Cadmium and Cadmium/BDE (47 or 209) Exposure Affect Mitochondrial Function, DNA Damage/Repair Mechanisms and Barrier Integrity in Airway Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. A549 Cell Cultures and Exposure
2.3. Cell Proliferation Assay
2.4. Detection of Intracellular ROS
2.5. Detection of JC-1 Mitochondrial Membrane Potential to Assess Mitochondrial Function
2.6. Cell Apoptosis
2.7. DNA Damage Analysis
2.8. Measurement of TJ Integrity by TEER
2.9. Total Protein Extraction
2.10. Western Blot Analyses
2.11. Western Blot Antibodies
2.12. Gel Images Evaluation
2.13. Immunofluorescence for γH2AX Foci Formation
2.14. RNA Isolation and Quantitative RT-PCR (ZO-1, Claudin-1 and E-Cadherin-1)
2.15. Morphological Evaluation
2.16. Statistical Analysis
3. Results
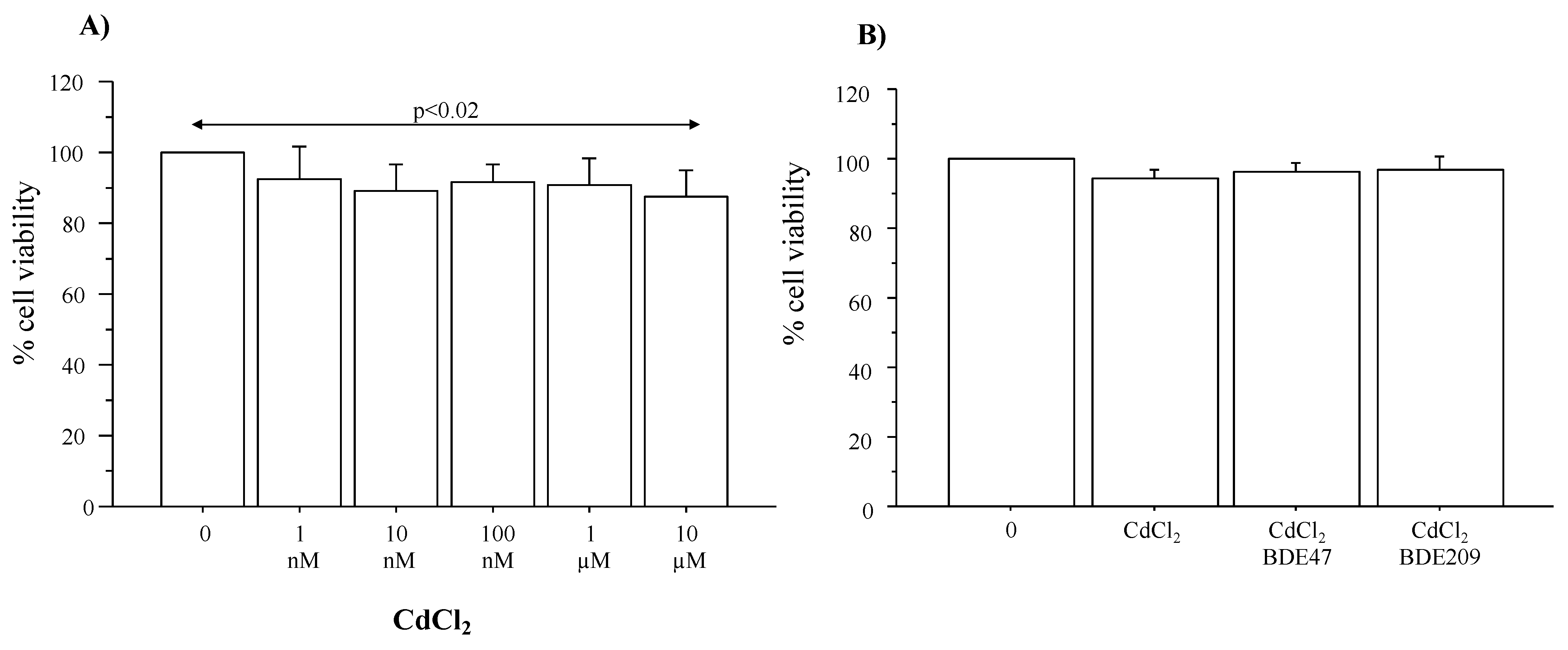
3.1. Cytotoxic Effects of CdCl2 and CdCl2/BDE Mixtures in Submerged Culture of A549 Cells
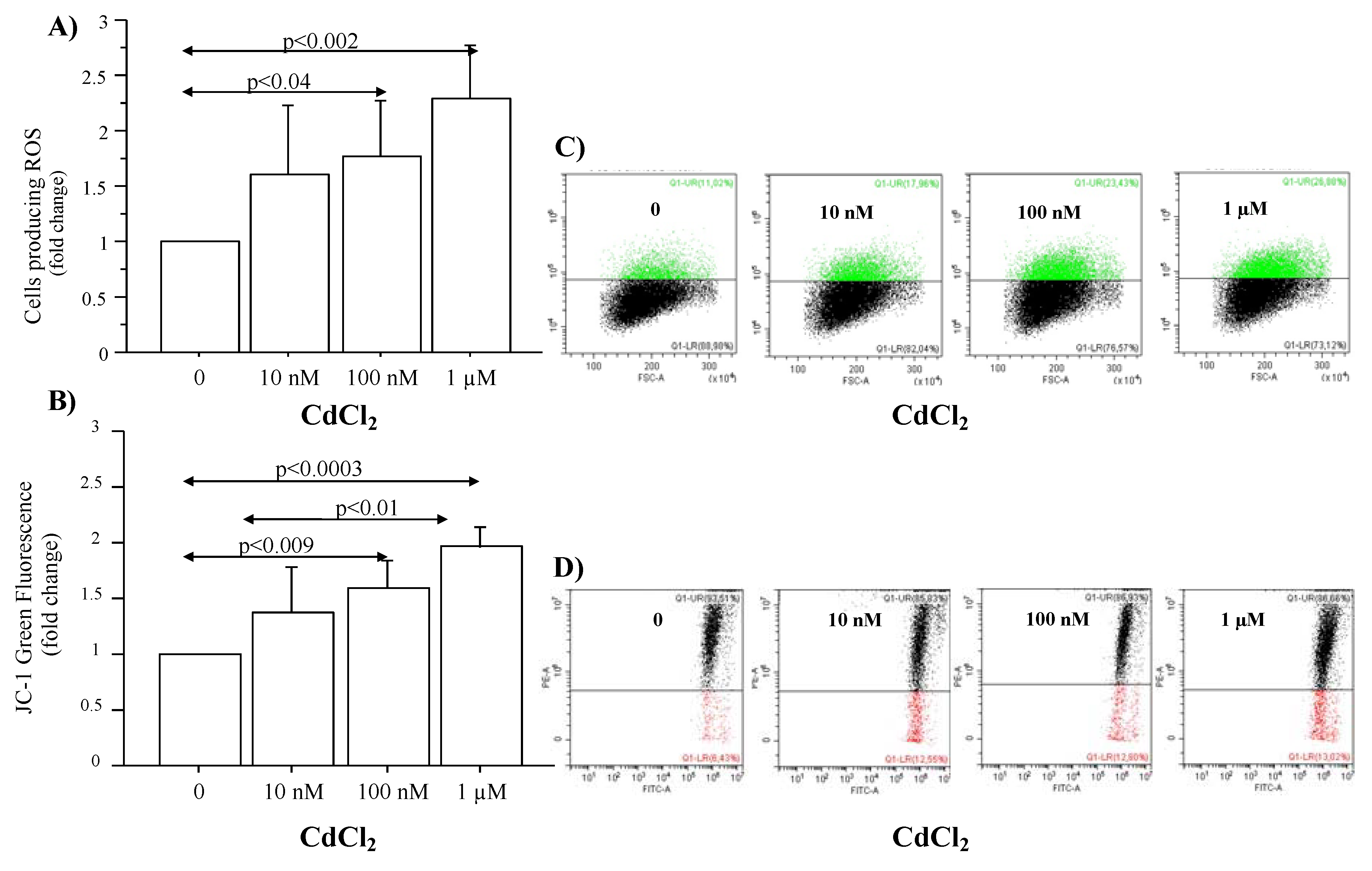
3.2. Effect of CdCl2 Treatment on ROS Production and Mitochondrial Injury in Submerged Culture of A549 Cells
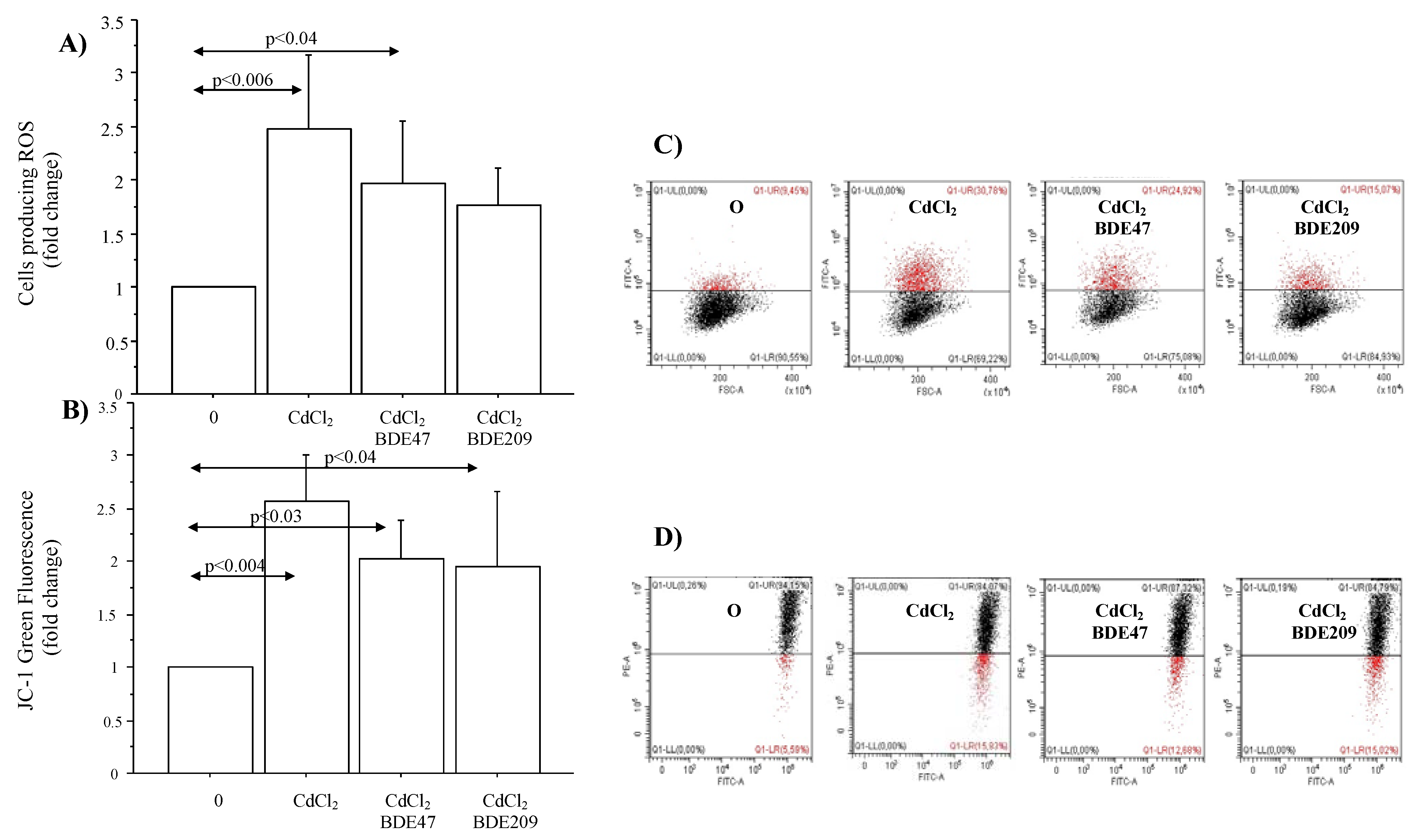
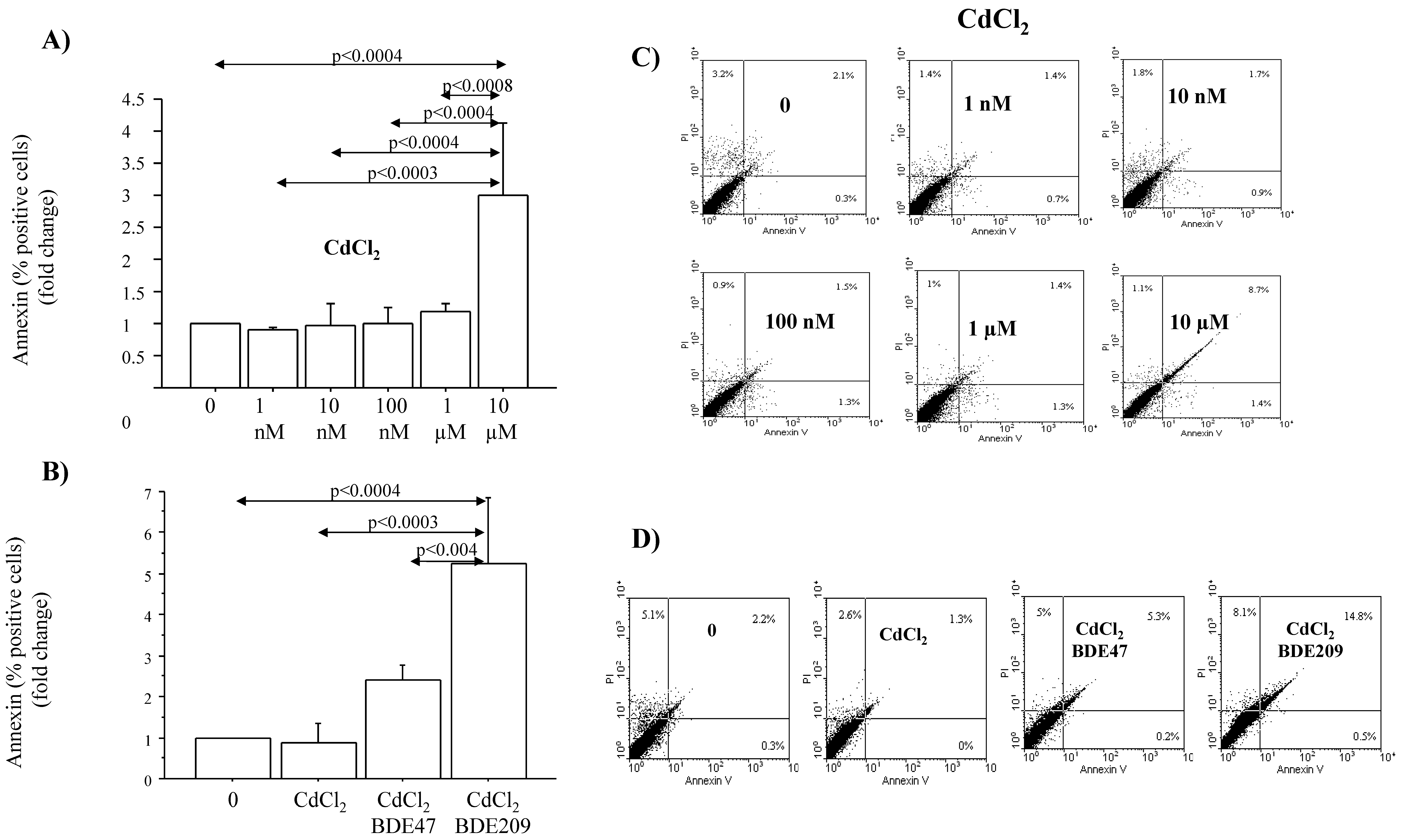
3.3. Effects of CdCl2 and CdCl2/BDE Mixtures on ROS Production, Mitochondrial Injury and Apoptosis in A549 Cells
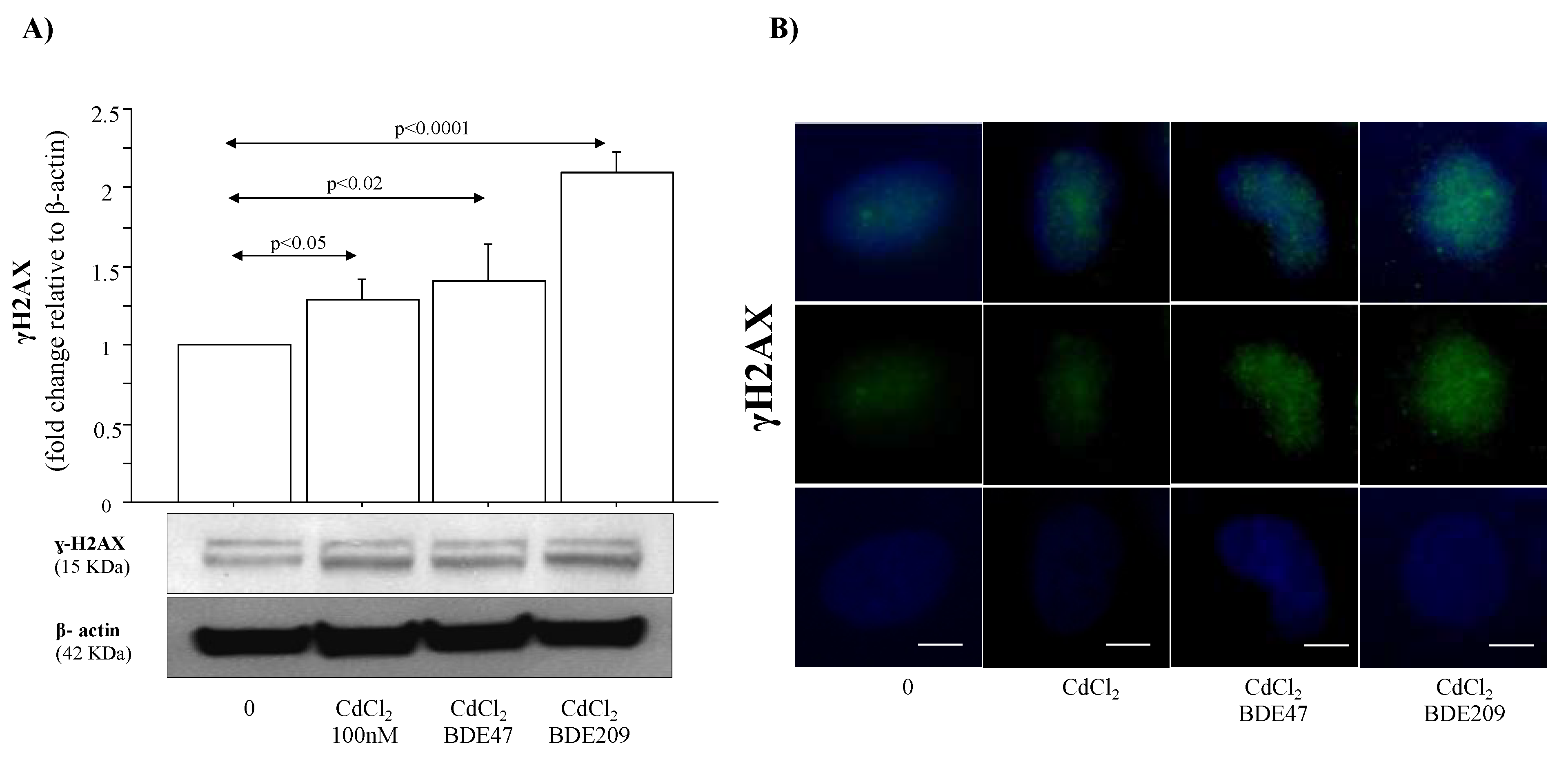
3.4. Effect of CdCl2 and CdCl2/BDE Mixtures on DNA Damage/Repair Mechanisms in A549 Cells
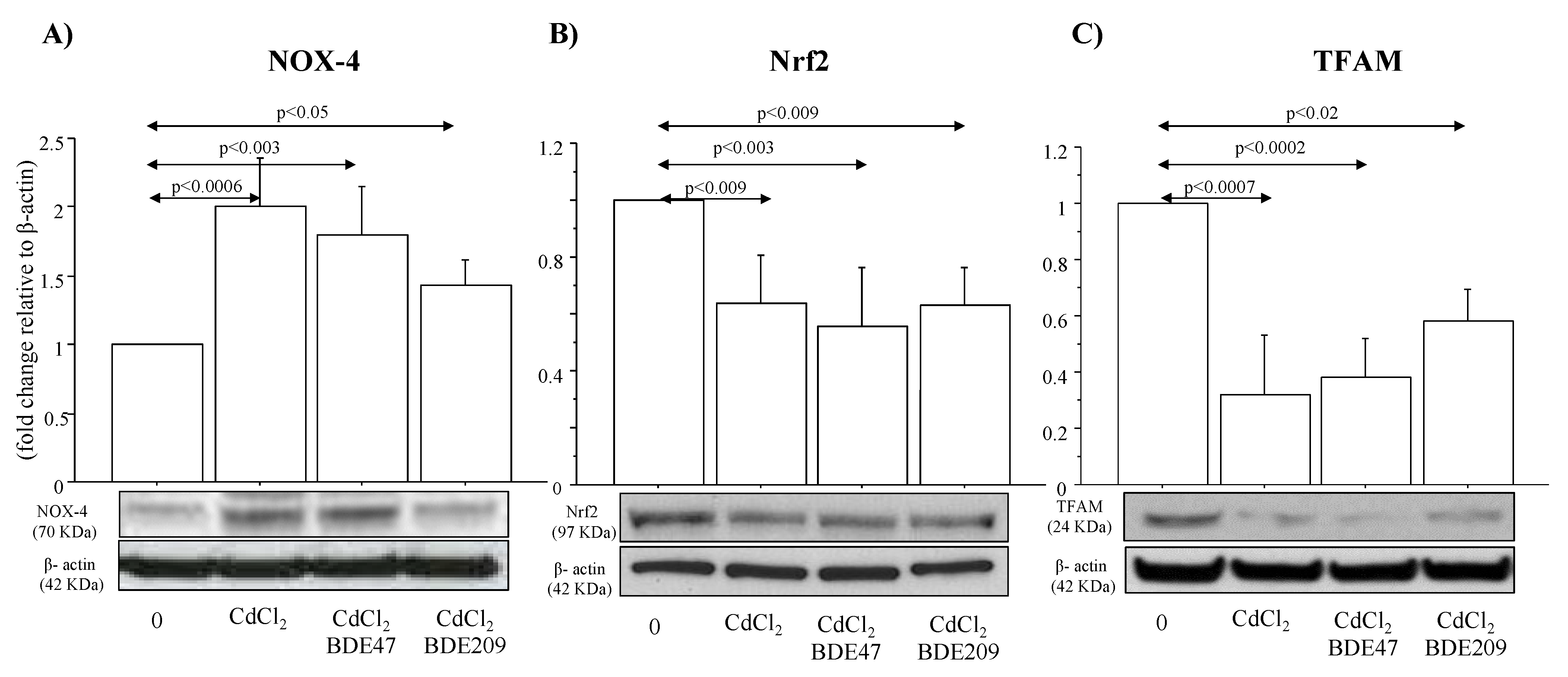
3.5. Cytotoxic Effects and NOX-4, Nrf2 and TFAM Protein Expression in ALI Culture of A549 Cells Exposed to CdCl2 and CdCl2/BDE Mixtures
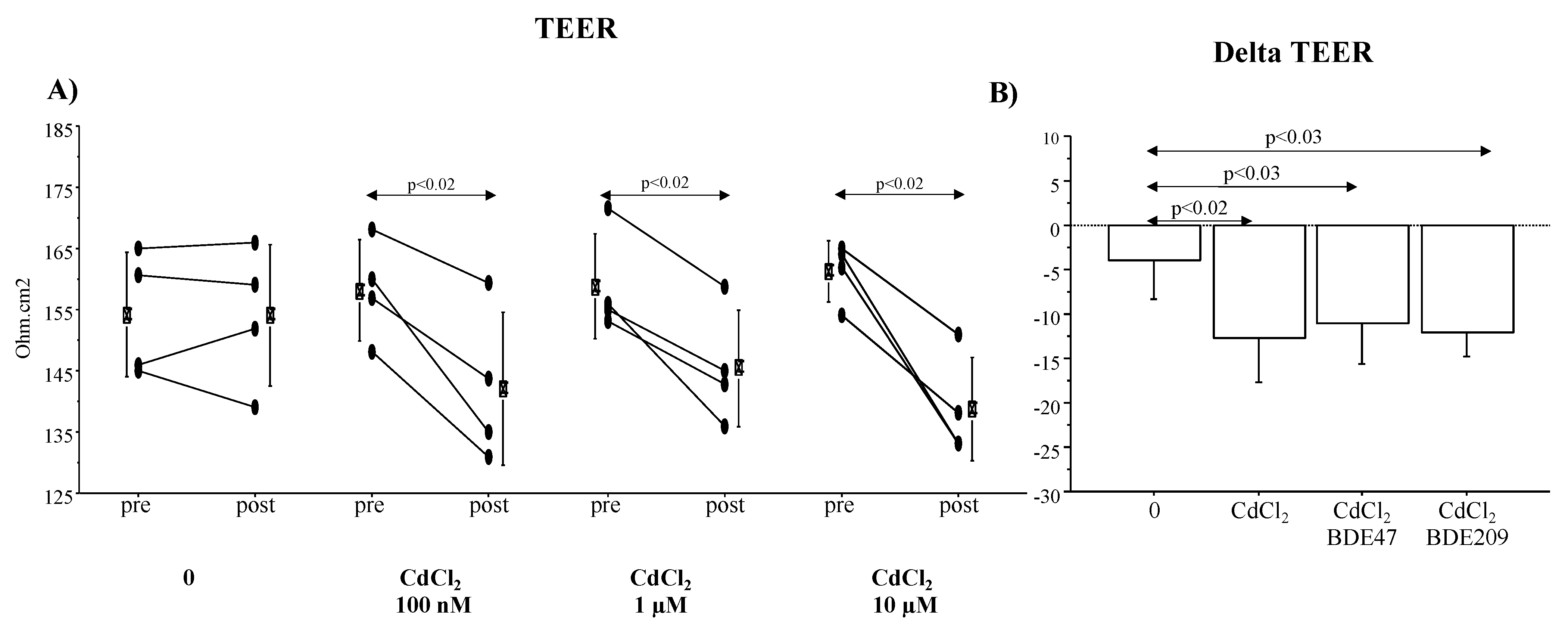
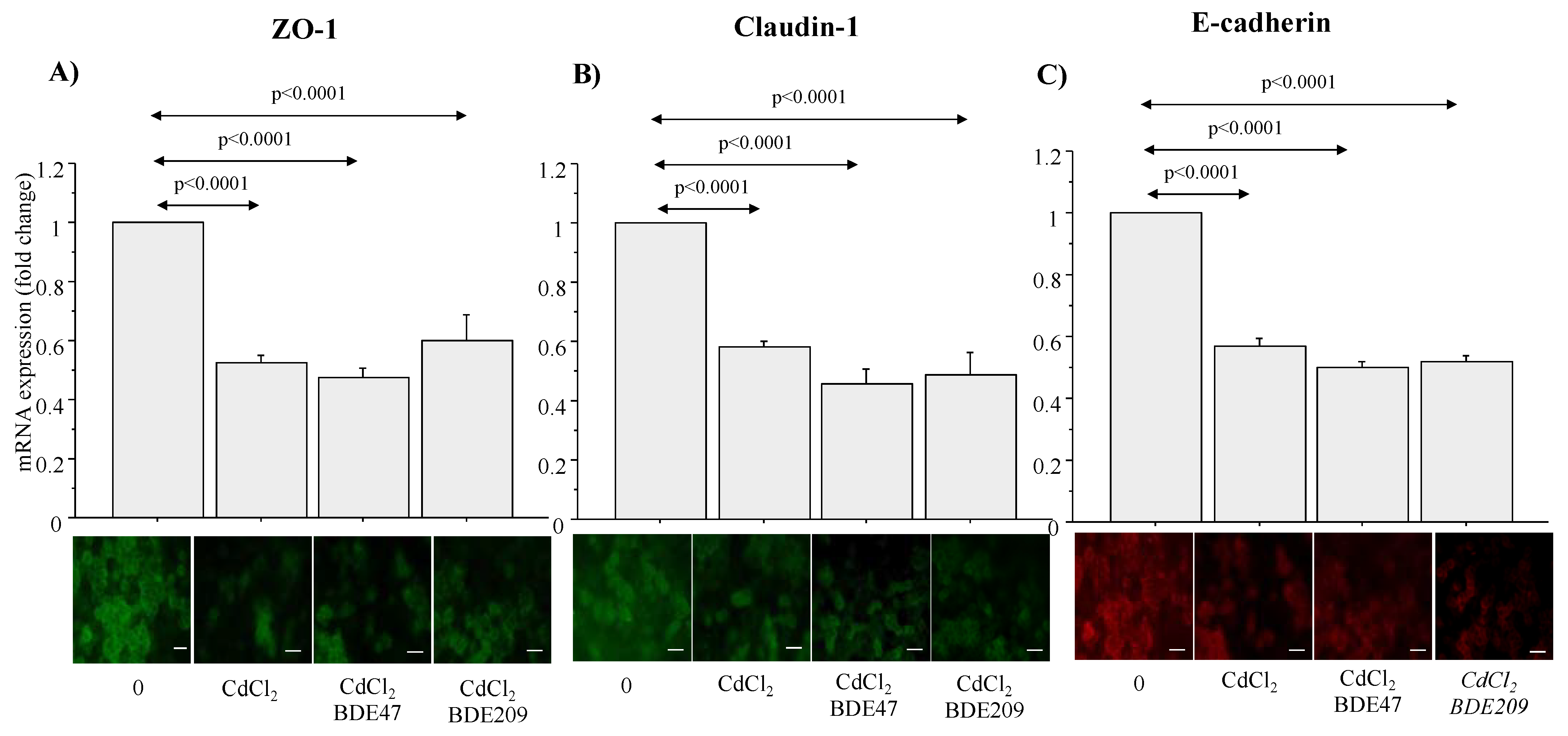
3.6. TEER and ZO-1, Claudin-1 and E-Cadherin mRNA and Protein Expression in ALI Culture of A549 Cells Exposed to CdCl2 and CdCl2/BDE Mixtures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
List of Abbreviations
| CdCl2 | (Cadmium Chloride) |
| BDEs | (Brominated Diphenyl Ether flame-retardants) |
| ALI | (Air–Liquid Interface) |
| ROS | (Reactive Oxygen Species) |
| ZO-1 | (Zonula Occludens-1) |
| NAPDH | Nicotinamide Adenine Dinucleotide Phosphate |
| NOX4 | (NADPH oxidase 4) |
| TFAM | (Transcription Factor A, Mitochondrial) |
