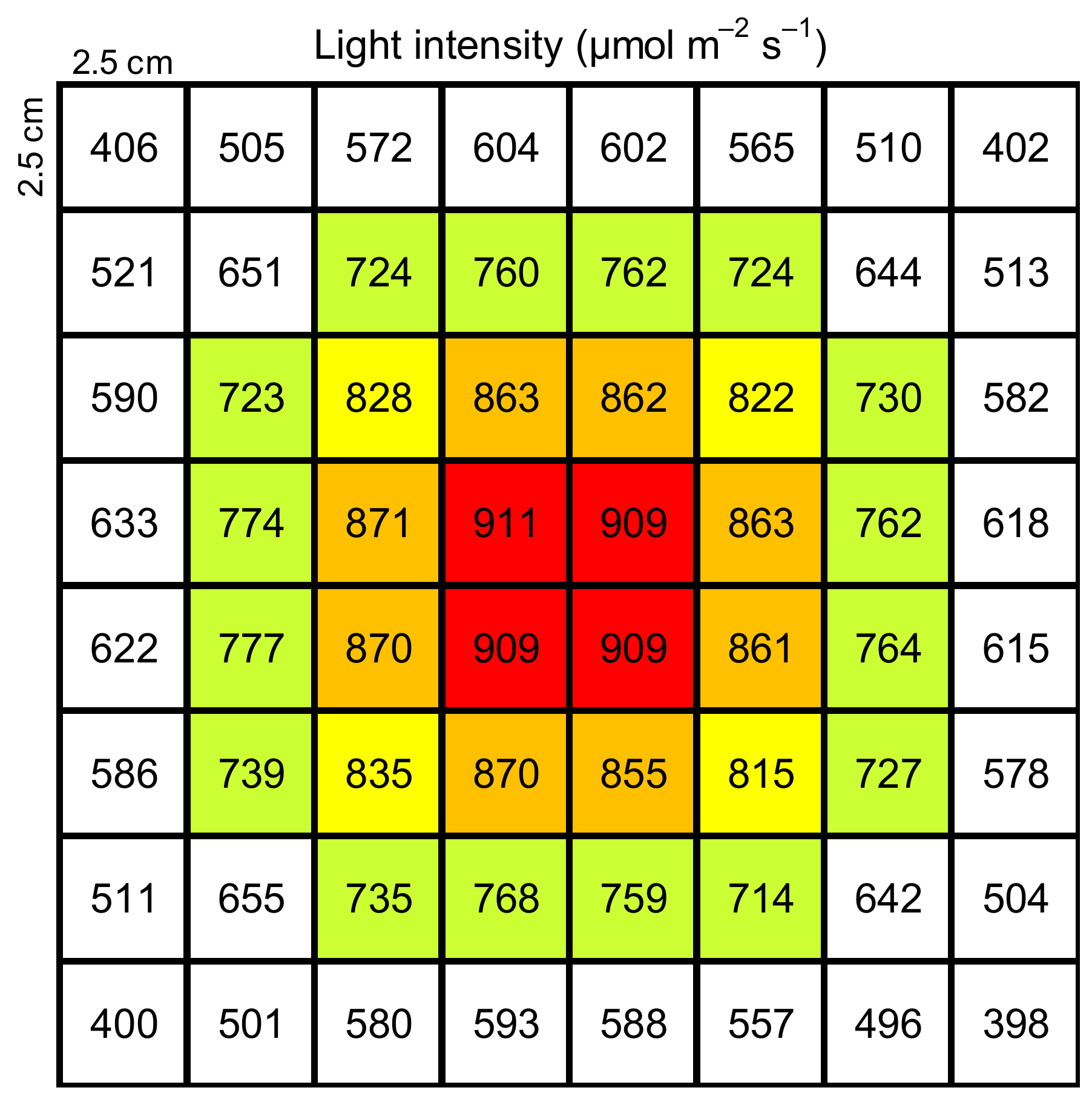
A method of measuring the isoprene emitted from excised palm leaves, which included incubation under a constant water temperature and light exposure, was developed. To determine a suitable incubation period, the isoprene emissions from leaves were determined using incubation periods of 10, 40, and 60 min. B. yatay, W. filifera, P. dactylifera ‘Piarom’, P. dactylifera ‘Major’, and P. canariensis were tested on 18–21 June and 2 November 2021; 24 November 2021; 25 November 2021; 6 December 2021; and 10 November 2021, respectively; four or five replications were performed for each species. A leaflet was first cut into pieces 1.5–2 cm in length. Then, each piece was sealed in a glass vial (10 mL in volume) with a screw cap having a silicone/PTFE septum. The glass vial was prepared by adding a hard PTFE net as a platform and 5 μL of distilled water to prevent water loss from the leaf. Next, the vial was incubated at a constant temperature for a certain period in a water bath tank (22 cm × 31cm × 14 cm) (Personal-11, Taitec Corporation, Nagoya, Japan), and stable photosynthetic active radiation was provided by a light-emitting diode (LED) light panel (TH2-211X200SW, CCS Inc., Kyoto, Japan). The LED light panel was located 10 cm above the base of the water bath tank. The base of the tank was split into 8 × 8 grids (2.5 cm × 2.5 cm). The PPFD of each grid was measured with a PPFD meter (LI-250A, Li-Cor Inc.) to verify the light intensity distribution; the result is shown in Figure 1. To maximize the number of vials that could be processed simultaneously, grids with a PPFD of 855–875 μmol m−2 s−1 were used, which allows the incubation of eight vials at once. Then, the leaf piece was moved and sealed in another vial and further incubated for 10 min. Note that vial exchange was not performed for measurements with an incubation period of 10 min. Finally, the vial was removed from the incubation system for isoprene collection. During collection, a filter tube was connected to the vial to introduce VOC-free air into the vial, and a collection tube was connected to the vial to capture the isoprene inside the vial. Both tubes were packed with 200 mg of Tenax TA and 100 mg of Carbotrap B (PerkinElmer Inc., Waltham, MA, USA) as adsorbents. Then, a syringe (30 mL) was connected to the other side of the collection tube to draw air from the vial. The collection tube was then analyzed to identify and quantify the VOCs, as described in Section 2.5.
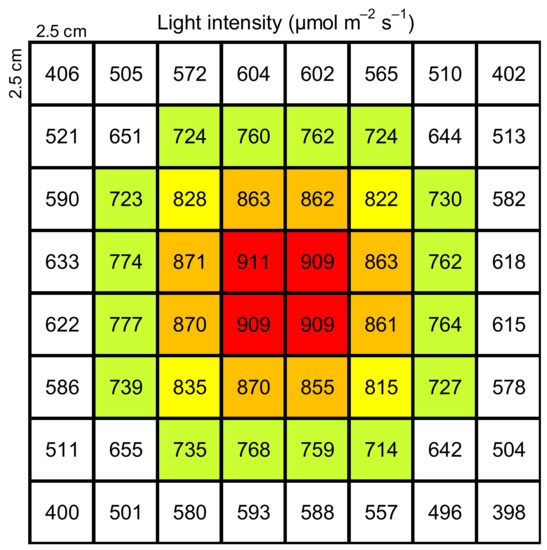
Figure 1.
Distribution of photosynthetic photon flux density (PPFD, μmol m−2 s−1) at the base of water bath tank during exposure to an LED light source. (Red-filled cells stand for PPFD larger than 900 μmol m−2 s−1; orange-filled cells stand for PPFD ranged from 850 to 900 μmol m−2 s−1; yellow-filled cells stand for PPFD ranged from 800 to 850 μmol m−2 s−1; green-filled cells stand for PPFD ranged from 700 to 800 μmol m−2 s−1; white-filled cells stand for PPFD less than 700 μmol m−2 s−1.
2.4. Comparison of Measurement Using Excised Leaves and Measurement Based on Enclosure Method
Four experiments were conducted to compare the isoprene emission rates obtained using excised leaves and the enclosure method on 25–31 May 2021, 20–21 July 2021, 7–11 October 2021, and 5–9 November 2021. In the first and second experiments, B. yatay, W. filifera (three individuals), P. dactylifera ‘Piarom’, and P. dactylifera ‘Major’ were observed. In the third and fourth experiments, B. yatay, W. filifera (three individuals), P. dactylifera ‘Piarom’, P. dactylifera ‘Major’, T. wagnerianus, and the sunlit and shaded P. canariensis individuals were observed.
