Association of Cardiovascular Disease and Long-Term Exposure to Fine Particulate Matter (PM2.5) in the Southeastern United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
- self-reported Black or White race,
- were recruited through a CHC, and
- we were able to model daily measures of PM2.5 exposure from ambient and satellite captured data for twelve months before their enrollment composed our analytic cohort.
2.2. Enrollment Instrument
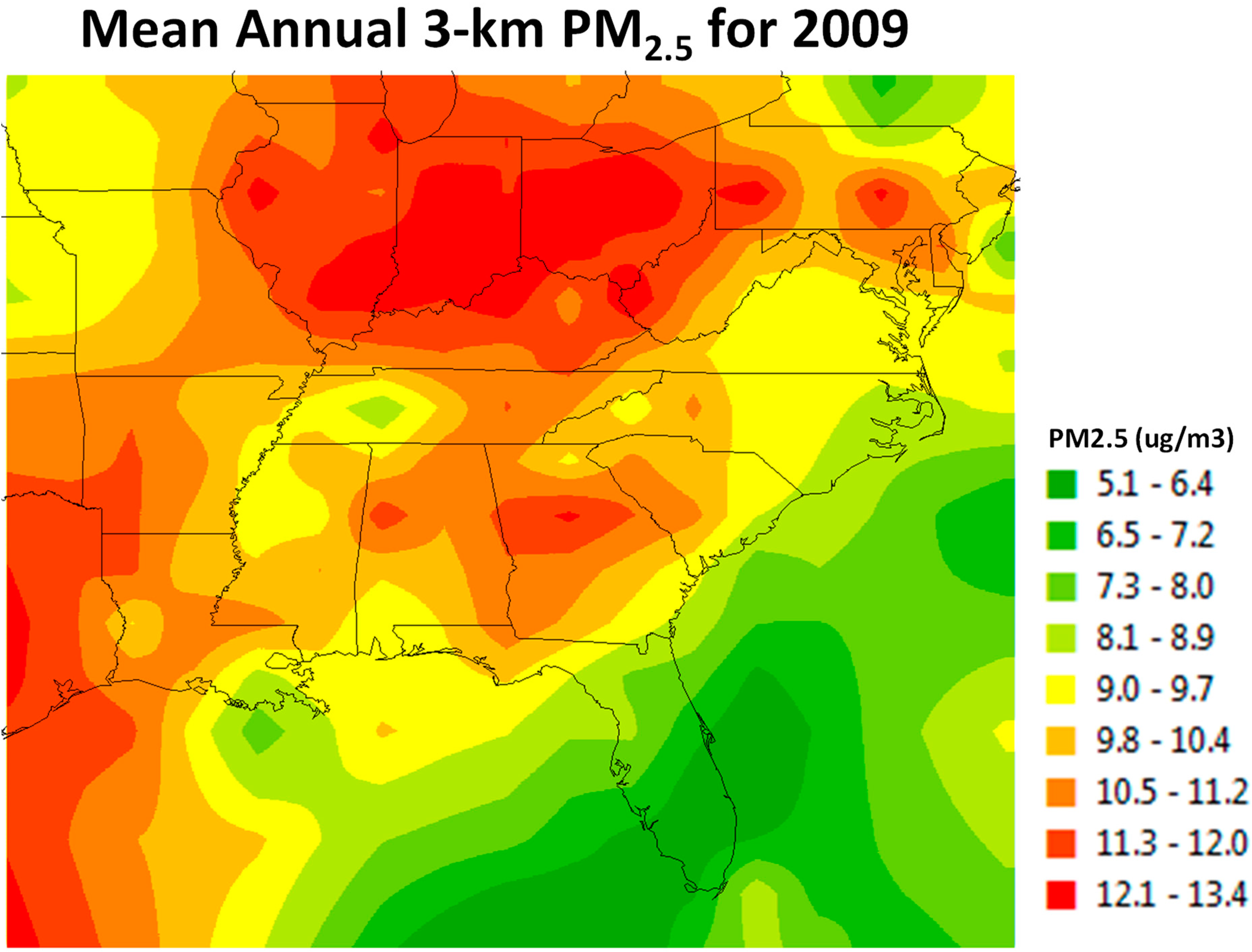
2.3. Air Pollution Exposure
2.4. Self-Report Cardiovascular Disease Ascertainment
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinelli, N.; Olivieri, O.; Girelli, D. Air particulate matter and cardiovascular disease: A narrative review. Eur. J. Intern. Med. 2013, 24, 295–302. [Google Scholar] [CrossRef]
- Lipfert, F.W. Long-term associations of morbidity with air pollution: A catalog and synthesis. J. Air Waste Manag. Assoc. 2018, 68, 12–28. [Google Scholar] [CrossRef] [PubMed]
- 2019 National Healthcare Quality and Disparities Report; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020; AHRQ Pub. No. 20(21)-0045-EF.
