1. Introduction
Risk assessment of COVID-19 transmission via airborne pathogen droplets (APDs) is essential for the development of safe distance guidelines [
1] and ventilation designs in various types of spaces [
2]. Early risk assessment models (RAMs) dominantly involved statistical analysis of disease propagations. These models are based on simplifying assumptions or use collected statistics to find key factors for various infectious diseases, which can be used to predict the effects of different interventions.
Examples of RAMs include the model proposed by Kermack and McKendrick [
3], which is a classical model for understanding the propagation of real-life epidemics. Another example is the Wells–Riley [
4] model, which is well known for the prediction of the risk of new infection within a group of people in a specific period of time. The Wells–Riley model [
4] is, however, limited by its steady-state and uniform distribution assumptions for the suspended infectious aerosols in the air. Rudnick et al. [
5] improved the Wells–Riley model to estimate the risk in unsteady conditions. This model was successfully embedded in some of the later research works [
6,
7,
8]. During the past decades, RAMs have been shifting from statistical approaches to deterministic approaches of tracing respiratory droplets’ trajectories towards a more detailed consideration of their movement in the background air. Such approaches became more popular since 2002, after the spread of the severe acute respiratory syndrome known as SARS-CoV-1. A summary of the existing models can be found in [
9,
10,
11,
12]. In this respect, computational fluid dynamics (CFD) has been broadly utilized to trace the spreading patterns of respiratory droplets in different environmental situations.
The existing CFD studies of respiratory air jets and droplets can be classified into two main categories. In the first group of studies, the formation of the respiratory droplets and their evaporation and breakdown are investigated, where the models have provided detailed insights into the thermodynamics of the droplets in different environmental conditions, such as at varying temperatures and humidity levels [
13]. Nonetheless, these models barely interpreted the results into the risk factors. In the second category, however, the focus is on the risk assessment associated with the spreading of particles of infectious diseases [
14]. In such studies, the risk assessment requires CFD simulations and experiments in a wide range of environmental conditions (e.g., varying temperature and humidity) as well as clinical information of APDs (e.g., size distribution, velocity magnitude, velocity profile, etc.). For example, Villafruela et al. [
15] and You et al. [
16] used the Wells–Riley-based method to calculate the infection risk in enclosed spaces based on the results of their CFD simulations. In another study, Watanabe et al. [
17] introduced two dose–response models for SARS-CoV based on exponential as well as beta-Poisson models. The former study used an exponential function to predict the probability of infection under a certain dosage of viral load, while the latter applied a beta distribution to demonstrate the probability of infection [
18]. In another work, Wang et al. employed a continuous random walk model (CRW) to solve the turbulent fluctuation of violent respiratory events. The CRW model was implemented into a Lagrangian model for particle dynamics and evaporation and a simple respiratory jet model to improve the prediction of median-sized droplets’ spreading [
19]. Moreover, Buonanno et al. [
20] presented a simple approach to estimate the viral load emitted by a contagious subject based on the type of respiratory event, inhalation rate, and activity level. This model hypothesized that the droplets emitted by an infected subject have the same viral load as sputum. Hence, by knowing the concentration of virus in sputum and the number of emitted droplets with a diameter of 10 µm or smaller, the viral load can be determined through a mass balance equation [
21]. This model has been further employed to estimate the change in infection risk by the ventilation and behaviors in public transportation spaces [
22]. In another research work, Guo et al. developed a mathematical approach to determine the spatial distribution of the probability of infection (PI) [
23]. Their method is based on combining the spatial flow impact factor (SFIF) and the Wells–Riley model to find the optimal arrangement of people and/or air purifiers in indoor spaces.
Since December 2019, the COVID-19 outbreak remarkably accelerated the efforts for introducing more accurate CFD-based RAMs. For example, only a few months after the pandemic, Vuorinen et al. [
24] proposed a multi-level approach with different degrees of complexity to investigate the airborne transmission of COVID-19. They studied susceptible and infected individuals in generic public places by Monte Carlo modeling. The droplet evaporation model was also applied to a CFD model to capture the shrinkage of large droplets to small nuclei. They showed that depending on the local concentration of aerosols, the recommended social distance at the time could be misleading. Instead, they calculated the time required to receive the sufficient dosage of pathogens as the exposure time, which can be in the order of a few seconds to an hour depending on the local concentration of aerosols. Furthermore, Mittal et al. [
25] combined fluid mechanics and a simplified mathematical technique to assess the risk of airborne transmission of a respiratory infection caused by the COVID-19 virus. The model was inspired by the Drake probabilistic model to estimate the number of active, communicative extraterrestrial civilizations in the Milky Way galaxy [
26]. Accounting for the factors affecting the generation of infectious droplets in the respiratory system of a host and the environmental parameters impacting the transmission of infectious droplets to the vicinity of a susceptible person, they provided a probabilistic model for the risk of infection that reasonably estimates the relative changes in the risk with some controversial behaviors and factors such as wearing a mask, physical distancing, and intensity of physical activity. In another work, Karimzadeh et al. [
27] suggested the minimum infective dosage of the disease be around 100 droplets. Some other studies have instead presented a more advanced description of the infective dosage as they used data of viral load in the sputum and determined infectious quantum by defining conversion factors [
24].
Many of the existing assessment models suffer from over-simplification of zero-dimensional steady-state assumptions, which do not take the spatial distribution of air velocity throughout the field, air–droplet interactions, and the time-dependent behavior of droplets into account. To overcome this shortage, this paper proposes a novel risk assessment model (RAM) to calculate the risk of virus transmission over time using an Eulerian–Lagrangian CFD approach. This model interprets different respiratory activities into a risk factor by calculation of the accumulated number of respiratory droplets passing a certain location within a period of exposure. The droplets’ temporal position and velocity are analyzed via a proposed algorithm to estimate the regions having more than the threshold of the number of droplets accounted as the minimum dose needed for infection of COVID-19; here, the suggested number is 100 following [
27]. The exhaled droplets have multiple components, including a non-evaporative nucleus with an evaporating hydro-surface to give a more realistic representation of respiratory droplets. The performance of the proposed prediction method is assessed under different relative humidity values and temperatures as well as different respiratory events, including sneeze and cough.
Section 2 introduces the governing equations of the Eulerian–Lagrangian CFD model and all the applied sub-models.
Section 3 then presents the proposed RAM and its underlying assumptions.
Section 4 demonstrates sample cases to assess the performance of the proposed method for different exhalation activities in various thermal conditions.
Section 5 presents the results, and
Section 6 provides conclusions and recommendations for future work.
2. Methodology
In this study, an Eulerian–Lagrangian CFD model is used to simulate the aerodynamics and thermodynamics of droplets released from respiratory events in several thermally conditioned spaces. The model solves the transport equations for the continuous phase (air) with the Eulerian approach while the trajectories of the droplets are determined by a Lagrangian method. Moreover, the CFD model includes the heat and mass transfer between droplets and the surrounding air to account for relative humidity and temperature alterations. Additionally, each droplet is assumed to be composed of a non-evaporative nucleus in a soluble evaporative liquid.
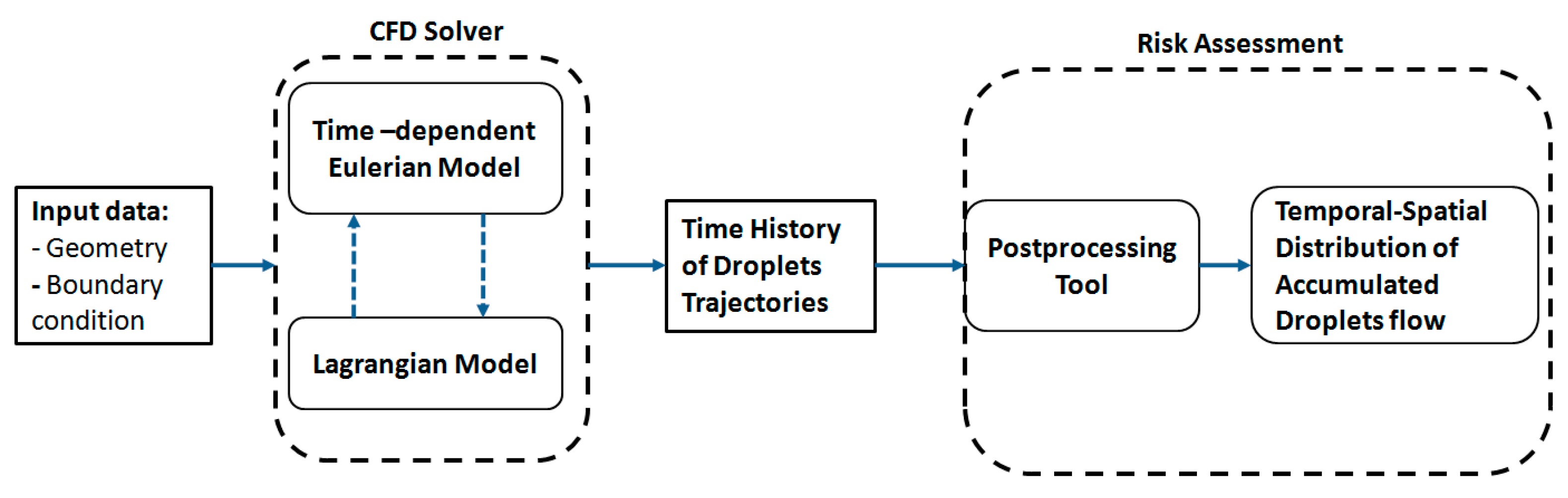
To predict the temporal accumulated distribution of droplets and, hence, calculate the associated risk of disease aerosol transmission, a novel framework was developed, as shown in
Figure 1. The input to the algorithm is the CFD simulation output data, including the diameter, position, and velocity of the droplets. The algorithm then predicts the accumulated number of droplets passing different locations within a fixed time interval. The computed concentrations will then be used to calculate the risk of disease transmission in a specific location in the space according to the available data for the minimum infective dosage.