The target leaflet was first measured using the enclosure method while it was attached to the plant. The enclosure measurement method was similar to the method described above in Section 2.2, except that instead of connecting the additional air pathway of the photosynthesis measuring system to the spectrometer, an adsorbent tube was connected to the additional air pathway for each sampling. The light intensity, leaf temperature, and CO2 concentration in the cuvette were set to 1000 μmol m−2 s−1, 30 °C, and 400 ppm, respectively. Before isoprene was collected, the leaf was enclosed in the cuvette for approximately 40–60 min until the net photosynthetic rate became steady. During isoprene collection, an adsorbent tube was plugged to the added air pathway of the photosynthesis measuring system, and a micropump (MP-Σ30NII, Sibata Inc., Tokyo, Japan) was connected to the other side of the adsorbent tube to draw air from the cuvette for 5 min at a rate of 0.2 L min−1; 1 L of air was passed through the adsorbent tube. After the enclosure measurement, the leaf inside the cuvette was cut for measurement using the process described in Section 2.3. Note that because the leaflet had been stabilized by the cuvette, the incubation period was set to 10 min, and vial exchange was omitted. The water temperature during incubation was set to 30 °C in the first and second experiments and 25 °C in the third and fourth experiments.
2.5. Gas Chromatography Analysis
The isoprene samples collected using an adsorbent tube were analyzed by a gas chromatography system. Two-stage desorption was performed by a thermal desorption system (TD-30, Shimadzu Corporation, Kyoto, Japan) to desorb the VOCs from the adsorbent tube, where the desorption, trap, and redesorption temperatures were 280, −20, and 280 °C, respectively. The redesorbed VOCs were then injected into a gas chromatography flame ionization detector system (Nexis GC-30, Shimadzu Corporation) with a SH-I-5MS capillary column (length: 60 m; φ: 0.25 mm; ID: 1.0 µm; Shimadzu Corporation) for identification and quantification. This system used helium as a carrier gas; the gas pressure, column flow rate, linear velocity, and split ratio were 125.0 kPa, 1.1 mL min−1, 21.6 cm s−1, and 18.7:1, respectively. During analysis, the column temperature was first held at 35 °C for 5 min; then, it was increased to 250 °C at a rate of 5 °C min−1 and held at 250 °C for 10 min. The isoprene quantity (N, nmol) was determined by an internal standard line, where toluene-d8 was used as the standard substance, and the coefficient of determination of the standard line was >0.999.
2.6. Calculation of Gas Exchange and Carbon Ratio
The isoprene emission rate (nmol m−2 s−1) was determined by different means depending on the measurement method. That of the excised leaves (Iexcised) was determined as follows:
where Aexcised (m2) is the area of the excised leaf, and t (s) is the incubation period after vial exchange (approximately 600 s). The isoprene emission rate for the enclosure method (Ienclose) was determined as follows:
where Cin and Cout (nmol mol−1) are the inflow and outflow isoprene concentrations of the cuvette, respectively; win and wout (mol mol−1) are the inflow and outflow water vapor concentrations of the cuvette, respectively; F (mol s−1) is the air inflow rate of the cuvette (5 × 10−4 mol s−1); and Acuvette and Aleaf (m2) are the planar area of the cuvette (0.0006 m2) and actual leaf area inside the cuvette, respectively. Cin was assumed to be 0 nmol m−2 s−1 because isoprene was eliminated from the inflow by the platinum catalyst, as described above. Cout was determined as follows:
