Understanding the Sources of Ambient Fine Particulate Matter (PM2.5) in Jeddah, Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
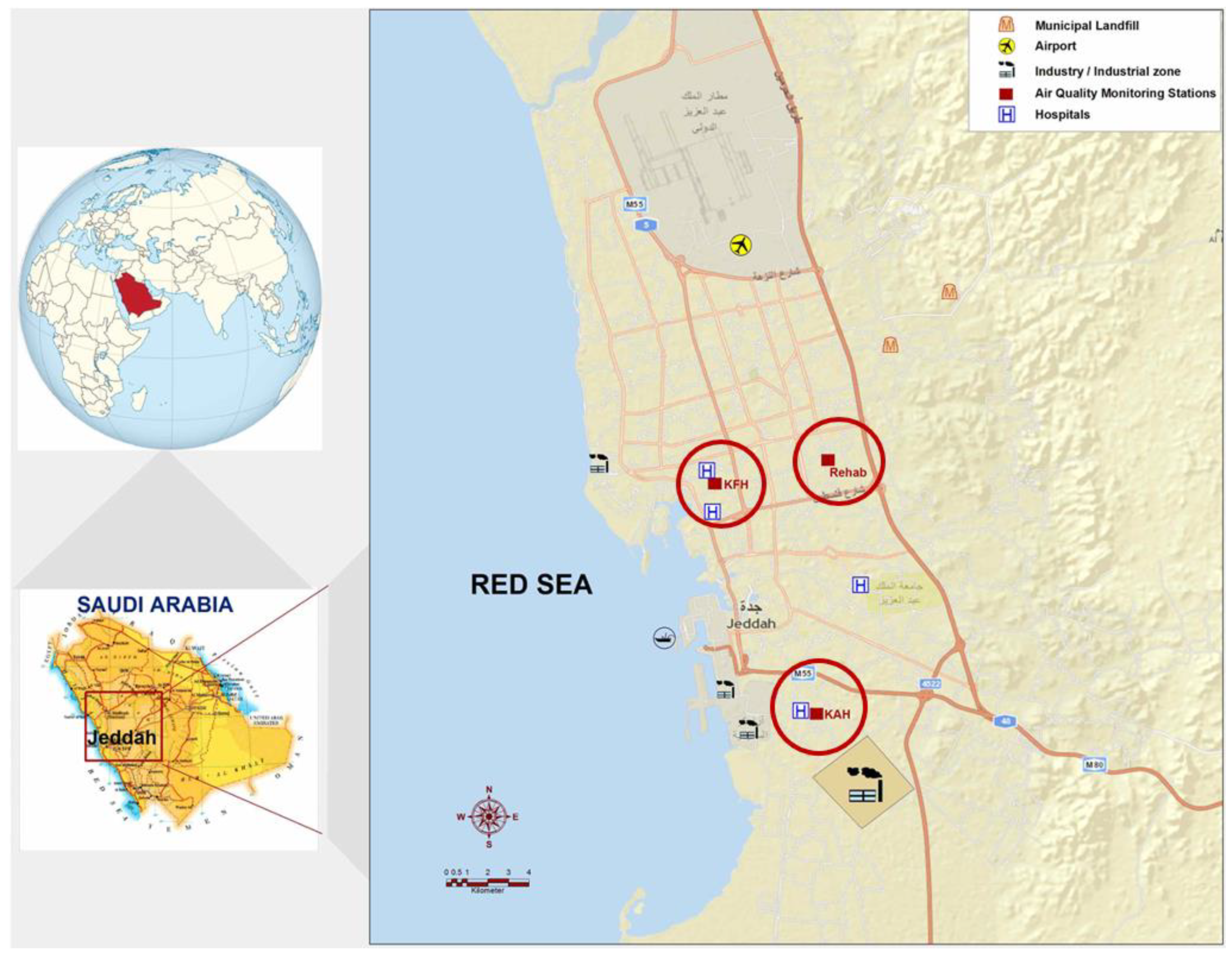
2.1. Study Area
2.2. PM2.5 Sampling and Analysis
2.3. PM2.5 Mass-Reconstruction
| [TE] = 1.31[V] + 1.46[Cr] + 1.29[Mn] + 1.27[Ni] + 1.13[Cu] + 1.24[Zn] + 1.32[As] + 1.41[Se] + 1.40[Br] + 1.37[Sr] + 1.14[Er] + 1.12[Ba] + 1.14[Lu] + 1.08[Pb] | (4) [7,37,38] |
2.4. PM2.5 Source Apportionment
3. Results and Discussion
3.1. PM2.5 Mass and Chemical Composition
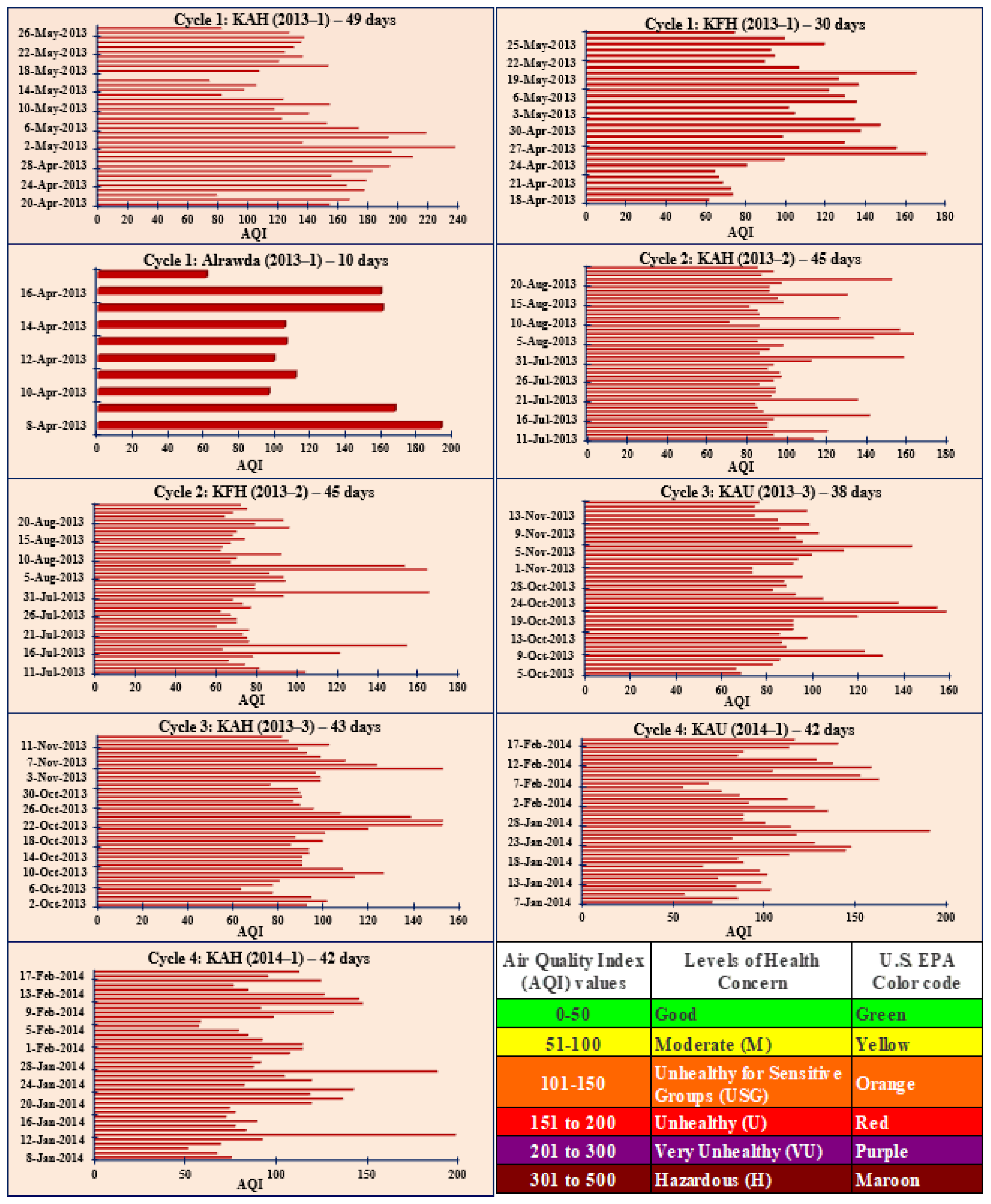
3.2. Air Quality Index (AQI) in Jeddah and Comparison with Other Studies
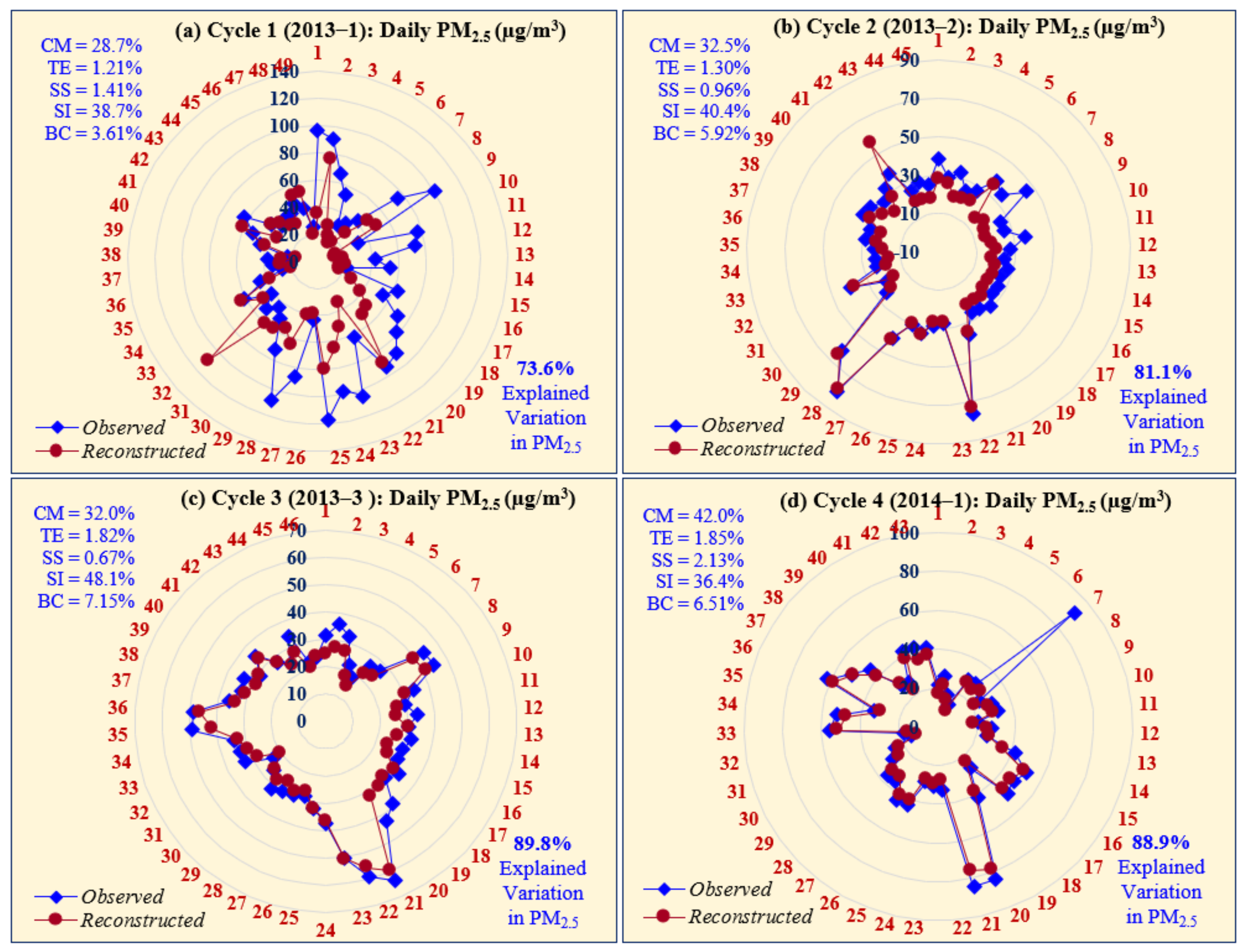
3.3. PM2.5 Mass-Reconstruction