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Walker, B., Jr.; Mouton, C.P. Environmental influences on cardiovascular health. J. Natl. Med. Assoc. 2008, 100, 98–102. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Burger, M.R.; Coull, B.A.; Schwartz, J.; Suh, H.H.; Koutrakis, P.; Schlaug, G.; Gold, D.R.; Mittleman, M.A. Ambient air pollution and the risk of acute ischemic stroke. Arch. Intern. Med. 2012, 172, 229–234. [Google Scholar] [CrossRef]
- Wang, Y.; Eliot, M.N.; Wellenius, G.A. Short-term Changes in Ambient Particulate Matter and Risk of Stroke: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014, 3, e000983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Wang, J.; Jiang, Q.; He, Z.; Huang, Y.; Li, Z.; Cai, L.; Cao, S. Long-term exposure to PM(2.5) and stroke: A systematic review and meta-analysis of cohort studies. Environ. Res. 2019, 177, 108587. [Google Scholar] [CrossRef]
- Scheers, H.; Jacobs, L.; Casas, L.; Nemery, B.; Nawrot, T.S. Long-Term Exposure to Particulate Matter Air Pollution Is a Risk Factor for Stroke: Meta-Analytical Evidence. Stroke 2015, 46, 3058–3066. [Google Scholar] [CrossRef] [Green Version]
- Signorello, L.B.; Hargreaves, M.K.; Blot, W.J. The Southern Community Cohort Study: Investigating Health Disparities. J. Health Care Poor Underserved 2010, 21, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamdan, M.; Crosson, W.; Limaye, A.; Rickman, D.; Quattrochi, D.; Estes, M.; Qualters, J.; Sinclair, A.; Tolsma, D.; Adeniyi, K.; et al. Methods for Characterizing Fine Particulate Matter Using Ground Observations and Satellite Remote-Sensing Data: Potential Use for Environmental Public Health Surveillance. J. Air Waste Manag. Assoc. 2009, 59, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Hargreaves, M.K.; Steinwandel, M.D.; Zheng, W.; Cai, Q.; Schlundt, D.G.; Buchowski, M.S.; Arnold, C.W.; McLaughlin, J.K.; Blot, W.J. Southern community cohort study: Establishing a cohort to investigate health disparities. J. Natl. Med. Assoc. 2005, 97, 972–979. [Google Scholar]
- Cannon, C.P. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone 2007, 8, 11–28. [Google Scholar] [CrossRef]
- Al-Hamdan, M.Z.; Crosson, W.L.; Economou, S.A.; Estes, M.G., Jr.; Estes, S.M.; Hemmings, S.N.; Kent, S.T.; Puckett, M.; Quattrochi, D.A.; Rickman, D.L.; et al. Environmental Public Health Applications Using Remotely Sensed Data. Geocarto Int. 2014, 29, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, M.; Halloway, T.; Choi, S.; O’Neill, S.M.; Al-Hamdan, M.Z.; Van Donkelaar, A.; Martin, R.V.; Jin, X.; Fiore, A.M.; Henze, D.K.; et al. Methods, availability, and applications of PM2.5 exposure estimates derived from ground measurements, satellite, and atmospheric models. J. Air Waste Manag. Assoc. 2019, 69, 1391–1414. [Google Scholar] [CrossRef] [PubMed]
- National Ambient Air Quality Standards for Particulate Matter. Federal Register 78 FR 3086: 1/15/2013; 15 January 2013. Available online: https://www.govinfo.gov/content/pkg/FR-2013-01-15/pdf/2012-30946.pdf (accessed on 20 July 2021).