2.1. Eulerian CFD Model
The governing equations of the Eulerian model for an unsteady incompressible flow include conservation of mass, momentum, and energy, as represented in Equations (1)–(3):
where
x,
u,
,
,
P, and
F represent direction, velocity, density, kinematic viscosity, pressure, and body force, respectively;
D denotes the material derivate; and
e and
T represent the internal energy and temperature of the flow, respectively. The simulations also consider the buoyancy force.
Furthermore, the realizable k-epsilon model was used to solve the turbulence inside the flow field.
The main simulations, regardless of the number of droplets and the droplet distributions for sneeze and cough events, included energy equations in the droplet phase as well. While the transport of the discrete phase (droplets) was solved using a Lagrangian approach, the realizable k-epsilon turbulence model was employed for simulation of the continuous phase (air).
2.2. Lagrangian Discrete Phase Model
The respiratory droplets were modeled as Lagrangian particles [
21] using the commercial CFD solver of Simcenter STAR-CCM+, where the conservation equations of mass, momentum, and energy of the discrete phase are derived for each droplet in a Lagrangian form to calculate their trajectories:
where
i symbolizes the coordinate direction (
i =
x,
y or
z), subscript
p represents particles,
is the force per unit particle mass (acceleration), and the term
represents the drag force (force per unit particle mass) calculated as:
where
is the molecular dynamic viscosity of the fluid, and
is the particle diameter.
is the particle Reynolds number, given as follows:
In the present study, the respiratory droplets released from the human mouth are considered as a discrete phase dispersed and carried out with the background airflow of the respiratory air jet. If the dispersed phase is volatile, soluble, or reactive, then mass transfer occurs between the phases accompanied by an inter-phase heat transfer. Hence, heat transfer can take place because of the inter-phase temperature differences. The inter-phase mass transfer causes a size change in the dispersed material particles as described in the following sections.
While collision between particles [
28,
29] is the main source of energy transfer in many two-phase flows—for instance, granular flows [
30,
31,
32]—past studies showed that collision and break-up of respiratory droplets can be safely skipped when simulations aim at characterizing the flow over a large distance and a long time [
33,
34]. Inter-droplet interactions such as collision [
28,
31,
35] or agglomeration are not considered because of the low concentration of the discrete phase as well as the rapid evaporation of released droplets, which drastically reduces the size of the droplets in a very short time and consequently reduces the chance of any interactions between the droplets.
2.3. Particle Mass Balance
The conservation of the mass of particles can be expressed as follows:
where
denotes the mass of the particle, and
represents the rate of mass transfer from the particle. The latter has a non-zero value due to the evaporation process.
2.4. Droplet Evaporation
In the case of internally homogeneous droplets having a single liquid, such as a chemical species, the rate of change of droplet mass due to quasi-steady evaporation can be formulated as follows:
where
represents the mass transfer conductance and
is known as the Spalding transfer number.
Similarly, the multi-component droplet evaporation model assumes that droplets are internally homogeneous, consisting of an ideal mixture of liquid components subject to vaporization. Moreover, the model assumes inert components in both the droplet and the gas. Regarding the evaporation of multi-component droplets,
is defined as the rate of change of mass of each transferred component due to quasi-steady evaporation:
where
i is the index of each component in the mixture, and
represents the fractional mass transfer rate, for which the sum of all
N components complies with the following equation:
2.5. Particle Energy Balance
By considering small-sized droplets and assuming that the particles are internally homogenous (i.e., low Biot number (<0.1)), the conservation of energy can be derived as follows:
where
is the rate of convective heat transfer to the particle from the continuous phase,
represents the rate of radiation heat transfer, and
is related to other heat sources. In this study, radiative heat transfer is considered negligible.
2.6. CFD Model Setup
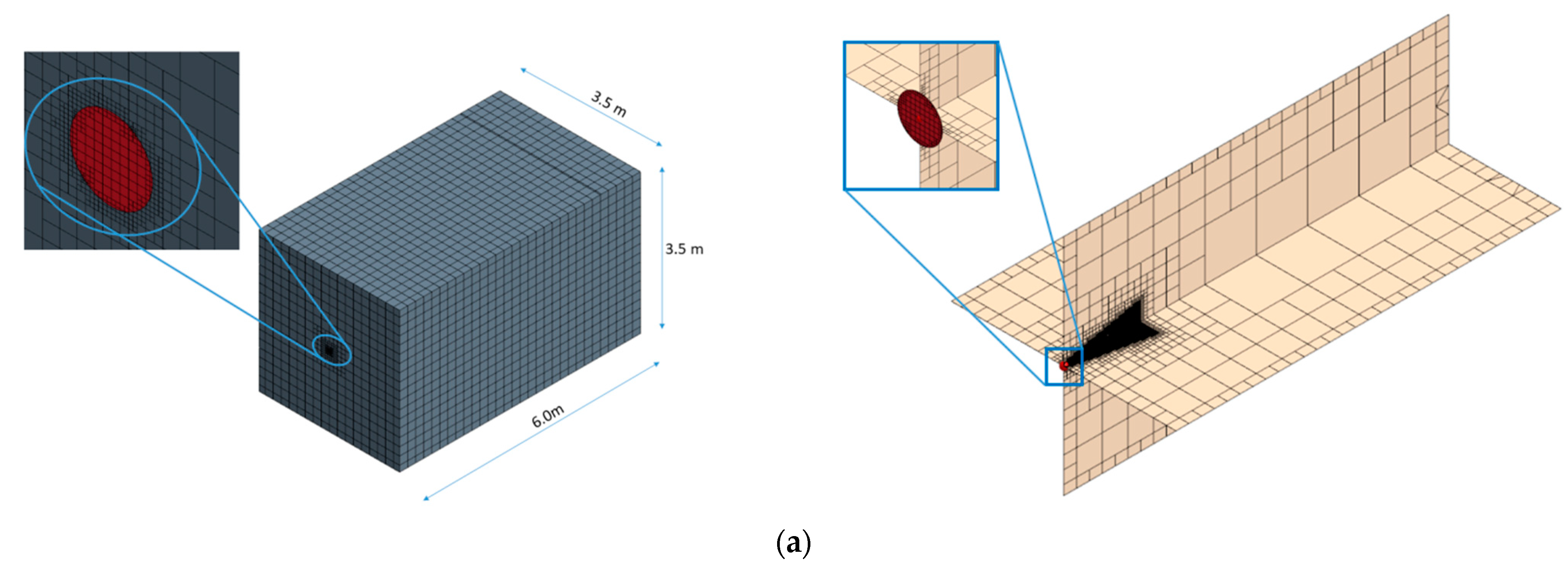
The computational domain was a 3.5 × 3.5 × 6 m room (
Figure 2a). The dimensions of the domain were selected after a series of preliminary simulations ensuring the adequacy of the room dimensions for analysis of the airborne behavior of droplets when the exhalation jet’s normalized velocity reaches the value of 0.001. It should be noted that since the measured velocities in all the sneeze or cough experiments depend on the patients’ characteristics (sex, age, weight, body type, etc.), a velocity profile normalized with respect to the sneeze or cough velocity at the location of the mouth can provide a better non-dimensional tool for comparison. In the present research, the exhalation jet velocity at the inlet was used to normalize the velocity field inside the domain. These simulations were performed for different droplet sizes from 0.1 to 100 μm. The results of the simulations, conducted within a wide time span, implied that after a long period of simulations, droplets with a diameter of 10 μm or below had become airborne, traveling up to 6 m from the emitting surface with a terminal velocity below 0.02 m·s
−1, while droplets with a diameter of 100 μm regimented at much smaller distances of about 1 m from the jet inlet. The size of the final domain together with the velocity contour of the simulated air jet is presented in
Figure 2b.
