Characteristics of Unorganized Hydrogen Sulfide Dispersion for Industrial Building Layout Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Experimental Measurement
2.3. Parameters Calculation
2.4. Simulation Scheme and Boundary Conditions
3. Results and Discussion
3.1. Subsection H2S Concentration Results and Model Validation
3.2. H2S Dispersion Simulation with Different Temperature
3.3. H2S Dispersion Simulation with Different Wind Speeds
3.4. Safe Distance for Outdoor Sedimentation Tank
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Yang, W.; Li, J. Characterization and prediction of odours from municipal sewage treatment plant. Water Sci. Technol. 2018, 2017, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Guarrasi, J.; Trask, C.; Kirychuk, S. A Systematic Review of Occupational Exposure to Hydrogen Sulfide in Livestock Operations. J. Agromed. 2015, 20, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Colomer, F.L.; Morató, H.E.; Iglesias, E.M. Estimation of hydrogen sulfide emission rates at several wastewater treatment plants through experimental concentration measurements and dispersion modeling. J. Air Waste Manag. Assoc. 2012, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, W.; Wang, H.; Gao, X. Odor impact assessment of trace sulfur compounds from working faces of landfills in Beijing, China. J. Environ. Manag. 2018, 220, 136–141. [Google Scholar] [CrossRef]
- Yalamanchili, C.; Smith, M.D. Acute hydrogen sulfide toxicity due to sewer gas exposure. Am. J. Emerg. Med. 2008, 26, 518.e5–518.e7. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, T.; He, Y.; Wu, Y.; Zhang, J.; Jiang, M. Simultaneous removal of hydrogen sulfide and ammonia in the gas phase: A review. Environ. Chem. Lett. 2022, 20, 1403–1419. [Google Scholar] [CrossRef]
- Bu, H.; Carvalho, G.; Yuan, Z.; Bond, P.; Jiang, G. Biotrickling filter for the removal of volatile sulfur compounds from sewers: A review. Chemosphere 2021, 277, 130333. [Google Scholar] [CrossRef]
- Jiang, Z.A.; Wang, Y.P.; Men, L.G. Ventilation control of tunnel drilling dust based on numerical simulation. J. Cent. South Univ. 2021, 25, 1342–1356. [Google Scholar] [CrossRef]
- Nuvolone, D.; Petri, D.; Pepe, P.; Voller, F. Health effects associated with chronic exposure to low-level hydrogen sulfide from geothermoelectric power plants. A residential cohort study in the geothermal area of Mt. Amiata in Tuscany. Sci. Total Environ. 2019, 659, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Tubbs, N.; Cheung, J.; Judenherc-Haouzi, A. Methylene Blue Administration During and After Life-Threatening Intoxication by Hydrogen Sulfide: Efficacy Studies in Adult Sheep and Mechanisms of Action. Toxicol. Sci. 2019, 168, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Selene, C.H.; Chou, J. Concise International Chemical Assessment Document 53: Hydrogen sulfide: Human health aspects. In IPCS Concise International Chemical Assessment Documents; World Health Organization: Geneva, Switzerland, 2003; p. 53. [Google Scholar]
- GBZ 2.1-2019; Occupational Exposure Limits for Hazardous Agents in the Workplace—Part 1: Chemical Hazardous Agents. Available online: http://www.nhc.gov.cn/wjw/pyl/202003/67e0bad1fb4a46ff98455b5772523d49.shtml (accessed on 13 September 2022).