where V (L) is the air volume passed through the adsorbent tube, and Vm (L mol−1) is the molar volume of air at 0 °C (22.4 L mol−1).
Iexcised = N/Aexcised × t
Ienclose = {Cout × [(1 − win)/(1 − wout)] − Cin} × F × (Aleaf/Acuvette)
Cout = N × (Vm/V)
The net photosynthetic rate (Pn, μmol m−2 s−1) was determined as follows:
where and (μmol mol−1) are the inflow and outflow CO2 concentrations of the cuvette, respectively.
The fraction of carbon emitted as isoprene in the carbon assimilated by photosynthesis (Risoprene, %) is defined as follows:
where CNisoprene is the carbon number of isoprene (CNisoprene = 5). Note that the steady-state values of Ienclose and Pn were used to calculate each leaf temperature.
Risoprene = (Ienclose × CNisoprene)/(Pn × 1000) × 100%
3. Results
3.1. Response of Isoprene Emission Rate to Leaf Temperature
The time plots showed clear stepwise increases in Ienclose with increasing leaf temperature for all four palm species, B. yatay, P. dactylifera ‘Piarom’, P. dactylifera ‘Major’, and W. filifera (Figure 2). B. yatay had the highest isoprene emissions, where the steady-state average Ienclose at 20, 25, 30, and 35 °C were 4.1, 12.9, 29.9, and 50.5 nmol m−2 s−1, respectively. P. dactylifera ‘Piarom’ had the second-largest Ienclose, where the values at each leaf temperature were 3.7, 9.6, 20.7, and 33.3 nmol m−2 s−1, respectively. Although it is in the same species, P. dactylifera ‘Major’ exhibited lower Ienclose, where the values at each leaf temperature were 3.1, 7.4, 13.4, and 21.7 nmol m−2 s−1, respectively. W. filifera exhibited the lowest Ienclose at 20, 25, and 30 °C (1.3, 4.1, and 10.8 nmol m−2 s−1, respectively) but showed a slightly higher rate at 35 °C (24.4 nmol m−2 s−1) than P. dactylifera ‘Major’. The steady-state Ienclose at each leaf temperature typically increased nonlinearly with increasing leaf temperature, where the net increase gradually became larger. However, the increase was not mathematically exponential because the growth ratio decreased with increasing leaf temperature (25 °C to 20 °C: 2.8 times; 30 °C to 25 °C: 2.2 times; and 35 °C to 30 °C: 1.8 times).
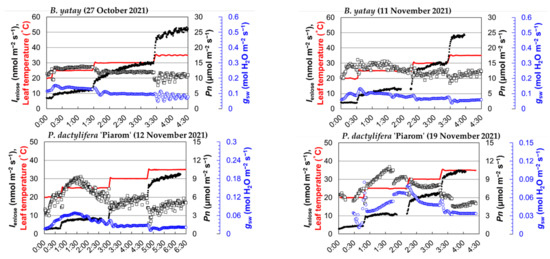
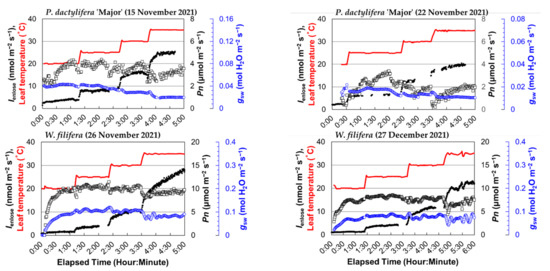
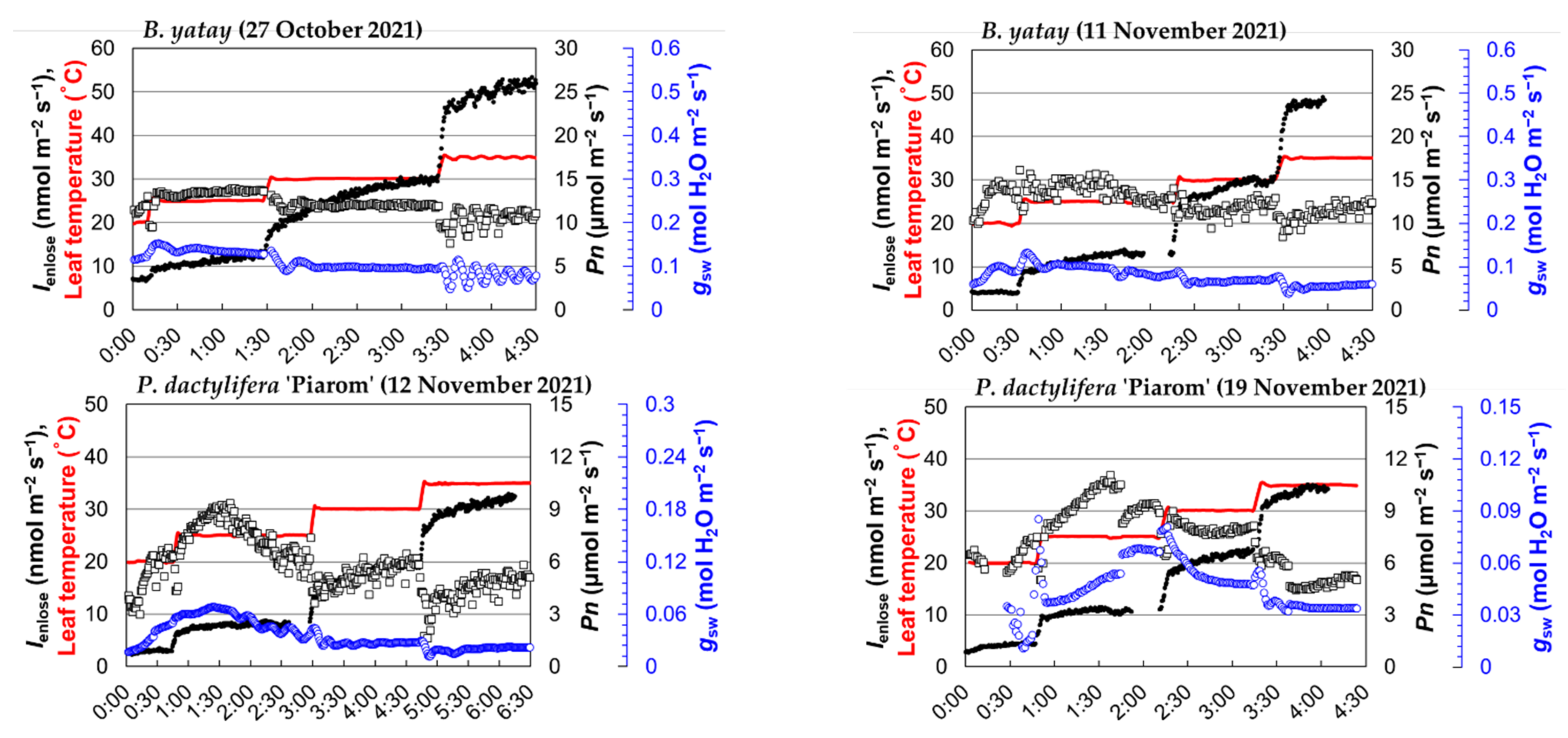
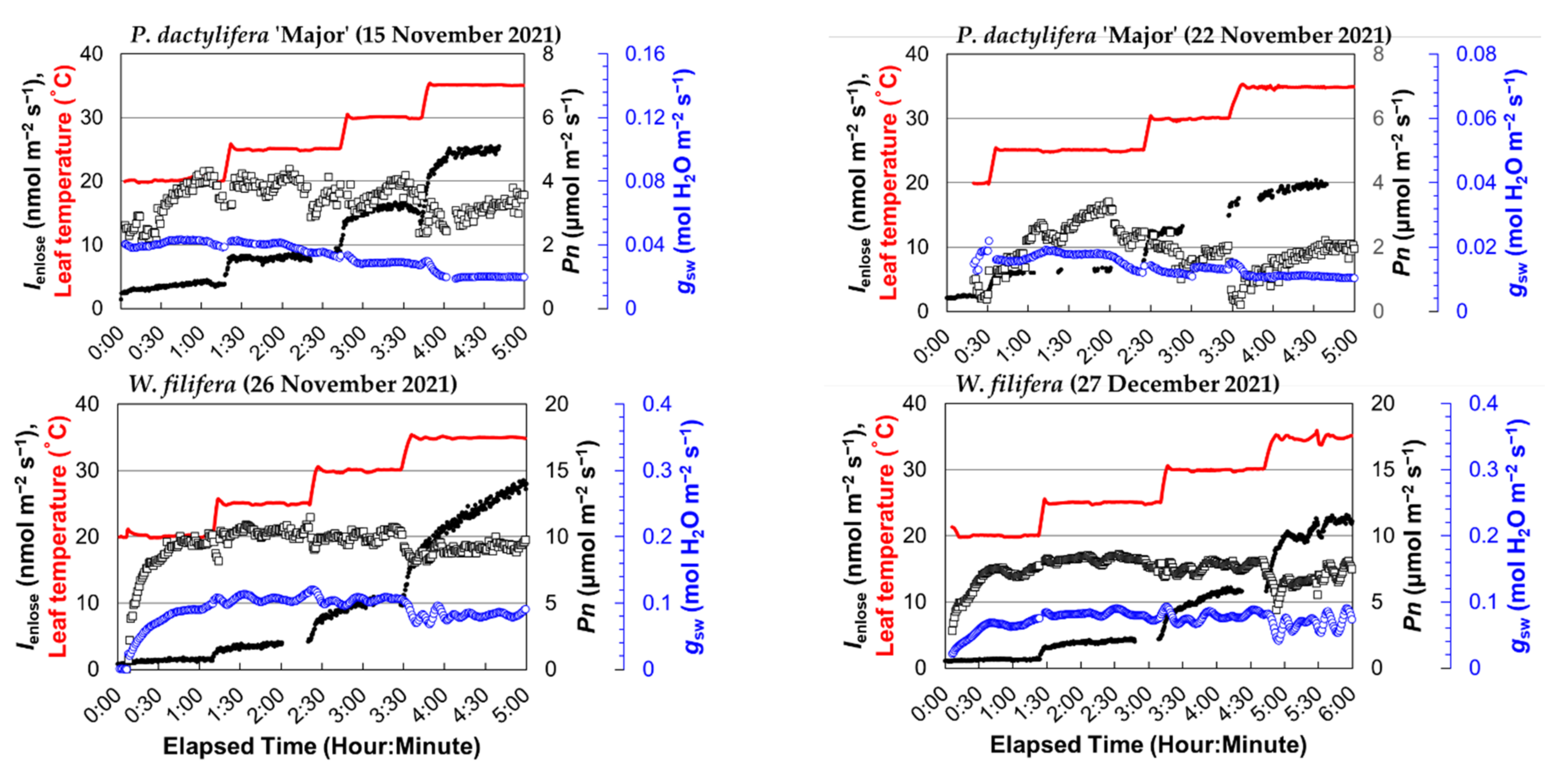
Figure 2.
The time plot of the isoprene emission rate obtained by the enclosure method (Ienclose), net photosynthetic rate (Pn), stomatal conductance (gsw), and leaf temperature for four palm species (B. yatay, P. dactylifera ‘Piarom’, P. dactylifera ‘Major’, and W. filifera). Solid dots: Ienclose; open squares: Pn; blue-colored open circles: gsw; red line: leaf temperature.
During heating, Ienclose generally responded instantaneously to leaf temperature. However, Ienclose continued to increase moderately after the leaf temperature stopped increasing and required ~40 min, on average, to reach the steady state. The period of moderate increase varied greatly among measurements. The shortest period was that of P. dactylifera ‘Major’ as leaf temperature increased from 20 to 25 °C (~10 min), and the longest was that of B. yatay as leaf temperature increased from 25 to 30 °C (~90 min).
By contrast, the palm species exhibited relatively stable variation in Pn, but large differences appeared between species. The Pn value of palm leaves typically reached a maximum at 20 or 25 °C and, then, decreased slightly at higher leaf temperatures. The steady-state Pn values of B. yatay were 10.9–13.8 and 12.2–13.8 μmol m−2 s−1 in the two measurements. Those of P. dactylifera ‘Piarom’ were 5.2–6.7 and 5.2–9.4 μmol m−2 s−1; those of P. dactylifera ‘Piarom’ were 3.6–4.1 and 0.6–2.4 μmol m−2 s−1; those of W. filifera were 9.2–10.5 and 7.1–8.2 μmol m−2 s−1.