- Reitz, C.; Schupf, N.; Luchsinger, J.A.; Brickman, A.M.; Manly, J.J.; Andrews, H.; Tang, M.X.; DeCarli, C.; Brown, T.R.; Mayeux, R. Validity of self-reported stroke in elderly African Americans, Caribbean Hispanics, and Whites. Arch. Neurol. 2009, 66, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Engstad, T.; Bonaa, K.H.; Viitanen, M. Validity of self-reported stroke: The Tromso Study. Stroke 2000, 31, 1602–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C.; Merlo, J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat. Med. 2017, 36, 3257–3277. [Google Scholar] [CrossRef]
- Goldstein, H.; Browne, W.; Rasbash, J. Partitioning variation in multilevel models. Stat. Issues Psychol. Educ. Soc. Sci. 2002, 1, 223–231. [Google Scholar] [CrossRef]
- Peters, A. Particulate matter and heart disease: Evidence from epidemiological studies. Toxicol. Appl. Pharmacol. 2005, 207, 477–482. [Google Scholar] [CrossRef]
- Li, C.; Grove, M.L.; Yu, B.; Jones, B.C.; Morrison, A.; Boerwinkle, E.; Liu, X. Genetic variants in microRNA genes and targets associated with cardiovascular disease risk factors in the African-American population. Hum. Genet. 2018, 137, 85–94. [Google Scholar] [CrossRef]
- Lee, C.R.; North, K.E.; Bray, M.S.; Avery, C.L.; Mosher, M.J.; Couper, D.J.; Coresh, J.; Folsom, A.R.; Boerwinkle, E.; Heiss, G.; et al. NOS3 polymorphisms, cigarette smoking, and cardiovascular disease risk: The atherosclerosis risk in communities’ study. Pharm. Genom. 2006, 16, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Juarez, P.D.; Tabatabai, M.; Valdez, R.B.; Hood, D.B.; Im, W.; Mouton, C.P.; Colen, C.; Al-Hamdan, M.Z.; Matthews-Juarez, P.; Lichtveld, M.Y.; et al. The Effects of Social, Personal, and Behavioral Risk Factors and PM2.5 on Cardio-Metabolic Disparities in a Cohort of Community Health Center Patients. Int. J. Environ. Res. Public Health 2020, 17, 3561. [Google Scholar] [CrossRef]
- Munzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef] [PubMed]
- EPA. Annual Report. Our Nation’s Air 2020. Available online: https://gispub.epa.gov/air/trendsreport/2020/#home (accessed on 13 August 2020).
- EPA. Particulate Matter (PM) Standards—Table of Historical PM NAAQS. In Reviewing National Ambient Air Quality Standards (NAAQS). 2020. Available online: https://www3.epa.gov/ttn/naaqs/standards/pm/s_pm_history.html (accessed on 13 August 2020).
- Stockfelt, L.; Andersson, E.M.; Molnár, P.; Gidhagen, L.; Segersson, D.; Rosengren, A.; Barregard, L.; Sallsten, G. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ. Res. 2017, 158, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Zheng, Y.; Li, X.; Liu, J.; Zhang, Q.; Zhu, T. A component-specific exposure-mortality model for ambient PM2.5 in China: Findings from a nationwide epidemiology based on outputs from a chemical transport model. Faraday Discuss. 2021, 226, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Wyzga, R.E.; Rohr, A.C. Long-term particulate matter exposure: Attributing health effects to individual PM components. J. Air Waste Manag. Assoc. 2015, 65, 523–543. [Google Scholar] [CrossRef]