- Walewska, A.; Szewczyk, A.; Koprowski, P. Gas Signaling Molecules and Mitochondrial Potassium Channels. Int. J. Mol. Sci. 2018, 19, 3227. [Google Scholar] [CrossRef] [PubMed]
- Somma, R.; Granieri, D.; Troise, C.; Terranova, C.; De Natale, G.; Pedone, M. Modelling of hydrogen sulfide dispersion from the geothermal power plants of Tuscany (Italy). Sci. Total Environ. 2017, 583, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Silva, C.; Calvo, D.C.; Torres, N.; Ayala, L.; Gaitán, M.; González, L.; Rincón, P.; Susa, M.R. Hydrogen sulphide emissions and dispersion modelling from a wastewater reservoir using flux chamber measurements and AERMOD® simulations. Atmos. Environ. 2020, 224, 117263. [Google Scholar] [CrossRef]
- Gulia, S.; Kumar, A.; Khare, M. Performance evaluation of CALPUFF and AERMOD dispersion models for air quality assessment of an industrial complex. J. Sci. Ind. Res. 2015, 74, 302–307. [Google Scholar]
- Wu, C.; Yang, F.; Brancher, M.; Liu, J.; Qu, C.; Piringer, M.; Schauberger, G. Determination of ammonia and hydrogen sulfide emissions from a commercial dairy farm with an exercise yard and the health-related impact for residents. Environ. Sci. Pollut. Res. 2020, 27, 37684–37698. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Mazinani, S.; Asadi, M.; Mirmohammadi, M. Mathematical and experimental study of hydrogen sulfide concentrations in the Kahrizak landfill, Tehran, Iran. Environ. Eng. Res. 2019, 24, 572–581. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, G. Hydrogen Sulfide Dispersion Consequences Analysis in Different Wind Speeds: A CFD Based Approach. In Proceedings of the International Conference on Energy and Environment Technology, Washington, DC, USA, 16–18 October 2009; pp. 365–368. [Google Scholar]
- Lin, X.; Barrington, S.; Gong, G.; Choinière, D. Simulation of odour dispersion downwind from natural windbreaks using the computational fluid dynamics standard k-ε model. Can. J. Civ. Eng. 2009, 36, 895–910. [Google Scholar] [CrossRef]
- Tominaga, Y.; Stathopoulos, T. Numerical simulation of dispersion around an isolated cubic building: Comparison of various types of k-ε models. Atmos. Environ. 2009, 43, 3200–3210. [Google Scholar] [CrossRef]
- Moen, A.; Mauri, L.; Narasimhamurthy, V.D. Comparison of k-ε models in gaseous release and dispersion simulations using the CFD code FLACS. Process. Saf. Environ. Prot. 2019, 130, 306–316. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Chan, K.; Elkamel, A.; Ahmadi, L. Effects of meteorological conditions on the concentration and dispersion of an accidental release of H2S in Canada. Atmos Environ. 2014, 82, 316–326. [Google Scholar] [CrossRef]
