Abstract
The fast development of large-scale intensive animal husbandry has led to an increased proportion of atmospheric pollution arising from livestock and poultry housing. Atmospheric pollutants, including particulate matter (PM), ammonia (NH3), hydrogen sulfide (H2S), and greenhouse gases (GHG), as well as other hazardous materials (e.g., gases, bacteria, fungi and viruses), have significant influences upon the local atmospheric environment and the health of animals and nearby residents. Therefore, it is imperative to develop livestock and poultry housing mitigation strategies targeting atmospheric pollution, to reduce its negative effects on the ambient atmosphere and to promote sustainable agricultural production. In this paper, we summarize the various strategies applied for reducing outlet air pollutants and purifying inlet air from mechanical ventilated livestock and poultry housing. This review highlights the current state of knowledge on the removal of various atmospheric pollutants and their relative performance. The potential optimization of processes and operational design, material selection, and other technologies, such as electrostatic spinning, are discussed in detail. The study provides a timely critical analysis to fill the main research gaps or needs in this domain by using practical and stakeholder-oriented evaluation criteria.
1. Introduction
The rising demand for food, especially for livestock products, has elicited great concerns over their negative environmental impacts [1,2,3]. In different parts of the world, animal production is highly concentrated, and intensive large-scale animal husbandry operations have expanded rapidly [4,5]. Not only are there more animals in the farms, but the farms themselves are becoming more specialized, consequently increasing their environmental load; this includes their contamination load upon soil and water, and their gaseous load upon air [5,6]. Aerial pollutants generated by the animal farms may affect the quality of the local atmosphere, the neighborhood, and the health of both animals and workers [7,8,9,10,11,12]. Further, airborne diseases, such as the porcine reproductive and respiratory syndrome virus, can be transmitted among intensive herds, and the incoming air from the atmosphere must be filtered to improve animal health and reduce bio-aerosol transmission [13,14].
Air emissions generated by animal industry harbor an abundant mixture of pollutants, mostly consisting of ammonia (NH3); greenhouse gases [carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O)]; odors; hydrogen sulfide (H2S); volatile organic compounds (VOCs); particulate matter (PM); and bio-aerosols [10,15,16,17]. They arise simultaneously from various farm components, namely: animal housing; yards; manure storage and treatment; and land spreading [6]. Concerns over the air pollution generated from livestock and poultry farming have spurred new legislation and large research programs. All those pollutants’ emissions are regulated, at present, by international protocols and national regulations aimed at addressing the problem of how to achieve emission reductions in intensive livestock and poultry farming [3,6]. Many cost-effective and environmentally friendly farming methods are already available, yet they are unlikely to be sufficient to ensure that current environmental targets are fully met [3]. Practically, stakeholder-oriented evaluation of existing and new best available technologies is key for the successful abatement of air pollution from the livestock and poultry sector [18].
Some abatement options available for intensive pig and poultry farming were brought together and listed as best available techniques (BAT). The efficacies of many of the currently BAT-listed options are both modest and difficult to regulate, and this list is still far from complete. Limiting emissions via the application of BATs alone may be insufficient to meet environmental goals; meanwhile, many other methods and treatment options are currently excluded [3,19]. Maurer et al. [18] established a literature database tab on air management practices and tools, providing an overview of the mitigation practices best suited to addressing the emissions of odor, gas, and PM from livestock operations. The data show that the target emission percentage reductions in nine air pollutants vary greatly with different technologies, some of which are inefficient and may increase other particular pollutants when reducing certain targeted pollutants. Although some management practices can be used to reduce emissions, a certain level of degraded air quality by animal operations is inevitable [20]. Each technology has both its advantages and disadvantages, and the corresponding mitigation technology implemented should be selected according to the pollutant type, ventilation rate, concentration range, and other site-specific factors, such that secondary pollution should also be avoided [20,21]. It is, thus, necessary to optimize the available technologies in terms of their process design and/or operation, with a view to targeting a specific pollution source to improve the mitigation efficiency of all pollutants of concern at a given site [22].
Housing systems are a very common feature in concentrated and intensive animal husbandry, especially for pig and poultry breeding. When the ventilation air from animal houses is released into the ambient atmosphere, the air pollutants are introduced into the environment [23]. Aspects of the housing’s construction and management, such as its ventilation and air treatment systems, may be improved to reduce aerial emissions [6,16]. By applying mechanical/forced or hybrid ventilation, which are now widely used, indoor conditions such as gas and PM concentrations can be controlled to ensure the well-being of workers and animals, and to maintain production performance [23,24,25,26]. A mechanical ventilation system can be used to bring in external fresh air and discharge the internal dirty gases, to realize the effective exchange of air inside and outside the livestock and poultry housing; this can improve the temperature, humidity and air quality in the livestock and poultry house [27,28]. This system has many benefits for animal welfare and productivity, which cannot be overemphasized in animal breeding [29,30]. Furthermore, these mechanically ventilated housing systems facilitate the utilization of an air-purification system to clean the outlet and inlet air, when compared with a pure natural ventilation system, thereby reducing the total emissions from livestock and poultry buildings and providing fresh air to the buildings [24,26].
This review has two aims: (1) To summarize the mitigation strategies of air pollutants for animal housing with mechanical or hybrid ventilation, by focusing on the end-of-pipe techniques used to purify inlet and outlet air from and into the atmosphere, respectively; (2) to identify knowledge gaps/needs and promising future research avenues, to provide timely guidance for housing systems when renovating, expanding or building new animal housing for integrated environmental permitting.
2. Ventilation of Livestock and Poultry Housing
According to the different internal and external pressures applied, mechanical/forced ventilation systems can be divided into those reliant on negative pressure ventilation, positive pressure ventilation, or isobaric ventilation [31,32]. Negative pressure ventilation refers to the use of exhaust fans to force the dirty air out of the farm, resulting in slight negative pressure in the livestock and poultry housing’s fresh air under the action of atmospheric pressure into the farm [33]. Positive pressure ventilation refers to the use of fans to force the outdoor air into the livestock and poultry housing; the outlet air volume can be adjusted, but is always less than the intake air volume, resulting in slight indoor positive pressure [34]. Isobaric ventilation refers to fans used at the same time in the inlet and outlet portals, leaving the air volume’s size unchanged, and the pressure in the livestock and poultry housing basically consistent with atmospheric pressure [30].
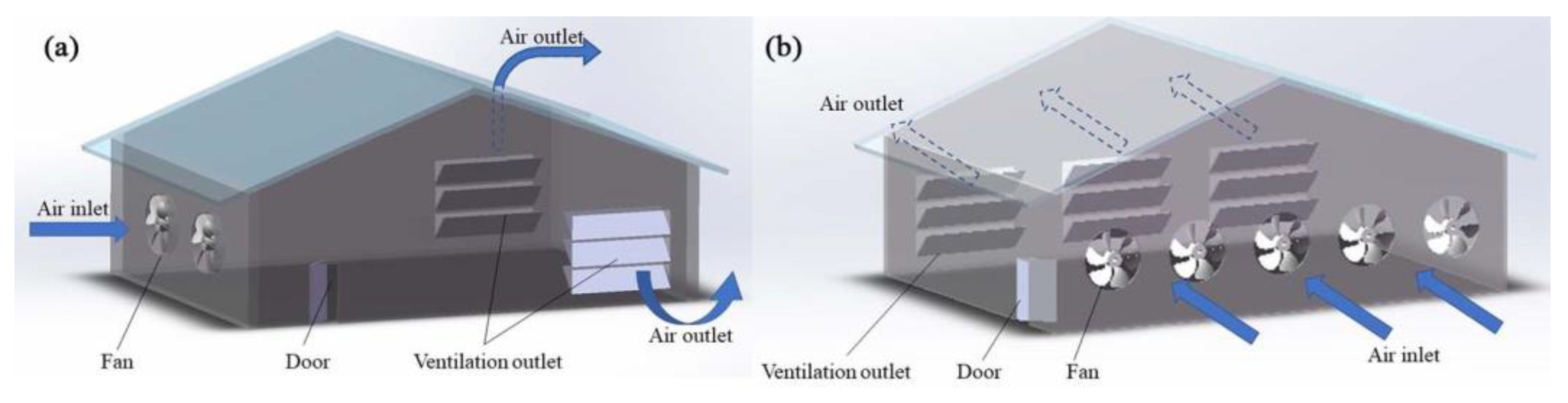
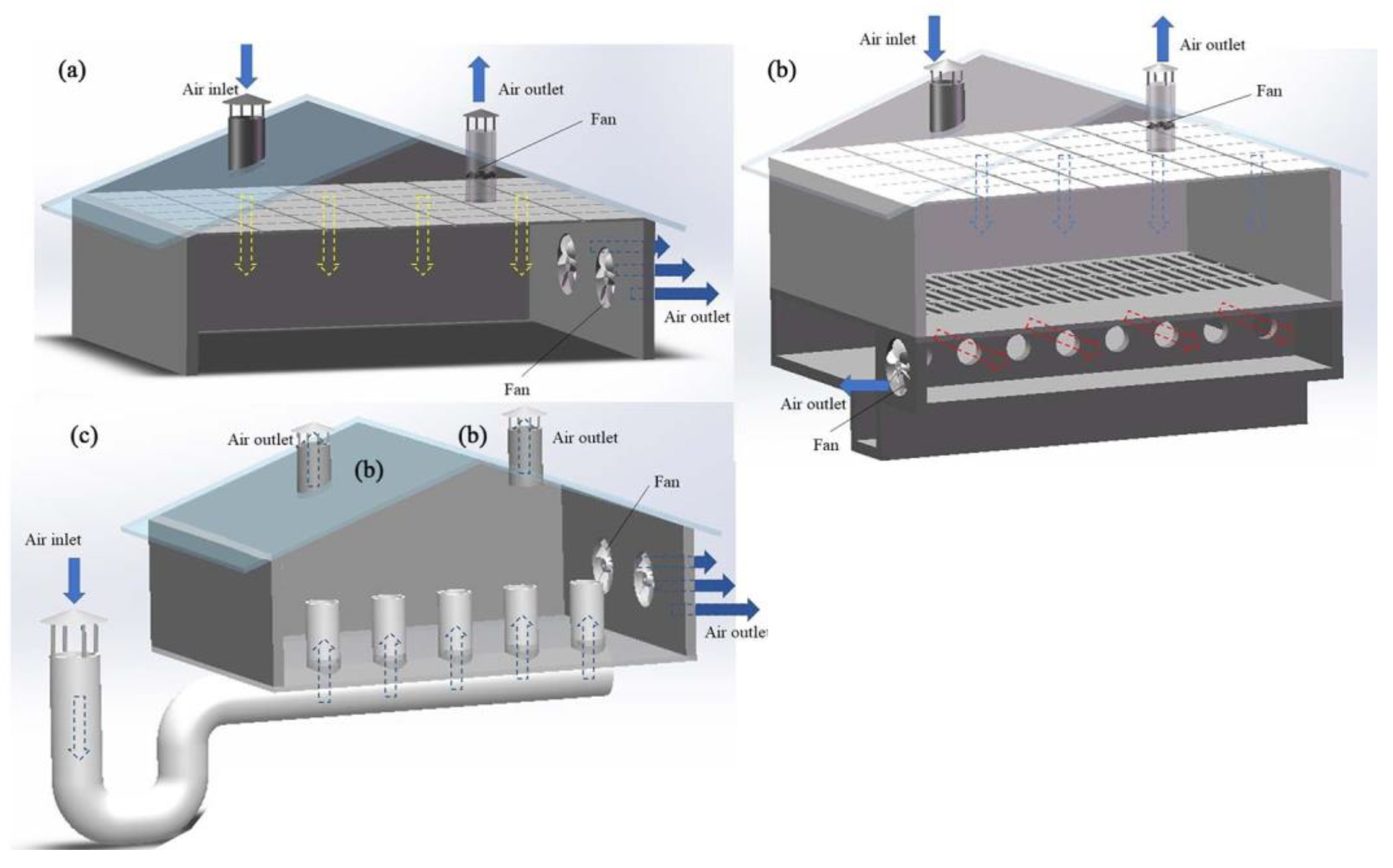
According to the different fans’ arrangements, mechanical ventilation can be further divided into longitudinal ventilation, transverse ventilation, and vertical ventilation [35,36,37]. Longitudinal ventilation means that the fan is installed on the gable and the air inside the house flows along the longer axis (Figure 1a). Transversal ventilation means that the fan is installed on the transverse wall, and flows along the shorter axis (Figure 1b) [38]. The diameters of fans used for transverse ventilation are generally small [39]. Vertical ventilation refers to applying a vertical flow of air in livestock and poultry housing. A common form of vertical ventilation is ceiling ventilation (Figure 2a), whereby air enters the ceiling from one side of the roof, diffuses into the room via the ceiling, and exits from the other side of the roof after circulating in the room [40]. In some cases, fans are installed at the lower position of the side wall to expel or inhale the air from the side wall, and then vent from the roof; however, this ventilation method is rarely used alone [26]. Pit ventilation is another form (mainly used in pig farming), in which fresh air first enters the ceiling of the attic from the cornice of the roof, then enters the room through the diffuse ceiling because of the negative pressure (Figure 2b). The dirty air passes through the slatted floor to the top of the manure pool and is pumped outside [23,26]. This ventilation method can prevent the floating of PM and volatile odorous compounds from the manure pool, to further improve the air quality in the livestock and poultry housing [41]. Another form is tunnel ventilation (Figure 2c); this is achieved by laying pipes or building cement pipes horizontally several meters underground. One end of the pipe is connected to a vertical pipe extending from the ground as the air inlet, and a fan is installed at the front end of the pipe to force the air input. After its heat exchange with the ground, the air is inputted into the livestock and poultry housing, eventually flowing outside via the roof outlet [42]. This ventilation method can effectively improve the temperature of livestock and poultry housing, by cooling it in the summer and heating it up in winter. Generally, in actual animal husbandry settings, mechanical ventilation does not take one form only, but rather, a mixture of many forms.
