Mitigation of Acute Hydrogen Sulfide and Ammonia Emissions from Swine Manure during Three-Hour Agitation Using Pelletized Biochar
Abstract
:1. Introduction
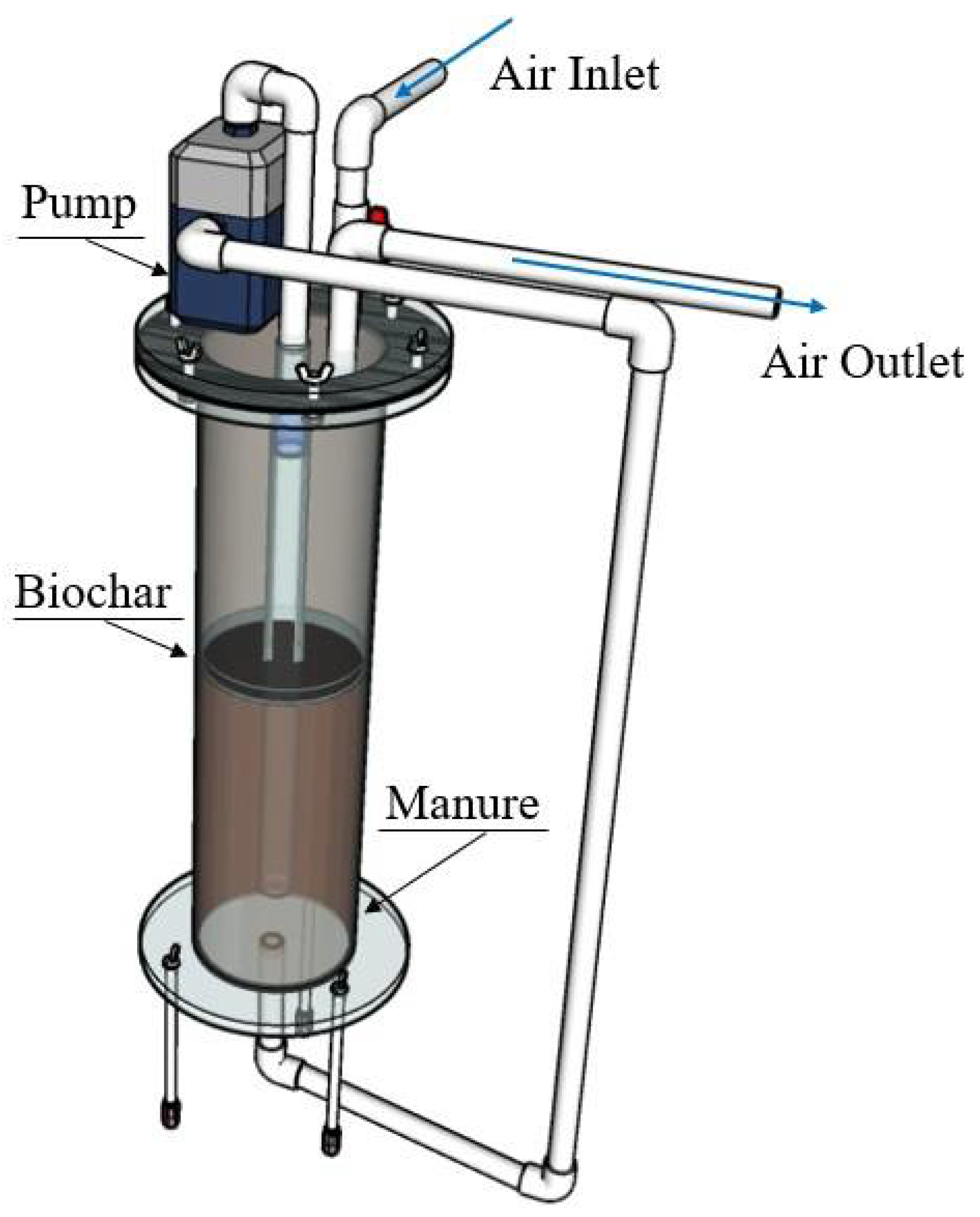
2. Materials and Methods
2.1. Pelletization of Biochar
2.2. NH3 and H2S Measurements
2.3. Manure Properties
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
- Biochar powder was significantly (p < 0.05) more effective than the biochar pellets.
