Can Green Plants Mitigate Ammonia Concentration in Piglet Barns?
Abstract
1. Introduction
2. Materials and Methods
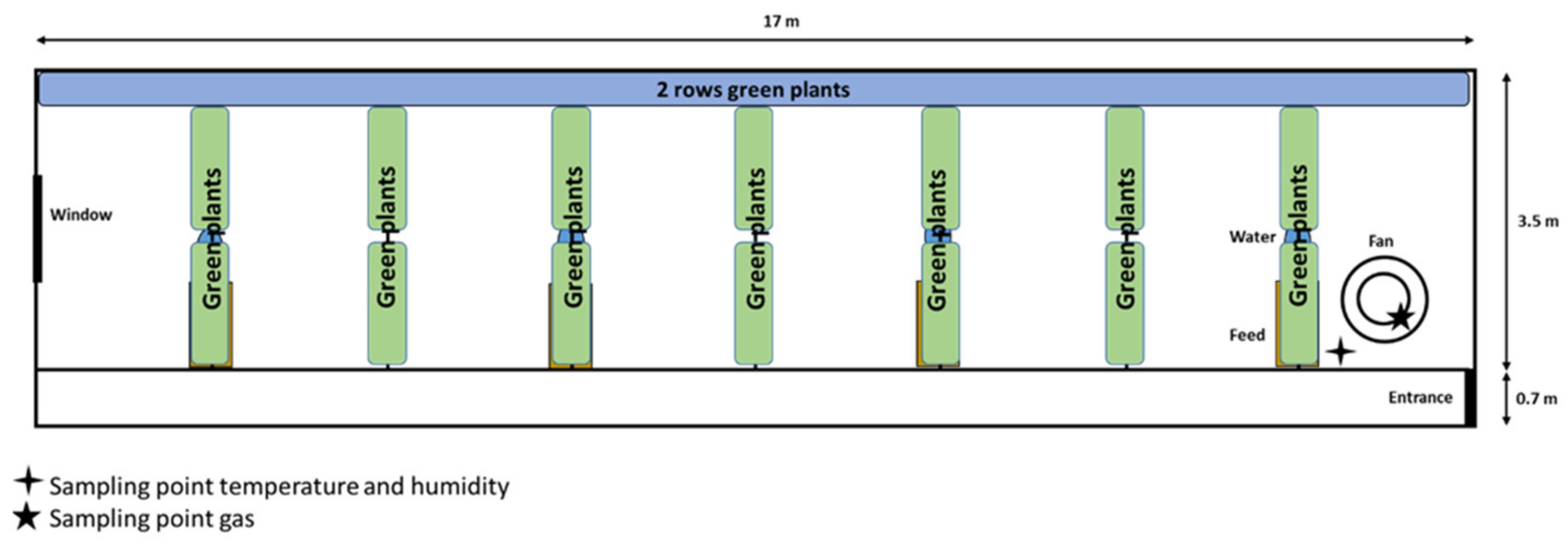
2.1. Pig Barn Description
2.2. Description of Green Plant Structure and Management
2.3. Measurement of Air Parameters
2.4. Estimation of Ventilation Rate and Gas Emission Rate
2.5. Statistical Analysis
3. Results and Discussion
3.1. Piglet Body Mass Gain and Mortality during the Entire Growing Period
3.1.1. Experiment 1, Summer–Autumn
3.1.2. Experiment 2, Winter
3.2. Gas Concentration and Ventilation Rate during the Last Two Weeks of the Growing Period
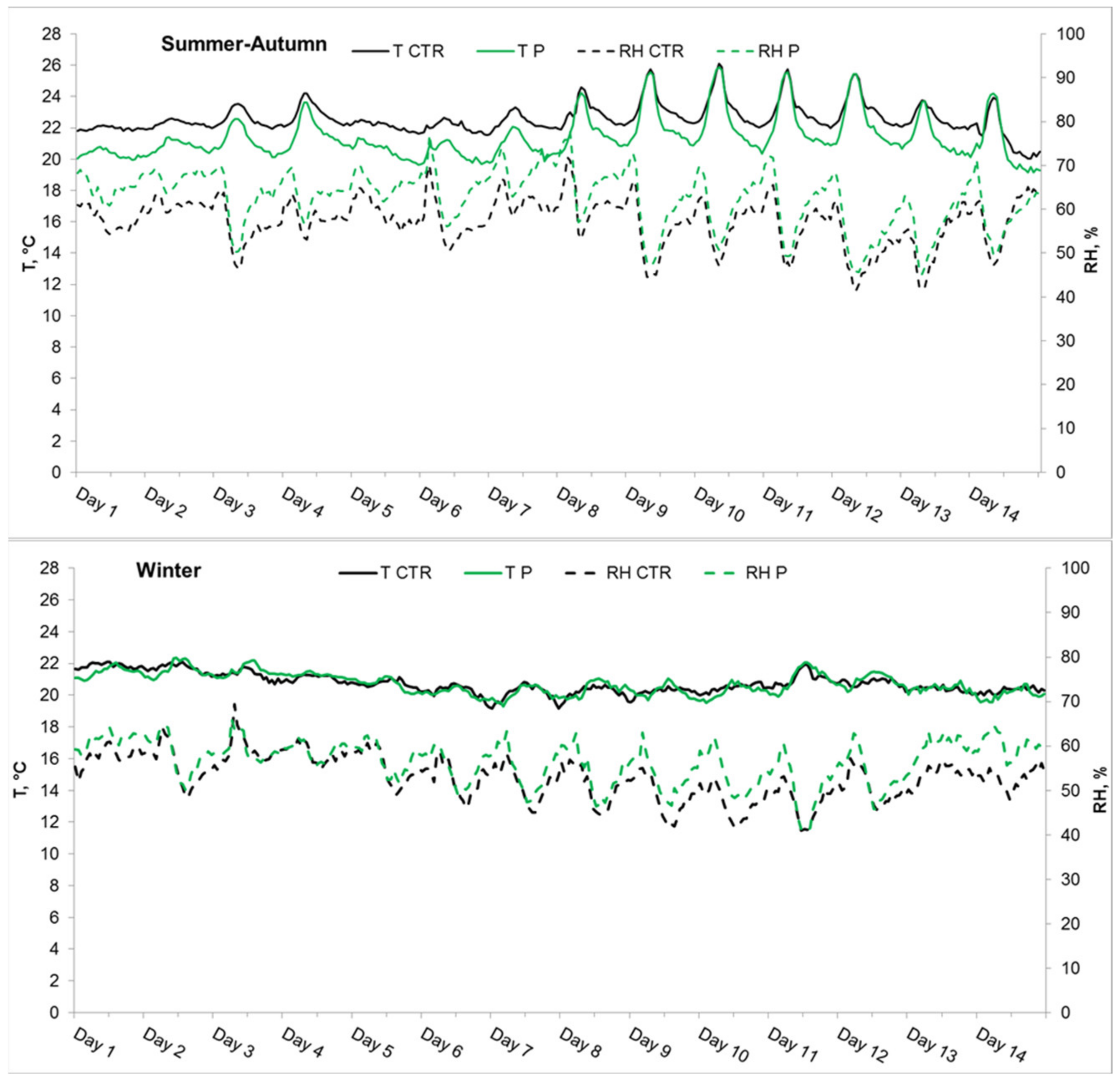
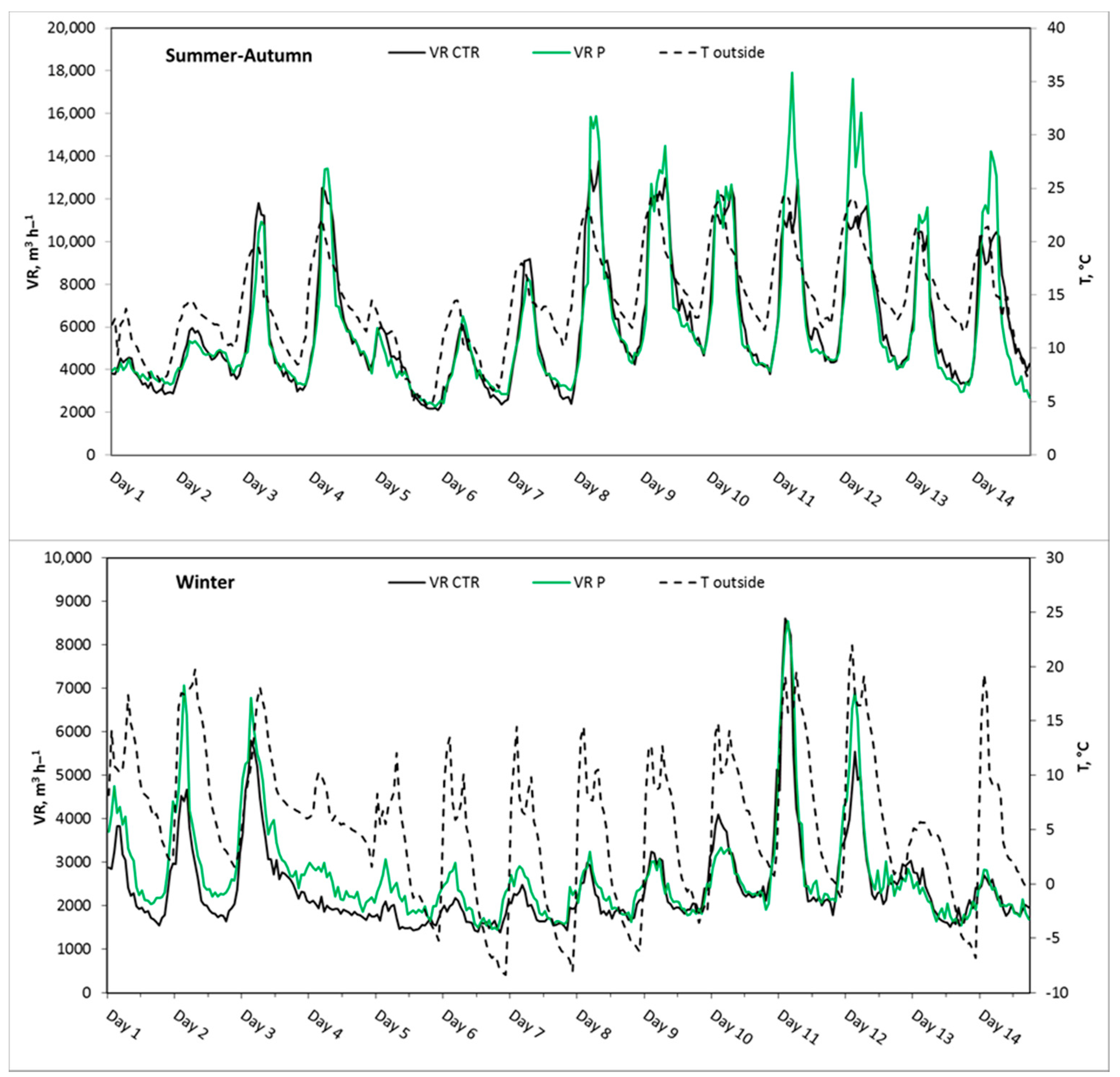
3.2.1. Experiment 1, Summer–Autumn
3.2.2. Experiment 2, Winter
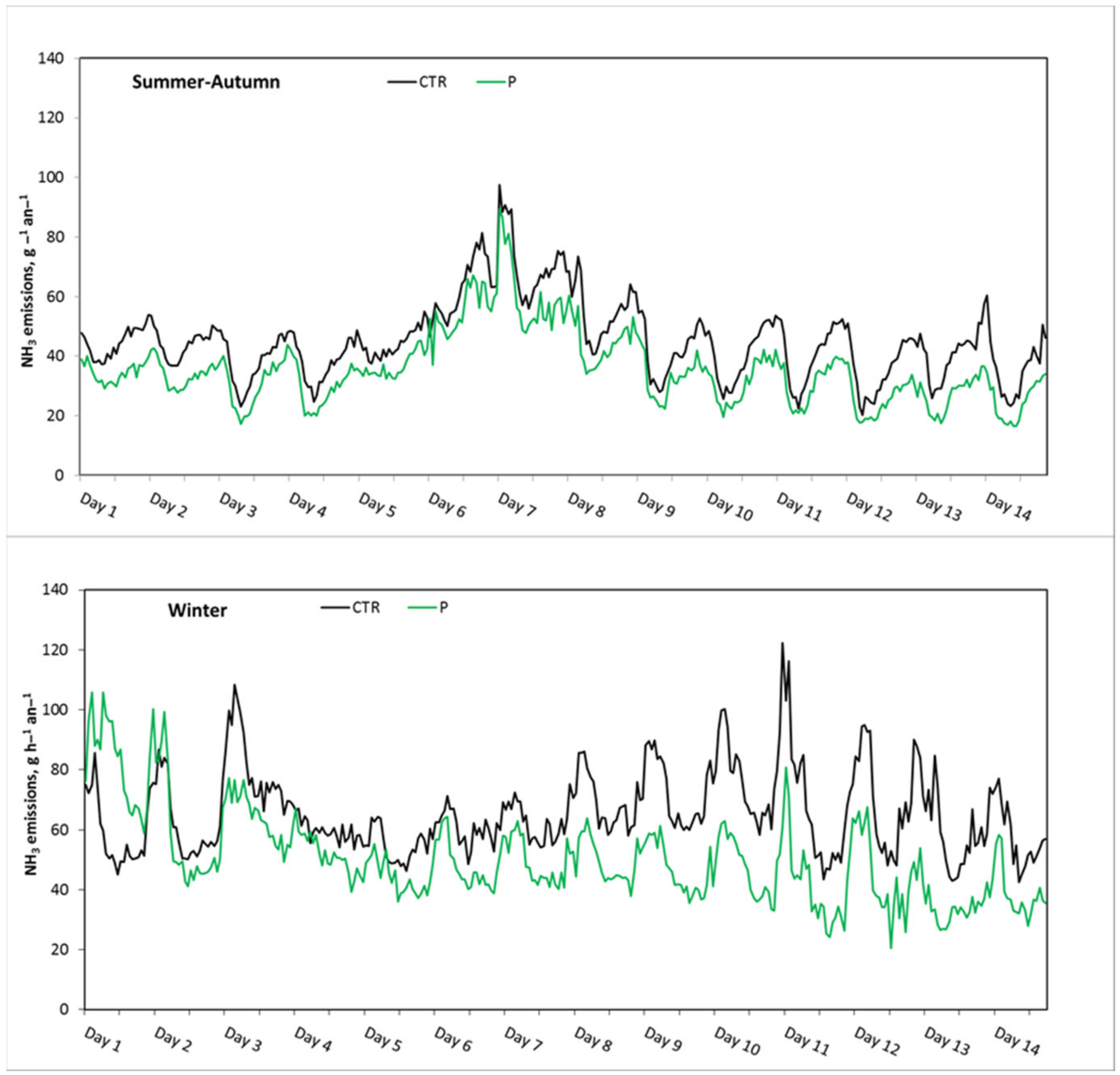
3.3. Ammonia Emission Rate during the Last Two Weeks of the Growing Period
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannakis, E.; Kushta, J.; Bruggeman, A.; Lelieveld, J. Costs and benefits of agricultural ammonia emission abatement options for compliance with European air quality regulations. Environ. Sci. Eur. 2019, 31, 93. [Google Scholar] [CrossRef]
- Oenema, O.; Velthof, G.L.; Amann, M.; Klimont, Z.; Winiwarter, W. Emissions from Agriculture and Their Control Potentials (Tsap Report 3); International Institute for Applied Systems Analysis (IIASA), European Commission: Laxenburg, Austria, 2012. [Google Scholar]
- Webb, J.; Menzi, H.; Pain, B.F.; Misselbrook, T.H.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing ammonia emissions from livestock production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, K.; Liu, X.; Meng, L.; Huaitalla, R.M.; Shen, J.; Hartung, E.; Gallmann, E.; Roelcke, M.; Zhang, F. Atmospheric NH3 dynamics at a typical pig farm in china and their implications. Atmos. Pollut. Res. 2014, 5, 455–463. [Google Scholar] [CrossRef]