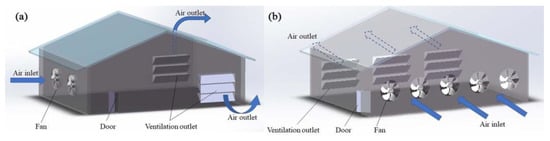
Figure 1.
Schematic of mechanical longitudinal ventilation (a), and transverse ventilation (b).
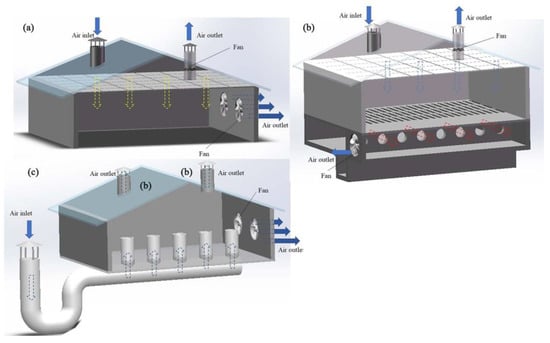
Figure 2.
Schematic of ceiling ventilation (a), pit ventilation (b), and tunnel ventilation (c).
There are often multiple ventilation fans used in livestock and poultry housing. The diameter of these fans is generally between 60 and 130 cm. The main fan is normally open and running for a long time, with auxiliary fans operating intermittently. As conveyed in Table 1, the ventilation rate depends on the ventilation mode and type of livestock and poultry housing. Different types of livestock housing are compatible (to varying degrees) with different types of ventilation systems. It is worth noting that high-rise layer hen houses are often used in conjunction with ceiling ventilation systems, perhaps due to the fact that this enables airflow containing contaminants to be exhausted more quickly from the house. The total ventilation rate and number of fans increase as the density of the housed animals increases, but the average ventilation tends to remain in a basic range. For a pig farm, the average ventilation rate of its room is generally not more than 150 m3 h−1 pig−1, and the ventilation rate of the pit exhaust is generally 10 m3 h−1 pig−1 [23,26,30,43,44,45,46]. For a poultry house, the ventilation rate of the room typically does not exceed 15 m3 h−1 hen−1 [32,38,47,48,49,50,51,52,53,54].

Table 1.
Status of mechanical ventilation in livestock and poultry houses.
Temperature and relative humidity could also affect the occurrence of respiratory diseases by influencing the respiratory and thermoregulatory behavior of the animals and the survival and spread of airborne pathogens [26,46]. As seen in Table 2, the range of longitude is 33°41′–64°50′ for mechanical livestock housing, which corresponds to a temperate climate zone. A proper temperature contributes to the health of the housed animals [23]. Not surprisingly, the ventilation requirement is lower in winter, when the temperatures are lower [26]. If nursery pigs are exposed to higher or lower temperatures than recommended for an extended period of time, they become very susceptible to respiratory disease. Humidification may be needed to increase indoor relative humidity during the winter months [30,46]. In this respect, modulating both the temperature and humidity ranges may play a guiding role, as per Table 2.

Table 2.
The longitude range, temperature, and humidity of the different livestock and poultry houses.
3. General Emissions of Air Pollutants
Air pollutants in pig and poultry housing pose a risk to the health of animals and farm staff. The concentrations of air pollutants are influenced by the design of the housing systems and their ventilation rate, as well as manure management practices. Table 3 provides an overview of the most common pollutants and their concentration ranges found in pig and poultry houses equipped with mechanical ventilation systems. Gas emissions of NH3 and H2S can cause various diseases, such as central paralysis, toxic liver disease, and myocardial strain [56]. The threshold for NH3 concentrations in livestock housing is 25 ppm for an 8 h working day [57]. Greenhouse gases such as methane (CH4), nitrous oxide (N2O) and carbon dioxide (CO2) contribute to global warming, thus constituting a major threat to the world’s environment [16]. Various species of airborne bacteria can occur in the buildings housing pigs. Most are Gram-positive bacteria whereas Gram-negative bacteria are generally present at very low concentrations [58]. Airborne bacteria in these pig buildings may be a major cause of decreased pig productivity and respiratory diseases in farm staff, such as asthma, rhinitis, and bronchitis [59]. The biological sources of PM from pig and poultry houses are numerous, including the feed used, and the feces, urine, dander, bedding, skin, and hair of the animals [60]. Particulate-matter-attaching microbes can expose farmers and pigs to infectious and allergic diseases, including pneumonia, asthma, and rhinitis [59]. Because high levels of aerial pollutants in barns adversely affect the health of animals and human beings, it is essential to install effective mitigating emission systems in pig and poultry barns.

Table 3.
The range of air pollutant concentration in pig and poultry houses.
Table 4 presents an overview of the concentration ranges of aerial pollutants according to season in pig and poultry houses equipped with mechanical ventilation systems. Seasonally, the indoor concentrations of all aerial pollutants change widely. For example, the NH3 concentration of poultry houses goes from 25 ppm in winter down to 2 ppm in summer, and the PM10 concentration of pig houses spans 5000 pm in winter to 1500 pm in summer. The main reason for the differences in harmful gases and particulate matter concentrations for summer versus winter is likely the magnitude of ventilation. The ventilation rate is smaller during winter than summer in order to maintain a comfortable indoor temperature. However, a decreasing ventilation rate leads to the accumulation of harmful gases and PM [62]. Hence, it is essential to tailor mitigating emission system, such as changing the trickling density of chemical and biological air scrubbers in different seasons, to improve the indoor environment year-round [22,79,80].

Table 4.
The range of air pollutant concentration by season in pig and poultry houses.
4. Mitigation Strategies
4.1. Inlet Air Filtration Systems
In 2015, an unprecedented outbreak of highly pathogenic H5N2 avian influenza devastated the U.S. poultry industry, causing economic losses totaling USD 3.3 billion [82,83]. That highly pathogenic avian influenza virus was transmitted through fine particles in the air into poultry houses [84]. In laying hen houses in Spain, 85% were infected with Mycoplasma gallisepticum. Although there were no clinical symptoms in the poultry, there was a decrease in the production of eggs, and their quality was reduced [85,86]. The presence of outdoor Mycoplasma suggests that inlet air could be a source of entry for this pathogen. Accordingly, the risk of airborne avian influenza transmission may be considerably reduced by inlet air filtration of inflected flocks [87]. To limit the airborne transmission of avian influenza and other pathogens, inlet air filtration systems have been used to reduce PM (the carrier of pathogens) in poultry and livestock housing [84], providing an effective way to prevent airborne microorganisms’ transmission; these are the main measures used to lessen the risk of airborne disease transmission between houses [88].
Over a 7-year period, the incidence of porcine reproductive and respiratory syndrome virus (PRRSV) in 20 filtered and 17 non-filtered control sow herds in North America showed that air filtration reduced the risk of a novel PRRSV introduction by approximately 80% [14]. Inlet air filtration has already been demonstrated to efficiently reduce PRRSV and Mycoplasma hyopneumoniae from inlet air [89,90]. The filtration of ventilated fresh air can control airborne disease transmission, and has been shown to have potential long-term economic impacts [91,92].
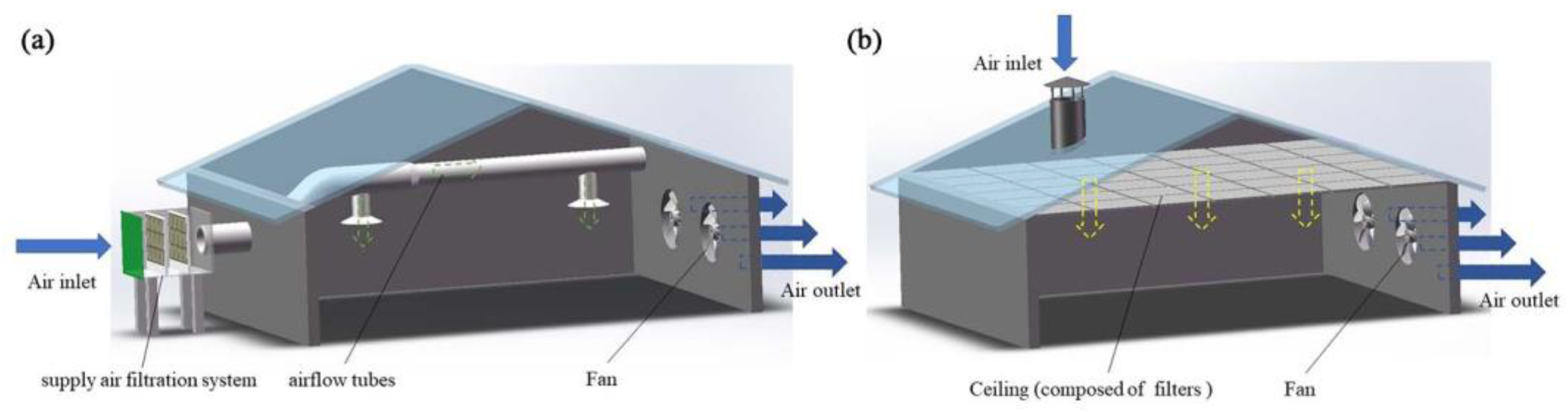
Inlet air purification measures used in mechanical ventilated livestock and poultry housing mainly include a supply air filtration system (SAFS) and a ceiling air filtration system (CAFS). The SAFS is composed of a filter and airflow tubes (Figure 3a). The filter is installed on the side wall and carries the filtered air, via the airflow tubes, into the house. The filter consists of a windscreen, pre-filter, secondary filter and an adiabatic cooling unit [44]. Each house can be equipped with multiple supply air filtration systems. The CAFS is installed in the attics of livestock and poultry houses with ceilings composed of coarse filters or sub-high-efficiency filters (Figure 3b). The fresh air is supplied to the house through the chimney air intake, as illustrated in Figure 3b. It should be noted that, for cost-effectiveness purposes, many manufacturers install only coarse-efficiency filters. Sub-efficient filters may be installed during high-epidemic periods. In addition, evaporative cooling pads may be installed to reduce the inlet air temperature, which can also serve as wet scrubbers to reduce the amount of inlet air pollutants in summer [88].