| Nrf2 | (Nuclear factor erythroid 2–Related Factor 2) |
| TEER | (Trans epithelial/transendothelial electrical resistance) |
| DMSO | (Dimethyl sulfoxide) |
| DCFH-DA | (2, 7- dichloro-difluorescein diacetate) |
| EDTA | (ethylenediaminetetraacetic acid) |
| BCA | (bicinchoninic acid) |
| SDS | (sodium dodecyl sulfate) |
| PAGE | (polyacrylamide gel electrophoresis) |
| A.D.U. | (arbitrary densitometric units) |
| H2AX | (H2A histone family member X) |
References
- Agency for Toxic Substances and Disease Registry. 2019 Substance Priority List; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2019. [Google Scholar]
- Rennolds, J.; Butler, S.; Maloney, K.; Boyaka, P.N.; Davis, I.C.; Knoell, D.L.; Parinandi, N.L.; Cormet-Boyaka, E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 116, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Gómara, B.; Herrero, L.; Ramos, J.J.; Mateo, J.R.; Fernández, M.A.; García, J.F.; González, M.J. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ. Sci. Technol. 2007, 41, 6961. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, Q.; Yang, Y.; Nie, Z.; Cheng, J.; Yang, J.; Wang, Y.; Chai, M. Polybrominated diphenyl ethers (PBDEs) and heavy metals in road dusts from a plastic waste recycling area in north China: Implications for human health. Environ. Sci. Pollut. Res. 2016, 23, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Hu, G.; Xu, Z.; Li, H.; Zhang, L.; Zheng, J.; Chen, L.; He, D. Characterization and distribution of heavy metals, polybrominated diphenyl ethers and perfluoroalkyl substances in surface sediment from the Dayan River, South China. Bull. Environ. Contam. Toxicol. 2015, 94, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-T.; Son, M.-H.; Lee, D.-H.; Seong, W.J.; Han, S.; Chang, Y.-S. Partitioning behavior of heavy metals and persistent organic pollutants among Feto–maternal bloods and tissues. Environ. Sci. Technol. 2015, 49, 7411–7422. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, M.; Gao, Y.; Cui, J. In vitro study on the joint hepatoxicity upon combined exposure of cadmium and BDE-209. Environ. Toxicol. Pharmacol. 2018, 57, 62–69. [Google Scholar] [CrossRef]