- Philippe, F.X.; Cabaraux, J.F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Dong, H.; Kang, G.; Zhu, Z.; Tao, X.; Chen, Y.; Xin, H.; Harmon, J.D. Ammonia, methane and carbon dioxide concentrations and emissions of a hoop grower-finischer swine barn. Trans. Asabe 2009, 52, 1741–1747. [Google Scholar] [CrossRef]
- Van der Heyden, C.; Demeyer, P.; Volcke, E.I.P. Mitigating emissions from pig and poultry housing facilities through air scrubbers and biofilters: State-of-the-art and perspectives. Biosys. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- Wathes, C.M.; Demmers, T.G.M.; Xin, H. Ammonia concentrations and emissions in livestock production facilities: Guidelines and limits in the USA and UK. In Proceedings of the ASAE Annual International Meeting, Las Vegas, NV, USA, 27–30 July 2003; pp. 1–11. [Google Scholar]
- Verordnung zum Schutz Landwirtschaftlicher Nutztiere und Anderer zur Erzeugung Tierischer Produkte Gehaltener Tiere bei Ihrer Haltung (Tierschutz-Nutztierhaltungsverordnung—TierschnutztV). Verbraucherschutz, Bundesministerium der Justiz und für Verbraucherschutz Ed., Germany. 2001. Available online: https://www.gesetze-im-internet.de/tierschnutztv/ (accessed on 12 June 2021).
- Wathes, C.M.; Jones, J.B.; Kristensen, H.H.; Jones, E.K.M.; Webster, A.J.F. Aversion of pigs and domestic fowl to atmospheric ammonia. Trans. Am. Soc. Agric. Eng. 2002, 45, 1605–1610. [Google Scholar]