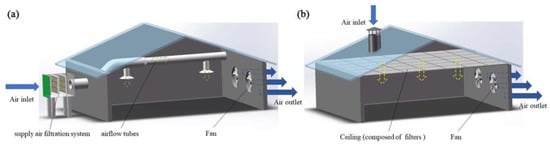
Figure 3.
Schematic diagram of inlet air filtration systems. (a): Supply air filtration system; (b): Ceiling air filtration system.
Both SAFS and CAFS were installed alongside existing ventilation equipment at a pig farm in Saxony, Germany [44]. Then, pollutant concentrations under either filtration system, and without any air filtration, in mechanically ventilated pig houses were compared; this revealed that PM1 and PM2.5 concentrations in the barns equipped with a SAFM decreased by 21.29% and 19.7%, respectively (Table 5). Methicillin-resistant Staphylococcus aureus (MRSA), coliform bacteria, and Escherichia coli all decreased under each of the two filtration systems compared with the barn lacking either. The reduction in coliform bacteria abundance in livestock houses equipped with SAFM and CAFM was 88.07% and 86.61%, respectively, due to these air filter systems preventing the direct entry of coliforms into the houses.

Table 5.
The reduction rate of various pollutants by inlet air filtration systems.
Furthermore, in the German study, there were no remarkable differences in total bacterial counts in barns between either air filtration system and without an air filtration system (p = 0.824) [44]. The NH3 concentration was slightly higher in barns equipped with either of the filter systems than those without the filter system in place, suggesting that the installation of an inlet air filtration system is not conducive to the emission of NH3, as shown in Table 6.

Table 6.
Average concentration (36 weeks) in pig houses with two filtration systems and without filtration system (Data from [44]).
These two air filtration systems are normally used in tandem with a filter of a certain filter grade. Minimum efficiency reporting values (MERVs) are a certified filter grade—as officially defined by the American Society of Heating, Refrigerating, and Air Conditioning Engineers (ASHRAE)—which represents the ability of a filter to capture particles between 0.3 µm and 10 µm in size. This value helps one to compare the performance of different filters: the higher the MERV rating, the better the filter’s ability to capture specific types of particles. Despite the fact that the PM-removal efficiencies of low-grade MERV filters installed at an air inlet were lower than those of high-grade MERV filters, improvement can be gained by combining other promising PM-precipitation technologies such as electrostatic particulate ionization (EPI) [84]. Zhao et al. evaluated, over a 1-year period, two rounds (round1: spring to summer; round2: late fall to spring) of PM-removal tests of an electrostatic air filtration system—consisting of a low-grade air filter and an electrostatic particle ionization system (EAFS) installed at the inlet of a commercial high-rise chicken house [84]. Compared with smaller particles, the PM-removal efficiency was higher for larger particles because these are more easily captured by the filter media (Table 5). The average PM-removal efficiency was significantly higher in round 1 than round 2. This is explained by more dust accumulating on the filter media in winter than summer, which reduces the porosity and permeability of the filter to some extent, resulting in a decreased PM filtration efficiency [93].
Since the adoption of filtration systems, the industrial production of porcine reproductive and respiratory syndrome virus (PRRS)-negative pigs has increased from 59% (unfiltered) to 93% (filtered), with an accompanying increased economic value estimated at USD 5 per pig weaned [91]. The US swine industry is shifting towards implementing filtered fresh-air ventilation systems that use pleated filters to improve breeding-herd health and reduce the frequency of airborne disease outbreak [13]. In summary, the positive removal efficiency of PM and other pathogens of the inlet air filtration system suggest promising potential to reduce the risk of animal infection in livestock houses.
4.2. Outlet Air Filtration Systems
4.2.1. Classification
Pollutants emitted into the atmosphere from livestock and poultry cause pollution of the surrounding ambient air. The air in the barn contains many pathogenic microorganisms, which spread through the air and may infect nearby animals and humans. One direct method of reducing air-pollutant emissions is to install filters in the ventilation openings of the barn. In the mechanically ventilated livestock and poultry houses, outlet filtration systems are often used in conjunction with negative-pressure ventilation so that any airflow containing pollutants are forced through the filter media by the mechanical ventilation system in place [94,95]. Filters are used to reduce the emissions of exhaust gases by physical, biological, and chemical processes. The outlet air filtration systems usually include air scrubbers, biofilters, and dry filters [96,97,98].
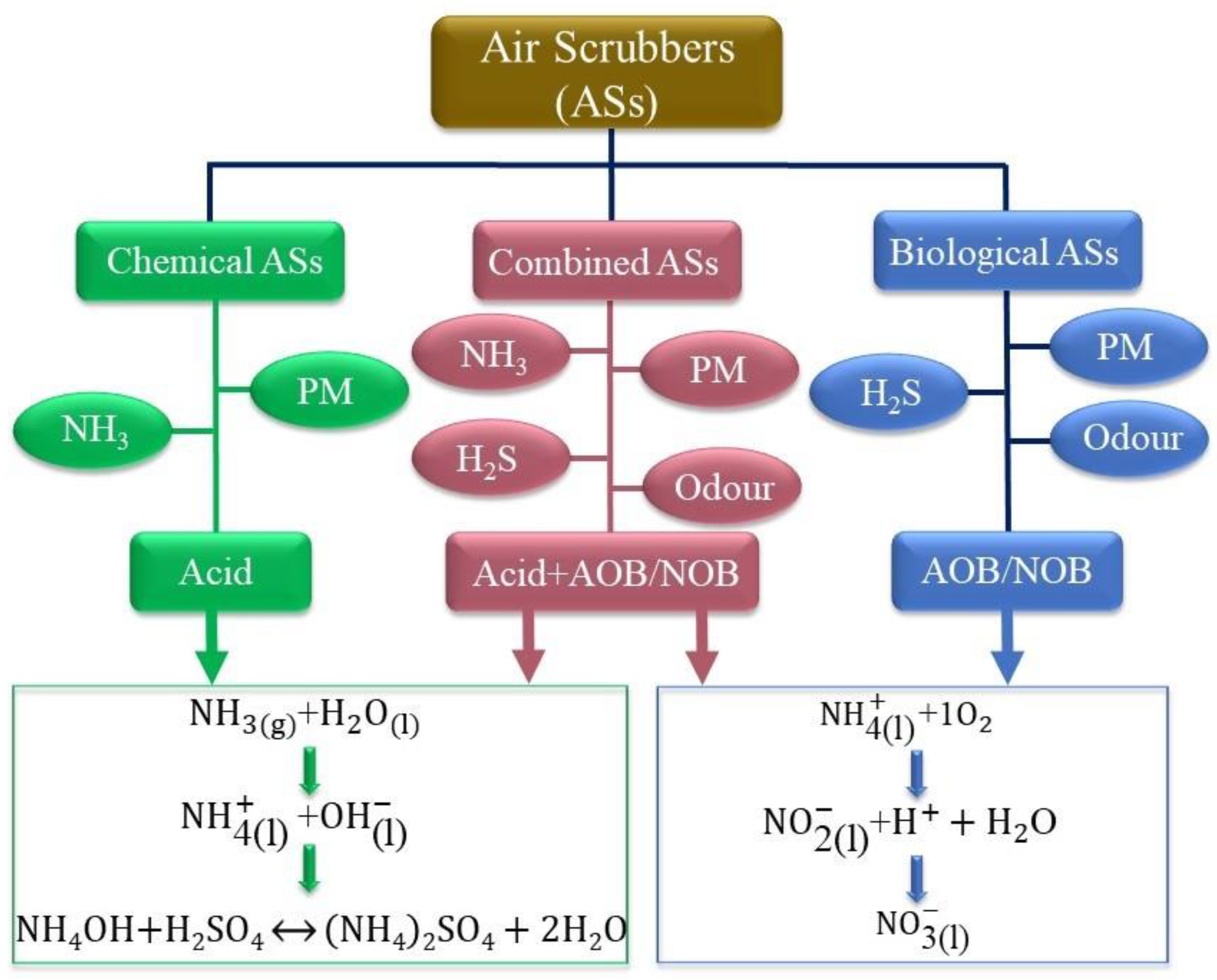
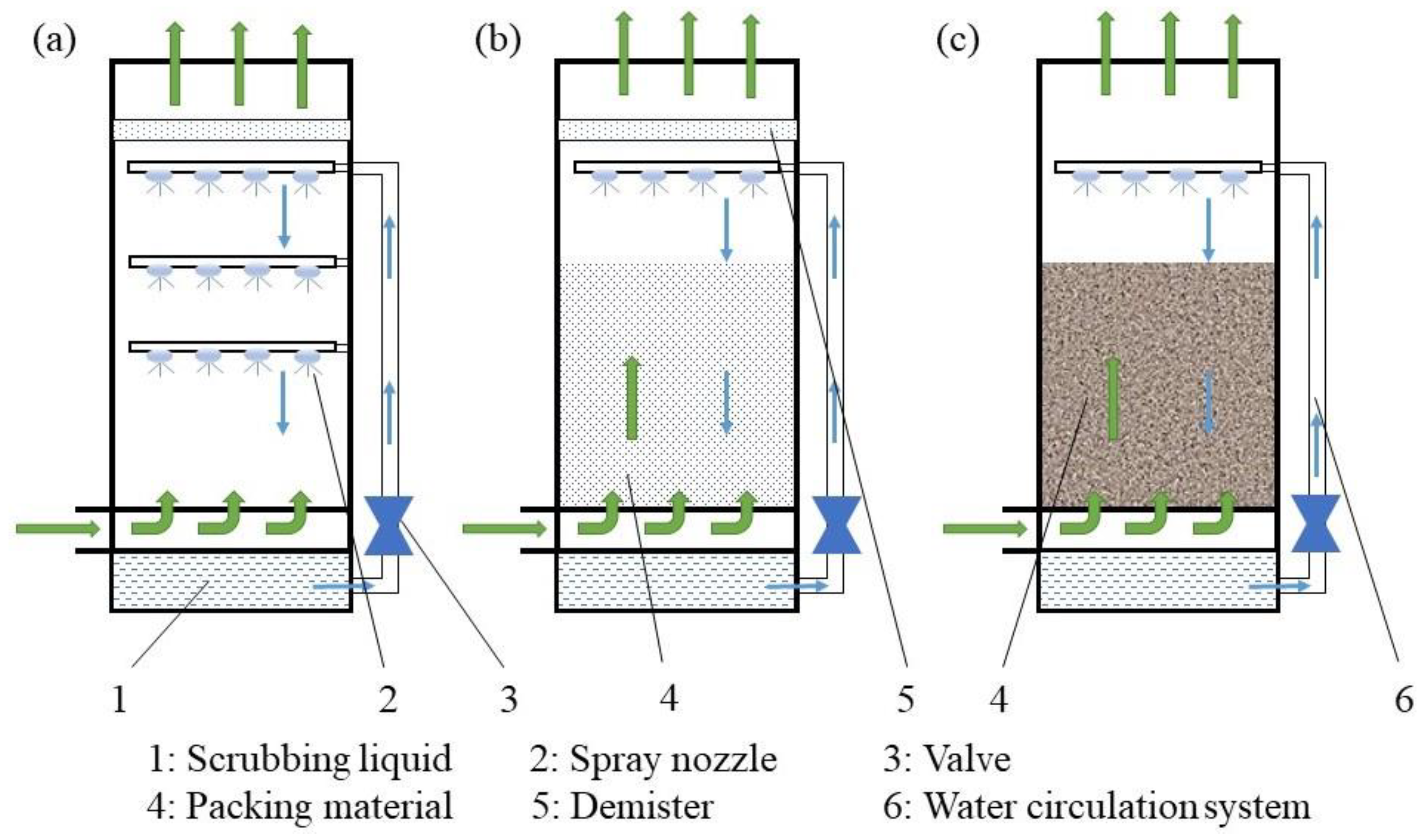
An air scrubber is an air-purification system that removes PM or other pollutants from the air by using moisture or filtering the airstream as it enters the scrubber. The data in the literature indicate that air scrubbers can effectively remove air pollutants in mechanically ventilated housing. They can be divided into three main groups—chemical, biological, and combined air scrubbers—as depicted in Figure 4. In chemical air scrubbers (‘chemical ASs’), an acid is added to the washing water to decrease the pH (to 1.5–4), shifting the equilibrium towards ammonium and thereby increasing the absorption capacity, for which sulfuric acid (H2SO4) is typically used. In biological air scrubbers (‘biological ASs’), air pollutants are captured in the washing water and adsorbed by microorganisms for the purpose of purifying the gas; for example, NH3 is oxidized by nitrifying bacteria to nitrite () and subsequently converted to nitrate () (nitrification). These conversion processes are carried out by ammonia-oxidizing bacteria (AOB) such as Nitrosomonas and nitrite oxidizing bacteria (NOB) such as Nitrobacter and Nitrospira, respectively [99]. A chemical AS is very efficient at removing ammonia, while a biological AS is more appropriate suitable for odor reduction. When two or more ASs are positioned behind each other, the overall system is known as a combined ASs. This type has evolved from the separate chemical and biological scrubbers for joint use in the combined removal of differing types of pollutants, such as NH3, odor and PM [22].