The respiratory droplets were released from a circular area located at the center of the 3.5 × 3.5 m wall with a diameter of nearly 1.2 cm, which aligned with the reported values in the literature [
29,
30,
31]. The front boundary against the source was an outlet and the rest of the surfaces were considered as solid walls with no-slip boundary conditions (see
Table 1). The Lagrangian droplets (discrete phase) were assumed to have spherical shapes and were initially composed of 3% non-evaporative and 97% evaporative mass fractions. The non-volatile fraction had a density of 1280.8 kg·m
−3 and a specific heat transfer of 2404.6 J·Kg
−1·K
−1 at the standard state temperature of 298.15 K. On the contrary, the evaporative fraction was assumed to have material properties equal to those of water (density of 997.6 kg·m
−3, specific heat transfer of 4181.7 J·Kg
−1·K
−1 at the same standard state temperature). In addition, the saturation pressure of the evaporative fraction was set to 3170.3 Pa. Having assumed the multi-component droplets, the mass-weighted mixture was used for the calculation of the density and specific heat of each droplet. In addition, regarding the boundary condition for each droplet’s outer surface, it was assumed that the droplets would stick to any wall surface of the room as they reached them. The Lagrangian model also included the Schiller–Naumann drag force coefficients and pressure gradient force to accurately simulate the droplets’ trajectories.
The background air was simulated as a non-reactive ideal gas, composed of standard air and some amount of water vapor depending on the relative humidity of each case. The dynamic viscosity, specific heat, and molecular weight of air were assumed to be 1.855 × 10−5 Pa·s, 1003.6 J·Kg−1·K−1, and 28.97 Kg·Kmol−1, respectively. Like the Lagrangian model, the weighted mixture method for the Eulerian model was employed for the calculation of the air–water mixture. With all the previous settings, the cases were solved on a computer cluster at Sogang University using 24 computational cores with Xeon(R) 2.20 GHz CPUs. The typical simulation time for 60 s was about 15 h.
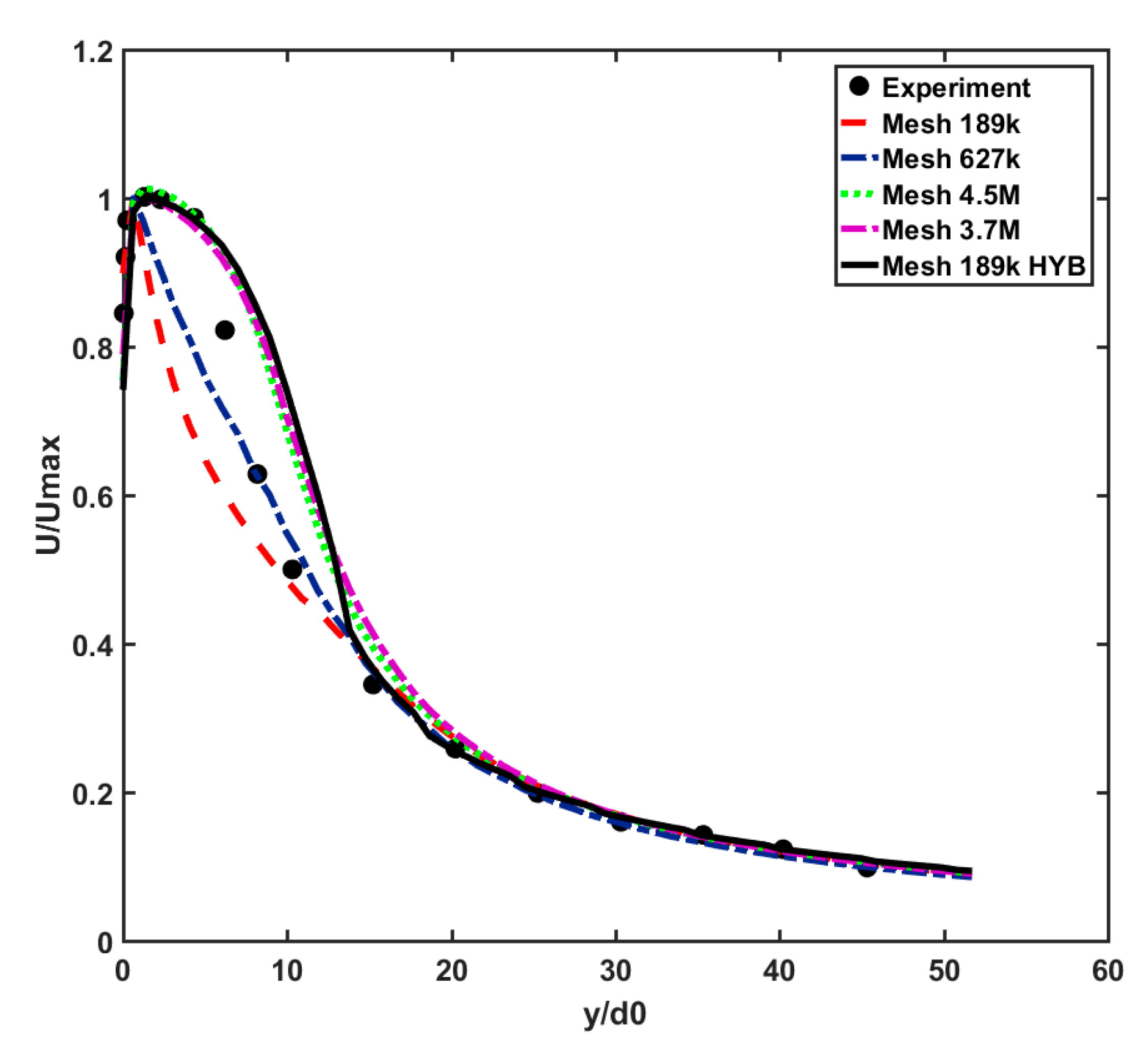
A mesh sensitivity analysis was performed to ensure the mesh independence of the CFD results and for obtaining the optimal computational grid to be used for modeling various cases. For this part, the flow velocity in the far-field zone (i.e., the distance where y/d
0 > 20 from the mouth) was investigated, and the normalized results were validated against the experimental results [
36]. The inlet velocity had spanwise (along with discharge hole radii) as well as streamwise (centerline) velocity profiles with the maximum value of 20 m·s
−1. It should be noted that the validation case of the Eulerian model was in the isothermal condition. Different mesh resolutions with hexahedral cells were investigated, ranging from 189 k cells to 4.5 M cells, as shown in
Figure 2a. The optimal mesh, 189k-HYBcase, has minimum and maximum cell sizes of 0.06 and 0.2 m, respectively, while its surface growth rate is 2.0. This results in a dense mesh of between 0.78 mm (minimum) near the mouth and 0.8 m (maximum) at the central part of the domain. To ensure the accuracy of the results near the walls, the “All Yplus” option was activated, enabling an automatic blending function between low and high Reynolds number wall treatment methods for the calculation of turbulence qualities, such as dissipation, production, and stress tensor [
37]. The summary of the applied boundary conditions to validate the Eulerian model is presented in
Table 1.
The CFD solver used a double-precision finite volume approach with a second-order discretization scheme. It also contained an energy coupling method (required for evaporation) with implicit integration.
To assess the accuracy of the centerline velocity, four validation metrics, including the factor of two observations (
FAC2), hit rate (
q), fractional bias (
FB), and normalized mean square error (
NMSE), were calculated. These metrics are defined as follows:
where
Ni is calculated as follows:
with
where
Qi and
Pi are the measured and computed values of a given variable, respectively;
n is the number of available data points, and
W and
D are the relative uncertainty and repeatability of the calculated data and their values for velocity assessments are 0.05 and 0.25, respectively. The angular brackets of the
FB and
NMSE equations represent the average of all measured points [
33,
34].
Table 2 shows the results of the validation metrics calculated at the centerline velocity diagrams of
Figure 3.
According to
Table 2, while
FAC2 and q give nearly the same values for different mesh sizes, the
FB and
NMSE values exhibit more sensitivity to the selected computational grids. For instance, applying a hybrid strategy to a mesh size of 189 k considerably improves the
FB and
NMSE values from 0.092 and 0.042 to −0.03 and 0.027, respectively, which are close to those of the case with 3.7 M cells. This implies that 189k-HYB is an optimal choice for conducting the simulations, providing the best accuracy while minimizing the CPU cost.