3.4. Correlation between Air Pollutants and Meteorology
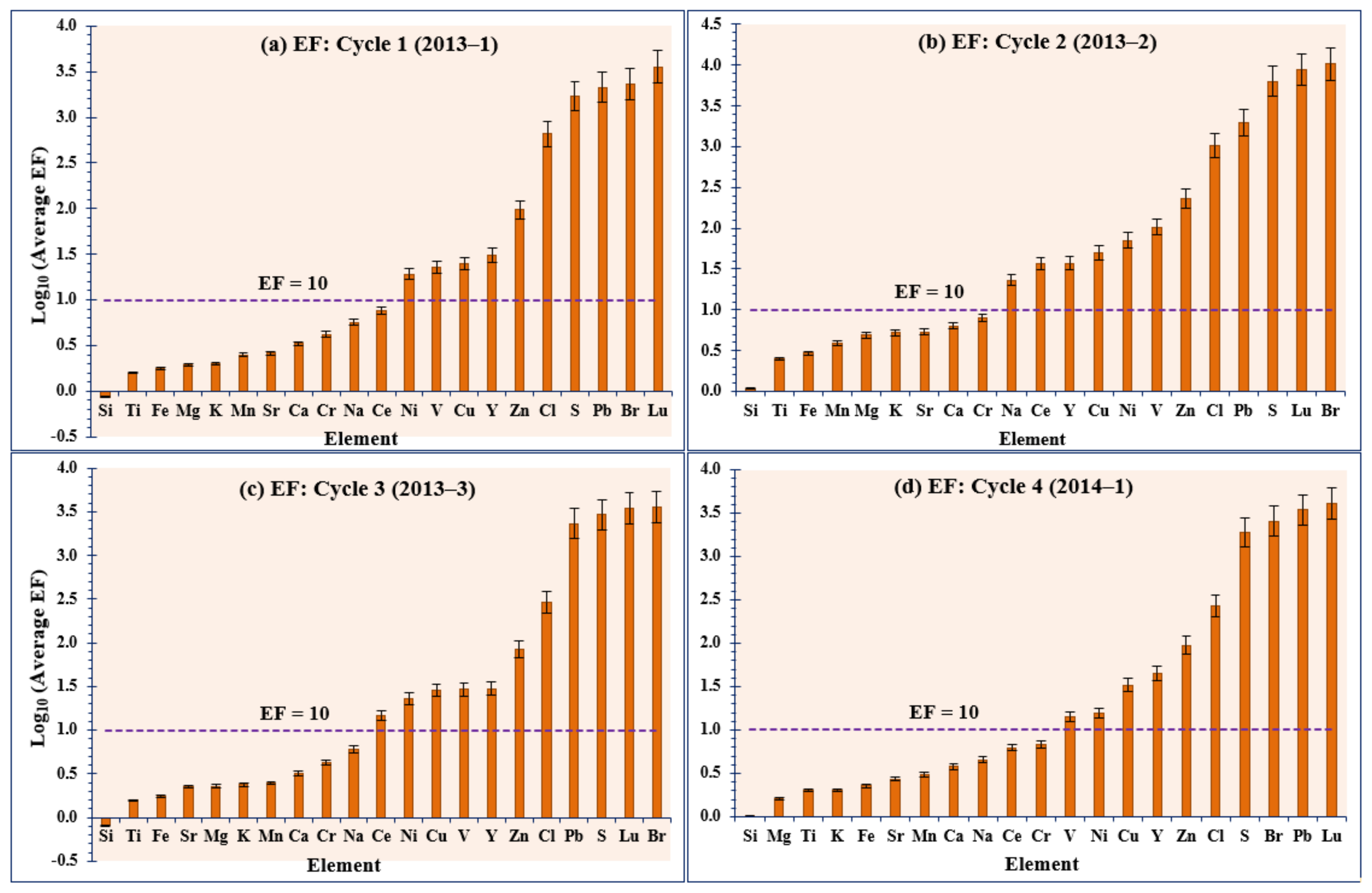
3.5. Elemental Enrichment Factor (EF)
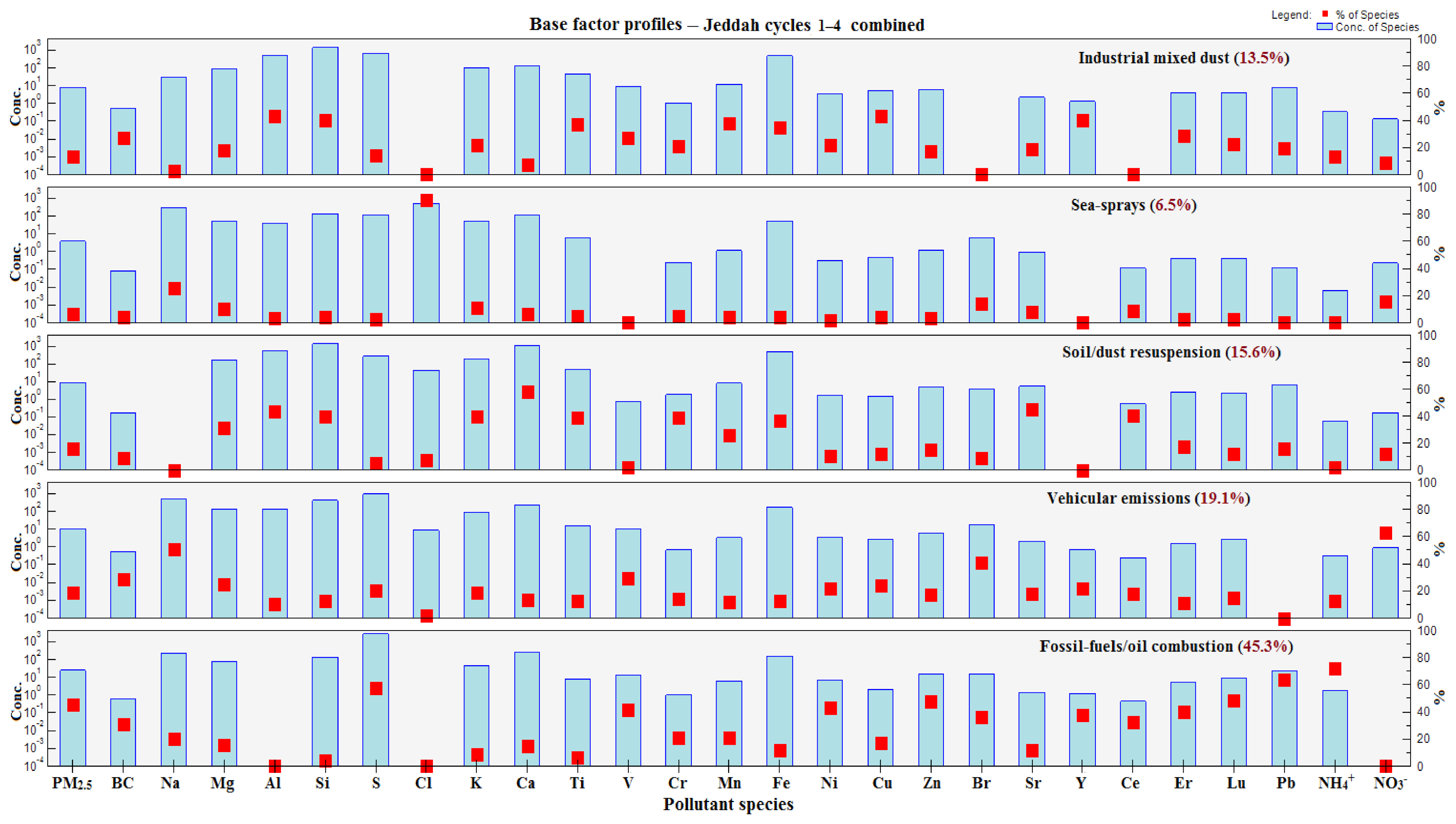
3.6. Positive Matrix Factorization (PMF)
3.7. Backward-In-Time Trajectories
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayebare, S.R.; Aburizaiza, O.S.; Khwaja, H.A.; Siddique, A.; Hussain, M.M.; Zeb, J.; Khatib, F.; Carpenter, D.O.; Blake, D.R. Chemical Characterization and Source Apportionment of PM2.5 in Rabigh, Saudi Arabia. Aerosol Air Qual. Res. 2016, 16, 3114–3129. [Google Scholar] [CrossRef]
- Alharbi, B.; Shareef, M.M.; Husain, T. Study of chemical characteristics of particulate matter concentrations in Riyadh, Saudi Arabia. Atmos. Pollut. Res. 2015, 6, 88–98. [Google Scholar] [CrossRef]
- Khodeir, M.; Shamy, M.; Alghamdi, M.; Zhong, M.; Sun, H.; Costa, M.; Chen, L.-C.; Maciejczyk, P. Source Apportionment and Elemental Composition of PM2.5 and PM10 in Jeddah City, Saudi Arabia. Atmos. Pollut. Res. 2012, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Habeebullah, T.M.; Seroji, A.R.; Gabr, S.S.; Mohammed, A.M.F.; Morsy, E.A. Modeling Particulate Matter Concentrations in Makkah, Applying a Statistical Modeling Approach. Aerosol Air Qual. Res. 2013, 13, 901–910. [Google Scholar] [CrossRef]
- WHO. The World Health Organization (WHO) Air Quality Guidelines-Global Update 2021: Ambient (Outdoor) Air Quality and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 29 March 2022).