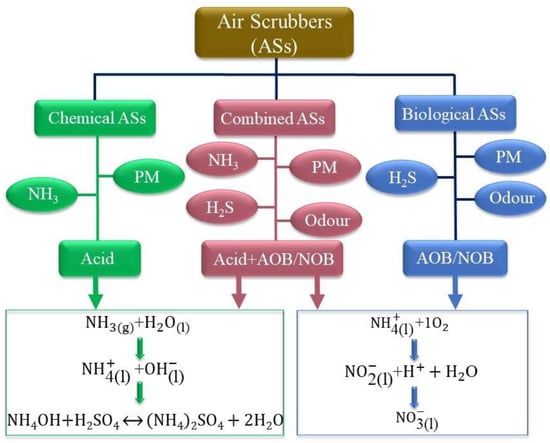
Figure 4.
Classification of currently used air scrubbers.
Biofilters mainly consist of a filter bed with a microorganism attachment. The use of inert packing materials inside provide the surfaces for gas–liquid contact [100]. Several materials (wood bark, wood chip, peat, compost, gravel, activated carbon, or plastic shapes) are used to prepare the bed media, which is kept humid but not necessarily subjected to continuous water spraying [77,101,102]. The same biological processes can take place as in biological air scrubbers. During the operation of a biofilter, a biofilm is formed by microorganisms growing on the packing material [99,103]. As polluted air flows through this packed bed, the harmful compounds are degraded by the active biofilm made of natural microorganisms that covers the bed. In addition, microbial degradation processes are normally oxidative in nature and produce compounds that are ecologically safe, such as CO2, water, sulfate, and nitrate. Typically, biofilters are not used directly, but in combination with air scrubbers.
A dry filter (DF) consists of a folding filter-plate and fiber filter, which are connected to form a filter wall between the dusty animal space and the outlet ventilators. While the pollutants pass through the dry filter, the airflow is forced by inertia separation to change its direction many times. Those particles heavier than the air will adhere to the wall of the folding plate, with some of the fine particles passing through the fiber filter for secondary filtration. Commonly used materials are polyethylene, polypropylene foil, glass fiber, etc. According to the folding shape of the dry filter, it can be divided into two types: a V-shaped dry filter and a pocket dry filter.
4.2.2. Structure
The reported descriptions of air cleaning systems such as air scrubbers and biofilters, have used wet packing media to purify the air from livestock buildings. Their filters remove pollutants (PM, NH3, H2S, and others) from outlet air through absorption in the media, followed by chemical and/or biological conversions and the removal of end products [104].
The structure of various air scrubbers and biofilters is shown in Figure 5. Air from the house is drawn through fans towards a central duct and blown as a counter-current into the air scrubber. A scrubbing liquid is distributed uniformly across the top of the scrubber. Intense contact between the air pollutants and liquid ensures mass transfer of water-soluble pollutants from the air into the washing water. The scrubbing water is recirculated to reduce excess water consumption. When the concentration of accumulated contaminants in the washing liquid becomes too high, fresh water must be added [22]. A demister behind the air scrubber prevents the escape of small droplets from the air scrubber [22]. Biological ASs and biofilters employ microorganisms immobilized within a biofilm affixed to a packing material to break down contaminants present in the air stream. These opposite flow directions of the water and exhaust air can provide intensive contact between the gas and liquid [105]. The process is relatively complex, involving dynamic interactions of absorption, adsorption, and biological degradation [106].
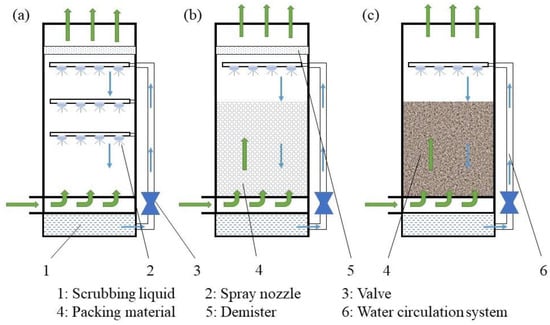
Figure 5.
Structural diagram of a chemical air scrubber (a), a combined air scrubber (b), and a biofilter (c).
Many factors should be considered in the design and application of an air scrubber. Chemical scrubbers typically have a relatively low air resistance and high NH3 removal potential. Acid concentration is one of the main factors affecting removal performance, with higher concentrations resulting in greater removal efficiencies [79,107,108]. However, during the application of the combined scrubber, the NH3 removal efficiency can be negatively affected by very high pH values and nitrifying bacteria inhibition that occurs in the biological air scrubber. Hence, the NH3 removal efficiency can probably be augmented by lowering the NH+4 concentration in the recirculation water by increasing the fresh water supply rate and the water discharge rate [109].
Biofiltration (i.e., biological air scrubbers and biofilters) have become a state-of-the-art technique applied on a large scale due to their robustness, cost-efficient installation/operation, and ecological characteristics [110]. However, their main drawback is that the pH is difficult to control, such that their long-term operation may cause the packing material to degrade and alter its microbial characteristics. Any clogging or fouling of the packing material can cause great pressure drops. To prevent the suppression of crucial bacteria, the pH of the biological scrubber must be maintained in a microbiologically favorable range, usually between 6.5 and 7.5. This implies a much larger volume of discharge produced compared to chemical air scrubbers. The advantages of combining a chemical scrubber with a biofiltration are, amongst others, the mitigation of NH3 and moisturizing of air before it enters the biofiltration unit, and thus, better conditions to sustain the microorganisms and their activity inside the biofiltration system [79].
Packing material is one of the main aspects affecting biofiltration’s efficacy, playing a pivotal role in the growth of biofilm. The physical and chemical properties of different packing media determine the gas flow speed and specific surface area, which are vital factors influencing the removal efficiency achieved via biofiltration [111,112]. The particle size, bulk density, porosity and moisture content of packing material are also important factors that affect the long-term operational stability. An overview of the characteristics of common fillers used in biofiltration is provided in Table 7 [21]. Air scrubbers and biofilters are affected by temperature, so they are not generally used in winter; however, dry filters can be used at any time of the year.

Table 7.
Characteristics of packing materials in biofiltration application [21].
4.2.3. Performance
The pollutant removal efficiency was calculated using the outlet concentration of pollutants from the house and the concentration after filtering via the filtration system, as follows:
where and are the outlet concentration of the house and the concentration after filtering through the filtration system, respectively.
The efficiency of reducing one or more pollutant’s emissions will vary depending on the type of filter used. Table 7 summarizes the removal efficiencies of various types of air scrubbers, biofilters and dry filters for PM2.5, PM10, ammonia, odor, nitrous oxide and hydrogen sulfide. The use of single-stage filters to clean exhaust from an animal house is limited because each filter targets a specific contaminant, as seen in Table 7. In general, acid scrubbers are more effective for removing NH3. The addition of acid to the scrubber water shifts the equilibrium toward ammonium, thereby improving its uptake. An NH3 mitigation efficiency of up to 96% can be achieved by maintaining the pH values between 1.5 and 4 [94,95]. Compared with chemical air scrubbers, the removal efficiency of NH3 by biofiltration is much lower, between 42% and 67% [21]. This is because the pH in biofiltration is kept higher to ensure the normal reproduction of microorganisms.
However, biofiltration is considered the most mature of odor-treatment technologies, and is now widely used in many livestock and poultry houses [94]. Nevertheless, the performance of biofilters varies with different packing materials. The average removal efficiency of biofilters in Table 8 was between 45% and 70% for odor. In particular, the removal efficiency of H2S was close to 100% when its outlet concentration was 2.5–3.5 mg/m3 [21].

Table 8.
The ventilation type and flow and the reduction rate of various pollutants of outlet air filtration systems.
As Table 8 shows, the biofilter has a filtration efficiency higher than 89% for low concentrations (0.148 mg/m3) of PM10, but removing high concentrations of dust with a biofilter alone entails a high risk of clogging [22]. Harmon et al. recommend against the use of biofilters at poultry facilities unless they have upstream dust filters. An upstream treatment of the exhaust air, especially for the removal of dust, is also recommended to help prevent the clogging of biofilters [79]. The removal efficiency of PM10 and PM2.5 is relatively high and more constant in combined air scrubbers, because more scrubber stages enable higher removal efficiency. Multi-stage packed scrubbers can effectively reduce the exhaust concentration of PM10 by 61–93%, and that of PM2.5 by 47–90%. A three-stage scrubber (water stage + acid stage + biofilter) was effective in reducing dust levels in the pig housing (93% reduction in PM10, 90% reduction in PM2.5) [129,131]. The multi-stage scrubbers are capable of reducing concentrations of airborne total bacteria by 46% to 85% of between 70% and 100% [129].