3. Risk Assessment Method (RAM)
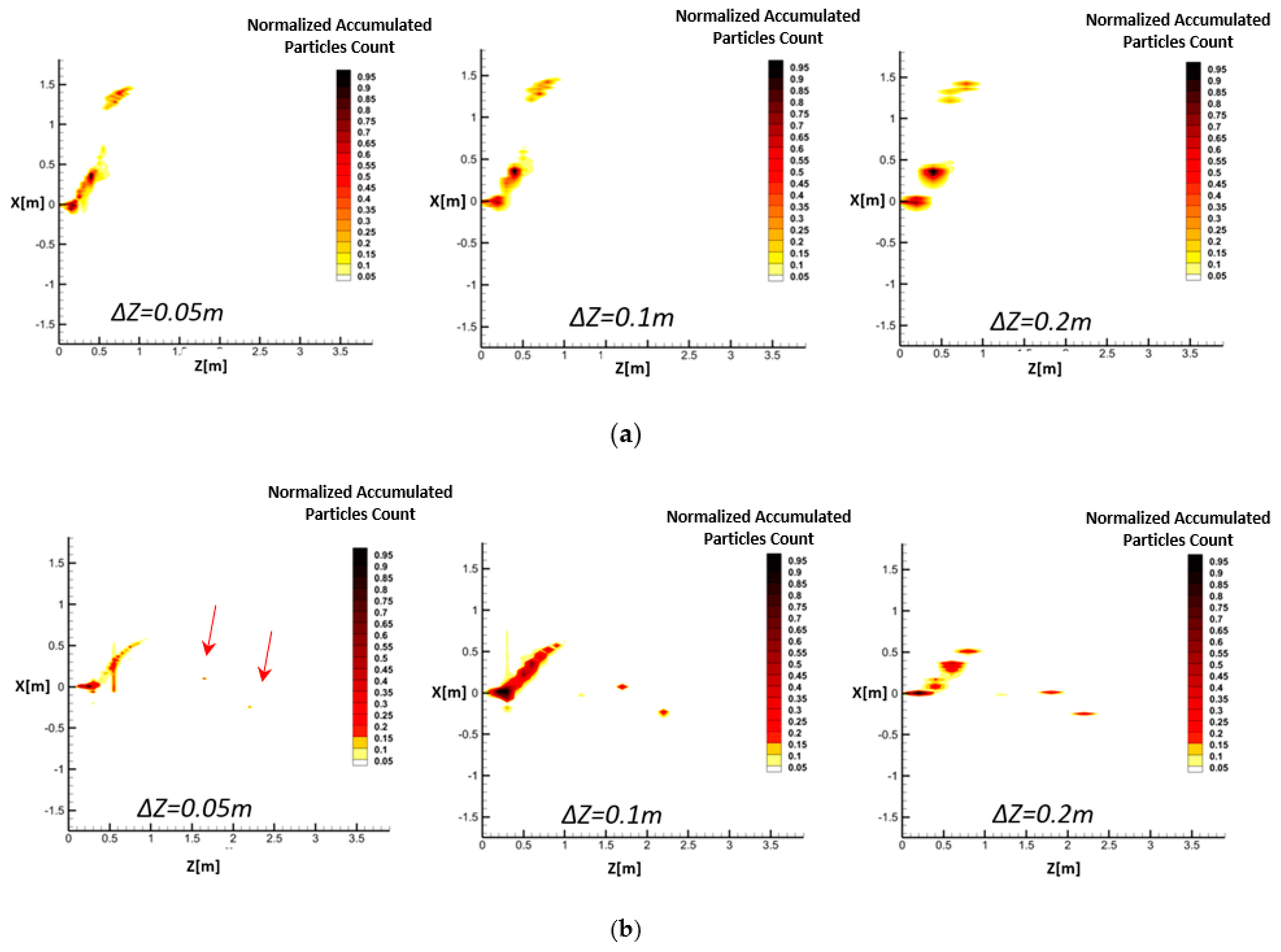
The risk of airborne disease transmission at each point of a domain is related to the summation of the virus-laden droplets passing through the point within a specific period. Thus, the time-dependent spatial risk of infection can be estimated by the temporal accumulative number of droplets passing through each point in the space. Therefore, in this section, an algorithm is developed to count the accumulated number of droplets at each location downstream of the released respiratory jet.
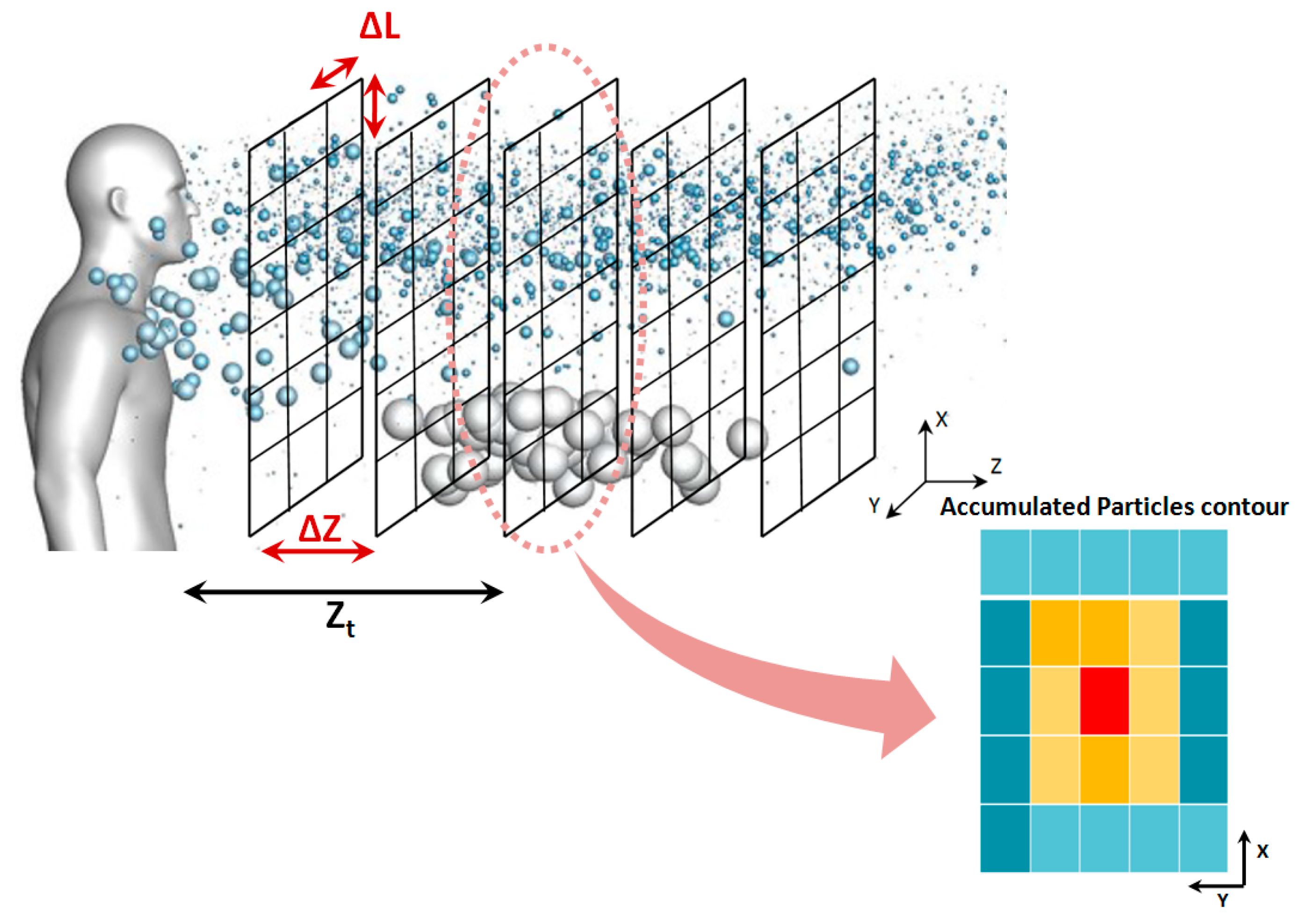
Since exhaled flows contain different droplet sizes, from sub-micron to hundreds of microns, the CFD solver may produce a table of information for each droplet size class at every time step, including position and velocity components as well as the computational grid coordinates. The length of this table depends on the number of defined droplet parcels. The algorithm defines multiple vertical planes at certain distances from the mouth (
Zt), as is shown in
Figure 4. The planes are then divided into multiple facial cells. In the next step, the algorithm counts the number of droplets passing through each cell at each time step. According to
Figure 4, calculation of the accumulated number of droplets in the field needs a separate 3D meshing with a coarser resolution than that of the CFD model, which is called a “secondary mesh”. Accordingly, the ∆
L and ∆
Z shown in
Figure 4 present the resolution of the secondary mesh.
After constructing the secondary mesh, by using the transient CFD data for the simulation period of 0 to t
1, the proposed algorithm calculates the accumulated number of droplets passing through each of the facial cells within the secondary mesh. As shown in
Figure 4, the droplets that pass the plane
at
(while not having reached it at
) should be identified and counted for each vertical plane of the secondary mesh. Here,
and
represent two consecutive times at which the CFD transient data are collected. For this purpose, the droplets’ positions at the previous time step are approximated using the following equation:
where
and
are the positions of each droplet at
and
in the Z-direction shown in
Figure 4, respectively, which are obtained from CFD results;
is the
z component of each droplet’s velocity vector at
, and
dt is the CFD solution time step. In other words,
and
are the instantaneous positions of droplets according to the CFD solver time step (
).
is known and
is predicted by Equation (16).