- PME. Presidency of Meteorology and Environment (PME), Ambient Air Quality Standards. Available online: http://www.pme.gov.sa/en/En_EnvStand19.pdf (accessed on 12 April 2016).
- Nayebare, S.R. Fine Particulate Air Pollution in Saudi Arabia–Implications for Cardiopulmonary Morbidity; State University of New York: Albany, NY, USA, 2016. [Google Scholar]
- Nasrallah, M.M.; Seroji, A.R. Particulates in the Atmosphere of Makkah and Mina Valley during Ramadhan and Hajj Season of 1424 and 1425 H (2004–2005). Arab Gulf J. Sci. Res. 2008, 26, 199–206. [Google Scholar]
- Khreis, H.; Cirach, M.; Mueller, N.; Hoogh, K.D.; Hoek, G.; Nieuwenhuijsen, M.J.; Rojas-Rueda, D. Outdoor air pollution and the burden of childhood asthma across Europe. Eur. Respir. J. 2019, 54, 1802194. [Google Scholar] [CrossRef]
- Guerreiro, C.B.B.; Foltescu, V.; De Leeuw, F. Air quality status and trends in Europe. Atmos. Environ. 2014, 98, 376–384. [Google Scholar] [CrossRef]
- Sillanpää, M.; Frey, A.; Hillamo, R.; Pennanen, A.S.; Salonen, R.O. Organic, elemental and inorganic carbon in particulate matter of six urban environments in Europe. Atmos. Chem. Phys. 2005, 5, 2869–2879. [Google Scholar] [CrossRef]
- Anderson, H.; Bremner, S.; Atkinson, R.; Harrison, R.; Walters, S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: Associations with fine and coarse particles, black smoke and sulphate. Occup. Environ. Med. 2001, 58, 504–510. [Google Scholar] [CrossRef]
- Sheffield, P.E.; Speranza, R.; Chiu, Y.-H.M.; Hsu, H.-H.L.; Curtin, P.C.; Renzetti, S.; Pajak, A.; Coull, B.; Schwartz, J.; Kloog, I.; et al. Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS ONE 2018, 13, e0195267. [Google Scholar] [CrossRef]
- Crouse, D.L.; Peters, P.A.; Van Donkelaar, A.; Goldberg, M.S.; Villeneuve, P.J.; Brion, O.; Khan, S.; Atari, D.O.; Jerrett, M.; Pope, C.A.; et al. Risk of Nonaccidental and Cardiovascular Mortality in Relation to Long-term Exposure to Low Concentrations of Fine Particulate Matter: A Canadian National-Level Cohort Study. Environ. Health Perspect. 2012, 120, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Puett, R.C.; Schwartz, J.; Yanosky, J.D.; Laden, F.; Grodstein, F. Exposure to Particulate Air Pollution and Cognitive Decline in Older Women. Arch. Intern. Med. 2012, 172, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and Temporal Variation in PM2.5 Chemical Composition in the United States for Health Effects Studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Turner, M.C.; Burnett, R.T.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; Krewski, D.; Brook, R.D. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res. 2015, 116, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.; Rajagopalan, S.; Pope, C.; Brook, J.; Bhatnagar, A.; Diez-Roux, A.; Holguin, F.; Hong, Y.; Luepker, R.; Mittleman, M.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Pope, C.A.I.; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution: Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef]
- Peden, D.B. The epidemiology and genetics of asthma risk associated with air pollution. J. Allergy Clin. Immunol. 2005, 115, 213–219. [Google Scholar] [CrossRef]
- Sunyer, J.; Schwartz, J.; Tobías, A.; Macfarlane, D.; Garcia, J.; Antó, J.M. Patients with Chronic Obstructive Pulmonary Disease Are at Increased Risk of Death Associated with Urban Particle Air Pollution: A Case-Crossover Analysis. Am. J. Epidemiol. 2000, 151, 50–56. [Google Scholar] [CrossRef]
- SADP. Saudi Arabia Demographic Profile 2014; PopulationPyramid.Net: Saudi Arabia, 2014. [Google Scholar]
- Hasanean, H.; Almazroui, M. Rainfall: Features and Variations over Saudi Arabia, A Review. Climate 2015, 3, 578. [Google Scholar] [CrossRef]
- Ahmed, T.; Dutkiewicz, V.A.; Shareef, A.; Tuncel, G.; Tuncel, S.; Husain, L. Measurement of black carbon (BC) by an optical method and a thermal-optical method: Intercomparison for four sites. Atmos. Environ. 2009, 43, 6305–6311. [Google Scholar] [CrossRef]
- Rattigan, O.V.; Civerolo, K.; Doraiswamy, P.; Felton, H.D.; Hopke, P.K. Long term black carbon measurements at two urban locations in New York. Aerosol Air Qual. Res. 2013, 13, 1181–1196. [Google Scholar] [CrossRef]
- Wang, Y.; Hopke, P.K.; Rattigan, O.V.; Chalupa, D.C.; Utell, M.J. Multiple-year black carbon measurements and source apportionment using Delta-C in Rochester, New York. J. Air Waste Manag. Assoc. 2012, 62, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, A.A.; Boman, J.; Al-Malawi, D.A.R.; Shehadeh, Z.F. Elemental Composition of PM2.5 Particles Sampled in Industrial and Residential Areas of Taif, Saudi Arabia. Aerosol Air Qual. Res. 2013, 13, 1356–1364. [Google Scholar] [CrossRef]
- Yatkin, S.; Gerboles, M.; Borowiak, A. Evaluation of standardless EDXRF analysis for the determination of elements on PM10 loaded filters. Atmos. Environ. 2012, 54, 568–582. [Google Scholar] [CrossRef]