- Juarez, P.D.; Hood, D.B.; Song, M.-A.; Ramesh, A. Use of an exposome approach to understand the effects of exposures from the natural, built, and social environments on cardio-vascular disease onset, progression, and outcomes. Front. Public Health 2020, 17, 3661. [Google Scholar] [CrossRef]
- Fitchen, J.M. Residential Mobility among the Rural Poor1. Rural. Sociol. 1994, 59, 416–436. [Google Scholar] [CrossRef]
| Variable | Percent |
|---|---|
| Over 12 ug/m3 PM2.5 | 83.0 |
| Male | 39.1 |
| Black | 67.1 |
| Employed | 36.3 |
| Senior | 10.1 |
| Education | |
| No High School | 30.4 |
| High School | 35.9 |
| Some College | 24.3 |
| College or more | 9.4 |
| Marital Status | |
| Married | 32.4 |
| Separated | 35.1 |
| Widowed | 9.6 |
| Single | 22.8 |
| Income | |
| Less than USD 15,000 | 60.0 |
| USD 15,000–25,000 | 21.7 |
| USD 25,000–50,000 | 12.7 |
| More than USD 50,000 | 5.7 |
| Inside Home Air Quality | |
| Poor | 6.1 |
| Fair | 28.8 |
| Good | 52.2 |
| Excellent | 12.9 |
| Cardio-Metabolic Disease Risk | |
| CMD Risk | 70.2 |
| Variable | PM2.5 < 12 µg/m3 | PM2.5 > 12 µg/m3 | p-Value |
|---|---|---|---|
| Education * | 0.799 | ||
| Less than High School | 30.2% | 30.4% | |
| High School | 36.4% | 35.8% | |
| Voc., Tech, or Some College | 24.0% | 24.3% | |
| College Graduate or Beyond | 9.3% | 9.5% | |
| Marital Status * | <0.0001 | ||
| Married or with Partner | 34.5% | 32.0% | |
| Divorced | 34.5% | 35.2% | |
| Widowed | 10.2% | 9.5% | |
| Single | 20.7% | 23.3% | |
| Household Income * | <0.0001 | ||
| Less than USD 15,000 | 59.1% | 60.2% | |
| USD 15,000–24,999 | 19.9% | 22.0% | |
| USD 25,000–49,999 | 13.5% | 12.5% | |
| USD 50,000 or more | 7.5% | 5.3% | |
| Air Quality Inside * | <0.0001 | ||
| Poor | 5.6% | 6.1% | |
| Fair | 26.4% | 29.3% | |
| Good | 52.2% | 52.2% | |
| Excellent | 15.8% | 12.3% | |
| Employed * | 36.7% | 36.2% | 0.414 |
| Elder (>65 years old) * | 12.2% | 9.7% | <0.0001 |
| CVD Risk (Present) * | 70.6% | 70.1% | <0.385 |
| Race—Black * | 65.1% | 67.5% | <0.0001 |
| Sex—Male * | 39.2% | 39.0% | 0.737 |
| PM2.5 More 12 µg/m3 ** | 10.3 µg/m3 | 14.0 µg/m3 | <0.0001 |
| Estimated Fixed Effects | |||||||
|---|---|---|---|---|---|---|---|
| Model Term | Coefficient | SE | t | p-Value | OR * | Lower 95% CI for OR | Upper 95% CI for OR |
| Intercept | −1.940 | 0.0848 | −22.885 | <0.001 | 0.144 | 0.122 | 0.170 |
| PM2.5 | |||||||
| >12 µg/m3 | 0.125 | 0.0425 | 2.950 | 0.003 | 1.134 | 1.043 | 1.232 |
| <12 µg/m3 | Ref | ||||||
| Sex | |||||||
| Male | 0.021 | 0.0237 | 0.879 | 0.379 | 1.021 | 0.975 | 1.070 |
| Female | Ref | ||||||
| Race | |||||||
| Black | 0.092 | 0.0281 | 3.287 | 0.001 | 1.097 | 1.038 | 1.159 |
| White | Ref | ||||||
| Employed | |||||||
| Yes | −0.593 | 0.0260 | −22.816 | <0.001 | 0.553 | 0.525 | 0.581 |
| No | Ref | ||||||
| Age | |||||||
| 65 or Older | 0.276 | 0.0357 | 7.747 | <0.001 | 1.318 | 1.229 | 1.414 |
| 64 or Younger | Ref | ||||||
| Education | |||||||
| College+ | −0.144 | 0.0462 | −3.120 | 0.002 | 0.866 | 0.791 | 0.948 |