- Leijs, M.M.; van Teunenbroek, T.; Olie, K.; Koppe, J.G.; ten Tusscher, G.W.; van Aalderen, W.M.; de Voogt, P. Assessment of current serum levels of PCDD/Fs, dl-PCBs and PBDEs in a Dutch cohort with known perinatal PCDD/F exposure. Chemosphere 2008, 73, 176–181. [Google Scholar] [CrossRef]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e2. [Google Scholar] [CrossRef]
- Albano, G.D.; Moscato, M.; Montalbano, A.M.; Anzalone, G.; Gagliardo, R.; Bonanno, A.; Giacomazza, D.; Barone, R.; Drago, G.; Cibella, F.; et al. Can PBDEs affect the pathophysiologic complex of epithelium in lung diseases? Chemosphere 2020, 241, 12. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; McCray, P.B., Jr.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015, 45, 1150–1162. [Google Scholar] [CrossRef] [Green Version]
- De Rose, V.; Molloy, K.; Gohy, S.; Pilette, C.; Greene, C.M. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediat. Inflamm. 2018, 2018, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, S.; Heijink, I.H.; Hesse, L.; Koo, H.K.; Shaheen, F.; Fouadi, M.; Kuchibhotla, V.; Lambrecht, B.N.; Van Oosterhout, A.; Hackett, T.L.; et al. Characterization of a lung epithelium specific E-cadherin knock-out model: Implications for obstructive lung pathology. Sci. Rep. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, R.R.; Nonavinakere, V.K.; Soliman, M.R. Intratracheal exposure of the guinea pig lung to cadmium and/or selenium: A histological evaluation. Toxicol. Lett. 2000, 114, 101–109. [Google Scholar] [CrossRef]
- Cao, X.; Lin, H.; Muskhelishvili, L.; Latendresse, J.; Richter, P.; Heflich, R.H. Tight junction disruption by cadmium in an in vitro human airway tissue model. Respir. Res. 2015, 16, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Chen, Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J. Transl. Med. 2017, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Białas, A.J.; Sitarek, P.; Miłkowska-Dymanowska, J.; Piotrowski, W.J.; Górski, P. The Role of Mitochondria and Oxidative/Antioxidative Imbalance in Pathobiology of Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2016, 2016, 7808576. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.; Pendyala, S.; Hecker, L.; Lee, P.J.; Natarajan, V.; Thannickal, V.J. NOX enzymes and pulmonary disease. Antioxid. Redox Signal. 2009, 11, 2505. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Kim, S.H.; Hamasaki, N. Mitochondrial transcription factor A (TFAM): Roles in maintenance of mtDNA and cellular functions. Mitochondrion 2007, 7, 39–44. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the antioxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.H.; Chi, G.Y.; Kim, G.Y.; Chang, Y.C.; Moon, S.K.; Nam, S.W.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol. Lett. 2012, 212, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.M.; Albano, G.D.; Anzalone, G.; Moscato, M.; Gagliardo, R.; Di Sano, C.; Bonanno, A.; Ruggieri, S.; Cibella, F.; Profita, M. Cytotoxic and genotoxic effects of the flame retardants (PBDE-47, PBDE-99 and PBDE-209) in human bronchial epithelial cells. Chemosphere 2020, 245, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Park, S.; Sung, J.H. Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model. Int. J. Environ. Res. Public Health 2020, 17, 9218. [Google Scholar] [CrossRef]
- Huff, M.O.; Todd, S.L.; Smith, A.L.; Elpers, J.T.; Smith, A.P.; Murphy, R.D.; Bleser-Shartzer, A.S.; Hoerter, J.E.; Radde, B.N.; Klinge, C.M. Arsenite and Cadmium Activate MAPK/ERK via Membrane Estrogen Receptors and G-Protein Coupled Estrogen Receptor Signaling in Human Lung Adenocarcinoma Cells. Toxicol Sci. 2016, 152, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albano, G.D.; Zhao, J.; Etling, E.B.; Park, S.Y.; Hu, H.; Trudeau, J.B.; Profita, M.; Wenzel, S.E. IL-13 desensitizes β2-adrenergic receptors in human airway epithelial cells through a 15-lipoxygenase/G protein receptor kinase 2 mechanism. J. Allergy Clin. Immunol. 2015, 135, 1144–1153.e539. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; White, C.C.; Dabrowski, M.J.; Kavanagh, T.J.; Eckert, M.L.; Gallagher, E.P. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 101, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Saquib, Q.; Al-Khedhairy, A.A.; Al-Arifi, S.; Dhawan, A.; Musarrat, J. Assessment of methyl thiophanate-Cu (II) induced DNA damage in human lymphocytes. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2009, 23, 848–854. [Google Scholar] [CrossRef]