- Xu, H.M.; Cao, J.J.; Ho, K.F.; Ding, H.; Han, Y.M.; Wang, G.H.; Chow, J.C.; Watson, J.G.; Khol, S.D.; Qiang, J.; et al. Lead concentrations in fine particulate matter after the phasing out of leaded gasoline in Xi’an, China. Atmos. Environ. 2012, 46, 217–224. [Google Scholar] [CrossRef]
- Korzhova, E.N.; Kuznetsova, O.V.; Smagunova, A.N.; Stavitskaya, M.V. Determination of inorganic pollutants in atmospheric aerosols. J. Anal. Chem. 2011, 66, 222–240. [Google Scholar] [CrossRef]
- Margui, E.; Van Grieken, R. X-Ray Fluorescence Spectrometry and Related Techniques; Momentum Press, LLC.: New York, NY, USA, 2013. [Google Scholar]
- Buhrke, V.E.; Jenkins, R.; Smith, D.K. Practical Guide for the Preparation of Specimens for X-ray Fluorescence and X-ray Diffraction Analysis; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Thermo-Scientific. Analysis of Air Filters by ARL QUANT’X High Performance Energy Dispersive X-ray Fluorescence Spectrometer (EDXRF). 2007. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A%2F%2Fassets.thermofisher.com%2FTFS-Assets%2FMSD%2FApplication-Notes%2FXRAN41964-air-filter-analysis-EDXRF.pdf (accessed on 29 March 2022).
- Chow, J.C.; Lowenthal, D.; Chen, L.W.A.; Wang, X.; Watson, J. Mass reconstruction methods for PM2.5: A review. Air Qual. Atmos. Health 2015, 8, 243–263. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Lowenthal, D.H.; Chen, L.-W.A.; Wang, X.; Biscay, P. Reformulation of PM2.5 Mass Reconstruction Assumptions for the San Joaquin Valley Final Report; Desert Research Institute, Nevada System of Higher Education: Reno, NV, USA, 2012. [Google Scholar]
- Macias, E.S.; Zwicker, J.O.; Ouimette, J.R.; Hering, S.V.; Friedlander, S.K.; Cahill, T.A.; Kuhlmey, G.A.; Richards, L.W. Regional haze case studies in the southwestern US—I. Aerosol chemical composition. Atmos. Environ. (1967) 1981, 15, 1971–1986. [Google Scholar] [CrossRef]
- Solomon, P.A.; Fall, T.; Salmon, L.; Cass, G.R.; Gray, H.A.; Davidson, A. Chemical characteristics of PM10 aerosols collected in the Los Angeles area. JAPCA 1989, 39, 154–163. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Fabretti, J.-F.; Sauret, N.; Gal, J.-F.; Maria, P.-C.; Schärer, U. Elemental characterization and source identification of PM2.5 using Positive Matrix Factorization: The Malraux road tunnel, Nice, France. Atmos. Res. 2009, 94, 320–329. [Google Scholar] [CrossRef]
- Sofowote, U.M.; McCarry, B.E.; Marvin, C.H. Source Apportionment of PAH in Hamilton Harbour Suspended Sediments: Comparison of Two Factor Analysis Methods. Environ. Sci. Technol. 2008, 42, 6007–6014. [Google Scholar] [CrossRef] [PubMed]
- Hopke, P.K. A Guide to Positive Matrix Factorization (PMF); Center for Air Resources Engineering and Science, Clarkson University: Potsdam, NY, USA, 2000; pp. 1–16. [Google Scholar]
- Vivone, G.; D’Amico, G.; Summa, D.; Lolli, S.; Amodeo, A.; Bortoli, D.; Pappalardo, G. Atmospheric Boundary Layer height estimation from aerosol lidar: A new approach based on morphological image processing techniques-(Preprint). Atmos. Chem. Phys. 2020, 21, 4249–4265. [Google Scholar] [CrossRef]
- Ramana, M.V.; Krishnan, P.; Nair, S.M.; Kunhikrishnan, P.K. Thermodynamic structure of the Atmospheric Boundary Layer over the Arabian Sea and the Indian Ocean during pre-INDOEX and INDOEX-FFP campaigns. Ann. Geophys. 2004, 22, 2679–2691. [Google Scholar] [CrossRef][Green Version]
- Li, W. Study of Diurnal Cycle Variability of Planetary Boundary Layer Characteristics over the Red Sea and Arabian Peninsula; King Abdullah University of Science and Technology (KAUST): Thuwal, Saudi Arabia, 2012. [Google Scholar] [CrossRef]
- Draxler, R.R.; Rolph, G.D. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model Access via NOAA ARL READY Website. NOAA Air Resources Laboratory, College Park, MD. Available online: http://www.arl.noaa.gov/HYSPLIT.php (accessed on 13 July 2021).
- Rolph, G.D. Real-Time Environmental Applications and Display sYstem (READY) Website. NOAA Air Resources Laboratory, College Park, MD. Available online: http://www.ready.noaa.gov (accessed on 13 July 2021).
- Jish Prakash, P.; Stenchikov, G.; Kalenderski, S.; Osipov, S.; Bangalath, H. The impact of dust storms on the Arabian Peninsula and the Red Sea. Atmos. Chem. Phys. 2015, 15, 199–222. [Google Scholar] [CrossRef]
- Singh, N.; Murari, V.; Kumar, M.; Barman, S.C.; Banerjee, T. Fine particulates over South Asia: Review and meta-analysis of PM2.5 source apportionment through receptor model. Environ. Pollut. 2017, 223, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, G.; Paul, K. Preliminary Examination of Trace Elements in Tyres, Brake Pads and Road Bitumen in New Zealand; Ministry of Transport: Wellington, New Zealand, 2003.
- U.S.EPA. The National Ambient Air Quality Standards for Particle Pollution. Revised Air Quality Standards for Particle Pollution and Updates to the Air Quality Index (AQI); U.S.EPA: Washington, DC, USA, 2012.