- Donham, K.J.; Thorne, P.S.; Breuer, G.M.; Powers, W.; Marquez, S.; Reynolds, S.J. Exposure limits related to air quality and risk assessment. In Iowa Concentrated Animal Feeding Operations Air Quality Study; Iowa State University and The University of Iowa Study Group: Iowa City, IA, USA, 2002; Chapter 8; pp. 164–183. [Google Scholar]
- Dourmad, J.Y.; Garcia-Launay, F.; Narcy, A. Pig nutrition: Impact on nitrogen, phosphorus, Cu and Zn in pig manure and on emissions of ammonia, greenhouse gas and odours. In Proceedings of the Batfarm European Workshop Reconciling Livestock Management to the Environment, Rennes, France, 19–20 March 2013. [Google Scholar]
- Philippe, F.X.; Nicks, B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric. Ecosyst. Environ. 2014, 199, 10–25. [Google Scholar] [CrossRef]
- ERDA. Atmosphere surface exchange of particulate and gaseous pollutants. In Proceedings of the Fourteenth ERDA Air Cleaning Conference, San Valley, ID, USA, 2 August 1974; Volume 38. [Google Scholar]
- Hill, A.R. Reproductive behaviour of metopolophium-festucae (theobald) at different temerpatures and on different host plants. Ann. Appl. Biol. 1971, 67, 289–295. [Google Scholar] [CrossRef]
- Rasmussen, K.H.; Taheri, M.K. Global emissions and antural processes for removal of gaseous pollutants. Water Air Soil Pollut. 1975, 4, 33–64. [Google Scholar] [CrossRef]
- Omasa, K.; Endo, R.; Tobe, K.; Kondo, T. Gas diffusion model analysis of foliar absorption of organic and inorganic air pollutants. Phyton Ann. Rei Bot. A 2002, 42, 135–148. [Google Scholar]
- Hörmann, V.; Brenske, K.R.; Ulrichs, C. Suitability of test chambers for analyzing air pollutant removal by plants and assessing potential indoor air purification. Water Air Soil Pollut. 2017, 228, 1–13. [Google Scholar] [CrossRef]