- Forti, E.; Bulgheroni, A.; Cetin, Y.; Hartung, T.; Jennings, P.; Pfaller, W.; Prieto, P. Characterisation of cadmium chloride induced molecular and functional alterations in airway epithelial cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2010, 25, 159–168. [Google Scholar] [CrossRef]
- Anzalone, G.; Albano, G.D.; Montalbano, A.M.; Riccobono, L.; Bonanno, A.; Gagliardo, R.; Bucchieri, F.; Marchese, R.; Moscato, M.; Profita, M. IL-17A-associated IKK-α signaling induced TSLP production in epithelial cells of COPD patients. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Leopardi, P.; Cordelli, E.; Villani, P.; Cremona, T.P.; Conti, L.; De Luca, G.; Crebelli, R. Assessment of in vivo genotoxicity of the rodent carcinogen furan: Evaluation of DNA damage and induction of micronuclei in mouse splenocytes. Mutagenesis 2010, 25, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Halicka, D.; Traganos, F.; Darzynkiewicz, Z. Cytometric analysis of DNA damage: Phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs). Methods Mol. Biol. 2009, 523, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, A. The gender-specific risk to liver toxicity and cancer of flounder (Platichthys flesus (L.)) at the German Wadden Sea coast. Aquat. Toxicol. 2004, 70, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health A Glob. Access Sci. Source 2011, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Soo-Jin, C.; Jae-Min, O.; Jin-Ho, C. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J. Inorg. Biochem. 2009, 103, 463–471. [Google Scholar] [CrossRef]
- Kim, J.S.; Peters, T.M.; O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Thorne, P.S. Validation of an in vitro exposure system for toxicity assessment of air-delivered nanomaterials. Toxicol. Vitr. 2013, 27, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. J. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.O.; Marnewick, J.L.; Ellis, E.M. Heme oxygenase-1 attenuates cadmium-induced mitochondrial-caspase 3- dependent apoptosis in human hepatoma cell line. BMC Pharmacol. Toxicol. 