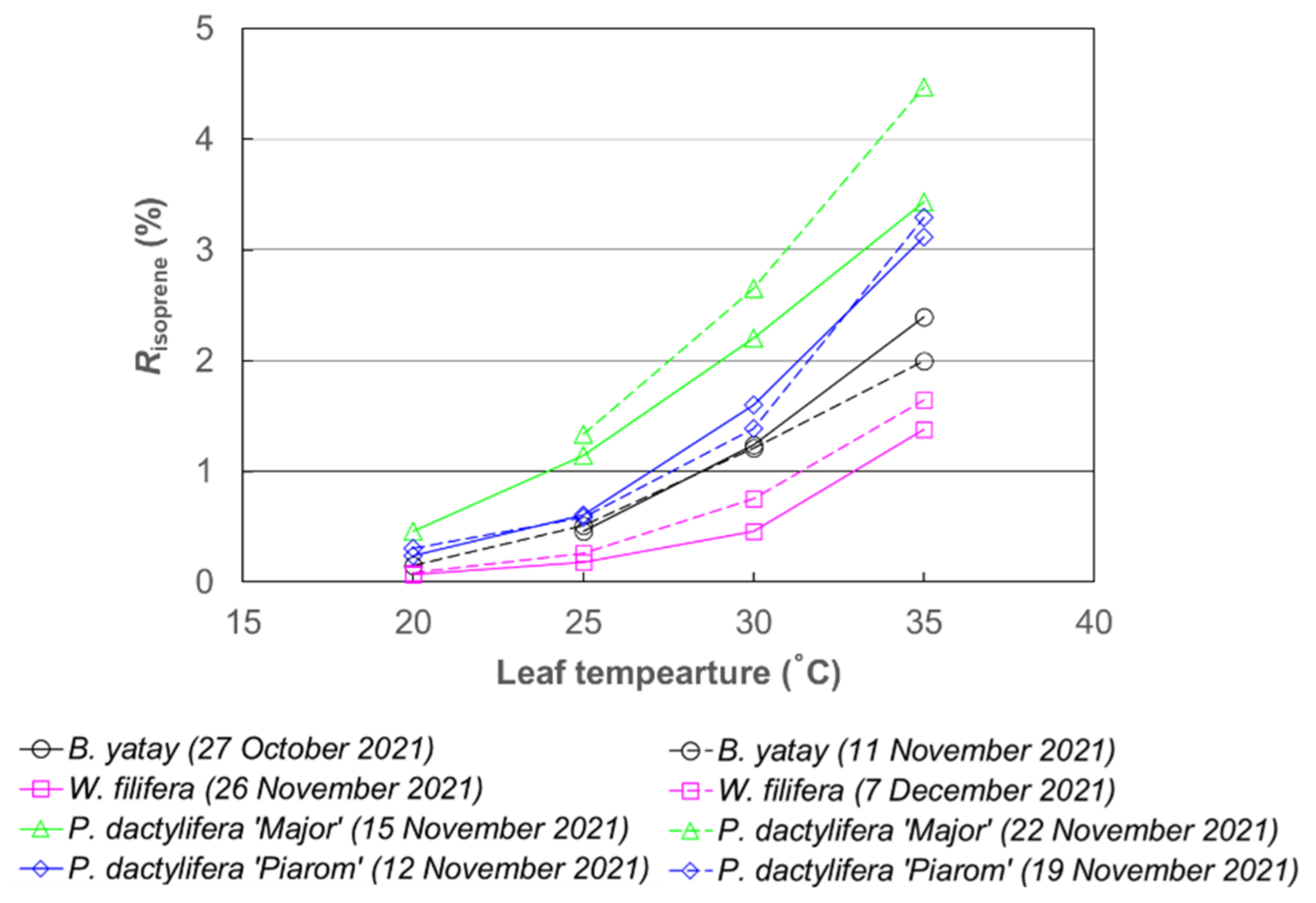
Risoprene varied widely among the palm species at each temperature; however, overall, it increased nonlinearly with increasing leaf temperature in a pattern similar to that of Ienclose (Figure 3). At 20 °C, Risoprene did not exceed 0.5% for any palm species. By contrast, at 35 °C, all the species had Risoprene values larger than 1%, and P. dactylifera ‘Major’ had an extremely large value (>4%).
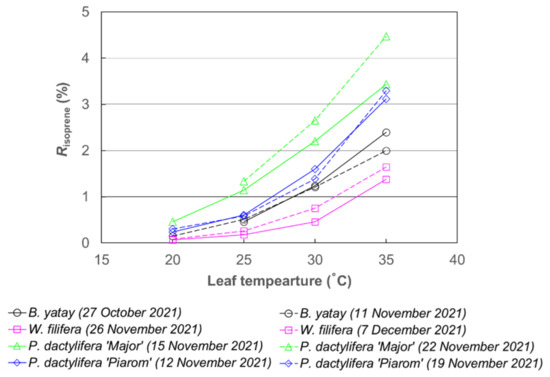
Figure 3.
Fraction of carbon emitted as isoprene in carbon assimilated by photosynthesis (Risoprene) of B. yatay, P. dactylifera ‘Piarom’, P. dactylifera ‘Major’, and W. filifera) at leaf temperatures of 20, 25, 30, and 35 °C.
3.2. Isoprene Emission Rate for Different Incubation Periods
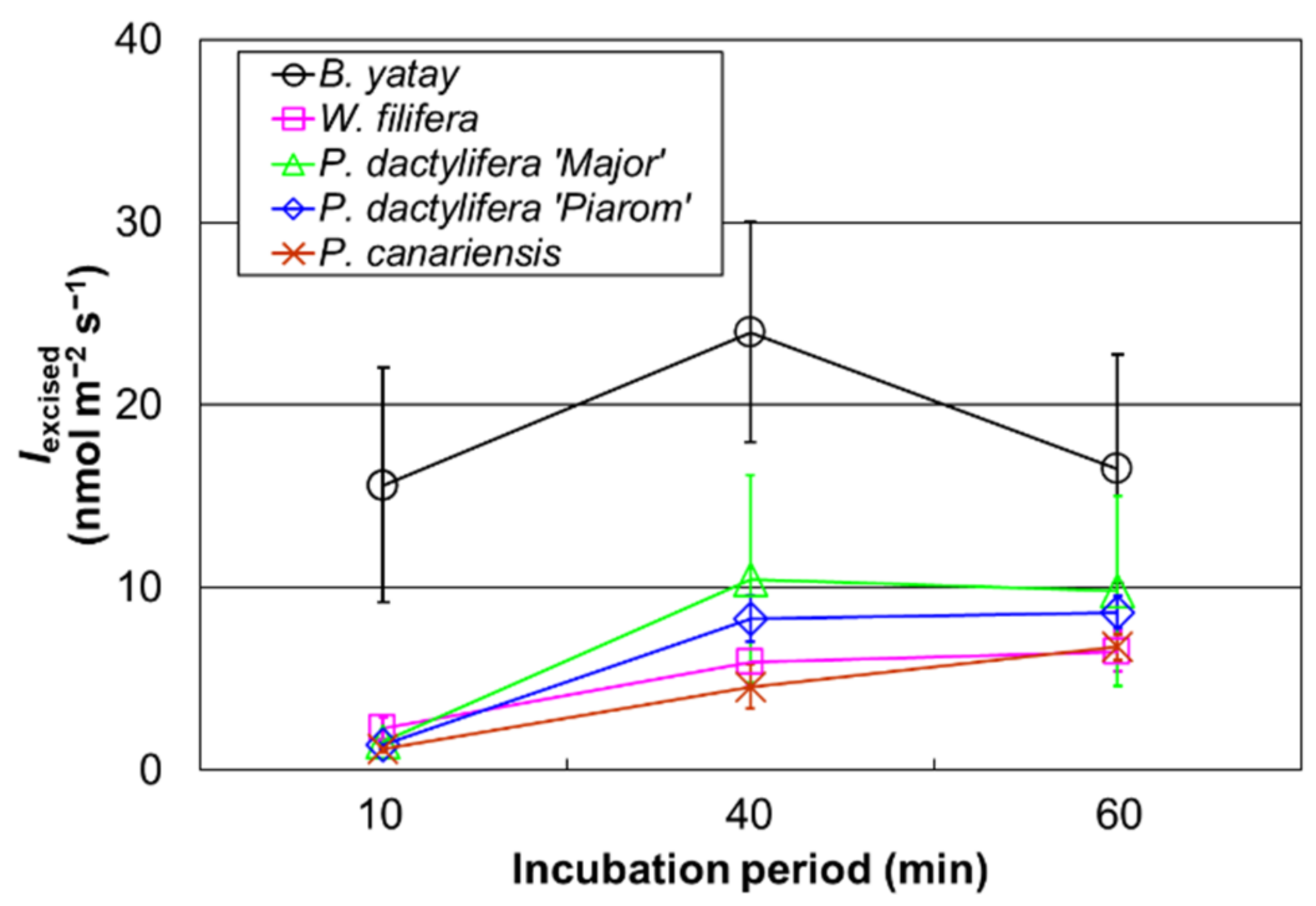
The Iexcised values of B. yatay, W. filifera, P. dactylifera ‘Piarom’, P. dactylifera ‘Major’, and the sunlit P. canariensis individual were measured for incubation periods of 10, 40, and 60 min (Figure 4). The isoprene emission rate of B. yatay increased as the incubation period increased from 10 to 40 min and, then, decreased at an incubation period of 60 min. The Iexcised values for periods of 10 min (15.6 ± 6.4 nmol m−2 s−1) and 60 min (16.5 ± 6.3 nmol m−2 s−1) were approximately 65% of that at 40 min (24.0 ± 6.0 nmol m−2 s−1). For W. filifera, P. dactylifera ‘Piarom’, and P. dactylifera ‘Major’, Iexcised increased as the incubation period increased from 10 min (2.3 ± 0.6, 1.4 ± 0.2, and 1.6 ± 0.2 nmol m−2 s−1, respectively) to 40 min (5.9 ± 1.2, 8.3 ± 1.3, and 10.5 ± 5.7 nmol m−2 s−1, respectively). The value for a period of 60 min was approximately the same level as that of 40 min (6.5 ± 1.1, 8.7 ± 0.9, and 9.8 ± 5.2 nmol m−2 s−1, respectively). P. canariensis exhibited a constant linear increase in Iexcised with increasing incubation period during the entire experiment, where Iexcised was 1.2 ± 0.1, 4.6 ± 1.2, and 6.7 ± 0.7 nmol m−2 s−1 after 10, 40, and 60 min, respectively. Iexcised was typically low in the initial stage and increased at 40 min for all the palm species; however, the tendency after further incubation depended on the species.