- Zhao, X.; Zhang, X.; Xu, X.; Xu, J.; Meng, W.; Pu, W. Seasonal and diurnal variations of ambient PM2.5 concentration in urban and rural environments in Beijing. Atmos. Environ. 2009, 43, 2893–2900. [Google Scholar] [CrossRef]
- Begum, B.A.; Hossain, A.; Nahar, N.; Markwitz, A.; Hopke, P.K. Organic and black carbon in PM2.5 at an urban site at Dhaka, Bangladesh. Aerosol Air Qual. Res. 2012, 12, 1062–1072. [Google Scholar] [CrossRef]
- Amarsaikhan, D.; Battsengel, V.; Nergui, B.; Ganzorig, M.; Bolor, G. A Study on Air Pollution in Ulaanbaatar City, Mongolia. J. Geosci. Env. Prot. 2014, 2, 123. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Satsangi, P.G.; Masih, J.; Taneja, A. Metal concentration of PM2.5 and PM10 particles and seasonal variations in urban and rural environment of Agra, India. Sci. Total Environ. 2009, 407, 6196–6204. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.H.; Schauer, J.J.; Heo, J.; Kadhim, A.K.H. Source apportionment of PM2.5 carbonaceous aerosol in Baghdad, Iraq. Atmos. Res. 2015, 156, 80–90. [Google Scholar] [CrossRef]
- Yatkin, S.; Bayram, A. Source apportionment of PM10 and PM2.5 using positive matrix factorization and chemical mass balance in Izmir, Turkey. Sci. Total Environ. 2008, 390, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Arfaeinia, H.; Nabizadeh, R.; Alimohammadi, M.; Aalamolhoda, A. Levels of PM2.5—associated heavy metals in the ambient air of Sina hospital district, Tehran, Iran. J. Air Pollut. Health 2015, 1, 1–6. [Google Scholar]
- Brown, K.W.; Bouhamra, W.; Lamoureux, D.P.; Evans, J.S.; Koutrakis, P. Characterization of Particulate Matter for Three Sites in Kuwait. J. Air Waste Manag. Assoc. 2008, 58, 994–1003. [Google Scholar] [CrossRef]
- Degobbi, C.; Lopes, F.D.; Carvalho-Oliveira, R.; Muñoz, J.E.; Saldiva, P.H. Correlation of fungi and endotoxin with PM2.5 and meteorological parameters in atmosphere of Sao Paulo, Brazil. Atmos. Environ. 2010, 45, 2277–2283. [Google Scholar] [CrossRef]
- Akyuz, M.; Cabuk, H. Meteorological variations of PM2.5/PM10 concentrations and particle-associated polycyclic aromatic hydrocarbons in the atmospheric environment of Zonguldak, Turkey. J. Hazard Mater. 2009, 170, 13–21. [Google Scholar] [CrossRef]
- Nakhle, M.M.; Farah, W.; Ziade, N.; Abboud, M.; Coussa-Koniski, M.L.; Annesi-Maesano, I. Beirut Air Pollution and Health Effects—BAPHE study protocol and objectives. Multidiscip. Respir. Med. 2015, 10, 21. [Google Scholar] [CrossRef]
- Hueglin, C.; Gehrig, R.; Baltensperger, U.; Gysel, M.; Monn, C.; Vonmont, H. Chemical characterization of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland. Atmos. Environ. 2005, 39, 637–651. [Google Scholar] [CrossRef]
- Pateraki, S.; Assimakopoulos, V.; Maggos, T.; Fameli, K.; Kotroni, V.; Vasilakos, C. Particulate matter pollution over a Mediterranean urban area. Sci. Total Environ. 2013, 463–464, 508–524. [Google Scholar] [CrossRef]
- Khan, M.F.; Shirasuna, Y.; Hirano, K.; Masunaga, S. Characterization of PM2.5, PM2.5–10 and PM10 in ambient air, Yokohama, Japan. Atmos. Res. 2010, 96, 159–172. [Google Scholar] [CrossRef]
- Zhuang, H.; Chan, C.K.; Fang, M.; Wexler, A.S. Formation of nitrate and non-sea-salt sulfate on coarse particles. Atmos. Environ. 1999, 33, 4223–4233. [Google Scholar] [CrossRef]
- Zhuang, H.; Chan, C.K.; Fang, M.; Wexler, A.S. Size distributions of particulate sulfate, nitrate, and ammonium at a coastal site in Hong Kong. Atmos. Environ. 1999, 33, 843–853. [Google Scholar] [CrossRef]
- Wioletta, R.-K.; Izabela, S.; Barbara, M.; Krzysztof, K.; Anna, Z.; Kornelia, K. Size-Resolved Water-Soluble Ionic Composition of Ambient Particles in an Urban Area in Southern Poland. J. Environ. Prot. 2013, 4, 371–379. [Google Scholar] [CrossRef]
- Bauer, S.E.; Koch, D.; Unger, N.; Metzger, S.M.; Shindell, D.T.; Streets, D.G. Nitrate aerosols today and in 2030: A global simulation including aerosols and tropospheric ozone. Atmos. Chem. Phys. 2007, 7, 5043–5059. [Google Scholar] [CrossRef]
- U.S.EPA. Atmospheric Ammonia: Sources and Fate: A Review of Ongoing Federal Research and Future Needs; U.S.EPA: Washington, DC, USA, 2000.
- Frank, N.H. Retained nitrate, hydrated sulfates, and carbonaceous mass in federal reference method fine particulate matter for six eastern U.S. cities. J. Air Waste Manag. Assoc. 2006, 56, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Lowenthal, D.H.; Magliano, K.L. Loss of PM2.5 nitrate from filter samples in central California. J. Air Waste Manag. Assoc. 2005, 55, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Raask, E. The mode of occurrence and concentration of trace elements in coal. Prog. Energy Combust. Sci. 1985, 11, 97–118. [Google Scholar] [CrossRef]
- Aburas, H.M.; Zytoon, M.A.; Abdulsalam, M.I. Atmospheric Lead in PM2.5 after Leaded Gasoline Phase-out in Jeddah City, Saudi Arabia. Clean-Soil Air Water 2011, 39, 711–719. [Google Scholar] [CrossRef]
- RSC. Royal Society of Chemistry (RSC): Uses and Properties of Lutetium (Lu). Available online: http://www.rsc.org/periodic-table/element/71/lutetium (accessed on 6 June 2016).
- Steinbach, C. Cerium Dioxide (CeO2) - Overview. nanopartikel.info (2011-02-02). Available online: http://nanopartikel.info/en/nanoinfo/materials/cerium-dioxide (accessed on 6 June 2016).