- Grundmann, G.L.; Lensi, R.; Chamalet, A. Delayed NH3 and N2O uptake by maize leaves. New Phytot. 1993, 124, 259–263. [Google Scholar] [CrossRef]
- Lockyer, D.R.; Whitehead, D.C. The uptake of gaseous ammonia by the leaves of italian ryegrass. J. Exp. Bot. 1986, 37, 919–927. [Google Scholar] [CrossRef]
- Rogers, H.H.; Aneja, V.P. Uptake of atmospheric ammonia by selected plant species. Environ. Exp. Bot. 1980, 20, 251–257. [Google Scholar] [CrossRef]
- Porter, L.K.; Viets, F.G.; Hutchinson, G.L. Air containing nitrogen-15 ammonia: Foliar absorption by corn seedlings. Science 1972, 175, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.L.; Millington, R.J.; Peters, D.B. Atmospheric ammonia: Absorption by plant leaves. Science 1972, 175, 771–772. [Google Scholar] [CrossRef] [PubMed]
- TÜV Rheinland Energie und Umwelt GmbH. Test Report 936/21220683/A; TÜV Rheinland Energie und Umwelt GmbH: Köln, Germany, 2013. [Google Scholar]
- Sage, R.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Guo, W.J.; Lee, N. Effect of leaf and plant age, and day/night temperature on net CO2 uptake in phalaenopsis amabilis var. Formosa. J. Am. Soc. Hort. Sci. 2006, 131, 320–326. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, Z.; Shang, B.; Kang, G.; Zhu, H.; Xin, H. Emissions of greenhouse gases from a typical chinese swine farrowing barn. Trans. Asabe 2007, 50, 1037–1044. [Google Scholar] [CrossRef][Green Version]
- Van Ouwerkerk, E.N.J.; Pedersen, S. Application of the Carbon Dioxide Mass Balance Method to Evaluate Ventilation Rates in Livestock Buildings. In Proceedings of the XII CIGR World Congress on Agricultural Engineering, Milan, Italy, 29 August–1 September 1994; pp. 516–529. [Google Scholar]