Assuming that
dt is small enough,
can be assumed to be constant from
to
. By approximation of the
Z location of each droplet in the previous time step (
) and its current location (
), the droplets passing through the plane
between
and
can be identified.
In Equation (17), is the time required for each droplet to pass the plane that is positioned at , which is different from the CFD solver . Therefore, possesses only discrete values depending on the resolution of the secondary mesh. and are in-plane estimated positions of droplets, while and are their instantaneous positions inside the domain.
In the next step, the algorithm finds the
and
coordinates of the point in the plane
where the droplet crossed the plane
. The
X and
Y positions of each droplet passing through the plane
can be determined as follows:
where
, represent the position and
and
are the velocity components of the droplets obtained from the transient CFD output data file at
in
X and
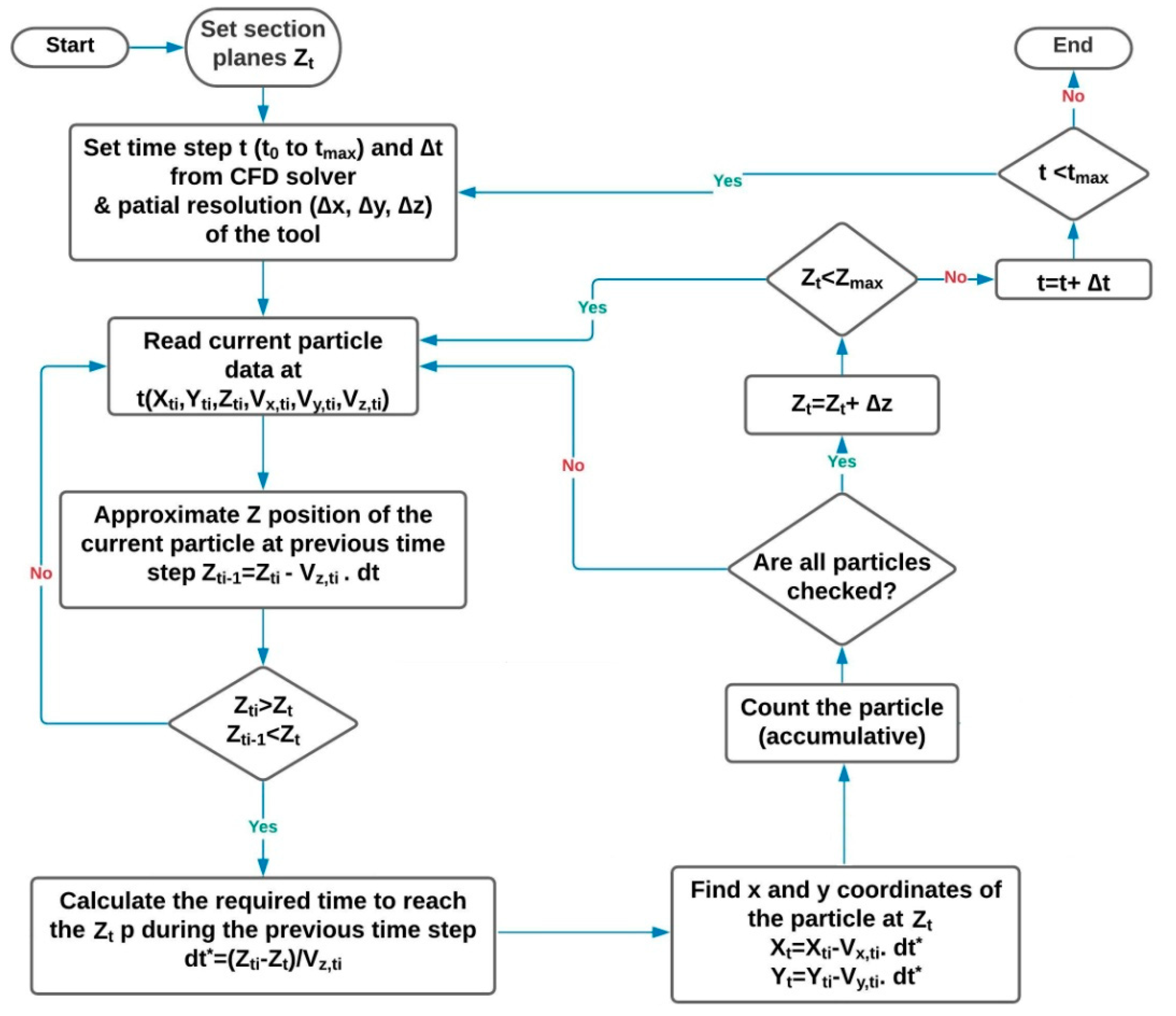
Y directions. The graphical representation of the abovementioned procedure for the RAM is presented as a flowchart in
Figure 5. A computer program code was written in MATLAB software for post-processing of the CFD simulations based on this flowchart.
In fact, according to
Figure 5,
is compared with
Zt at each time step to see if it passes the plane
Zt within
dt. If yes, Equation (17) is used to estimate the time required for that (i.e.,
dt*). Then, the calculated
dt* is applied to calculate the in-plane positions of droplets (
Xt and
Yt) using Equations (18) and (19).
By following this procedure, one can understand how many droplets passed through different facial cells in a certain plane
from
to
By repeating this process for all the time steps between the beginning of a respiratory event CFD simulation and an arbitrary time, the temporal accumulated number of droplets passing through different facial cells can be resolved. This generates droplet number contours similar to those schematically shown in
Figure 4. Eventually, following a selected clinical threshold for the minimum number of droplets in an epidemic model (here, it is 100 droplets [
27]), it is possible to perform a risk assessment of safe distances. Therefore, only regions with an accumulated number of passing droplets higher than 100 are considered risky areas.
4. Case Studies
Different exhalation activities are modeled using boundary conditions describing pathogen bio-sources, which are referred to in several related clinical research works [
35,
38,
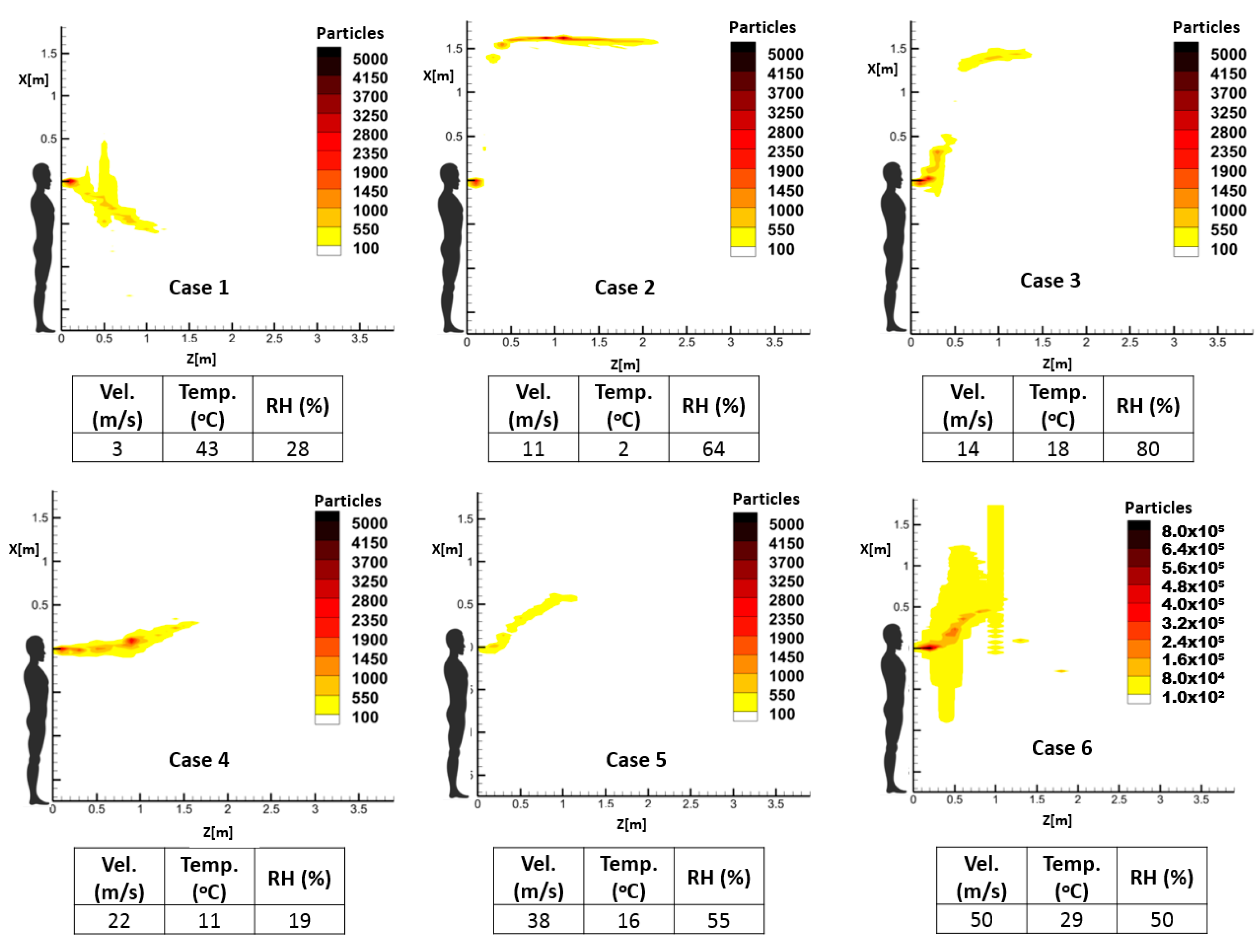
39]. This information includes the temporal velocity profile, flow rate, and distribution of droplet size in different modes of respiratory events, namely cough, and sneeze. Since respiratory events are modeled as exhaled droplets that immediately evaporate when they are discharged to the environment, the temperature and relative humidity of the space can deeply influence the evaporation and thus the dispersion pattern of the droplets. Therefore, droplets’ velocity, the temperature and relative humidity of the space for some sample case studies are introduced in
Table 3. These parameters were selected to represent different spreading patterns. It should be noted that in these cases, the background velocity of the target environment is not considered. The CFD results of the case studies were then processed using the novel proposed risk assessment algorithm. It should be noted that the exhalation jet temperature of all the following cases was set as 37 °C. According to the reported velocity ranges in the literature [
36,
40], different scenarios were defined as shown in
Table 3 to represent a range of respiratory events to evaluate the performance of the proposed RAM. Moreover, the droplet number and size distribution of the scenarios were adapted from experimental studies [
36,
37].