- Trovarelli, A. Catalysis by Ceria and Related Materials; Imperial College Press: London, UK, 2002. [Google Scholar]
- Bleiwas, D.I. Potential for Recovery of Cerium Contained in Automotive Catalytic Converters: U.S. Department of the Interior; U.S. Geological Survey Open-File Report 2013–1037; U.S. Geological Survey: Reston, VA, USA, 2013; p. 10.

| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± S.D | Min | Max | Mean ± S.D | Min | Max | Mean ± S.D | Min | Max | Mean ± S.D | Min | Max |
| PM2.5 (μg/m3) | 58.8 ± 25 | 22.3 | 118 | 36.2 ± 12.3 | 21.3 | 80 | 33.9 ± 9.1 | 18.5 | 63 | 38 ± 17.7 | 12.4 | 90.8 |
| BCIR (μg/m3) | 1.9 ± 0.6 | 0.8 | 2.9 | 1.8 ± 0.4 | 0.8 | 2.7 | 2.4 ± 0.6 | 1.4 | 4.0 | 2.3 ± 0.8 | 0.9 | 4.6 |
| BCUV (μg/m3) | 1.6 ± 0.5 | 0.7 | 2.6 | 1.2 ± 0.3 | 0.6 | 1.8 | 1.6 ± 0.3 | 0.1 | 2.3 | 1.6 ± 0.5 | 0.7 | 3.3 |
| Delta–C (μg/m3) | −0.3 ± 0.2 | −0.9 | 0.1 | −0.6 ± 0.2 | −1.02 | −0.2 | −0.8 ± 0.3 | −1.7 | −0.3 | −0.6 ± 0.3 | −1.3 | −0.2 |
| RH (%) | 53.6 ± 8.1 | 38.0 | 71 | 56.2 ± 8.0 | 30.0 | 71 | 63.8 ± 6.8 | 47 | 78 | 51.9 ± 12 | 26 | 71.0 |
| Temp. (°C) | 29.6 ± 2.6 | 24.4 | 34.4 | 27.2 ± 0.8 | 31.1 | 35.6 | 29.6 ± 1.2 | 27.8 | 31.7 | 25 ± 1.8 | 20 | 27.8 |
| WS (m/s) | 6.5 ± 2.1 | 3.0 | 11 | 6.6 ± 1.5 | 4.0 | 10 | 5.4 ± 1.6 | 3.0 | 10 | 7.5 ± 3.0 | 3.0 | 15 |
| Trace Elements, TEs (ng/m3) | ||||||||||||
| Sulfur (S) | 4972 ± 2577 | 1323 | 10,935 | 4434 ± 1301 | 1271 | 7973 | 6838 ± 2714 | 2629 | 15,224 | 4348 ± 2216 | 1214 | 9549 |
| Silicon (Si) | 3427 ± 2076 | 727 | 12,733 | 2727 ± 3783 | 397 | 16,469 | 2255 ± 931 | 578 | 4197 | 3748 ± 2739 | 723 | 14,151 |
| Calcium (Ca) | 1780 ± 1404 | 660 | 10,015 | 1049 ± 565 | 455 | 2730 | 1216 ± 367 | 628 | 2037 | 1863 ± 1342 | 500 | 7380 |
| Iron (Fe) | 1367 ± 772 | 354 | 4760 | 1252 ± 1622 | 283 | 7295 | 945 ± 340 | 318 | 1665 | 1565 ± 1117 | 347 | 5549 |
| Aluminum (Al) | 1227 ± 832 | 177 | 5116 | 1052 ± 1662 | 53.1 | 7191 | 830 ± 361 | 152 | 1568 | 1193 ± 988 | 96.8 | 4812 |
| Sodium (Na) | 1065 ± 516 | 434 | 2919 | 1151 ± 721 | 172 | 2552 | 953 ± 456 | 480 | 2090 | 735 ± 388 | 237 | 1723 |
| Chlorine (Cl) | 547 ± 997 | DL | 5621 | 261 ± 477 | DL | 1674 | 108 ± 364 | DL | 2150 | 219 ± 389 | DL | 1882 |
| Magnesium (Mg) | 516 ± 232 | 227 | 1688 | 397 ± 234 | 169 | 1181 | 454 ± 121 | 241 | 767 | 439 ± 305 | 108 | 1663 |
| Potassium (K) | 490 ± 287 | 259 | 2160 | 412 ± 234 | 208 | 1236 | 412 ± 76.8 | 286 | 558 | 460 ± 292 | 153 | 1622 |
| Lead (Pb) | 318 ± 401 | 2.73 | 1783 | 89.2 ± 190 | DL | 1157 | 294 ± 304 | 4.71 | 1142 | 361 ± 441 | 16.1 | 2298 |
| Titanium (Ti) | 126 ± 77.9 | 28.8 | 498 | 112 ± 146 | 25 | 628 | 85.3 ± 30 | 26.9 | 152 | 141 ± 94 | 30.2 | 458 |
| Zinc (Zn) | 90.5 ± 149 | 11.6 | 696 | 60.9 ± 94.6 | 9.7 | 494 | 52.1 ± 44.5 | 12.1 | 206 | 53.3 ± 49.9 | 12.1 | 224 |
| Bromine (Br) | 50.2 ± 14.8 | 24.5 | 92.5 | 64 ± 22.9 | 21.2 | 114 | 68.2 ± 18.4 | 36.5 | 130 | 51.4 ± 20.3 | 25.7 | 136 |
| Vanadium (V) | 36.5 ± 19.8 | DL | 80.9 | 41.6 ± 14.2 | 22.1 | 74.7 | 32.8 ± 14.4 | 8.5 | 67.9 | 15.1 ± 13.1 | DL | 50.4 |
| Manganese (Mn) | 31.3 ± 16.9 | 9.5 | 77.7 | 26.2 ± 32.4 | 3.4 | 144 | 22.6 ± 8.7 | 6.1 | 41.5 | 35.3 ± 28.9 | 7.7 | 148 |
| Lutetium (Lu) | 21.4 ± 15.5 | 5.0 | 71.5 | 16.7 ± 11.4 | 6.2 | 56.6 | 15.8 ± 7.4 | 3.5 | 35 | 18.5 ± 9.9 | 6.2 | 46 |
| Nickel (Ni) | 17.3 ± 8.8 | 3.9 | 40.3 | 17.1 ± 5.7 | 9.0 | 37.1 | 15.0 ± 5.6 | 5.1 | 28.1 | 11.3 ± 6.2 | 2.8 | 31.4 |
| Copper (Cu) | 17 ± 13.8 | 3.2 | 59.9 | 10.4 ± 5.8 | 3.6 | 26.2 | 13.6 ± 13.3 | 5.2 | 89.3 | 17.4 ± 12.9 | 3.6 | 70.5 |
| Erbium (Er) | 16.1 ± 12.7 | 4.3 | 65.8 | 12.1 ± 12 | 3.9 | 55.8 | 10.9 ± 5.7 | DL | 28.2 | 14.6 ± 8.8 | 2.2 | 43.5 |