- Liu, Z.; Powers, W.; Harmon, J.D. Estimating ventilation rates of animal houses through CO2 balance. Trans. Asabe 2016, 59, 321–328. [Google Scholar]
- CIGR. Heat and moisture production at animal and house levels. In CIGR Section II, 4th Report of Working Group on Climatisation of Animal Houses; Danish Institute of Agricultural Sciences: Slagelse, Denmark; Research Centre Bygholm: Horsens, Denmark, 2002. [Google Scholar]
- Carr, J. Garth Pig Stockmanship Standards; 5m Publishing: Sheffield, UK, 1998. [Google Scholar]
- Brown-Brandl, T.M.; Nienaber, J.A.; Xin, H.; Gates, R.S. A literature review of swine heat production. Trans. ASAE 2004, 47, 259–270. [Google Scholar] [CrossRef]
- Maderbacher, R.S.G.; Winter, P.; Baumgartner, W. Abgangsursachen in einem schweinezuchtbetrieb [causes of mortality in a swine breeding establishment]. Dtsch Tierarztl Wochenschr. 1993, 100, 468–473. [Google Scholar]
- Gebhardt, J.T.; Tokach, M.D.; Dritz, S.S.; DeRouchey, J.M.; Woodworth, J.C.; Goodband, R.D.; Henry, S.C. Postweaning mortality in commercial swine production II: Review of non-infectious contributing factors. Transl. Anim. Sci. 2020, 4, 462–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhao, X.Y.; He, Y.; Wang, K.Y. Cough sound analysis to assess air quality in commercial weaner barns. Comput. Electron. Agric. 2019, 160, 8–13. [Google Scholar] [CrossRef]
- Han, K.S.; Ruan, L.W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. R. 2020, 27, 16019–16051. [Google Scholar] [CrossRef]
- Xu, K.; Zheng, C.M.; Ye, H. The transpiration characteristics and heat dissipation analysis of natural leaves grown in different climatic environments. Heat Mass Transfer. 2020, 56, 95–108. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Aarnink, A.J.A.; Gerrits, W.J.J.; Heetkamp, M.J.H.; Canh, T.T.; Spoolder, H.A.M.; Kemp, B.; Verstegen, M.W.A. Thermal behaviour of growing pigs in response to high temperature and humidity. Appl. Anim. Behav. Sci. 2005, 91, 1–16. [Google Scholar] [CrossRef]