2015, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Jiang, Y.L.; Fei, J.; Cao, P.; Zhuang, J.; Nie, G.; Yang, F.; Dai, X.; Cao, H.; Xing, C.; et al. Cadmium induces cytotoxicity through oxidative stress-mediated apoptosis pathway in duck renal tubular epithelial cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yin, H.; Peng, H.; Lu, G.; Liu, Z.; Dang, Z. OPFRs and BFRs induced A549 cell apoptosis by caspase-dependent mitochondrial pathway. Chemosphere 2019, 221, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, H.; Zhang, M.; Zhang, T.; Frank, J.; Chen, G. Autophagy in arsenic carcinogenesis. Exp. Toxicol. Pathol. Off. J. Ges. fur Toxikol. Pathol. 2014, 66, 163–168. [Google Scholar] [CrossRef]
- Son, Y.O.; Pratheeshkumar, P.; Roy, R.V.; Hitron, J.A.; Wang, L.; Zhang, Z.; Shi, X. Nrf2/p62 signaling in apoptosis resistance and its role in cadmium-induced carcinogenesis. J. Biol. Chem. 2014, 289, 28660–28662, Retraction published J. Biol. Chem. 2018, 293, 15455. [Google Scholar] [CrossRef] [Green Version]
- Nyunoya, T.; Mebratu, Y.; Contreras, A.; Delgado, M.; Chand, H.S.; Tesfaigzi, Y. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am. J. Respir. Cell Mol. Biol. 2014, 50, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Tran, I.; Ji, C.; Ni, I.; Min, T.; Tang, D.; Vij, N. Role of Cigarette Smoke-Induced Aggresome Formation in Chronic Obstructive Pulmonary Disease-Emphysema Pathogenesis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 159–173. [Google Scholar] [CrossRef]
- de Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885. [Google Scholar] [CrossRef]
- Pesonen, M.; Vähäkangas, K. Autophagy in exposure to environmental chemicals. Toxicol. Lett. 2019, 305, 1–9. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [Green Version]
- Doherty, J.; Baehrecke, E.H. Life, Death and Autophagy. Nat. Cell Biol. 2018, 20, 1110. [Google Scholar] [CrossRef]