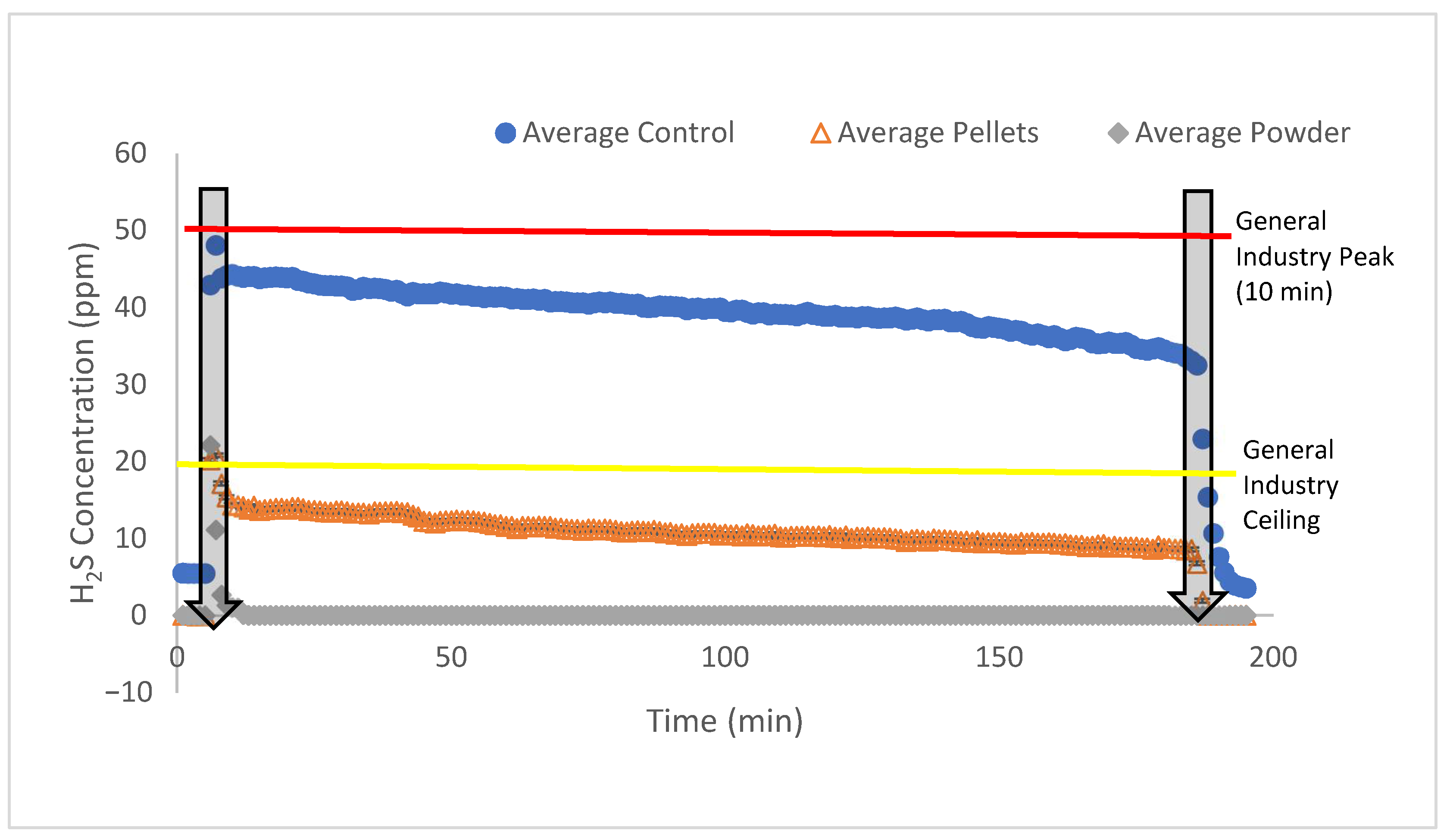
- Pellets reduced total H2S and NH3 emissions by ~72% and ~68%, respectively (p = 0.001), compared with ~99% by powder (p = 0.001).