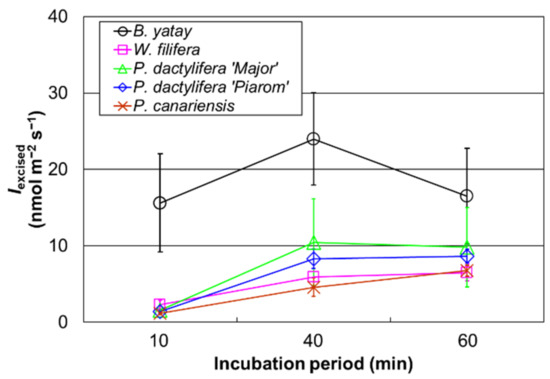
Figure 4.
The isoprene emission rate obtained using excised leaves (Iexcised) for incubation periods of 10, 40, and 60 min. Open circles, squares, triangles, diamonds, and crosses represent the mean Iexcised values of B. yatay (n = 5), W. filifera (n = 4), P. dactylifera ‘Major’ (n = 4), P. dactylifera ‘Piarom’ (n = 4), and P. canariensis, respectively. Vertical bars represent the standard deviation.
3.3. Comparison of Isoprene Emission Rates Obtained Using Excised Leaves and Enclosure Method
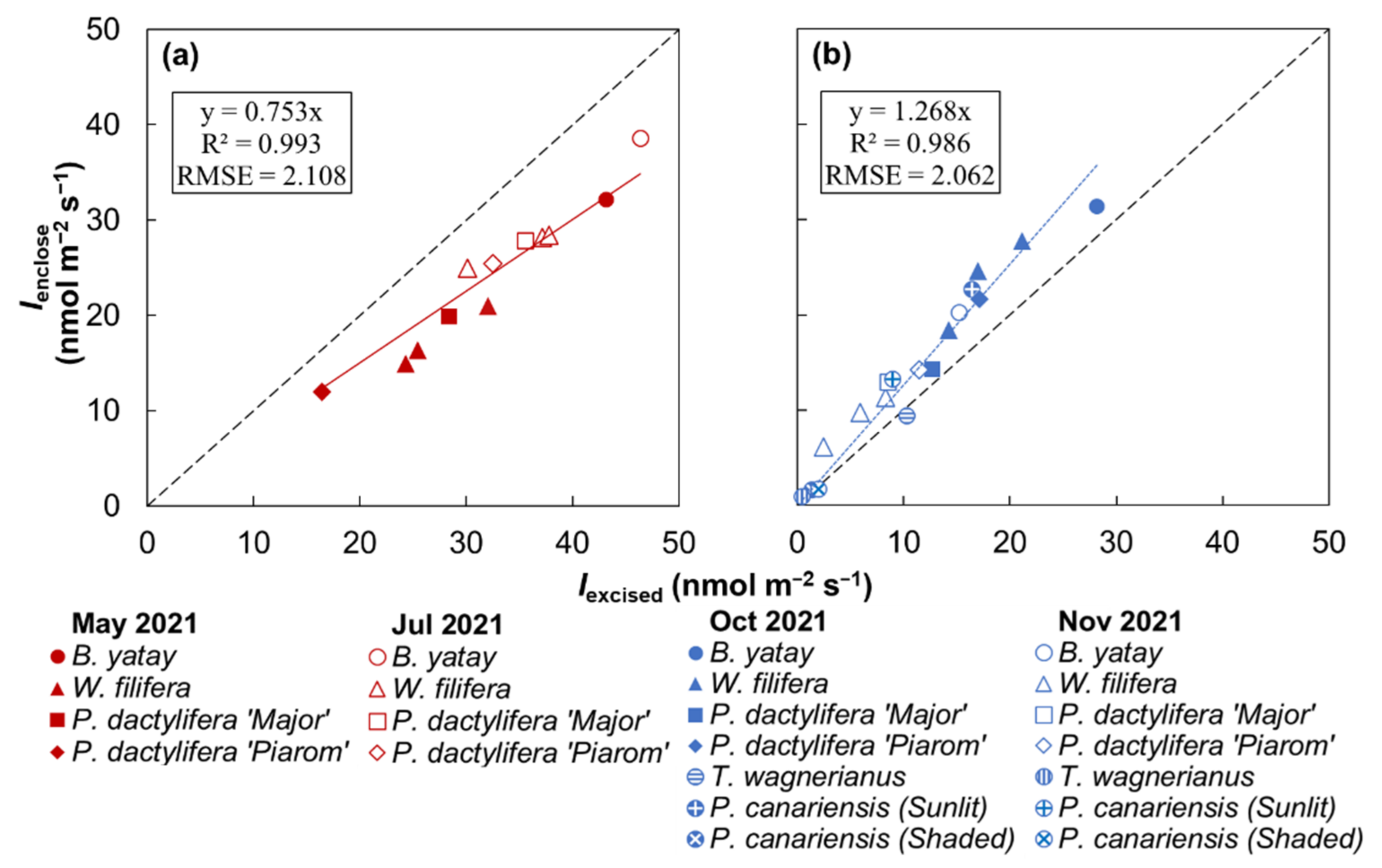
Figure 5 shows the relationship between Iexcised and Ienclose for the palm species. B. yatay showed the highest isoprene emission rates in all the experiments. T. wagnerianus and the shaded P. canariensis individual had the lowest rates. W. filifera, P. dactylifera ‘Major’, P. dactylifera ‘Piarom’, and the sunlit P. canariensis individual had similar rates.
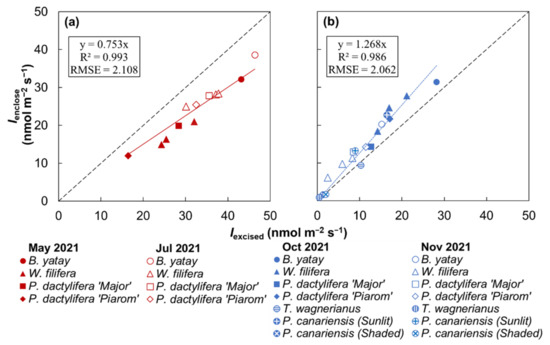
Figure 5.
Comparison of isoprene emission rates obtained by the enclosure method (Ienclose) and the method using excised leaves (Iexcised) for multiple palm species at water temperatures of (a) 30 °C and (b) 25 °C. Solid lines represent linear regression between Ienclose and Iexcised, where the intercept value was set to zero. (RMSE: root mean square error).
During all the experiments, Ienclose ranged from 1.0 to 38.6 nmol m−2 s−1. Iexcised had a wider range from 0.4 to 46.3 nmol m−2 s−1. Overall, the Ienclose values in the first and third experiments were very similar, and those in the second and fourth experiments were higher and lower, respectively. By contrast, Iexcised was higher in the first and second experiments and lowest in the fourth experiment.
Strong positive linear correlations between Ienclose and Iexcised were identified separately at incubation temperatures of 25 °C (R2 = 0.986) and 30 °C (R2 = 0.993). The slopes of the correlations at 30 and 25 °C were 0.75 and 1.27, respectively. The slope at 25 °C was 1.7 times higher than that at 30 °C. According to the slope of the two correlations, the Iexcised values obtained at 25 °C were generally 0.8 times the Ienclose values, and those obtained at 30 °C were generally 1.3 times the Ienclose values.
4. Discussion
4.1. Temperature Response of Isoprene Emission Rate of Palm Species
The isoprene emission measurement at different leaf temperatures revealed considerable differences in the isoprene emission rates of the palm species. B. yatay, which was reported for the first time, exhibited extremely high Ienclose values under standard conditions, which were comparable to those of species known to be strong emitters, e.g., Populus tremuloides (59 nmol m−2 s−1), Quercus alba (79 nmol m−2 s−1), and Salix nigra (37 nmol m−2 s−1) [41,42,43]. W. filifera and P. dactylifera reportedly showed moderate isoprene emission under standard conditions [35,44], whereas our observation revealed high Ienclose under standard conditions. Seasonality might be a factor, as a decreasing trend in Ienclose with time (27 October–7 December 2021) was observed. In addition, the lower PPFD in the indoor environment starting on 1 November 2021, might have decreased Ienclose, because lower isoprene emission capacity has been reported in plants acclimated to lower light intensity [45,46,47].