| Some College | −0.085 | 0.0315 | −2.692 | 0.007 | 0.919 | 0.864 | 0.977 |
| High School | −0.149 | 0.0274 | −5.443 | <0.001 | 0.861 | 0.816 | 0.909 |
| No High School | Ref | ||||||
| Marital Status | |||||||
| Single | −0.259 | 0.0610 | −6.956 | <0.001 | 0.654 | 0.581 | 0.737 |
| Widowed | 0.166 | 0.0386 | −3.639 | <0.001 | 0.869 | 0.806 | 0.937 |
| Separated | −0.079 | 0.0283 | −3.569 | 0.005 | 0.901 | 0.850 | 0.954 |
| Married | Ref | ||||||
| Income | |||||||
| >USD 50,000 | −0.424 | 0.0610 | −6.956 | <0.001 | 0.654 | 0.581 | 0.737 |
| USD 25,000–50,000 | −0.140 | 0.0386 | −3.639 | <0.001 | 0.869 | 0.806 | 0.937 |
| USD 15,000–25,000 | −0.105 | 0.0293 | −3.569 | <0.001 | 0.901 | 0.850 | 0.954 |
| <USD 15,000 | Ref | ||||||
| Air Quality Inside | |||||||
| Excellent | 0.158 | 0.0553 | 2.859 | 0.004 | 1.171 | 1.051 | 1.305 |
| Good | 0.049 | 0.0483 | 1.014 | 0.311 | 1.050 | 0.955 | 1.155 |
| Fair | 0.031 | 0.0500 | 0.622 | 0.534 | 1.032 | 0.935 | 1.138 |
| Poor | Ref | ||||||
| CVD Risk | |||||||
| Present | 1.580 | 0.0320 | 49.322 | <0.001 | 4.854 | 4.558 | 5.168 |
| Absent | Ref | ||||||
| Estimated Random Effects | |||||||
| Estimate | SE | Z | p-Value | Lower | Upper | ||
| Variance of STATE | 0.014 | 0.012 | 1.160 | 0.246 | 0.003 | 0.076 | |
| Variance of Clinic nested in STATE | 0.062 | 0.015 | 4.077 | 0.000 | 0.039 | 0.101 | |
| PM2.5 Only (Univariate Model Not Shown) | Multiple Variables with PM2.5 (Table 3) | |
|---|---|---|
| CVD ICC State Level | 0.003499697 | 0.004159402 |
| CVD ICC State(Clinic) | 0.040538159 | 0.022579613 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez, R.B.; Al-Hamdan, M.Z.; Tabatabai, M.; Hood, D.B.; Im, W.; Wilus, D.; Nori-Sarma, A.; Ramesh, A.; Donneyong, M.M.; Langston, M.A.; et al. Association of Cardiovascular Disease and Long-Term Exposure to Fine Particulate Matter (PM2.5) in the Southeastern United States. Atmosphere 2021, 12, 947. https://doi.org/10.3390/atmos12080947
Valdez RB, Al-Hamdan MZ, Tabatabai M, Hood DB, Im W, Wilus D, Nori-Sarma A, Ramesh A, Donneyong MM, Langston MA, et al. Association of Cardiovascular Disease and Long-Term Exposure to Fine Particulate Matter (PM2.5) in the Southeastern United States. Atmosphere. 2021; 12(8):947. https://doi.org/10.3390/atmos12080947
Chicago/Turabian StyleValdez, R. Burciaga, Mohammad Z. Al-Hamdan, Mohammad Tabatabai, Darryl B. Hood, Wansoo Im, Derek Wilus, Amruta Nori-Sarma, Aramandla Ramesh, Macarius M. Donneyong, Michael A. Langston, and et al. 2021. "Association of Cardiovascular Disease and Long-Term Exposure to Fine Particulate Matter (PM2.5) in the Southeastern United States" Atmosphere 12, no. 8: 947. https://doi.org/10.3390/atmos12080947
APA StyleValdez, R. B., Al-Hamdan, M. Z., Tabatabai, M., Hood, D. B., Im, W., Wilus, D., Nori-Sarma, A., Ramesh, A., Donneyong, M. M., Langston, M. A., Mouton, C. P., & Juárez, P. D. (2021). Association of Cardiovascular Disease and Long-Term Exposure to Fine Particulate Matter (PM2.5) in the Southeastern United States. Atmosphere, 12(8), 947. https://doi.org/10.3390/atmos12080947