- The maximum H2S and NH3 concentrations were reduced from 48.1 ± 4.8 ppm and 1810 ± 850 ppm to 20.8 ± 2.95 ppm and 775 ± 182 ppm by the pellets, and to 22.1 ± 16.9 ppm and 40.3 ± 57 ppm by powder, respectively. These reductions are equivalent to reducing the maximum concentrations of H2S and NH3 during the 3-h manure agitation by 57% and 57% (pellets) and 54% and 98% (powder), respectively.
- The changes in manure properties treated with biochar showed less loss of nitrogen and more carbon compared with the control, albeit not significant due to variability. This early observation should be further explored as the biochar treatment of manure hints at improved manure quality and, therefore, the potential for improved sustainability of the nexus of animal and crop production.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


| Manure Property (g/L) | Δ Control | Δ Pellet | Δ Powder |
|---|---|---|---|
| Moisture | 7.112 | −10.79 | 3.95 |
| %Diff | −252 (0.11) | −44 (0.35) | |
| Mineral Matter | −0.619 | −0.614 | 0.123 |
| %Diff | 1 (0.50) | 120 (0.03) | |
| Total Nitrogen | −0.143 | −0.101 | −0.062 |
| %Diff | 29 (0.39) | 57 (0.31) | |
| Ammonium-N (NH4-N) | −0.135 | −0.075 | −0.072 |
| %Diff | 45 (0.33) | 47 (0.30) | |
| Organic-N | −0.008 | −0.027 | 0.010 |
| %Diff | −219 (0.31) | 214 (0.39) | |
| Phosphorus (P) | −0.038 | −0.076 | −0.023 |
| %Diff | −99 (0.29) | 41 (0.32) | |
| Potassium (K) | 0.028 | −0.065 | 0.071 |
| %Diff | −338 (0.14) | 158 (0.16) | |
| Calcium (Ca) | −0.036 | −0.038 | −0.037 |
| %Diff | −6 (0.48) | −2 (0.49) | |
| Sodium (Na) | 0.010 | −0.036 | −0.004 |
| %Diff | −446 (0.047) | −142 (0.12) | |
| Sulfur (S) | −0.028 | −0.058 | 0.001 |
| %Diff | −109 (0.106) | 104 (0.23) | |
| Carbon (C) | −0.010 | 0.886 | 0.149 |
| %Diff | 9346 (0.166) | 1650 (0.36) |
References
- Barker, J.; Curtis, S.; Hogsett, O.; Humenik, F. Safety in Swine Production Systems; Cooperative Extension Service: Raleigh, NC, USA, 1986. [Google Scholar]
- Donham, J.K.; Yeggy, J.; Dague, R.R. Production Rates of Toxic Gases from Liquid Swine Manure: Health Implications for Workers and Animals in Swine Confinement Buildings. Biol. Wastes 1988, 24, 161–173. [Google Scholar] [CrossRef]
- Hoff, S.J.; Bundy, D.S.; Nelson, M.A.; Zelle, B.C.; Jacobson, L.D.; Heber, A.J.; Ni, J.Q.; Zhang, Y.; Koziel, J.A.; Beasley, D.B. Emissions of ammonia, hydrogen sulfide, and odor before, during and after slurry removal from a deep-pit swine finisher. J. Air Waste Manag. Assoc. 2006, 56, 581–590. [Google Scholar] [CrossRef] [Green Version]
- OSHA. 29 CFR 1910.1000, Table Z-2: Toxic and Hazardous Substances; Occupational Safety and Health Administration: Washington, DC, USA, 2017.
- Chénard, L.; Lemay, S.P.; Laguë, C. Hydrogen sulfide assessment in shallow-pit swine housing and outside manure storage. J. Agric. Saf. Health 2003, 9, 285–302. [Google Scholar] [CrossRef]
- Pesce, E.P.; Zhao, J.; Manbeck, H.B.; Murphy, D.J. Screening Ventilation Strategies for Confined-Space Manure Storage Facilities. J. Agric. Saf. Health 2008, 14, 283–308. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Lim, T.T. Ammonia and hydrogen sulfide in swine production. In Air Quality and Livestock Farming; CRC Press: London, UK, 2018; pp. 69–88. [Google Scholar]
- NIOSH Pocket Guide to Chemical Hazards; U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 1997.