For all the palm species (B. yatay, W. filifera, P. dactylifera ‘Major’, and P. dactylifera ‘Piarom’), Ienclose clearly depended on temperature. Differences among species were relatively small at 20 °C and increased at higher temperatures. The nonlinear increases in Ienclose from 20 to 35 °C for the palm species were generally consistent with the temperature dependence reported in previous studies [48,49,50]. By contrast, the Pn values of the palms exhibited varying responses to leaf temperature, increasing when the leaf temperature increased from 20 to 25 °C but decreasing at higher temperatures. The decrease at high temperatures is attributed mainly to decreasing gsw. Risoprene of the palm species increased significantly with leaf temperature because of the decrease in Pn and substantial increase in Ienclose, which is consistent with the pattern of hybrid aspen (Populus tremula × Populus tremuloides) reported by Rasulov et al. [51]. In addition, species such as P. dactylifera ‘Major’ and P. dactylifera ‘Piarom’ exhibited extremely high Risoprene (>3%) at a leaf temperature of 35 °C in this study compared to those recorded from hybrid aspen.
Although isoprene emission is not directly related to stomatal status [52,53], water loss at high temperatures is still a concern. Extreme water loss could cause folding of the excised leaves and thus reduce the amount of light they receive, causing large errors in the measured isoprene emission rate.
Our measurements demonstrated that Ienclose responded very rapidly to fluctuations in leaf temperature. The relationship was almost the same as that between Ienclose and leaf temperature during the initial heating process. However, Ienclose continued to increase moderately even after warming stopped. An average of 40 min was required to reach the steady-state value of Ienclose. This subsequent increase may be associated with the time required for dimethylallyl diphosphate (DMADP), one of the precursors for isoprene synthesis, to reach equilibrium after a temperature change [54]. This process varied greatly over our observations; the shortest periods were less than 5 min, whereas the longest periods exceeded 90 min.
4.2. Determination of Appropriate Incubation Period for Isoprene Measurement Using Excised Palm Leaves
The effect of cutting on the leaves may increase with time. However, the stabilization period must be long enough for the isoprene emission rate of palm leaves to become steady. There should be an optimal incubation period considering the opposing effects of time on stabilization and disturbance. The use of different incubation periods for B. yatay, W. filifera, P. dactylifera ‘Major’, P. dactylifera ‘Piarom’, and P. canariensis revealed different trends in Iexcised. B. yatay exhibited a peak at 40 min. An optimal incubation period for accurate Iexcised measurement was not identified for W. filifera, P. dactylifera ‘Major’, and P. dactylifera ‘Piarom’. A 10 min incubation period was too short for the excised leaves to adapt to a water temperature of 30 °C and PPFD of 870 μmol m−2 s−1 from an air temperature of ~25 °C and PPFD of ~200 μmol m−2 s−1. In addition, B. yatay showed a very large decrease in Iexcised when the incubation period was increased from 40 to 60 min.
The low isoprene emission rate in the initial stage of incubation can be explained by the time required to increase the DMADP pool. However, the reason for the decrease in emission rate after the peak is not clear. We can only hypothesize that intense water loss after cutting resulted in either lower PPFD received because of deformation or decreased ATP due to inhibited photophosphorylation [55]. The production pathway (the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway) of isoprene-related DMADP is highly dependent on ATP and NADPH produced by the light reactions [56]. Therefore, a smaller DMADP pool can be expected with water loss. Because a few decreases in Iexcised with time were observed, Iexcised may be underestimated for long incubation periods. According to our results, 40 min could be a moderately suitable incubation period for obtaining the isoprene emission rate of excised palm leaves.
4.3. Suitability of Measurement Using Excised Leaves to Evaluate Isoprene Emission from Palm Leaves
To further examine the validity of the method using excised leaves to obtain the isoprene emission rate, we applied both the enclosure method and this method to the same sections of leaves of B. yatay, W. filifera, P. dactylifera ‘Major’, P. dactylifera ‘Piarom’, T. wagnerianus, and P. canariensis. Four experiments conducted under different conditions revealed different ranges of Ienclose. A previous observational study of an oak indicated that the isoprene emission rate can be affected by seasonal temperature variation [57]; it was also reported that P. dactylifera exposed to 35 °C for 14 days showed a larger isoprene emission rate than those exposed to 20 °C for 14 days. Our measurements of Ienclose also varied with average monthly temperature. The second experiment, with an average monthly temperature of 26.3 °C, yielded the largest Ienclose values overall. By contrast, the first and third experiments, with moderate average monthly temperatures (19.6 and 19.9 °C, respectively) revealed the second-largest Ienclose values in similar ranges. In the fourth experiment, which had the lowest average monthly temperature (14.5 °C), T. wagnerianus and P. canariensis, which were planted outside, had much lower Ienclose values. Although the pot seedlings were moved indoors and exposed to a relatively warm temperature (25 °C), decreases in Ienclose were also detected. The reason might be the lower intensity of growing light supplied by horticultural lights (less than 300 μmol m−2 s−1 PPFD), as explained in Section 4.1. Despite the different seasonal patterns, strong correlations between Iexcised and Ienclose were detected regardless of seasonal temperature. For instance, the observed isoprene emission rates were much lower in the fourth experiment than in the third experiment, but the slopes between Iexcised and Ienclose were the same. This result proves that the Ienclose value of palm leaves is well correlated with Iexcised under any growth conditions if the incubation temperature is controlled.
Nevertheless, in the first and second experiments, Iexcised was significantly larger than Ienclose, regardless of the similar temperature setting and lower light intensity in the water bath (~860 μmol m−2 s−1) compared to that in the enclosure (1000 μmol m−2 s−1). This result is unexpected, because the isoprene emission rate should increase with increasing light intensity because of light-induced growth of the DMADP pool [58]. Although the effect of cutting was not ruled out, another possible reason is that the leaf pieces were above ambient temperature (i.e., the water temperature in the water bath tank) because of non-photochemical quenching [59]. Iexcised was 1.7 times larger on average at a water temperature of 30 °C compared to that at 25 °C. This increase with temperature seems reasonable but is slightly smaller than the ratio of Ienclose at a leaf temperature of 35 °C to that at 30 °C (~1.8 times) in our measurement of the temperature dependence of the enclosure method. Because observations at lower temperatures usually showed much higher values (30 to 25 °C: ~2.2 times; 25 to 20 °C: ~2.7 times), this result implies that the temperature of the leaf pieces could have been higher than the temperature of the water bath.
4.4. Development of Method Using Excised Leaves to Measure Isoprene Emission Rate
Enclosure measurement of VOC emissions from plant leaves has been recommended to obtain an accurate isoprene emission inventory. However, this method is unsuitable for large-scale sampling under controlled conditions because there is a tradeoff between time efficiency and accurate environmental control. Leaf discs have been used for multiple samplings of isoprene emissions with control or treatment in previous studies (e.g., [60,61,62]). However, most of these studies focused on clarifying the effect of isoprene-related factors inferred from changes in isoprene emission rate rather than obtaining an isoprene emission rate inventory. Several studies have reported increased or decreased isoprene emission rates from leaves of a vine (Pueraria lobaia) and grasses (Phragmites australis, Mucuna deeringeniana) resulting from wounds [63,64]. However, studies have also found no significant change after aspen (Populus tremuloides) and hybrid aspen leaves were excised [65,66]. In addition, previous studies indicated that water loss from a leaf disc significantly disturbed the water status of leaves [67,68]. Although the thick cuticle of palm leaves prevents severe water loss from the leaf surface [69,70], it is better to minimize the water loss from the severed part of the leaf.
To reduce water loss, we used rectangular leaflets with a ratio of cut length (cm) to area (cm2) of only 1.0–1.4. By contrast, the ratios of typical leaf discs (1–1.5 cm2) can be as high as 4.2–6.3. In addition, the excised leaves were sealed in a vial containing a drop of water to reduce the vapor pressure deficit between the excised leaf and air in the vial. By using a water bath tank and a tested light source, we can simply implement a unified environmental control of multiple samples at once. Here, the time efficiency was at least eight times higher than that of enclosure measurement. The efficiency could be further improved by increasing the number of water bath systems.