Dry filters are mainly used to capture the PM in livestock and poultry houses. While the removal efficiency of PM by the a dry filter was found to increase with increasing particle diameter (PM2.5: 41%, PM10: 64%), it was not affected by the upstream PM concentration (p < 0.001) [96]. Given that particles are carriers of bacteria, dry filters also have a certain filtration effect on fungi, for which a filtration efficiency of 20% is attainable [96]. However, dry filters negligibly affect the NH3 concentration, and thus, do little to alter the NH3 emissions from animal housing [97]. Integrating NH3 reduction techniques in the dry filter method is very difficult, although it should be possible to integrate a simple wet scrubber to remove NH3 into the dry filter, potentially improving the overall dust and NH3 removal efficiencies [97].
4.2.4. Monitoring Strategy
The monitoring of pollutants in livestock housing contributes immensely to an effective reduction in air pollution. The monitoring instruments for pollutants in livestock and poultry housing and their working form are presented in Table 9. Concentrations of NH3, H2S, CH4, and PM can be measured in real time with specialized instruments. However, the composition of odor pollutants is complicated, and different pollutants might interact with each other during the detection process. In general, odor samples are collected into Tedlar bags, then analyzed using a gas chromatography–mass spectrometer (GC-MS) or by an olfactometer. Nevertheless, an electronic nose can monitor the odor concentration in real time. The response pattern of a gas sensor array to identify odors has the advantages of good selectivity, continuous monitoring, and fast detection, but is hindered by its poor stability, need for regular calibration, and narrow testing range. It is critical to identify the components and quantities of odor pollution in the livestock farm promptly and accurately, for robust odor component analysis [21,134].

Table 9.
Characteristics of packing materials in biofiltration application.
In order to monitor indoor concentrations (IC), monitor instruments were generally placed in the middle of the central pen of the barn, and some were placed in front of the filter in order to compare the filtration efficiency of the filter [25]. The height of the instruments was related to the height of the adult pigs (0.8 m) or the breathing height of nursery pigs (0.5 m) [8,16]. As for emission concentrations (EC), the best position to achieve a representative average gas concentration was measured at the exhaust ventilator of the barns [134]. When monitoring the PM concentrations, the measurement interval was set larger to minimize the risk of being blocked, for example, making measurements every 15 min [16].
5. Perspectives
The use of air scrubbers and biofilters is limited by the seasons, but dry filters can be used year-round. However, the high filtration efficiency and low-pressure drop in current dry filters cannot be simultaneously satisfied, and the filtration effect on air pollutants in livestock houses, such as NH3, is low. Some promising technologies present possible solutions to reduce NH3 emissions from livestock housing, such as gas-permeable membranes (GPMs) and electrostatic spinning technology (EST) [19]. Specifically, the NH3 in the house is captured by reaction with an acidic solution flowing inside the membranes prepared by GPM [19]. Membranes prepared by EST (Table 10) have also proven effective at filtering PM; toxic and harmful gases; and bacteria and viruses.

Table 10.
The removal efficiency of pollutants by electrostatic spinning technology.
The filter membranes prepared by EST feature several notable characteristics: controllable pore size distribution, good fiber uniformity, an adjustable structure, small pore size, high porosity, high filtration efficiency, and a low basic weight, making this an ideal air filtration technology [142]. A hybrid membrane of poly(vinyl alcohol) NF and Fe-BTC on a macroporous nonwoven material (MOF + NF/NW) was shown to achieve outstanding gas-capturing efficiencies at 100 ppm of NH3 and H2S (≈60% and ≈35% from their initial concentrations, respectively) [136]. Electrospinning membranes have very high potential for filtering livestock barn inlet and outlet pollutants due to their excellent filtration performance.
6. Conclusions
Mitigation strategies of air pollutants in mechanically ventilated livestock and poultry housing are of great significance for their production performance and atmosphere quality. The ventilation systems, types of pollutant emissions, and strategies for purifying inlet air and reducing pollutants in the air were summarized for mechanically ventilated livestock and poultry houses. The following conclusions were drawn:
(1) The concentration of pollutants in such houses are generally higher in winter than in summer, and energy consumption can be effectively reduced by undertaking different suitable measures (modifying the ventilation rate and temperature).
(2) Inlet air filtration systems can effectively reduce the amount of airborne PM (the carrier of pathogens) and microorganism transmission, especially SAFS, which should reduce the potential risk of animal infections in a given house.
(3) Single-stage filter for mitigating emissions at the outlet is limited because each filter targets a different specific contaminant. By contrast, combination filters can remove multiple pollutants effectively. The monitoring location of IC and EC is also very critical for pollutant mitigation.
Currently, there are many problems with inlet air purification and outlet air filtration measures. The requirements of inlet and outlet cannot be satisfied by a single universal-measure applicable to all livestock and poultry houses, making it necessary to consider the reasonable use of multiple measures in concert.
Author Contributions
Conceptualization, L.G., W.C. and B.Z.; methodology, L.G. and W.C.; formal analysis, L.G., W.C. and B.Z.; data curation, B.Z., Y.J. and F.H.; writing—original draft preparation, L.G., B.Z., Y.J. and F.H.; writing—review and editing, L.G., B.Z. and W.C.; visualization, B.Z. and Y.J.; super-vision, L.G. and W.C.; project administration, L.G.; funding acquisition, L.G. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Foundation of Science and Technology Department of Jilin Province, grant number 20190302040GX; the Key Research Program of Frontier Sciences, Chinese Academy of Sciences (No. QYZDB-SSW-DQC045); and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28080201, XDA2307050103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request to corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, H.; Chang, J.; Havlík, P.; van Dijk, M.; Valin, H.; Janssens, C.; Ma, L.; Bai, Z.; Herrero, M.; Smith, P.; et al. China’s Future Food Demand and Its Implications for Trade and Environment. Nat. Sustain. 2021, 4, 1042–1051. [Google Scholar] [CrossRef]
- Herrero, M.; Henderson, B.; Havlík, P.; Thornton, P.K.; Conant, R.T.; Smith, P.; Wirsenius, S.; Hristov, A.N.; Gerber, P.; Gill, M.; et al. Greenhouse Gas Mitigation Potentials in the Livestock Sector. Nat. Clim. Chang. 2016, 6, 452–461. [Google Scholar] [CrossRef]
- Loyon, L.; Burton, C.H.; Misselbrook, T.; Webb, J.; Philippe, F.X.; Aguilar, M.; Doreau, M.; Hassouna, M.; Veldkamp, T.; Dourmad, J.Y.; et al. Best Available Technology for European Livestock Farms: Availability, Effectiveness and Uptake. J. Environ. Manag. 2016, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, K.; Meng, L.; Liu, X.; Hartung, E.; Roelcke, M.; Zhang, F. Concentrations and Emissions of Particulate Matter from Intensive Pig Production at a Large Farm in North China. Aerosol. Air Qual. Res. 2017, 16, 79–90. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; Verstegen, M.W.A. Nutrition, Key Factor to Reduce Environmental Load from Pig Production. Livest. Sci. 2007, 109, 194–203. [Google Scholar] [CrossRef]
- Tullo, E.; Finzi, A.; Guarino, M. Review: Environmental Impact of Livestock Farming and Precision Livestock Farming as a Mitigation Strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Domingo, N.G.G.; Hunt, N.D.; Gittlin, M.; Colgan, K.K.; Marshall, J.D.; Robinson, A.L.; Azevedo, I.M.L.; Thakrar, S.K.; Clark, M.A.; et al. The Food We Eat, the Air We Breathe: A Review of the Fine Particulate Matter-Induced Air Quality Health Impacts of the Global Food System. Environ. Res. Lett. 2021, 16, 103004. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, K.; Liu, J.; Jin, X.; Li, C. Distribution Characteristics of Bioaerosols inside Pig Houses and the Respiratory Tract of Pigs. Ecotoxicol. Environ. Saf. 2021, 212, 112006. [Google Scholar] [CrossRef]
- Costantino, A.; Fabrizio, E.; Villagrá, A.; Estellés, F.; Calvet, S. The Reduction of Gas Concentrations in Broiler Houses through Ventilation: Assessment of the Thermal and Electrical Energy Consumption. Biosyst. Eng. 2020, 199, 135–148. [Google Scholar] [CrossRef]
- Dumont, É. Impact of the Treatment of NH3 Emissions from Pig Farms on Greenhouse Gas Emissions. Quantitative Assessment from the Literature Data. New Biotechnol. 2018, 46, 31–37. [Google Scholar] [CrossRef]
- Michiels, A.; Piepers, S.; Ulens, T.; Van Ransbeeck, N.; Del Pozo Sacristán, R.; Sierens, A.; Haesebrouck, F.; Demeyer, P.; Maes, D. Impact of Particulate Matter and Ammonia on Average Daily Weight Gain, Mortality and Lung Lesions in Pigs. Prev. Vet. Med. 2015, 121, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Fu, J.; Liu, X.-F.; Chen, W.-W.; Hao, J.-L.; Li, X.-L.; Pant, O.P. Air Pollution and Meteorological Conditions Significantly Contribute to the Worsening of Allergic Conjunctivitis: A Regional 20-City, 5-Year Study in Northeast China. Light Sci. Appl. 2021, 10, 190. [Google Scholar] [CrossRef]
- Smith, B.C.; Ramirez, B.C.; Hoff, S.J.; Harmon, J.D.; Stinn, J.P. Design and Validation of a Mobile Laboratory for Testing Air Inlet Filter Loading at Animal Houses. AgricEngInt CIGR J. 2019, 21, 39–50. [Google Scholar]