- Alvarado, A.C.; Predicala, B.Z. Occupational Exposure Risk for Swine Workers in Confined Housing Facilities. J. Agric. Saf. Health 2019, 25, 37–50. [Google Scholar] [CrossRef]
- CIGR. Climatization of Animal Houses, 2nd ed.; Commission Internationale du Génie Rurale, Faculty of Agricultural Sciences, State University of Gent: Gent, Belgium, 1992. [Google Scholar]
- Busse, F.W. Comparison measurements of the house climate in swine stables with and without respiratory diseases or cannibalism. In Livestock Environment. Fourth International Symposium; Collins, E., Boon, C., Eds.; University of Warwick: Coventry, UK, 1993; pp. 904–908. [Google Scholar]
- Nour, M.M.; Cheng, Y.; Ni, J.; Sheldon, E.; Field, W. Summary of Injuries and Fatalities Involving Livestock Manure Storage, Handling, and Transport Operations in Seven Central States: 1976-2019. J. Agric. Saf. Health 2021, 27, 105–122. [Google Scholar] [CrossRef]
- Mitloehner, F.M.; Calvo, M.S. Worker Health and Safety in Concentrated Animal Feeding Operations. J. Agric. Saf. Health 2008, 14, 163–187. [Google Scholar] [CrossRef]
- Predicala, B.Z.; Cortus, E.L.; Lemay, S.P.; Laguë, C. Effectiveness of a Manure Scraper System for Reducing Concentrations of Hydrogen Sulfide and Ammonia in a Swine Grower-Finisher Room. Trans. ASABE 2007, 50, 999–1006. [Google Scholar] [CrossRef]
- Alvarado, A.; Predicala, B. Control of Gas and Odor Levels in Swine Facilities Using Filters with Zinc Oxide Nanoparticles. Trans. ASABE 2017, 60, 943–956. [Google Scholar] [CrossRef]
- Chen, L.; Hile, M.; Fabian, E.E.; Xu, Z.; Bruns, M.; Brown, V. Iron Oxide to Mitigate Hydrogen Sulfide Gas Release from Gypsum-Bedded Dairy Manure Storages. Trans. ASABE 2018, 61, 1101–1112. [Google Scholar] [CrossRef]
- Barber, E.M.; McQuitty, J.B. Chemical control of hydrogen sulphide from anaerobic swine manure I: Oxidizing agents. Can. Agric. Eng. 1975, 17, 90–96. [Google Scholar]
- Clanton, C.J.; Nicolai, R.E.; Schmidt, D.R. Chemical additions to swine manure to reduce hydrogen sulfide losses: A laboratory study. In Proceedings of the ASAE Annual International Meeting 1999, No. 994007, Toronto, ON, Canada, 18–22 July 1999. [Google Scholar]
- Smith, S.; Nicolai, D. Hydrogen sulfide reduction of swine manure using potassium permanganate and hydrogen peroxide. In Proceedings of the ASAE Midwest Regional Meeting 2005, No. SD05-801, Brookings, South Dakota, September–October 2005. [Google Scholar]
- Chen, B.; Koziel, J.A.; Banik, C.; Ma, H.; Lee, M.; Wi, J.; Meiirkhanuly, Z.; O’Brien, S.C.; Li, P.; Andersen, D.S.; et al. Mitigation of Odor, NH3, H2S, GHG, and VOC Emissions With Current Products for Use in Deep-Pit Swine Manure Storage Structures. Front. Environ. Sci. 2020, 8, 613646. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Banik, C.; Ma, H.; Lee, M.; O’Brien, S.C.; Li, P.; Andersen, D.S.; Białowiec, A.; Brown, R.C. Mitigation of Gaseous Emissions from Stored Swine Manure with Biochar: Effect of Dose and Reapplication on a Pilot-Scale. Atmosphere 2021, 12, 96. [Google Scholar] [CrossRef]