- Pennisi, B.V. Growing indoor plants with success. In Bulletin University of Georgia; Fort Valley State University: Fort Valley, GA, USA; University of Georgia: Athens, GA, USA; U.S. Department of Agriculture: Washington, DC, USA, 2020; pp. 1–28. Available online: https://extension.uga.edu/publications/detail.html?number=B1318&title=Growing%20Indoor%20Plants%20with%20Success (accessed on 15 May 2021).
- Pluske, J.R.; Le Dividich, J.; Verstegen, M.W.A. Weaning the Pig: Concepts and Consequences; Wageningen Academic Pub.: Wageningen, The Netherlands, 2003. [Google Scholar]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W.; Zhang, J. Diseases of Swine, 11th ed.; Wiley-Blackwall: Hoboken, NJ, USA, 2019; p. 1136. [Google Scholar]
- Ni, J.Q.; Heber, A.J.; Lim, T.T.; Tao, P.C.; Schmidt, A.M. Methane and carbon dioxide emission from two pig finishing barns. J. Environ. Qual. 2008, 37, 2001–2011. [Google Scholar] [CrossRef]
- Hinz, T.; Linke, S. A comprehensive experimental study of aerial pollutants in and emissions from livestock buildings. Part 1. Methods. J. Agric. Econ. Res. 1998, 70, 111–118. [Google Scholar] [CrossRef]
- Zhuang, S.; Brusselman, E.; Sonck, B.; Demeyer, P. Validation of Five Gas Analysers for Application in Ammonia Emission Measurements at Livestock Houses According to the VERA Test Protocol. Appl. Sci. 2020, 10, 5034. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Van der Weerden, T.J.; Pain, B.F.; Jarvis, S.C.; Chambers, B.J.; Smith, K.A.; Phillips, V.R.; Demmers, T.G.M. Ammonia emission factors for UK agriculture. Atmos. Environ. 