5. Conclusions
We examined the temperature response and effect of incubation period on the isoprene emission rate of palm leaves to identify the appropriate incubation parameters for the rapid measurement of isoprene emissions from palm leaves. We demonstrated that leaf temperatures of 25 to 30 °C and an incubation period of 40 min were suitable for measuring isoprene emissions from excised leaves of several palm species. After the method was established, the isoprene emission rates obtained by this method were compared with those of a widely used enclosure method. A strong consistent linear correlation was found between the results of these two methods. This result implies that measurement using excised leaves is appropriate for evaluating the isoprene emission capacity of palm species. Moreover, this method enables multiple samplings at the same time using a water bath system and an LED light panel, and, thus, enables highly efficient isoprene measurement. Therefore, this study successfully established a rapid protocol for measuring the isoprene emission rates of palm species.
Author Contributions
Conceptualization, A.T.; methodology, H.O., A.T. and T.-W.C.; software, T.-W.C.; validation, A.T.; formal analysis, T.-W.C. and H.O.; investigation, H.O. and T.-W.C.; resources, A.T.; data curation, T.-W.C.; writing—original draft preparation, T.-W.C.; writing—review and editing, A.T.; visualization, T.-W.C.; supervision, A.T.; project administration, A.T.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by JSPS KAKENHI, grant Number 19H04257 and 19H05666.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The authors would like to thank the employees of the Medicinal Botanical Garden of University of Shizuoka for technical support in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature Version 2.1 (MEGAN2.1): An Extended and Updated Framework for Modeling Biogenic Emissions. Geosci. Model Dev. 2012, 15, 1471–1492. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Huang, G.; Brook, R.; Crippa, M.; Janssens-Maenhout, G.; Schieberle, C.; Dore, C.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Friedrich, R. Speciation of anthropogenic emissions of non-methane volatile organic compounds: A global gridded data set for 1970–2012. Atmos. Chem. Phys. 2017, 17, 7683–7701. [Google Scholar] [CrossRef]
- Teng, A.P.; Crounse, J.D.; Wennberg, P.O. Isoprene Peroxy Radical Dynamics. JACS 2017, 139, 5367–5377. [Google Scholar] [CrossRef]
- Tani, A.; Mochizuki, T. Review: Exchanges of volatile organic compounds between terrestrial ecosystems and the atmosphere. J. Agric. Meteorol. 2021, 77, 66–80. [Google Scholar] [CrossRef]
- Dunker, A.M.; Koo, B.; Yarwood, G. Ozone sensitivity to isoprene chemistry and emissions and anthropogenic emissions in central California. Atmos. Environ. 2016, 145, 326–337. [Google Scholar] [CrossRef]
- Hewitt, C.N.; MacKenzie, A.R.; Di Carlo, P.; Di Marco, C.F.; Dorsey, J.R.; Evans, M.; Fowler, D.; Gallagher, M.W.; Hopkins, J.R.; Jones, C.E.; et al. Nitrogen management is essential to prevent tropical oil palm plantations from causing ground-level ozone pollution. Proc. Natl. Acad. Sci. USA 2009, 106, 18447–18451. [Google Scholar] [CrossRef]
- Duane, M.; Poma, B.; Rembges, D.; Astorga, C.; Larsen, B.R. Isoprene and its degradation products as strong ozone precursors in Insubria, Northern Italy. Atmos. Environ. 2002, 36, 3867–3879. [Google Scholar] [CrossRef]
- Pang, X.; Mu, Y.; Zhang, Y.; Lee, X.; Yuan, J. Contribution of isoprene to formaldehyde and ozone formation based on its oxidation products measurement in Beijing, China. Atmos. Environ. 2009, 43, 2142–2147. [Google Scholar] [CrossRef]
- Kaser, L.; Peron, A.; Graus, M.; Striednig, M.; Wohlfahrt, G.; Juráň, S.; Karl, T. Interannual Variability of BVOC Emissions in an Alpine City. Atmos. Chem. Phys. Discuss. 2021, 22, 5603–5618. [Google Scholar] [CrossRef]
- Fowler, D.; Amann, M.; Anderson, R.; Ashmore, M.; Cox, P.; Depledge, M.; Derwent, D.; Grennfelt, P.; Hewitt, N.; Hov, O.; et al. Ground-Level Ozone in the 21st Century: Future Trends, Impacts and Policy Implications, RS1276 ed.; The Royal Society: London, UK, 2008; Volume 15/08, pp. 57–66. [Google Scholar]
- Agyei, T.; Juráň, S.; Edwards-Jonášová, M.; Fischer, M.; Švik, M.; Komínková, K.; Ofori-Amanfo, K.K.; Marek, M.V.; Grace, J.; Urban, O. The Influence of Ozone on Net Ecosystem Production of a Ryegrass-Clover Mixture under Field Conditions. Atmosphere 2021, 12, 1629. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from isoprene photooxidation under high-NOx conditions. Geophys. Res. Lett. 2005, 32, L18808. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from isoprenephotooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef]
- Collins, W.J.; Derwent, R.G.; Johnson, C.E.; Stevenson, D.S. The Oxidation of Organic Compounds in the Troposphere and their Global Warming Potentials. Clim. Change 2002, 52, 453–479. [Google Scholar] [CrossRef]
- Pike, R.C.; Young, P.J. How plants can influence tropospheric chemistry: The role of isoprene emissions from the biosphere. Weather 2009, 64, 332–336. [Google Scholar] [CrossRef]
- Archibald, A.T.; Levine, J.G.; Abraham, N.L.; Cooke, M.C.; Edwards, P.M.; Heard, D.E.; Jenkin, M.E.; Karunaharan, A.; Pike, R.C.; Monks, P.S.; et al. Impacts of HOx regeneration and recycling in the oxidation of isoprene: Consequences for the composition of past, present and future atmospheres. Geophys. Res. Lett. 2011, 38, L05804. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Guenther, A.; Zimmerman, P.R.; Klinger, L.; Greenberg, J.P.; Ennis, C.; Davis, K.; Pollock, W.; Westberg, H.; Allwine, G.; Geron, C.D. Estimates of regional natural volatile organic compound fluxes from enclosure and ambient measurements. J. Geophys. Res. 1996, 101, 1345–1359. [Google Scholar] [CrossRef][Green Version]
- Tani, A.; Fushimi, K. Effects of temperature and light intensity on isoprene emission of Edgeworthia chrysantha. J. Agric. Meteorol. 2005, 61, 113–122. [Google Scholar] [CrossRef][Green Version]
- Tani, A.; Kawawata, Y. Isoprene emission from the major native Quercus spp. in Japan. Atmos. Environ. 2008, 42, 4540–4550. [Google Scholar] [CrossRef]
- Okumura, M.; Kosugi, Y.; Tani, A. Biogenic volatile organic compound emissions from bamboo species in Japan. J. Agric. Meteorol. 2018, 74, 40–44. [Google Scholar] [CrossRef]
- Chang, T.; Kume, T.; Okumura, M.; Kosugi, Y. Characteristics of isoprene emission from moso bamboo leaves in a forest in central Taiwan. Atmos. Environ. 2019, 211, 288–295. [Google Scholar] [CrossRef]
- Chang, T.W.; Kosugi, Y.; Okumura, M.; Jiao, L.; Chen, S.; Xu, D.; Liu, Z.; Shibata, S.; Chang, K.H. Isoprene emission characteristics of tall and dwarf bamboos. Atmos. Environ. X 2021, 12, 100136. [Google Scholar] [CrossRef]
- Day, M.E. Influence of temperature and leaf-to-air vapor pressure deficit on net photosynthesis and stomatal conductance in red spruce (Picea rubens). Tree Physiol. 2000, 20, 57–63. [Google Scholar] [CrossRef]
- Pimentel, C.; Rafael, V.R.; Santos, M.G.; Oliveira, R.F.; Machado, E.C. Effects of changes in the photosynthetic photon flux density on net gas exchange of Citrus limon and Nicotiana tabacum. Braz. J. Plant Physiol. 2004, 16, 77–82. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shiraiwa, T.; Nakajima, A.; Sato, J.; Nakazaki, T. Leaf Gas Exchange Activity in Soybean as Related to Leaf Traits and Stem Growth Habit. Crop Sci. 2008, 48, 1925–1932. [Google Scholar] [CrossRef]
- Tanaka, Y. Measurements and its Applications of Leaf Gas Exchange. Jpn. J. Crop Sci. 2016, 85, 339–346. [Google Scholar] [CrossRef][Green Version]
- Baker, W.J.; Dransfield, J. Beyond Genera Palmarum: Progress and prospects in palm systematics. Bot. J. Linn. 2016, 182, 207–233. [Google Scholar] [CrossRef]
- Eiserhardt, W.L.; Svenning, J.C.; Kissling, W.D.; Balslev, H. Geographical ecology of the palms (Arecaceae): Determinants of diversity and distributions across spatial scales. Ann. Bot. 2011, 108, 1391–1416. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The uniqueness of palms. Bot. J. Linn. 2006, 151, 5–14. [Google Scholar] [CrossRef]
- Harley, P.C.; Monson, R.K.; Lerdau, M.T. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia 1999, 118, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Dani, K.G.S.; Jamie, I.M.; Prentice, I.C.; Atwell, B.J. Evolution of isoprene emission capacity in plants. Trends Plant Sci. 2014, 19, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Baker, C.R.; Walker, A.P.; McDowell, N.; Alistair Rogers, A.; Higuchi, N.; Chambers, J.Q.; Jardine, K.J. Stimulation of isoprene emissions and electron transport rates as key mechanisms of thermal tolerance in the tropical species Vismia guianensis. Glob. Change. Biol. 2020, 26, 5928–5941. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.T.; Sudol, M.; Bloch, L.; Winer, A.M. Low-emitting urban forests: A taxonomic methodology for assigning isoprene and monoterpenes emission rates. Atmos. Environ. 1996, 30, 1437–1452. [Google Scholar] [CrossRef]
- Geron, C.; Owen, S.; Guenther, A.; Greenberg, J.; Rasmussen, R.; Bai, J.H.; Li, Q.J.; Baker, B. Volatile organic compounds from vegetation in southern Yunnan Province, China: Emission rates and some potential regional implications. Atmos. Environ. 2006, 40, 1759–1773. [Google Scholar] [CrossRef]
- Jardine, K.J.; Zorzanelli, R.F.; Gimenez, B.O.; Oliveira Piva, L.R.D.; Teixeira, A.; Fontes, C.G.; Robles, E.; Higuchi, N.; Chambers, J.Q.; Martin, S.T. Leaf isoprene and monoterpene emission distribution across hyperdominant tree genera in the Amazon basin. Phytochemistry 2020, 175, 112366. [Google Scholar] [CrossRef]
- Barfod, A.S.; Balhara, M.; Dransfield, J.; Balslev, H. SE Asian Palms for Agroforestry and Home Gardens. Forests 2015, 6, 4607–4616. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 2 April 2022).