- Banik, C.; Koziel, J.; De, M.; Bonds, D.; Chen, B.; Singh, A.; Licht, M. Soil nutrients and carbon dynamics in the presence of biochar-swine manure mixture under controlled leaching experiment using a Midwestern Mollisols. Front. Environ. Sci. 2021, 9, 66. [Google Scholar] [CrossRef]
- Banik, C.; Koziel, J.A.; Bonds, D.; Singh, A.; Licht, M. Comparing biochar-swine manure mixture to conventional manure impact on soil nutrient availability and plant uptake—A greenhouse study. Land 2021, 10, 372. [Google Scholar] [CrossRef]
- Kalus, K.; Koziel, J.A.; Opaliński, S. A Review of Biochar Properties and Their Utilization in Crop Agriculture and Livestock Production. Appl. Sci. 2019, 9, 3494. [Google Scholar] [CrossRef] [Green Version]
- Meiirkhanuly, Z.; Koziel, J.A.; Chen, B.; Białowiec, A.; Lee, M.; Wi, J.; Banik, C.; Brown, R.C.; Bakshi, S. Mitigation of Gaseous Emissions from Swine Manure with the Surficial Application of Biochars. Atmosphere 2020, 11, 1179. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Białowiec, A.; Lee, M.; Ma, H.; Li, P.; Meiirkhanuly, Z.; Brown, R.C. The Impact of Surficial Biochar Treatment on Acute H2S Emissions during Swine Manure Agitation before Pump-Out: Proof-of-the-Concept. Catalysts 2020, 10, 940. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Lee, M.; Ma, H.; Meiirkhanuly, Z.; Li, P.; Białowiec, A.; Brown, R.C. Mitigation of Acute Ammonia Emissions with Biochar during Swine Manure Agitation before Pump-Out: Proof-of-the-Concept. Front. Environ. Sci. 2020, 9, 98. [Google Scholar] [CrossRef]
- Dzonzi-undi, J.; Masek, O.; Abass, O. Determination of spontaneous ignition behavior of biochar accumulations. Int. J. Sci. Res. 2014, 3, 656–661. [Google Scholar]
- Fulhage, D.C. Solids Removal from Livestock Manure Lagoons. University of Missouri Extension. 1994. Available online: https://extension.missouri.edu/publications/wq324 (accessed on 21 June 2021).
- Test. Methods for the Examination of Composting and Compost (TMECC); USDA and U.S. Composting Council: Raleigh, NC, USA, 2002.
- Peters, J.; Combs, S.M.; Hoskins, B.; Jarman, J.; Kovar, J.L.; Watson, M.E.; Wolf, A.M.; Wolf, N. Recommended Methods of Manure Analysis. Publ. A3769 2003; Cooperative Extension University of Wisconsin: Madison, WI, USA, 2003. [Google Scholar]
- US EPA. Method 351.2. Determination of Total Kjeldahl Nitrogen by Semi-Automated Colorimetry; Environmental Monitoring Systems Laboratory Office of Research and Development: Cincinnati, OH, USA, 1993.
- Brown, R.C. The Role of Pyrolysis and Gasification in a Carbon Negative Economy. Processes 2021, 9, 882. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Gan, J.; Wang, Y.; Hu, B. Simulation of Hydrogen Sulfide Emission from Deep-Pit Manure Storage During Agitation. Trans. ASABE 2018, 61, 1951–1967. [Google Scholar] [CrossRef]
- USDA. Iowa Production Costs Report; USDA Agricultural Marketing Service: Washington, DC, USA. Available online: http://www.ams.usda.gov/mnreports/nw_gr210.txt (accessed on 18 June 2021).