- Godoi, A.; Grasel, A.M.; Polezer, G.; Brown, A.; Potgieter-Vermaak, S.; Scremim, D.C.; Yamamoto, C.I.; Godoi, R.H.M. Human exposure to hydrogen sulphide concentrations near wastewater treatment plants. Sci. Total Environ. 2018, 610–611, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Yu, J.; Abdalla, A.A.A.; Zhong, X.-P.; Ghazanfar, M.A. An assessment of k-ε turbulence models for gas distribution analysis. Nucl. Sci. Tech. 2017, 28, 146. [Google Scholar] [CrossRef]
- Mirzaei, F.; Mirzaei, F.; Kashi, E. Turbulence Model Selection for Heavy Gases Dispersion Modeling in Topographically Complex Area. J. Appl. Fluid. Mech. 2019, 12, 1745–1755. [Google Scholar] [CrossRef]
- Ben Ramoul, L.; Korichi, A.; Popa, C.; Zaidi, H.; Polidori, G. Numerical study of flow characteristics and pollutant dispersion using three RANS turbulence closure models. Environ. Fluid. Mech. 2019, 19, 379–400. [Google Scholar] [CrossRef]
- Lateb, M.; Masson, C.; Stathopoulos, T.; Bédard, C. Comparison of various types of k–ε models for pollutant emissions around a two-building configuration. J. Wind. Eng. Ind. Aerodyn. 2013, 115, 9–21. [Google Scholar] [CrossRef]
- Zwain, H.M.; Nile, B.K.; Faris, A.M.; Vakili, M.; Dahlan, I. Modelling of hydrogen sulfide fate and emissions in extended aeration sewage treatment plant using TOXCHEM simulations. Sci. Rep. 2020, 10, 22209. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, A.; Sattler, M.L. Correlating emissions with time and temperature to predict worst-case emissions from open liquid area sources. Air Repair 2005, 55, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Alakalabi, A.; Liu, W. Numerical investigation into the effects of obstacles on heavy gas dispersion in the atmosphere. In Proceedings of the XII International Conference on Computational Heat, Mass and Momentum Transfer (ICCHMT 2019), Preston, UK, 8 November 2019. [Google Scholar]
- Maïzi, A.; Dhaouadi, H.; Bournot, P.; Mhiri, H. CFD prediction of odorous compound dispersion: Case study examining a full scale waste water treatment plant. Biosyst. Eng. 2010, 106, 68–78. [Google Scholar] [CrossRef]
- Shen, S.; Wu, B.; Xu, H.; Zhang, Z.-Y. Assessment of Landfill Odorous Gas Effect on Surrounding Environment. Adv. Civ. Eng. 2020, 2020, 8875393. [Google Scholar] [CrossRef]