2000, 34, 871–880. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; Keen, A.; Metz, J.H.M.; Speelman, L.; Verstegen, M.W.A. Ammonia emission patterns during the growing periods of pigs housed on partially slatted floors. J. Agric. Econ. Res. 1995, 62, 105–116. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; van den Berg, A.J.; Keen, A.; Hoeksma, P.; Verstegen, M.W.A. Effect of slatted floor area on ammonia emissions and on the excretory and lying behavious of growing pigs. J. Agric. Econ. Res. 1996, 64, 299–310. [Google Scholar]
- Givan, G.V. Metabolic detoxification of ammonia in tissues of higher plants. Phytochemistry 1979, 18, 375–382. [Google Scholar] [CrossRef]
- Husted, S.; Schjoerring, J.K. Ammonia flux between oilseed rape plants and the atmosphere in response to changes in leaf temperature, light intensity, and air humidity—Interactions with leaf conductance and apoplastic NH4+ and H+ concentrations. Plant Physiol. 1996, 112, 67–74. [Google Scholar] [CrossRef] [PubMed]

| No. Pigs | Age | Body Mass | ||||
|---|---|---|---|---|---|---|
| Days | kg | |||||
| Start | End | Start | End | Start | End | |
| Experiment 1 (summer–autumn) | ||||||
| CTR | 147 | 141 | 28 | 83 | 6.5 ± 1.3 | 30.2 ± 4.6 |
| P | 148 | 142 | 28 | 83 | 6.3 ± 1.5 | 32.1 * ± 5.1 |
| Experiment 2 (winter) | ||||||
| CTR | 88 | 80 | 28 | 82 | 6.9 ± 1.3 | 31.2 ± 4.2 |
| P | 88 | 85 | 28 | 82 | 6.9 ± 1.6 | 33.1 * ± 4.7 |
| Summer- Autumn | CTR | P | Outside | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | NH3 | VR | T | RH | CO2 | NH3 | VR | T | RH | CO2 | NH3 | T | |
| ppm | ppm | m3 h−1 | °C | % | ppm | ppm | m3 h−1 | °C | % | ppm | ppm | °C | |