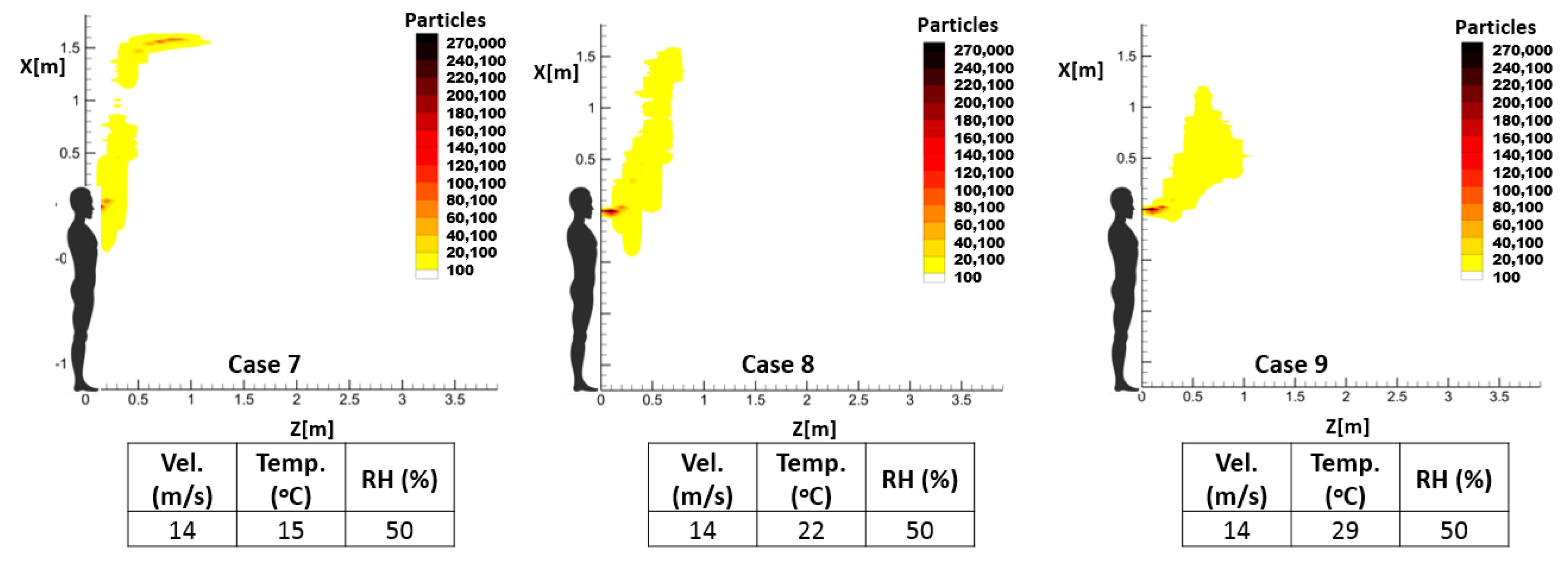
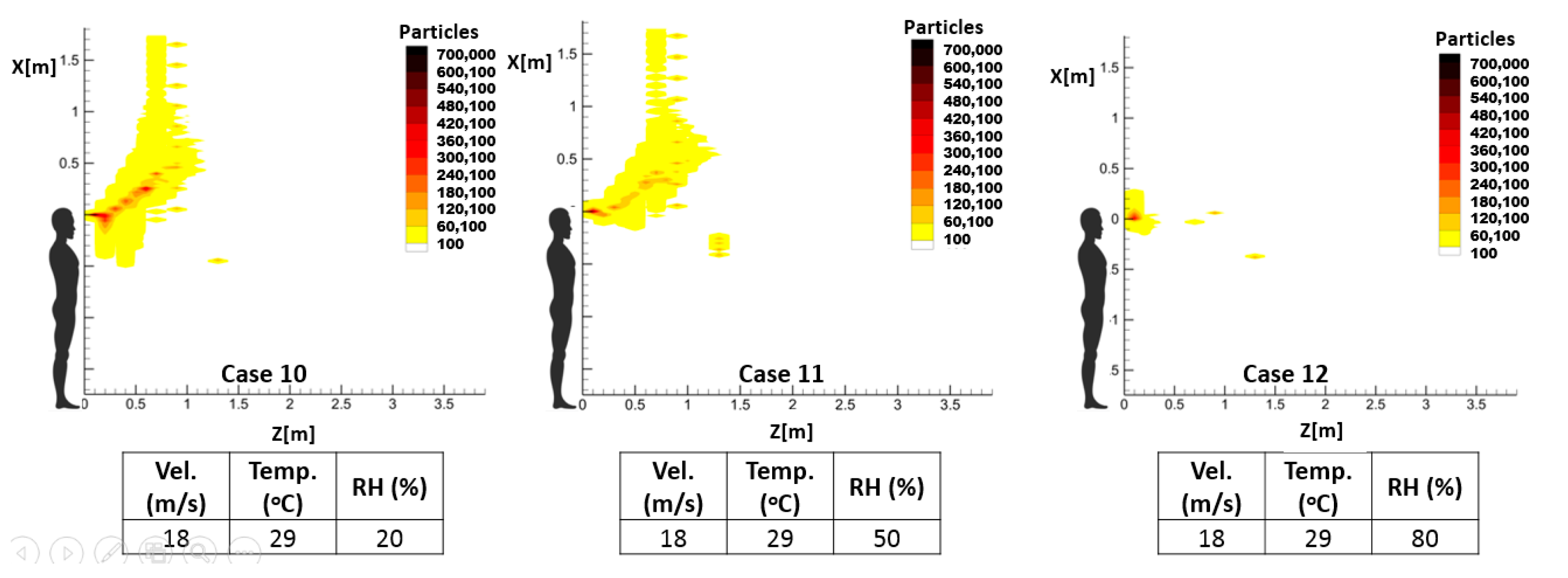
As mentioned earlier, the temperature and relative humidity of the environment has an important influence on the spread pattern and consequently the propagation of infective pathogens. Thus, investigating the impact of these environmental parameters on the accumulative distribution of droplets and, consequently, the high-risk zone will demonstrate one notable application of the proposed prediction method. For this purpose, three sample cough and sneeze cases were defined to demonstrate the respective impacts of the temperature and relative humidity of the environment, while keeping the other parameters unchanged.
Table 4 illustrates the characteristics of these exhalation activities.
Table 5 includes experimental data regarding the number and distribution of exhaled droplets during different exhalation activities.