| Strontium (Sr) | 12.2 ± 7.3 | 3.4 | 52.0 | 8.0 ± 5.1 | 2.8 | 25.9 | 7.6 ± 2.6 | 4.2 | 14 | 12.9 ± 9.9 | 1.4 | 49.3 |
| Yttrium (Y) | 11.3 ± 13 | DL | 58.3 | 5.0 ± 7.3 | DL | 39.6 | 10.2 ± 10.2 | DL | 37.3 | 12.2 ± 14.4 | DL | 75 |
| Cerium (Ce) | 6.2 ± 5.8 | DL | 27.8 | 9.4 ± 7.9 | DL | 40.4 | 8.5 ± 3.9 | DL | 14.1 | 3.4 ± 3.0 | DL | 12.7 |
| Chromium (Cr) | 5.2 ± 2.6 | 1.1 | 14.1 | 3.9 ± 3.7 | 1.1 | 19.1 | 3.6 ± 1.8 | DL | 9.3 | 6.4 ± 3.2 | 2.1 | 19.5 |
| Water-soluble ions IS (μg/m3) | ||||||||||||
| Sulfate (SO42−) | 17.8 ± 17 | 2.9 | 85.9 | 8.5 ± 3.2 | 1.9 | 22.9 | 12.1 ± 4.9 | 4.6 | 29.3 | 8.6 ± 4.7 | 2.1 | 20.2 |
| Ammonium (NH4+) | 2.5 ± 1.9 | 0.2 | 8.3 | 2.4 ± 0.9 | 0.6 | 4.9 | 3.2 ± 1.3 | 1.2 | 6.5 | 3.2 ± 1.7 | 0.44 | 7.3 |
| Nitrate (NO3−) | 1.5 ± 0.9 | 0.1 | 4.1 | 1.1 ± 0.8 | 0.2 | 3.9 | 0.9 ± 0.6 | 0.1 | 2.3 | 1.2 ± 0.6 | 0.4 | 2.6 |
| Oxalate (C2O42−) | 0.3 ± 0.1 | DL | 0.5 | 0.1 ± 0.1 | 0.04 | 0.2 | 0.1 ± 0.1 | 0.04 | 0.3 | 0.4 ± 0.5 | 0.02 | 1.9 |
| Pollutant Species | Category | R2 | Normal Residuals | S/N | Modeled Samples (%) |
|---|---|---|---|---|---|
| PM2.5 | Weak | 0.39 | Yes | 10 | 100 |
| Black carbon (BC) | Strong | 0.62 | Yes | 10 | 100 |
| Sodium (Na) | Strong | 0.83 | Yes | 10 | 100 |
| Magnesium (Mg) | Strong | 0.95 | Yes | 10 | 100 |
| Aluminum (Al) | Strong | 1.00 | Yes | 10 | 100 |
| Silicon (Si) | Strong | 1.00 | No | 10 | 100 |
| Sulfur (S) | Strong | 0.98 | No | 10 | 100 |
| Chlorine (Cl) | Strong | 1.00 | No | 6.4 | 100 |
| Potassium (K) | Strong | 0.97 | Yes | 10 | 100 |
| Calcium (Ca) | Strong | 1.00 | Yes | 10 | 100 |
| Titanium (Ti) | Strong | 0.99 | Yes | 10 | 100 |
| Vanadium (V) | Strong | 0.74 | No | 9.5 | 100 |
| Chromium (Cr) | Strong | 0.71 | Yes | 8.8 | 100 |
| Manganese (Mn) | Strong | 0.87 | Yes | 10 | 100 |
| Iron (Fe) | Strong | 0.99 | Yes | 10 | 100 |
| Nickel (Ni) | Strong | 0.76 | Yes | 10 | 100 |
| Copper (Cu) | Strong | 0.32 | Yes | 10 | 100 |
| Zinc (Zn) | Strong | 0.24 | No | 10 | 100 |
| Bromine (Br) | Strong | 0.03 | Yes | 9.3 | 100 |
| Strontium (Sr) | Strong | 0.98 | Yes | 10 | 100 |
| Yttrium (Y) | Strong | 0.08 | Yes | 7.8 | 100 |
| Cerium (Ce) | Weak | 0.19 | Yes | 3.0 | 100 |
| Erbium (Er) | Strong | 0.51 | No | 7.3 | 100 |
| Lutetium (Lu) | Strong | 0.49 | No | 9.9 | 100 |
| Lead (Pb) | Strong | 0.08 | Yes | 9.3 | 100 |
| Ammonium (NH4+) | Strong | 0.89 | No | 9.8 | 100 |
| Nitrate (NO3-) | Strong | 1.00 | Yes | 10 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayebare, S.R.; Aburizaiza, O.S.; Siddique, A.; Hussain, M.M.; Zeb, J.; Khatib, F.; Carpenter, D.O.; Blake, D.R.; Khwaja, H.A. Understanding the Sources of Ambient Fine Particulate Matter (PM2.5) in Jeddah, Saudi Arabia. Atmosphere 2022, 13, 711. https://doi.org/10.3390/atmos13050711
Nayebare SR, Aburizaiza OS, Siddique A, Hussain MM, Zeb J, Khatib F, Carpenter DO, Blake DR, Khwaja HA. Understanding the Sources of Ambient Fine Particulate Matter (PM2.5) in Jeddah, Saudi Arabia. Atmosphere. 2022; 13(5):711. https://doi.org/10.3390/atmos13050711
Chicago/Turabian StyleNayebare, Shedrack R., Omar S. Aburizaiza, Azhar Siddique, Mirza M. Hussain, Jahan Zeb, Fida Khatib, David O. Carpenter, Donald R. Blake, and Haider A. Khwaja. 2022. "Understanding the Sources of Ambient Fine Particulate Matter (PM2.5) in Jeddah, Saudi Arabia" Atmosphere 13, no. 5: 711. https://doi.org/10.3390/atmos13050711
APA StyleNayebare, S. R., Aburizaiza, O. S., Siddique, A., Hussain, M. M., Zeb, J., Khatib, F., Carpenter, D. O., Blake, D. R., & Khwaja, H. A. (2022). Understanding the Sources of Ambient Fine Particulate Matter (PM2.5) in Jeddah, Saudi Arabia. Atmosphere, 13(5), 711. https://doi.org/10.3390/atmos13050711