| Mean SD | 965 248 | 2.4 1.2 | 6050 3330 | 22.6 0.9 | 57.2 5.5 | 980 274 | 2.0 * 1.1 | 6060 2910 | 21.4 * 1.4 | 62.5 * 6.5 | 425 39 | 0.5 0.1 | 14.4 4.6 |
| Min | 526 | 0.5 | 2260 | 20.0 | 41.6 | 581 | 0.5 | 2100 | 19.1 | 45.2 | 377 | 0.2 | 4.6 |
| Max | 1630 | 6.0 | 17,900 | 26.1 | 71.9 | 1790 | 5.9 | 13,800 | 25.9 | 77.7 | 563 | 0.9 | 24.4 |
| Winter | CTR | P | Outside | ||||||||||
| CO2 | NH3 | VR | T | RH | CO2 | NH3 | VR | T | RH | CO2 | NH3 | T | |
| ppm | ppm | m3 h−1 | °C | % | ppm | ppm | m3 h−1 | °C | % | ppm | ppm | °C | |
| Mean SD | 1177 183 | 4.0 0.5 | 2390 1060 | 20.7 0.6 | 53.0 5.0 | 1162 203 | 3.2 * 0.4 | 2670 * 1170 | 20.7 0.7 | 56.4 * 5.0 | 482 57 | 1.0 0.3 | 5.4 6.6 |
| Min | 638 | 2.3 | 970 | 19.2 | 40.9 | 657 | 1.9 | 1330 | 19.3 | 40.7 | 423 | 0.5 | −8.3 |
| Max | 1600 | 5.2 | 8610 | 22.1 | 69.4 | 1630 | 5.3 | 8540 | 22.3 | 66.1 | 680 | 1.7 | 22.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menardo, S.; Berg, W.; Grüneberg, H.; Jakob, M. Can Green Plants Mitigate Ammonia Concentration in Piglet Barns? Atmosphere 2021, 12, 1150. https://doi.org/10.3390/atmos12091150
Menardo S, Berg W, Grüneberg H, Jakob M. Can Green Plants Mitigate Ammonia Concentration in Piglet Barns? Atmosphere. 2021; 12(9):1150. https://doi.org/10.3390/atmos12091150
Chicago/Turabian StyleMenardo, Simona, Werner Berg, Heiner Grüneberg, and Martina Jakob. 2021. "Can Green Plants Mitigate Ammonia Concentration in Piglet Barns?" Atmosphere 12, no. 9: 1150. https://doi.org/10.3390/atmos12091150
APA StyleMenardo, S., Berg, W., Grüneberg, H., & Jakob, M. (2021). Can Green Plants Mitigate Ammonia Concentration in Piglet Barns? Atmosphere, 12(9), 1150. https://doi.org/10.3390/atmos12091150