6. Concluding Remarks
A new assessment method has been presented to find the time-dependent accumulative concentration of infectious exhaled droplets based on CFD simulations. The CFD calculation results encompass the instantaneous position of droplets, while the infection is associated with receiving a sufficient infective dosage during the exposure time, which necessitates the calculation of the accumulated particles within an arbitrary time interval. For this purpose, transient CFD output data files including the position and velocity vectors of each released droplet are generated. By implementing a secondary mesh for post-processing of the transient CFD data, temporospatial droplet trajectories can be traced via a computational code. The CFD results of different respiratory events in terms of exhaled jet maximum velocity as well as environmental conditions were applied to the proposed framework to calculate droplets’ spreading lengths. The following remarks can be concluded:
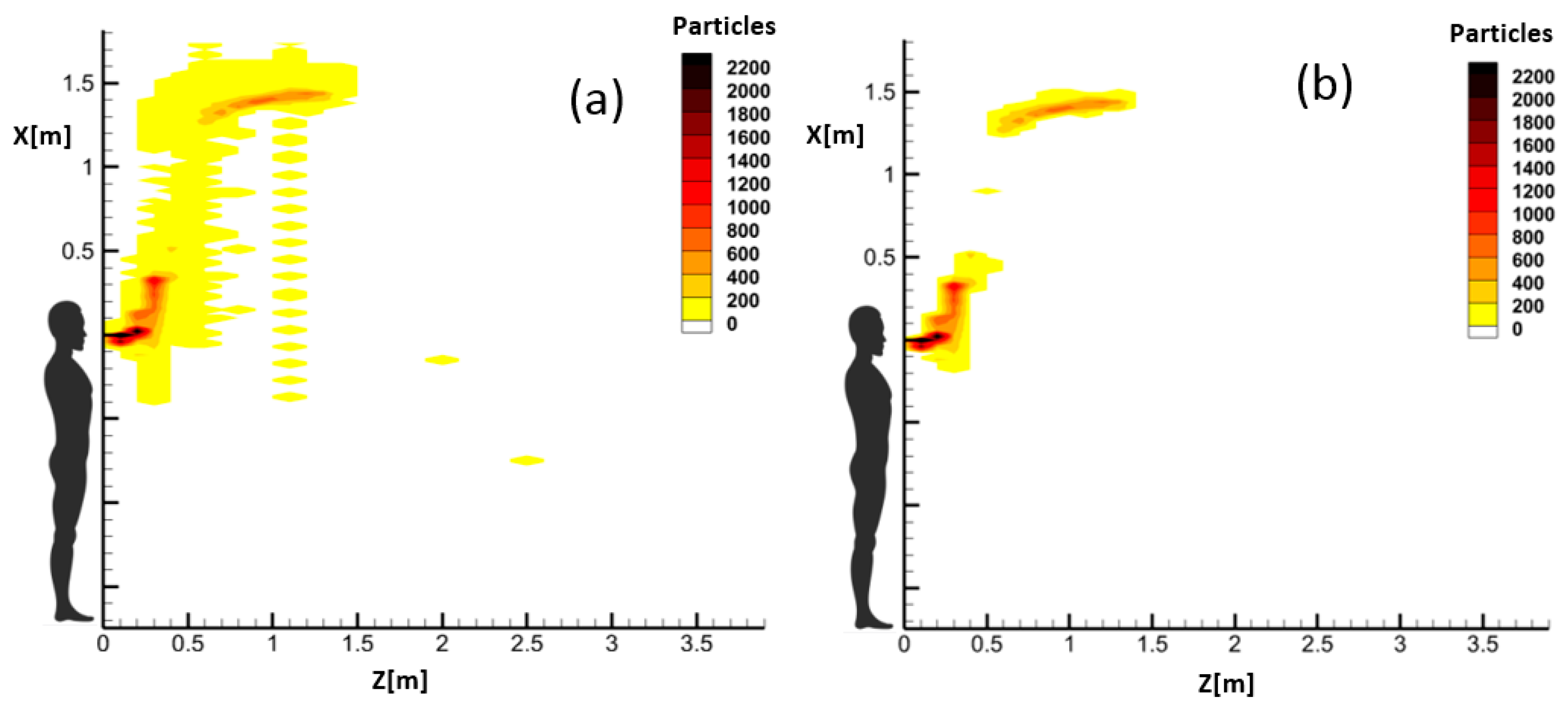
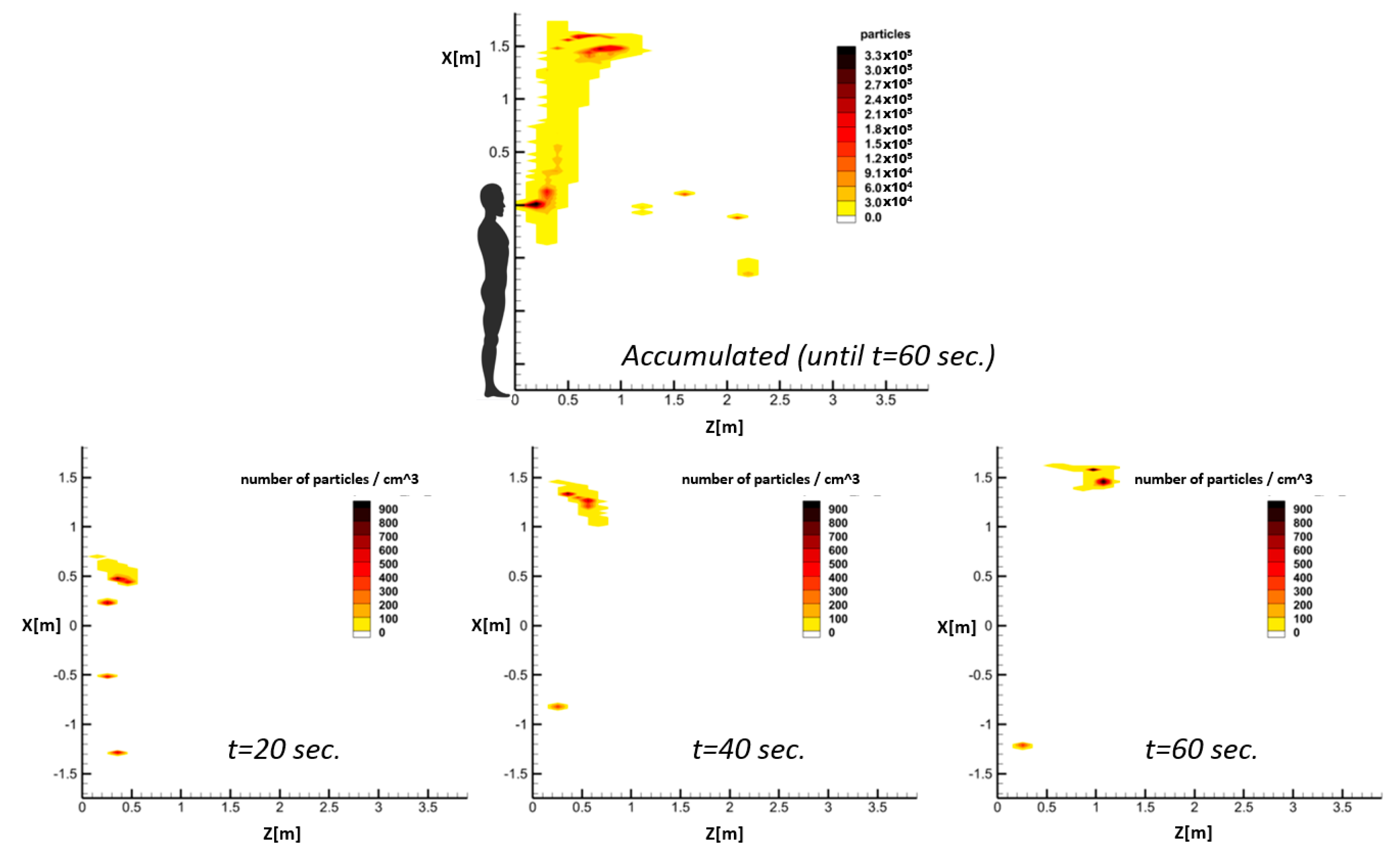
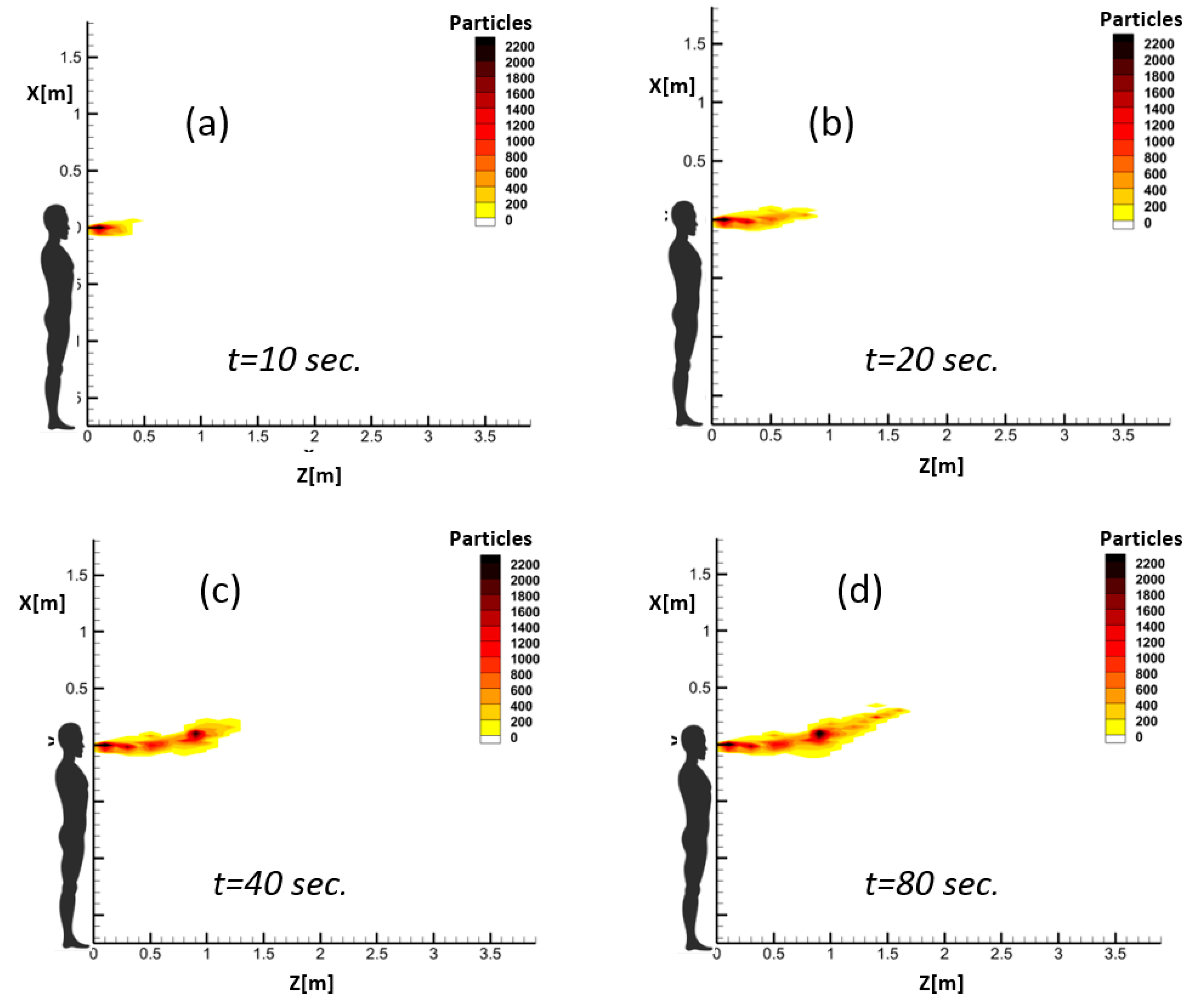
The obtained results indicate that the proposed RAM method can successfully capture different respiratory events. Hence, the length and height of spread as well as the overall behavior of different exhalation jets can be studied using the proposed RAM.
While the instantaneous CFD output data of droplets can barely provide any information regarding risky and safe zones inside a domain, the proposed model can represent the evolution of risky zones in time. Comparison of the instantaneous and accumulated droplets indicated that most of the heavy falling particles do not pass through the Z-constant planes through the field, which is used in the accumulated representation. Since these particles do not contribute to the dispersion of droplets in the domain, this adds no error to the calculated spreading length and height of airborne droplets.
The proposed RAM can be used to derive safe social distances in terms of exposure time. This is especially important in short-duration respiratory activities such as coughing or sneezing in which considerable viral loads are released by an infected person and the safe distance for susceptible persons depends on their exposure time.