- Alonso, C.; Murtaugh, M.P.; Dee, S.A.; Davies, P.R. Epidemiological Study of Air Filtration Systems for Preventing PRRSV Infection in Large Sow Herds. Prev. Vet. Med. 2013, 112, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Sun, Z.; Müller, D. Driving Factors of Direct Greenhouse Gas Emissions from China’s Pig Industry from 1976 to 2016. J. Integr. Agric. 2021, 20, 319–329. [Google Scholar] [CrossRef]
- Ulens, T.; Millet, S.; Van Ransbeeck, N.; Van Weyenberg, S.; Van Langenhove, H.; Demeyer, P. The Effect of Different Pen Cleaning Techniques and Housing Systems on Indoor Concentrations of Particulate Matter, Ammonia and Greenhouse Gases (CO2, CH4, N2O). Livest. Sci. 2014, 159, 123–132. [Google Scholar] [CrossRef]
- Moussavi, G.; Khavanin, A.; Sharifi, A. Ammonia Removal from a Waste Air Stream Using a Biotrickling Filter Packed with Polyurethane Foam through the SND Process. Bioresour. Technol. 2011, 102, 2517–2522. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of Performance Data for Technologies to Control Gaseous, Odor, and Particulate Emissions from Livestock Operations: Air Management Practices Assessment Tool (AMPAT). Data Brief 2016, 7, 1413–1429. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology. Membranes 2021, 11, 859. [Google Scholar] [CrossRef]
- Ullman, J.L.; Mukhtar, S.; Lacey, R.E.; Carey, J.B. A Review of Literature Concerning Odors, Ammonia, and Dust from Broiler Production Facilities: 4. Remedial Management Practices. J. Appl. Poult. Res. 2004, 13, 521–531. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, Measurement, and Control of Odor in Livestock Farms: A Review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Van der Heyden, C.; Demeyer, P.; Volcke, E.I.P. Mitigating Emissions from Pig and Poultry Housing Facilities through Air Scrubbers and Biofilters: State-of-the-Art and Perspectives. Biosyst. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- Van Huffel, K.; Hansen, M.J.; Feilberg, A.; Liu, D.; Van Langenhove, H. Level and Distribution of Odorous Compounds in Pig Exhaust Air from Combined Room and Pit Ventilation. Agric. Ecosyst. Environ. 2016, 218, 209–219. [Google Scholar] [CrossRef]
- Yeo, U.-H.; Lee, I.-B.; Kim, R.-W.; Lee, S.-Y.; Kim, J.-G. Computational Fluid Dynamics Evaluation of Pig House Ventilation Systems for Improving the Internal Rearing Environment. Biosyst. Eng. 2019, 186, 259–278. [Google Scholar] [CrossRef]
- Mostafa, E.; Hoelscher, R.; Diekmann, B.; Ghaly, A.E.; Buescher, W. Evaluation of Two Indoor Air Pollution Abatement Techniques in Forced-Ventilation Fattening Pig Barns. Atmos. Pollut. Res. 2017, 8, 428–438. [Google Scholar] [CrossRef]
- Saha, C.K.; Zhang, G.; Kai, P.; Bjerg, B. Effects of a Partial Pit Ventilation System on Indoor Air Quality and Ammonia Emission from a Fattening Pig Room. Biosyst. Eng. 2010, 105, 279–287. [Google Scholar] [CrossRef]
- Ni, J.-Q. Factors Affecting Toxic Hydrogen Sulfide Concentrations on Swine Farms―Sulfur Source, Release Mechanism, and Ventilation. J. Clean. Prod. 2021, 322, 129126. [Google Scholar] [CrossRef]
- Choi, H.L.; Han, S.H.; Albright, L.D.; Chang, W.K. The Correlation between Thermal and Noxious Gas Environments, Pig Productivity and Behavioral Responses of Growing Pigs. Int. J. Environ. Res. Public Health 2011, 8, 3514–3527. [Google Scholar] [CrossRef]
- Mulholland, K. FM and Milk Production: Herd Ventilation Systems in Hot Climates. J. Facil. Manag. 2013, 11, 284–288. [Google Scholar] [CrossRef]
- Chantziaras, I.; De Meyer, D.; Vrielinck, L.; Van Limbergen, T.; Pineiro, C.; Dewulf, J.; Kyriazakis, I.; Maes, D. Environment-, Health-, Performance- and Welfare-Related Parameters in Pig Barns with Natural and Mechanical Ventilation. Prev. Vet. Med. 2020, 183, 105150. [Google Scholar] [CrossRef]
- Saleeva, I.; Sklyar, A.; Marinchenko, T.; Postnova, M.; Ivanov, A. Efficiency of Poultry House Heating and Ventilation Upgrading. IOP Conf. Ser. Earth Environ. Sci. 2020, 433, 012041. [Google Scholar] [CrossRef]
- Costa, A.; Guarino, M. Particulate Matter Concentration and Emission Factor in Three Different Laying Hen Housing Systems. J. Agric. Eng. 2009, 40, 15. [Google Scholar] [CrossRef]
- Belote, B.L.; Soares, I.; Tujimoto-Silva, A.; Tirado, A.G.C.; Martins, C.M.; Carvalho, B.; Gonzalez-Esquerra, R.; Rangel, L.F.S.; Santin, E. Field Evaluation of Feeding Spray-Dried Plasma in the Starter Period on Final Performance and Overall Health of Broilers. Poult. Sci. 2021, 100, 101080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Choi, C.; Li, D.; Yan, G.; Li, H.; Shi, Z. Effects of Airspeed on the Respiratory Rate, Rectal Temperature, and Immunity Parameters of Dairy Calves Housed Individually in an Axial-Fan-Ventilated Barn. Animals 2021, 11, 354. [Google Scholar] [CrossRef]
- Du, L.; Yang, C.; Dominy, R.; Yang, L.; Hu, C.; Du, H.; Li, Q.; Yu, C.; Xie, L.; Jiang, X. Computational Fluid Dynamics Aided Investigation and Optimization of a Tunnel-Ventilated Poultry House in China. Comput. Electron. Agric. 2019, 159, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, W.; Tong, Q.; Li, B. Reducing Dust Deposition and Temperature Fluctuations in the Laying Hen Houses of Northwest China Using a Surge Chamber. Biosyst. Eng. 2018, 175, 206–218. [Google Scholar] [CrossRef]
- Calvet, S.; Cambra-López, M.; Blanes-Vidal, V.; Estellés, F.; Torres, A.G. Ventilation Rates in Mechanically-Ventilated Commercial Poultry Buildings in Southern Europe: Measurement System Development and Uncertainty Analysis. Biosyst. Eng. 2010, 106, 423–432. [Google Scholar] [CrossRef]
- Alberdi, O.; Arriaga, H.; Calvet, S.; Estellés, F.; Merino, P. Ammonia and Greenhouse Gas Emissions from an Enriched Cage Laying Hen Facility. Biosyst. Eng. 2016, 144, 1–12. [Google Scholar] [CrossRef]
- Samadpour, E.; Zahmatkesh, D.; Nemati, M.; Shahir, M. Determining the Contribution of Ventilation and Insulation of Broiler Breeding Houses in Production Performance Using Analytic Hierarchy Process (AHP). Braz. J. Poult. Sci. 2018, 20, 211–218. [Google Scholar] [CrossRef]
- Jongbo, A.O.; Moorcroft, I.; White, D.; Norton, T.; Okunola, A.A. Evaluation of Airflow Movement within a Broiler Shed with Roof Ventilation System during Summer. IOP Conf. Ser. Earth Environ. Sci. 2020, 445, 012028. [Google Scholar] [CrossRef]
- Zhang, G.; Bjerg, B.; Zong, C. Partial Pit Exhaust Improves Indoor Air Quality and Effectiveness of Air Cleaning in Livestock Housing: A Review. Appl. Eng. Agric. 2017, 33, 243–256. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.; Wang, T.; Xi, L.; Cheng, P.; Fang, M.; Liu, W. Application Effects of Three Ventilation Methods on Swine in Winter. Agron.J. 2021. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Dong, H.; Tao, X.; Yao, H. Particulate Matter Concentrations and Emissions of a Fattening Pig Facility in Northern China. Atmos. Pollut. Res. 2020, 11, 1902–1911. [Google Scholar] [CrossRef]
- Wenke, C.; Pospiech, J.; Reutter, T.; Altmann, B.; Truyen, U.; Speck, S. Impact of Different Supply Air and Recirculating Air Filtration Systems on Stable Climate, Animal Health, and Performance of Fattening Pigs in a Commercial Pig Farm. PLoS ONE 2018, 13, e0194641. [Google Scholar] [CrossRef]
- Blunden, J.; Aneja, V.P.; Westerman, P.W. Measurement and Analysis of Ammonia and Hydrogen Sulfide Emissions from a Mechanically Ventilated Swine Confinement Building in North Carolina. Atmos. Environ. 2008, 42, 3315–3331. [Google Scholar] [CrossRef]
- Choi, H.L.; Song, J.I.; Lee, J.H.; Albright, L.D. Albright Comparison of Natural and Forced Ventilation System in Nursery Pig House. Appl. Eng. Agric. 2010, 26, 1023–1033. [Google Scholar] [CrossRef]
- Kelleghan, D.B.; Hayes, E.T.; Everard, M.; Curran, T.P. Predicting Atmospheric Ammonia Dispersion and Potential Ecological Effects Using Monitored Emission Rates from an Intensive Laying Hen Facility in Ireland. Atmos. Environ. 2021, 247, 118214. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Ramirez, B.C.; Xin, H.; Wang, Y.; Hoff, S.J. Ventilation Performance and Bioenergetics of Dekalb White Hens in a Modern Aviary System. Biosyst. Eng. 2020, 199, 149–161. [Google Scholar] [CrossRef]
- Rosa, E.; Arriaga, H.; Calvet, S.; Merino, P. Assessing Ventilation Rate Measurements in a Mechanically Ventilated Laying Hen Facility. Poult. Sci. 2019, 98, 1211–1221. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, R.; Jiang, S.; El-Mashad, H.M.; Xin, H. Fan and Ventilation Rate Monitoring of Cage-Free Layer Houses in California. Trans. ASABE 2018, 61, 1939–1950. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Liu, S.; Diehl, C.A.; Lim, T.-T.; Bogan, B.W.; Chen, L.; Chai, L.; Wang, K.; Heber, A.J. Emission Factors and Characteristics of Ammonia, Hydrogen Sulfide, Carbon Dioxide, and Particulate Matter at Two High-Rise Layer Hen Houses. Atmos. Environ. 2017, 154, 260–273. [Google Scholar] [CrossRef]
- Zheng, W.; Kang, R.; Wang, H.; Li, B.; Xu, C.; Wang, S. Airborne Bacterial Reduction by Spraying Slightly Acidic Electrolyzed Water in a Laying-Hen House. J. Air Waste Manag. Assoc. 2013, 63, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Dekker, S.E.M.; Aarnink, A.J.A.; de Boer, I.J.M.; Koerkamp, P.W.G.G. Emissions of Ammonia, Nitrous Oxide, and Methane from Aviaries with Organic Laying Hen Husbandry. Biosyst. Eng. 2011, 110, 123–133. [Google Scholar] [CrossRef]
- Chai, L.; Ni, J.-Q.; Diehl, C.A.; Kilic, I.; Heber, A.J.; Chen, Y.; Cortus, E.L.; Bogan, B.W.; Lim, T.T.; Ramirez-Dorronsoro, J.-C.; et al. Ventilation Rates in Large Commercial Layer Hen Houses with Two-Year Continuous Monitoring. Br. Poult. Sci. 2012, 53, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lim, T.-T.; Jin, Y.; Heber, A.J.; Ni, J.-Q.; Cortus, E.L.; Kilic, I. Ventilation Rate Measurements at a Mechanically-Ventilated Pig Finishing Quad Barn. Biosyst. Eng. 2014, 121, 96–104. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Zhang, H.; Meng, L.; Pang, M. Ammonia Production, Hazards and Mitigation Measures in a Pig House. Chin. J. Anim. Nutr. 2015, 27, 2328–2334. [Google Scholar]