- Valverde, M.; Trejo, C.; Rojas, E. Is the capacity of lead acetate and cadmium chloride to induce genotoxic damage due to direct DNA-metal interaction? Mutagenesis 2001, 16, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Monteiro, C.; Pinho, F.; Pinho, S.; Ferreira de Oliveira, J.M.; Santos, C. Cadmium-induced genotoxicity in human osteoblast-like cells. Mutation research. Genet. Toxicol. Environ. Mutagenesis 2014, 775–776, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleaver, J.E. γH2Ax: Biomarker of damage or functional participant in DNA repair “all that glitters is not gold!”. Photochem. Photobiol. 2011, 87, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Canton, C.; Anadón, A.; Meredith, C. γH2AX as a novel endpoint to detect DNA damage: Applications for the assessment of the in vitro genotoxicity of cigarette smoke. Toxicol. Vitr. Int. J. Assoc. BIBRA 2012, 26, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- Riches, L.C.; Lynch, A.M.; Gooderham, N.J. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis 2008, 23, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Cann, K.L.; Dellaire, G. Heterochromatin and the DNA damage response: The need to relax. Biochem. Cell Biol. 2011, 89, 45–60. [Google Scholar] [CrossRef]
- Xu, Y.; Price, B.D. Chromatin dynamics and the repair of DNA double strand breaks. Cell Cycle 2011, 10, 261–267. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative stress, redox signaling, and autophagy: Cell death versus survival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef] [Green Version]
- Shafique, E.; Choy, W.C.; Liu, Y.; Feng, J.; Cordeiro, B.; Lyra, A.; Arafah, M.; Yassin-Kassab, A.; Zanetti, A.V.; Clements, R.T.; et al. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging 2013, 5, 515–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciarretta, S.; Zhai, P.; Shao, D.; Zablocki, D.; Nagarajan, N.; Terada, L.S.; Volpe, M.; Sadoshima, J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ. Res. 2012, 113, 1253–1264. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88 Pt B, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloonan, S.M.; Choi, A.M. Mitochondria in lung disease. J. Clin. Investig. 2016, 126, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- Rezaee, F.; Georas, S.N. Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am. J. Respir. Cell Mol. Biol. 2014, 50, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.; Sin, D.D. The airway epithelium: More than just a structural barrier. Ther. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef]