The calculated results confirm that the sensitivity of the predicted spreading patterns to environmental temperature and relative humidity can be investigated by the proposed RAM. According to selected sample cases, both parameters can influence the propagation length and even the mechanism by which droplets are transmitted inside the environment.
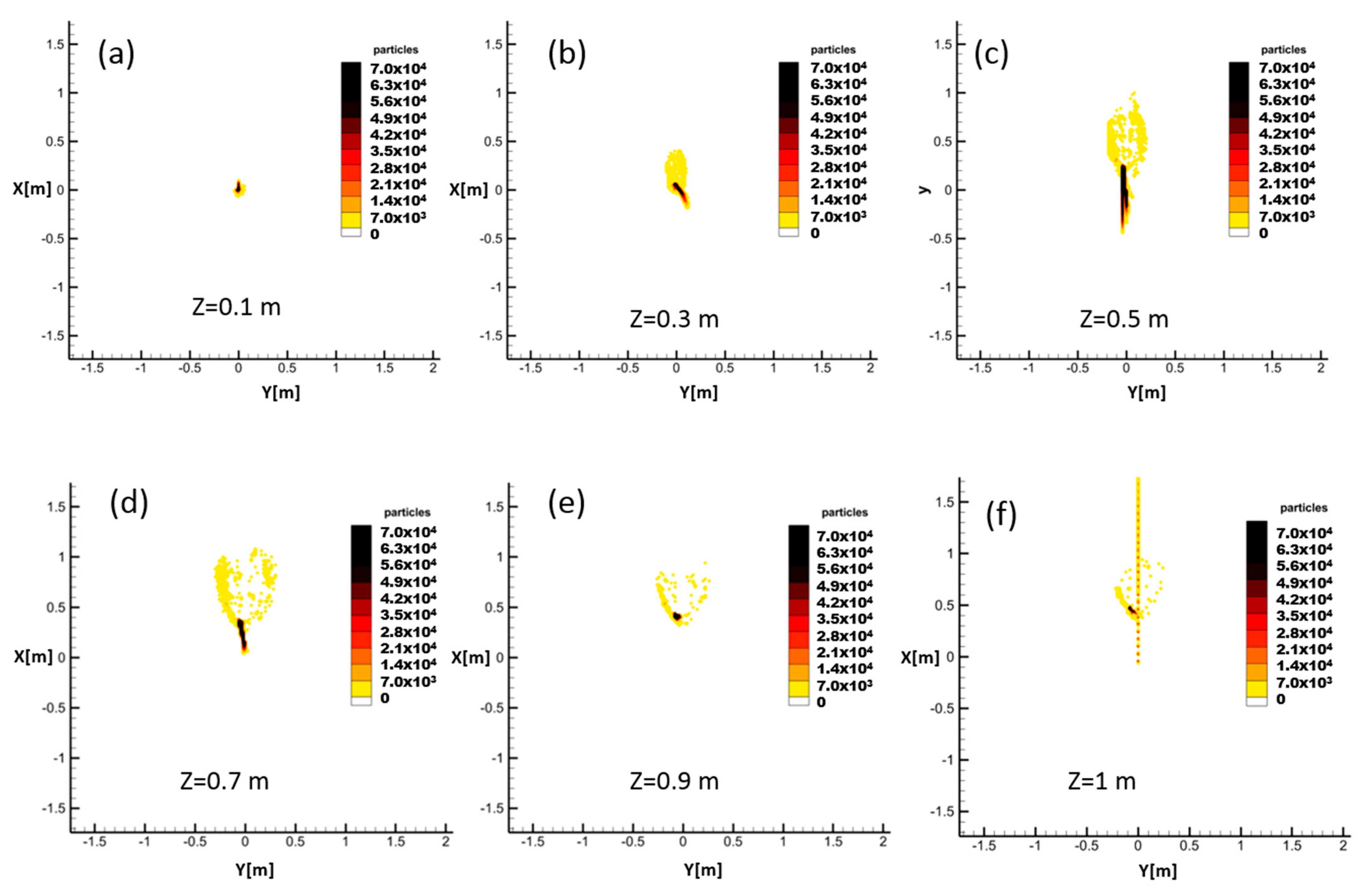
Finally, since this method is a 3D prediction model, it is possible to investigate the cross-section of the spreading pattern of droplets through Z-constant planes. These data can be used to define 3D risky regions. This will be the subject of our future work to add a lateral dimension to risk evaluation and further improve the resolution of social distance calculations. This will be particularly useful for spaces where a high density of people is inevitable. Additionally, this code can be adapted to specify risky volumes, as opposed to safe horizontal distances, including complex flow patterns caused by advection, buoyancy, and gravitational settling of particles.
Among the restrictions of this research work, one can identify the insufficient clinical data on exhalation activities as well as the infective dosage of COVID-19, which has a crucial impact on safe social distance calculations. Another limitation of this work is that the instantaneous position of droplets was approximated based on the available data of the previous time step of the CFD calculations. If the time-dependent position of droplets on each streamline is known, it would result in more precise predictions.
Future work can include an adaptation of this code to risky volumes, as opposed to safe horizontal distance. With complex flow patterns caused by advection, buoyancy and gravitational settling of particles, the risky environment can become complex. Perhaps the entire 3D space around the infection source can be broken down to 20–50 volumes and the risk in each volume can be calculated. In monitoring volumes, you would simply consider the time integral of the number of droplets in each volume at any given time.