- Feng, Y.; Eun, J.; Moon, S.; Nam, Y. Assessment of gas dispersion near an operating landfill treated by different intermediate covers with soil alone, low-density polyethylene (LLDPE), or ethylene vinyl alcohol (EVOH) geomembrane. Environ. Sci. Pollut. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Bayatian, M.; Azari, M.R.; Ashrafi, K.; Jafari, M.J.; Mehrabi, Y. CFD simulation for dispersion of benzene at a petroleum refinery in diverse atmospheric conditions. Environ. Sci. Pollut. Res. 2021, 28, 32973–32984. [Google Scholar] [CrossRef] [PubMed]
- List of Highly Hazardous Chemicals, Toxics and Reactives (Mandatory). Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.119AppA (accessed on 13 September 2022).
- Costigan, M.G. Hydrogen sulfide: UK occupational exposure limits. Occup. Environ. Med. 2003, 60, 308–312. [Google Scholar] [CrossRef] [PubMed]
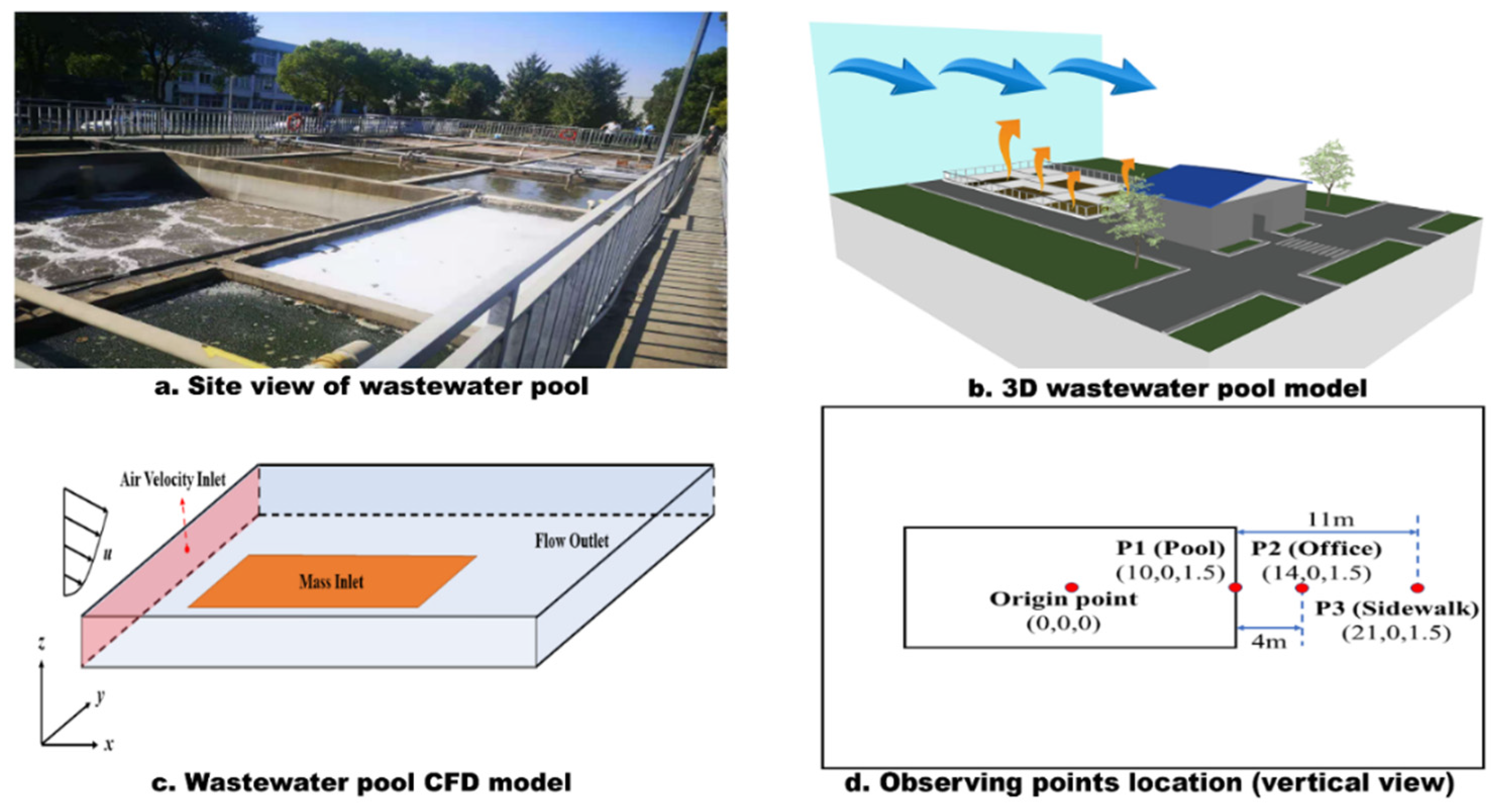









| Measurement No. | Collection Date | Sampling Locations | Temperature | Wind Speed |
|---|---|---|---|---|
| 1 | 21 September 2020 | P1, P2, P3 | 302.5–303.3 K | 3.2–4.6 m/s |
| 2 | 8 March 2021 | P1, P2, P3 | 287.8–288.4 K | 15.8–16.2 m/s |
| Wind Speed (m/s) | Temperature (K) | H2S Mass Flow Rate (kg/s) |
|---|---|---|
| 1.0 | 288 | 1.50 |
| 1.5 | 288 | 1.51 |
| 2.0 | 288 | 1.62 |
| 4.0 | 288 | 2.18 |
| 293 | 2.23 | |
| 298 | 2.29 | |
| 303 | 2.35 | |
| 6.0 | 288 | 2.62 |
| 8.0 | 288 | 3.07 |
| 10.0 | 288 | 3.49 |
| 12.0 | 288 | 3.88 |
| 14.0 | 288 | 4.25 |
| 16.0 | 288 | 4.61 |
| 18.0 | 288 | 4.95 |
| 20.0 | 288 | 5.29 |
| H2S Concentration (ppm) | P1 | P2 | P3 |
|---|---|---|---|
| Mean value | 7.88 | 3.48 | 1.45 |
| Mean deviation | 0.055 | 0.063 | 0.051 |
| Ratio | 0.7% | 1.8% | 3.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Guo, J.; Du, W.; Zeng, Z.; Li, L. Characteristics of Unorganized Hydrogen Sulfide Dispersion for Industrial Building Layout Optimization. Atmosphere 2022, 13, 1822. https://doi.org/10.3390/atmos13111822
Ma W, Guo J, Du W, Zeng Z, Li L. Characteristics of Unorganized Hydrogen Sulfide Dispersion for Industrial Building Layout Optimization. Atmosphere. 2022; 13(11):1822. https://doi.org/10.3390/atmos13111822
Chicago/Turabian StyleMa, Weiwu, Jiaxin Guo, Weiqiang Du, Zheng Zeng, and Liqing Li. 2022. "Characteristics of Unorganized Hydrogen Sulfide Dispersion for Industrial Building Layout Optimization" Atmosphere 13, no. 11: 1822. https://doi.org/10.3390/atmos13111822
APA StyleMa, W., Guo, J., Du, W., Zeng, Z., & Li, L. (2022). Characteristics of Unorganized Hydrogen Sulfide Dispersion for Industrial Building Layout Optimization. Atmosphere, 13(11), 1822. https://doi.org/10.3390/atmos13111822