- Li, J.X.H.; Tang, V.W.; Brieher, W.M. Actin protrusions push at apical junctions to maintain E-cadherin adhesion. Proc. Natl. Acad. Sci. USA 2020, 117, 432–438. [Google Scholar] [CrossRef]

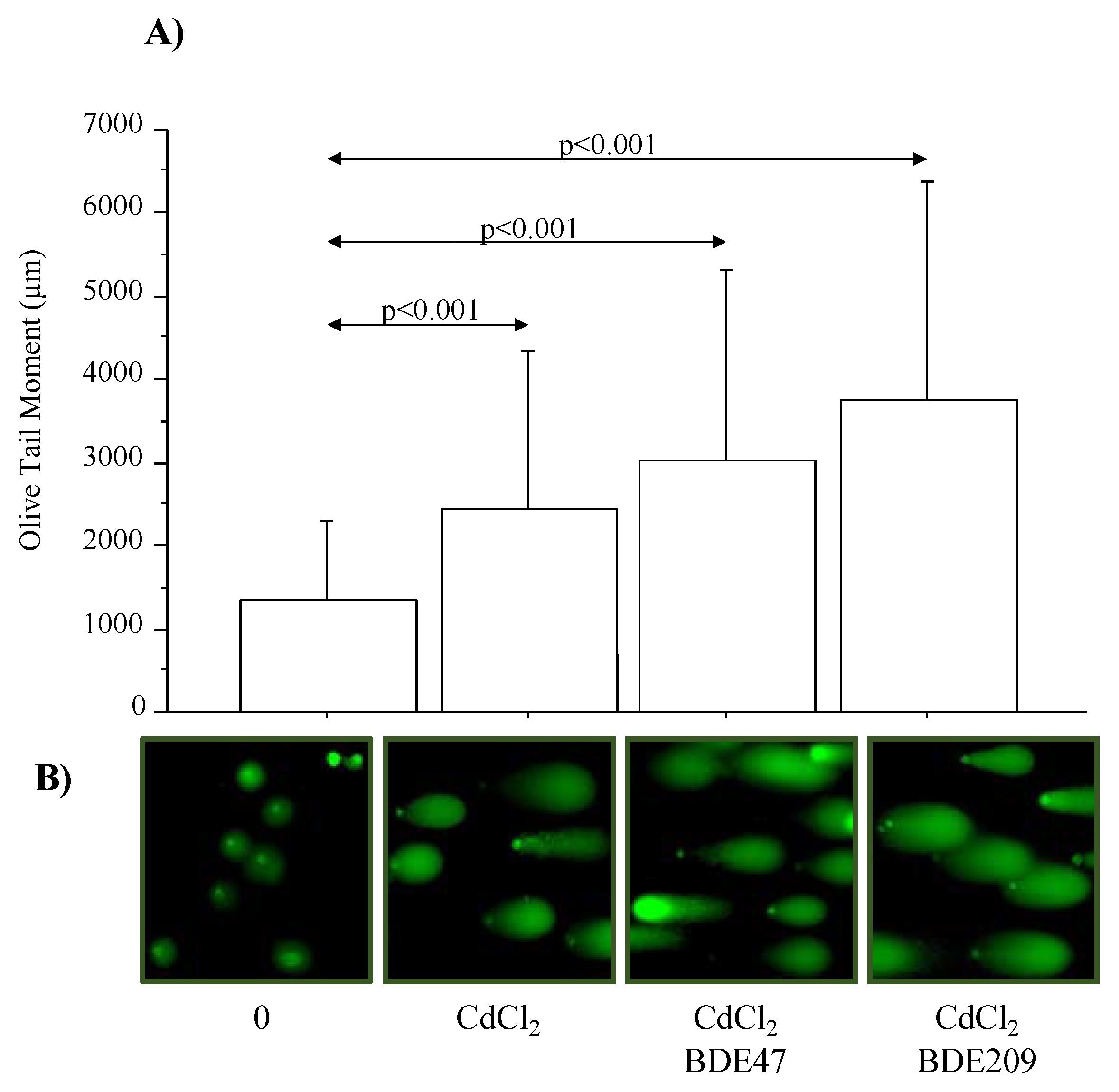
| A549 Cells | 1 | 2 | 3 | 4 | 5 | Frequency Classes Lenght (μm) |
|---|---|---|---|---|---|---|
| 1 = 0 - 20 | ||||||
| 0 | 117 | 32 | 1 | 0 | 0 | 2 = 21 - 40 |
| CdCl2 | 95 | 27 | 21 | 7 | 0 | 3 = 41 - 60 |
| CdCl2 + BDE 47 | 81 | 29 | 21 | 19 | 0 | 4 = 61 - 80 |
| CdCl2 + BDE 209 | 69 | 27 | 24 | 28 | 2 | 5 = 81 - 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, G.D.; Bonanno, A.; Montalbano, A.M.; Di Sano, C.; Anzalone, G.; Gagliardo, R.; Ruggieri, S.; Profita, M. Cadmium and Cadmium/BDE (47 or 209) Exposure Affect Mitochondrial Function, DNA Damage/Repair Mechanisms and Barrier Integrity in Airway Epithelial Cells. Atmosphere 2022, 13, 201. https://doi.org/10.3390/atmos13020201
Albano GD, Bonanno A, Montalbano AM, Di Sano C, Anzalone G, Gagliardo R, Ruggieri S, Profita M. Cadmium and Cadmium/BDE (47 or 209) Exposure Affect Mitochondrial Function, DNA Damage/Repair Mechanisms and Barrier Integrity in Airway Epithelial Cells. Atmosphere. 2022; 13(2):201. https://doi.org/10.3390/atmos13020201
Chicago/Turabian StyleAlbano, Giusy Daniela, Anna Bonanno, Angela Marina Montalbano, Caterina Di Sano, Giulia Anzalone, Rosalia Gagliardo, Silvia Ruggieri, and Mirella Profita. 2022. "Cadmium and Cadmium/BDE (47 or 209) Exposure Affect Mitochondrial Function, DNA Damage/Repair Mechanisms and Barrier Integrity in Airway Epithelial Cells" Atmosphere 13, no. 2: 201. https://doi.org/10.3390/atmos13020201
APA StyleAlbano, G. D., Bonanno, A., Montalbano, A. M., Di Sano, C., Anzalone, G., Gagliardo, R., Ruggieri, S., & Profita, M. (2022). Cadmium and Cadmium/BDE (47 or 209) Exposure Affect Mitochondrial Function, DNA Damage/Repair Mechanisms and Barrier Integrity in Airway Epithelial Cells. Atmosphere, 13(2), 201. https://doi.org/10.3390/atmos13020201