- Groot Koerkamp, P.W.G.; Metz, J.H.M.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.P.; Hartung, J.; Seedorf, J.; et al. Concentrations and Emissions of Ammonia in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Cormier, Y.; Tremblay, G.; Meriaux, A.; Brochu, G.; Lavoie, J. Airborne Microbial Contents in Two Types of Swine Confinement Buildings in Quebec. Am. Ind. Hyg. Assoc. J. 1990, 51, 304–309. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J. Indoor Distribution Characteristics of Airborne Bacteria in Pig Buildings as Influenced by Season and Housing Type. Asian-Australas. J. Anim. Sci. 2019, 32, 742–747. [Google Scholar] [CrossRef]
- Cambra-López, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne Particulate Matter from Livestock Production Systems: A Review of an Air Pollution Problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Z.; Misselbrook, T.; Wang, X.; Ma, L. Ammonia Emissions from Different Pig Production Scales and Their Temporal Variations in the North China Plain. J. Air Waste Manag. Assoc. 2021, 71, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Rong, X.; Zhu, J.; Zeng, Y.; Yue, J.; Lim, T.; Long, D. Short-Term Aerial Pollutant Concentrations in a Southwestern China Pig-Fattening House. Atmosphere 2021, 12, 103. [Google Scholar] [CrossRef]
- Zhang, Y.; Tanaka, A.; Dosman, J.A.; Senthilselvan, A.; Barber, E.M.; Kirychuk, S.P.; Holfeld, L.E.; Hurst, T.S. Acute Respiratory Responses of Human Subjects to Air Quality in a Swine Building. J. Agric. Eng. Res. 1998, 70, 367–373. [Google Scholar] [CrossRef]
- Van Ransbeeck, N.; Van Langenhove, H.; Demeyer, P. Indoor Concentrations and Emissions Factors of Particulate Matter, Ammonia and Greenhouse Gases for Pig Fattening Facilities. Biosyst. Eng. 2013, 116, 518–528. [Google Scholar] [CrossRef]
- Hayes, E.T.; Curran, T.P.; Dodd, V.A. Odour and Ammonia Emissions from Intensive Pig Units in Ireland. Bioresour. Technol. 2006, 97, 940–948. [Google Scholar] [CrossRef]
- Kim, K.Y. Exposure Level and Emission Characteristics of Ammonia and Hydrogen Sulphide in Poultry Buildings of South Korea. Indoor Built Environ. 2017, 26, 1168–1176. [Google Scholar] [CrossRef]
- Hong, E.-C.; Kang, H.-K.; Jeon, J.-J.; You, A.-S.; Kim, H.-S.; Son, J.-S.; Kim, H.-J.; Yun, Y.-S.; Kang, B.-S.; Kim, J.-H. Studies on the Concentrations of Particulate Matter and Ammonia Gas from Three Laying Hen Rearing Systems during the Summer Season. J. Environ. Sci. Health Part B 2021, 56, 753–760. [Google Scholar] [CrossRef]
- Almuhanna, E.A. Characteristics of Air Contaminants in Naturally and Mechanically Ventilated Poultry Houses in Al-Ahsa, Saudi Arabia. Trans. ASABE 2011, 54, 1433–1443. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Chai, L.; Chen, L.; Bogan, B.W.; Wang, K.; Cortus, E.L.; Heber, A.J.; Lim, T.-T.; Diehl, C.A. Characteristics of Ammonia, Hydrogen Sulfide, Carbon Dioxide, and Particulate Matter Concentrations in High-Rise and Manure-Belt Layer Hen Houses. Atmos. Environ. 2012, 57, 165–174. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, T.; Jiang, Z.; Min, Y.; Mo, J.; Gao, Y. Effect of Ventilation on Distributions, Concentrations, and Emissions of Air Pollutants in a Manure-Belt Layer House. J. Appl. Poult. Res. 2014, 23, 763–772. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Chen, B.; Li, P.; Banik, C. Mitigation of Odor and Gaseous Emissions from Swine Barn with UV-A and UV-C Photocatalysis. Atmosphere 2021, 12, 585. [Google Scholar] [CrossRef]
- Aunsa-Ard, W.; Pobkrut, T.; Kerdcharoen, T.; Prombaingoen, N.; Kijpreedaborisuthi, O. Electronic Nose for Monitoring of Livestock Farm Odors (Poultry Farms). In Proceedings of the 2021 13th International Conference on Knowledge and Smart Technology (KST), Bangsaen, Thailand, 21 January 2021; pp. 176–180. [Google Scholar] [CrossRef]
- Gallmann, E.; Hartung, E.; Brose, G.; Jungbluth, T. Determination of the Dynamics of the Odour Release from a Pig House, Using an Electronic Odour Sensor. Water Sci. Technol. 2004, 50, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Nikiema, J.; Brzezinski, R.; Buelna, G.; Heitz, M. A Review of the Environmental Pollution Originating from the Piggery Industry and of the Available Mitigation Technologies: Towards the Simultaneous Biofiltration of Swine Slurry and Methane. J. Environ. Eng. Sci. 2014, 9, 80–92. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, D.; Zhao, B.; Ma, S.; Liu, X.; Li, M.; Liu, X. Seasonal Variations and Spatial Distribution of Particulate Matter Emissions from a Ventilated Laying Hen House in Northeast China. Int. J. Agric. Biol. Eng. 2020, 13, 57–63. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, C.N.; Kim, Y.S. Assessment of Airborne Bacteria and Fungi in Pig Buildings in Korea. Biosyst. Eng. 2008, 99, 565–572. [Google Scholar] [CrossRef]
- Yang, W.; Guo, M.; Liu, G.; Yu, G.; Wang, P.; Wang, H.; Chai, T. Detection and Analysis of Fine Particulate Matter and Microbial Aerosol in Chicken Houses in Shandong Province, China. Poult. Sci. 2018, 97, 995–1005. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Ma, C. Characterization of Odorous Pollution and Health Risk Assessment of Volatile Organic Compound Emissions in Swine Facilities. Atmos. Environ. 2020, 223, 117233. [Google Scholar] [CrossRef]
- Hadlocon, L.S.; Manuzon, R.B.; Zhao, L.Y. Optimization of Ammonia Absorption Using Acid Spray Wet Scrubbers. Trans. ASABE 2014, 57, 647–659. [Google Scholar] [CrossRef]
- Hadlocon, L.S.; Zhao, L.Y.; Manuzon, R.B.; Elbatawi, I.E. An Acid Spray Scrubber for Recovery of Ammonia Emissions from a Deep-Pit Swine Facility. Trans. ASABE 2014, 57, 949–960. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Suh, H.-J.; Kim, J.-M.; Jung, Y.-H.; Moon, K.-W. Evaluation of Environmental Circumstance Within Swine and Chicken Houses in South Korea for the Production of Safe and Hygienic Animal Food Products. Korean J. Food Sci. Anim. Resour. 2008, 28, 623–628. [Google Scholar] [CrossRef][Green Version]
- Salaheldin, A.H.; Veits, J.; Abd El-Hamid, H.S.; Harder, T.C.; Devrishov, D.; Mettenleiter, T.C.; Hafez, H.M.; Abdelwhab, E.M. Isolation and Genetic Characterization of a Novel 2.2.1.2a H5N1 Virus from a Vaccinated Meat-Turkeys Flock in Egypt. Virol. J. 2017, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.L. Update on the Highly-Pathogenic Avian Influenza Outbreak of 2014–2015; Congressional Research Service: Washington, DC, USA, 2015; pp. 1–15.
- Zhao, Y.; Chai, L.; Richardson, B.; Xin, H. Field Evaluation of an Electrostatic Air Filtration System for Reducing Incoming Particulate Matter of a Hen House. Trans. ASABE 2018, 61, 295–304. [Google Scholar] [CrossRef]
- Sagardía, J. Factores que afectan a la prevalencia de mg en el sector de avicultura de puesta. Sel. Avícolas 2008, 50, 15–17. [Google Scholar]
- Peebles, E.D.; Park, S.W.; Branton, S.L.; Gerard, P.D.; Womack, S.K. Influence of Supplemental Dietary Poultry Fat, Phytase, and 25-Hydroxycholecalciferol on the Egg Characteristics of Commercial Layers Inoculated before or at the Onset of Lay with F-Strain Mycoplasma Gallisepticum. Poult. Sci. 2010, 89, 2078–2082. [Google Scholar] [CrossRef]
- Zhao, Y.; Richardson, B.; Takle, E.; Chai, L.; Schmitt, D.; Xin, H. Airborne Transmission May Have Played a Role in the Spread of 2015 Highly Pathogenic Avian Influenza Outbreaks in the United States. Sci. Rep. 2019, 9, 11755. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Wei, Y.; Li, B.; Wang, Y.; Zheng, H. Prevention of Particulate Matter and Airborne Culturable Bacteria Transmission between Double-Tunnel Ventilation Layer Hen Houses. Poult. Sci. 2019, 98, 2392–2398. [Google Scholar] [CrossRef]
- Spronk, G.; Otake, S.; Dee, S. Prevention of PRRSV Infection in Large Breeding Herds Using Air Filtration. Vet. Rec. 2010, 166, 758–759. [Google Scholar] [CrossRef]
- Pitkin, A.; Deen, J.; Dee, S. Use of a Production Region Model to Assess the Airborne Spread of Porcine Reproductive and Respiratory Syndrome Virus. Vet. Microbiol. 2009, 136, 1–7. [Google Scholar] [CrossRef]
- Alonso, C.; Davies, P.R.; Polson, D.D.; Dee, S.A.; Lazarus, W.F. Financial Implications of Installing Air Filtration Systems to Prevent PRRSV Infection in Large Sow Herds. Prev. Vet. Med. 2013, 111, 268–277. [Google Scholar] [CrossRef]
- Dee, S.; Otake, S.; Deen, J. Use of a Production Region Model to Assess the Efficacy of Various Air Filtration Systems for Preventing Airborne Transmission of Porcine Reproductive and Respiratory Syndrome Virus and Mycoplasma Hyopneumoniae: Results from a 2-Year Study. Virus Res. 2010, 154, 177–184. [Google Scholar] [CrossRef]
- Kanaoka, C.; Amornkitbamrung, M. Effect of Filter Permeability on the Release of Captured Dust from a Rigid Ceramic Filter Surface. Powder Technol. 2001, 118, 113–120. [Google Scholar] [CrossRef]
- Melse, R.W.; Ogink, N.W.M. Ogink Air Scrubbing Techniques for Ammonia and Odor Reduction at Livestock Operations: Review of on-Farm Research in the Netherlands. Trans. ASABE 2005, 48, 2303–2313. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, W.; Zhu, Z.; Yang, J.; Li, X.; Tian, Z.; Dong, H.; Zou, G. Mitigating Ammonia Emissions from Typical Broiler and Layer Manure Management—A System Analysis. Waste Manag. 2019, 93, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Mosquera, J.; Aarnink, A.J.A.; Groot Koerkamp, P.W.G.; Ogink, N.W.M. Evaluation of a Dry Filter and an Electrostatic Precipitator for Exhaust Air Cleaning at Commercial Non-Cage Laying Hen Houses. Biosyst. Eng. 2015, 129, 212–225. [Google Scholar] [CrossRef]
- Strohmaier, C.; Krommweh, M.S.; Büscher, W. Suitability of Different Filling Materials for a Biofilter at a Broiler Fattening Facility in Terms of Ammonia and Odour Reduction. Atmosphere 2019, 11, 13. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; Landman, W.J.M.; Melse, R.W.; Zhao, Y.; Ploegaert, J.P.M.; Huynh, T.T.T. Huynh Scrubber Capabilities to Remove Airborne Microorganisms and Other Aerial Pollutants from the Exhaust Air of Animal Houses. Trans. ASABE 2011, 54, 1921–1930. [Google Scholar] [CrossRef]
- Ottosen, L.D.M.; Juhler, S.; Guldberg, L.B.; Feilberg, A.; Revsbech, N.P.; Nielsen, L.P. Regulation of Ammonia Oxidation in Biotrickling Airfilters with High Ammonium Load. Chem. Eng. J. 2011, 167, 198–205. [Google Scholar] [CrossRef]