| Control | Pellets | Powder | |
|---|---|---|---|
| Maximum H2S Concentration, ppm (letter of groups) | 48.1 ± 4.84 (A) | 20.8 ± 2.95 (B) | 22.1 ± 16.9 (B) |
| %R (p-value) | - | 57% (0.02) | 54% (0.052) |
| Maximum NH3 Concentration, ppm (letter of groups) | 1811 ± 852 (A) | 775 ± 182 (B) | 40.3 ± 57.0 (C) |
| %R (p-value) | - | 57% (0.08) | 98% (0.001) |
| Control | Pellets | Powder | |
|---|---|---|---|
| Total Emission of H2S, mg/m2 (letter of groups) | 1.31 ± 0.305 (A) | 0.361 ± 0.0453 (B) | 0.0071 ± 0.005 (C) |
| %R (p-value) | - | 72% (0.001) | 99% (0.001) |
| Total Emission of NH3, mg/m2 (letter of groups) | 28.0 ± 12.3 (A) | 8.93 ± 1.70 (B) | 0.152 ± 0.216 (C) |
| %R (p-value) | - | 68% (0.001) | 99% (0.001) |
| Manure Property | Δ Control (% Wet Basis) | Δ Pellet (% Wet Basis) | Δ Powder (% Wet Basis) |
|---|---|---|---|
| Moisture | 0.073 | 0.043 | −0.020 |
| %Diff | - | −41 (0.41) | −127 (0.07) |
| Mineral Matter | −0.067 | −0.053 | 0.010 |
| %Diff | - | 20 (0.43) | 115 (0.03) |
| Total Nitrogen | −0.016 | −0.008 | −0.007 |
| %Diff | - | 49 (0.28) | 55 (0.28) |
| Ammonium-N (NH4-N) | −0.015 | −0.005 | −0.008 |
| %Diff | - | 64 (0.22) | 45 (0.26) |
| Nitrate-N (NO3-N) | 0.000 | 0.000 | 0.000 |
| %Diff | - | 0 | 0 |
| Organic-N | −0.001 | −0.003 | 0.001 |
| %Diff | −167 (0.33) | 200 (0.38) | |
| Phosphorus (P) | −0.006 | −0.007 | −0.002 |
| %Diff | −22 (0.41) | 61 (0.11) | |
| Potassium (K) | 0.002 | −0.005 | 0.006 |
| %Diff | −380 (0.15) | 280 (0.12) | |
| Calcium (Ca) | −0.004 | −0.004 | −0.004 |
| %Diff | 0 | 0 | |
| Magnesium (Mg) | −0.003 | −0.005 | 0.000 |
| %Diff | −60 (0.34) | 100 (0.09) | |
| Sodium (Na) | 0.001 | −0.003 | −0.001 |
| %Diff | −550 (0.04) | −200 (0.12) | |
| Sulfur (S) | −0.003 | −0.005 | 0.000 |
| %Diff | −78 (0.09) | 100 (0.22) | |
| Carbon (C) | −0.003 | 0.093 | 0.013 |
| %Diff | 2900 (0.15) | 500 (0.35) | |
| pH | 0.003 | −0.020 | 0.007 |
| %Diff | −700 (0.06) | 100 (0.47) | |
| C/N ratio | −0.053 | 0.560 | 0.040 |
| %Diff | 1150 (0.14) | 175 (0.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Koziel, J.A.; Lee, M.; O’Brien, S.C.; Li, P.; Brown, R.C. Mitigation of Acute Hydrogen Sulfide and Ammonia Emissions from Swine Manure during Three-Hour Agitation Using Pelletized Biochar. Atmosphere 2021, 12, 825. https://doi.org/10.3390/atmos12070825
Chen B, Koziel JA, Lee M, O’Brien SC, Li P, Brown RC. Mitigation of Acute Hydrogen Sulfide and Ammonia Emissions from Swine Manure during Three-Hour Agitation Using Pelletized Biochar. Atmosphere. 2021; 12(7):825. https://doi.org/10.3390/atmos12070825
Chicago/Turabian StyleChen, Baitong, Jacek A. Koziel, Myeongseong Lee, Samuel C. O’Brien, Peiyang Li, and Robert C. Brown. 2021. "Mitigation of Acute Hydrogen Sulfide and Ammonia Emissions from Swine Manure during Three-Hour Agitation Using Pelletized Biochar" Atmosphere 12, no. 7: 825. https://doi.org/10.3390/atmos12070825
APA StyleChen, B., Koziel, J. A., Lee, M., O’Brien, S. C., Li, P., & Brown, R. C. (2021). Mitigation of Acute Hydrogen Sulfide and Ammonia Emissions from Swine Manure during Three-Hour Agitation Using Pelletized Biochar. Atmosphere, 12(7), 825. https://doi.org/10.3390/atmos12070825