- Litvak, M.E.; Loreto, F.; Harley, P.C.; Sharkey, T.D.; Monson, R.K. The response of isoprene emission rate and photosynthetic rate to photon flux and nitrogen supply in aspen and white oak trees. Plant Cell Environ. 1996, 19, 549–559. [Google Scholar] [CrossRef]
- Geron, C.; Nie, D.; Arnts, R.R.; Sharkey, T.D.; Singsaas, E.L.; Vanderveer, P.J.; Guenther, A.; Sickles, J.E.; Kleindienst, T.E. Biogenic isoprene emission: Model evaluation in a southeastern United States bottomland deciduous forest. J. Geophys. Res. 1997, 102, 18889–18901. [Google Scholar] [CrossRef]
- Geron, C.; Harley, P.; Guenther, A. Isoprene emission capacity for US tree species. Atmos. Environ. 2001, 35, 3341–3352. [Google Scholar] [CrossRef]
- Arab, L.; Kreuzwieser, J.; Kruse, J.; Zimmer, I.; Ache, P.; Alfarraj, S.; Al-Rasheid, K.A.S.; Schnitzler, J.; Hedrich, R.; Rennenberg, H. Acclimation to heat and drought—Lessons to learn from the date palm (Phoenix dactylifera). Environ. Exp. Bot. 2016, 125, 20–30. [Google Scholar] [CrossRef]
- Harley, P.; Guenther, A.; Zimmerman, P. Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiol. 1997, 17, 705–714. [Google Scholar] [CrossRef]
- Hanson, D.T.; Sharkey, T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001, 24, 929–936. [Google Scholar] [CrossRef]
- Brilli, F.; Dani, K.G.S.; Pasqualini, S.; Costarelli, A.; Cannavò, S.; Paolocci, F.; Zittelli, G.C.; Mugnai, G.; Baraldi, R.; Loreto, F. Exposure to different light intensities affects emission of volatiles and accumulations of both pigments and phenolics in Azolla filiculoides. Physiol. Plant. 2022, 174, e13619. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. The influence of light and temperature on isoprene emission rates from live oak. Physiol. Plant. 1979, 47, 112–118. [Google Scholar] [CrossRef]
- Guenther, A.B.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Observations with eucalyptus and emission rate algorithm development. J. Geophys. Res. 1991, 96, 10799–10808. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Rasulov, B.; Bichele, I.; Hüve, K.; Vislap, V.; Niinemets, Ü. Acclimation of isoprene emission and photosynthesis to growth temperature in hybrid aspen: Resolving structural and physiological controls. Plant Cell Environ. 2015, 38, 751–766. [Google Scholar] [CrossRef]
- Monson, R.K.; Fall, R. Isoprene Emission from Aspen Leaves: Influence of Environment and Relation to Photosynthesis and Photorespiration. Plant Physiol. 1989, 90, 267–274. [Google Scholar] [CrossRef]
- Fall, R.; Monson, R.K. Isoprene emission rate and intercellular isoprene concentration as influenced by stomatal distribution and conductance. Plant Physiol. 1992, 100, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Hüve, K.; Bichele, I.; Laisk, A.; Niinemets, Ü. Temperature Response of Isoprene Emission in Vivo Reflects a Combined Effect of Substrate Limitations and Isoprene Synthase Activity: A Kinetic Analysis. Plant Physiol. 2010, 154, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Tezara, W.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Fall, R. Light-Dependent Isoprene Emission (Characterization of a Thylakoid-Bound Isoprene Synthase in Salix discolor Chloroplasts). Plant Physiol. 1996, 112, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Pétron, G.; Harley, P.; Greenberg, J.; Guenther, A. Seasonal temperature variations influence isoprene emission. Geophys. Res. Lett. 2001, 28, 1707–1710. [Google Scholar] [CrossRef]
- Rasulov, B.; Hüve, K.; Valbe, M.; Laisk, A.; Niinemets, U. Evidence That Light, Carbon Dioxide, and Oxygen Dependencies of Leaf Isoprene Emission Are Driven by Energy Status in Hybrid Aspen. Plant Physiol. 2009, 151, 448–460. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Kuzma, J.; Fall, R. Leaf Isoprene Emission Rate Is Dependent on Leaf Development and the Level of Isoprene Synthase. Plant Physiol. 1993, 101, 435–440. [Google Scholar] [CrossRef]
- Logan, B.A.; Monson, R.K. Thermotolerance of Leaf Discs from Four Isoprene-Emitting Species Is Not Enhanced by Exposure to Exogenous Isoprene. Plant Physiol. 1999, 120, 821–826. [Google Scholar] [CrossRef]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone Quenching Properties of Isoprene and Its Antioxidant Role in Leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef]
- Loreto, F.; Sharkey, T.D. Isoprene emission by plants is affected by transmissible wound signals. Plant Cell Environ. 1993, 16, 563–570. [Google Scholar] [CrossRef]
- Loreto, F.; Barta, C.; Brilli, F.; Nogues, I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006, 29, 1820–1828. [Google Scholar] [CrossRef]
- Monson, R.K.; Harley, P.C.; Litvak, M.E.; Wildermuth, M.; Guenther, A.B.; Zimmerman, P.R.; Fall, R. Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia 1994, 99, 260–270. [Google Scholar] [CrossRef]
- Rasulov, B.; Copolovici, L.; Laisk, A.; Niinemets, Ü. Postillumination Isoprene Emission: In Vivo Measurements of Dimethylallyldiphosphate Pool Size and Isoprene Synthase Kinetics in Aspen Leaves. Plant Physiol. 2009, 149, 1609–1618. [Google Scholar] [CrossRef]
- Van Gardingen, P.R.; Grace, J. Vapour Pressure Deficit Response of Cuticular Conductance in Intact Leaves of Fagus sylvatica L. J. Exp. Bot. 1992, 43, 1293–1299. [Google Scholar] [CrossRef]
- Hoad, S.P.; Grace, J.; Jeffree, C.E. A leaf disc method for measuring cuticular conductance. J. Exp. Bot. 1996, 47, 431–437. [Google Scholar] [CrossRef]
- Schuster, A.; Burghardt, M.; Alfarhan, A.; Bueno, A.; Hedrich, R.; Leide, J.; Thomas, J.; Riederer, M. Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB Plants 2016, 8, plw027. [Google Scholar] [CrossRef]
- Bueno, A.; Alfarhan, A.; Arand, K.; Burghardt, M.; Deininger, A.; Hedrich, R.; Leide, J.; Seufert, P.; Staiger, S.; Riederer, M. Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies. J. Exp. Bot. 2019, 70, 1613–1625. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).