- Hadlocon, L.J.S.; Manuzon, R.B.; Zhao, L. Development and Evaluation of a Full-Scale Spray Scrubber for Ammonia Recovery and Production of Nitrogen Fertilizer at Poultry Facilities. Environ. Technol. 2015, 36, 405–416. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L.; Neibling, H.; Brian He, B. Field Evaluation of Wood Bark-Based down-Flow Biofilters for Mitigation of Odor, Ammonia, and Hydrogen Sulfide Emissions from Confined Swine Nursery Barns. J. Environ. Manag. 2015, 147, 164–174. [Google Scholar] [CrossRef]
- Luo, J.; Lindsey, S. The Use of Pine Bark and Natural Zeolite as Biofilter Media to Remove Animal Rendering Process Odours. Bioresour. Technol. 2006, 97, 1461–1469. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, R.K.; Singh, D. Chapter 20-Microbe-Based Bioreactor System for Bioremediation of Organic Contaminants: Present and Future Perspective. In Microbe Mediated Remediation of Environmental Contaminants; Kumar, A., Singh, V.K., Singh, P., Mishra, V.K., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 241–253. ISBN 978-0-12-821199-1. [Google Scholar]
- Van der Heyden, C.; Brusselman, E.; Volcke, E.I.P.; Demeyer, P. Continuous Measurements of Ammonia, Nitrous Oxide and Methane from Air Scrubbers at Pig Housing Facilities. J. Environ. Manag. 2016, 181, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fiencke, C.; Guo, J.; Rieth, R.; Dong, R.; Pfeiffer, E.-M. Performance Evaluation and Optimization of Field-Scale Bioscrubbers for Intensive Pig House Exhaust Air Treatment in Northern Germany. Sci. Total Environ. 2017, 579, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hansen, M.J.; Guldberg, L.B.; Feilberg, A. Kinetic Evaluation of Removal of Odorous Contaminants in a Three-Stage Biological Air Filter. Environ. Sci. Technol. 2012, 46, 8261–8269. [Google Scholar] [CrossRef]
- Codolo, M.C.; Bizzo, W.A. Experimental Study of the SO2 Removal Efficiency and Volumetric Mass Transfer Coefficients in a Pilot-Scale Multi-Nozzle Spray Tower. Int. J. Heat Mass Transf. 2013, 66, 80–89. [Google Scholar] [CrossRef]
- Opaliński, S.; Korczyński, M.; Szołtysik, M.; Dobrzański, Z.; Kołacz, R. Application of Aluminosilicates for Mitigation of Ammonia and Volatile Organic Compound Emissions from Poultry Manure. Open Chem. 2015, 13, 967–973. [Google Scholar] [CrossRef]
- Melse, R.W.; Ploegaert, J.P.M.; Ogink, N.W.M. Biotrickling Filter for the Treatment of Exhaust Air from a Pig Rearing Building: Ammonia Removal Performance and Its Fluctuations. Biosyst. Eng. 2012, 113, 242–252. [Google Scholar] [CrossRef]
- Estelles, F.; Melse, R.W.; Ogink, N.W.M.; Calvet, S. Calvet Evaluation of the NH3 Removal Efficiency of an Acid Packed Bed Scrubber Using Two Methods: A Case Study in a Pig Facility. Trans. ASABE 2011, 54, 1905–1912. [Google Scholar] [CrossRef]
- Nabatilan, M.M.; Harhad, A.; Wolenski, P.R.; Moe, W.M. Activated Carbon Load Equalization of Transient Concentrations of Gas-Phase Toluene: Effect of Gas Flow Rate during Pollutant Non-Loading Intervals. Chem. Eng. J. 2010, 157, 339–347. [Google Scholar] [CrossRef]
- Cheng, Z.; Feng, K.; Xu, D.; Kennes, C.; Chen, J.; Chen, D.; Zhang, S.; Ye, J.; Dionysiou, D.D. An Innovative Nutritional Slow-Release Packing Material with Functional Microorganisms for Biofiltration: Characterization and Performance Evaluation. J. Hazard. Mater. 2019, 366, 16–26. [Google Scholar] [CrossRef]
- Yu, G.; Xu, X.; He, P. Isolates Identification and Characteristics of Microorganisms in Biotrickling Filter and Biofilter System Treating H2S and NH3. J. Environ. Sci. 2007, 19, 859–863. [Google Scholar] [CrossRef]
- Chen, J.; Su, Q.; Pan, H.; Wei, J.; Zhang, X.; Shi, Y. Influence of Balance Gas Mixture on Decomposition of Dimethyl Sulfide in a Wire-Cylinder Pulse Corona Reactor. Chemosphere 2009, 75, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, R.E.; Thaler, R. Vertical Biofilter Construction and Performance; American Society of Agricultural and Biological Engineers: Broomfield, CO, USA, 2007. [Google Scholar]
- Liu, T.; Dong, H.; Zhu, Z.; Shang, B.; Yin, F.; Zhang, W.; Zhou, T. Effects of Biofilter Media Depth and Moisture Content on Removal of Gases from a Swine Barn. J. Air Waste Manag. Assoc. 2017, 67, 1288–1297. [Google Scholar] [CrossRef]
- Park, S.-J.; Nam, S.-I.; Choi, E.-S. Removal of Odor Emitted from Composting Facilities Using a Porous Ceramic Biofilter. Water Sci. Technol. 2001, 44, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Koe, L.C.C.; Yan, R.; Chen, X. Biological Treatment of H2S Using Pellet Activated Carbon as a Carrier of Microorganisms in a Biofilter. Water Res. 2006, 40, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.L.; Garrepalli, D.R.; Nawal, C.S. Carbonyl Sulfide Removal with Compost and Wood Chip Biofilters, and in the Presence of Hydrogen Sulfide. J. Air Waste Manag. Assoc. 2009, 59, 1458–1467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rene, E.R.; Murthy, D.V.S.; Swaminathan, T. Performance Evaluation of a Compost Biofilter Treating Toluene Vapours. Process Biochem. 2005, 40, 2771–2779. [Google Scholar] [CrossRef]
- Morgan-Sagastume, J.M.; Noyola, A.; Revah, S.; Ergas, S.J. Changes in Physical Properties of a Compost Biofilter Treating Hydrogen Sulfide. J. Air Waste Manag. Assoc. 2003, 53, 1011–1021. [Google Scholar] [CrossRef]
- Kim, N.J.; Sugano, Y.; Hirai, M.; Shoda, M. Removal Characteristics of High Load Ammonia Gas by a Biofilter Seeded with a Marine Bacterium, Vibrio Alginolyticus. Biotechnol. Lett. 2000, 22, 1295–1299. [Google Scholar] [CrossRef]
- Lee, S.; Li, C.; Heber, A.J.; Ni, J.; Huang, H. Biofiltration of a Mixture of Ethylene, Ammonia, n-Butanol, and Acetone Gases. Bioresour. Technol. 2013, 127, 366–377. [Google Scholar] [CrossRef]
- Chitwood, D.E.; Devinny, J.S. Treatment of Mixed Hydrogen Sulfide and Organic Vapors in a Rock Medium Biofilter. Water Environ. Res. 2001, 73, 426–435. [Google Scholar] [CrossRef]
- Dumont, E.; Cabral, F.D.S.; Le Cloirec, P.; Andrès, Y. Biofiltration Using Peat and a Nutritional Synthetic Packing Material: Influence of the Packing Configuration on H2S Removal. Environ. Technol. 2013, 34, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Allievi, M.J.; Silveira, D.D.; Cantão, M.E.; Filho, P.B. Bacterial Community Diversity in a Full Scale Biofilter Treating Wastewater Odor. Water Sci. Technol. 2018, 77, 2014–2022. [Google Scholar] [CrossRef]
- Melse, R.W.; Hol, J.M.G. Biofiltration of Exhaust Air from Animal Houses: Evaluation of Removal Efficiencies and Practical Experiences with Biobeds at Three Field Sites. Biosyst. Eng. 2017, 159, 59–69. [Google Scholar] [CrossRef]
- Hansen, M.J.; Liu, D.; Guldberg, L.B.; Feilberg, A. Application of Proton-Transfer-Reaction Mass Spectrometry to the Assessment of Odorant Removal in a Biological Air Cleaner for Pig Production. J. Agric. Food Chem. 2012, 60, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Aarnink, A.J.A.; De Jong, M.C.M.; Ogink, N.W.M.; Koerkamp, P.G. Groot Koerkamp Effectiveness of Multi-Stage Scrubbers in Reducing Emissions of Air Pollutants from Pig Houses. Trans. ASABE 2011, 54, 285–293. [Google Scholar] [CrossRef]
- Clark, O.; Edeogu, I.; Feddes, J.; Coleman, R.; Abolghasemi, A.; Feddes, I. Effects of Operating Temperature and Supplemental Nutrients in a Pilot-Scale Agricultural Biofilter. Can. Biosyst. Eng. Genie Biosyst. Can. 2004, 46, 7–16. [Google Scholar]
- Melse, R.W.; Hofschreuder, P.; Ogink, N.W.M. Ogink Removal of Particulate Matter (PM10) by Air Scrubbers at Livestock Facilities: Results of an On-Farm Monitoring Program. Trans. ASABE 2012, 55, 689–698. [Google Scholar] [CrossRef]
- Demmers, T.G.M.; Saponja, A.; Thomas, R.; Phillips, G.J.; Mcdonald, A.G.; Stagg, S.; Bowry, A.; Nemitz, E. Dust and Ammonia Emissions from Uk Poultry Houses. In Proceedings of the XVIIth World Congress of the International Commission of Agricultural and Biosystems Engineering (CIGR), Quebec City, QC, Canada, 13–17 June 2010; p. 10. [Google Scholar]
- Mostafa, E.; Buescher, W. Indoor Air Quality Improvement from Particle Matters for Laying Hen Poultry Houses. Biosyst. Eng. 2011, 109, 22–36. [Google Scholar] [CrossRef]
- Mendes, L.; Ogink, N.; Edouard, N.; van Dooren, H.; Tinôco, I.; Mosquera, J. NDIR Gas Sensor for Spatial Monitoring of Carbon Dioxide Concentrations in Naturally Ventilated Livestock Buildings. Sensors 2015, 15, 11239–11257. [Google Scholar] [CrossRef]
- Jha, S.K.; Hayashi, K. A Novel Odor Filtering and Sensing System Combined with Regression Analysis for Chemical Vapor Quantification. Sens. Actuators B Chem. 2014, 200, 269–287. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, D.; Son, D.; Doh, S.J.; Kim, M.; Lee, H.; Yoon, K.R. Rational Process Design for Facile Fabrication of Dual Functional Hybrid Membrane of MOF and Electrospun Nanofiber towards High Removal Efficiency of PM2.5 and Toxic Gases. Macromol. Rapid Commun. 2022, 43, 2100648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, L.; Yang, Z.; Kong, E.S.-W.; Zhu, X.; Zhang, Y. Graphene Oxide-Modified Polyacrylonitrile Nanofibrous Membranes for Efficient Air Filtration. ACS Appl. Nano Mater. 2019, 2, 3916–3924. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Liu, L.; Yu, J.; Ding, B. A Fluffy Dual-Network Structured Nanofiber/Net Filter Enables High-Efficiency Air Filtration. Adv. Funct. Mater. 2019, 29, 1904108. [Google Scholar] [CrossRef]
- Souzandeh, H.; Wang, Y.; Zhong, W.-H. “Green” Nano-Filters: Fine Nanofibers of Natural Protein for High Efficiency Filtration of Particulate Pollutants and Toxic Gases. RSC Adv. 2016, 6, 105948–105956. [Google Scholar] [CrossRef]
- Souzandeh, H.; Johnson, K.S.; Wang, Y.; Bhamidipaty, K.; Zhong, W.-H. Soy-Protein-Based Nanofabrics for Highly Efficient and Multifunctional Air Filtration. ACS Appl. Mater. Interfaces 2016, 8, 20023–20031. [Google Scholar] [CrossRef]
- Tian, H.; Fu, X.; Zheng, M.; Wang, Y.; Li, Y.; Xiang, A.; Zhong, W.-H. Natural Polypeptides Treat Pollution Complex: Moisture-Resistant Multi-Functional Protein Nanofabrics for Sustainable Air Filtration. Nano Res. 2018, 11, 4265–4277. [Google Scholar] [CrossRef]
- Souzandeh, H.; Wang, Y.; Netravali, A.N.; Zhong, W.-H. Towards Sustainable and Multifunctional Air-Filters: A Review on Biopolymer-Based Filtration Materials. Polym. Rev. 2019, 59, 651–686. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).