Association between Polycyclic Aromatic Hydrocarbon Exposure and Diarrhea in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample Characteristics
2.3. Urinary Polycyclic Aromatic Hydrocarbon Analysis
2.4. Diarrhea Assessment
2.5. Covariate Assessment
2.6. Statistical Analysis
3. Results
3.1. Urinary PAH Data List Characteristics
3.2. Associations between PAHs and Diarrhea
3.3. Gender Difference in Association between PAHs and Diarrhea
3.4. Association between PAHs and Diarrhea Divided by Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
IUPAC Name of Polycyclic Aromatic Hydrocarbons
| 1-hydroxynaphthalene | naphthalen-1-ol |
| 2-hydroxynaphthalene | naphthalen-2-ol |
| 3-hydroxyfluorene | 9H-fluoren-3-ol |
| 2-hydroxyfluorene | 9H-fluoren-2-ol |
| 3-hydroxyphenanthrene | phenanthren-3-ol |
| 1-hydroxyphenanthrene | phenanthren-1-ol |
| 2-hydroxyphenanthrene | phenanthren-2-ol |
| 1-hydroxypyrene | pyren-1-ol |
| 9-hydroxyfluorene | 9H-fluoren-9-ol |
References
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Jahedi, F.; Rad, H.D.; Goudarzi, G.; Birgani, Y.T.; Babaei, A.A.; Angali, K.A. Polycyclic aromatic hydrocarbons in PM1, PM2.5 and PM10 atmospheric particles: Identification, sources, temporal and spatial variations. J. Environ. Health Sci. Eng. 2021, 19, 851–866. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Z.; Wu, H.; Zhang, G.; Zhang, H.; Yang, M. Polycyclic Aromatic Hydrocarbons in Sediments from Typical Algae, Macrophyte Lake Bay and Adjoining River of Taihu Lake, China: Distribution, Sources, and Risk Assessment. Water 2021, 13, 470. [Google Scholar] [CrossRef]
- Xu, X.; Cook, R.L.; Ilacqua, V.; Kan, H.; Talbott, E.; Kearney, G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci. Total Environ. 2010, 408, 4943–4948. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Razis, A.F.A. Polycyclic Aromatic Hydrocarbons (PAHs) and their Bioaccessibility in Meat: A Tool for Assessing Human Cancer Risk. Asian Pac. J. Cancer Prev. 2016, 17, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, K.G.; Antunes, M.C.; Da Silva, G.A.P. Acute diarrhea: Evidence-based management. J. Pediatr. 2015, 91, S36–S43. [Google Scholar] [CrossRef] [Green Version]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- An, L.; Shi, Q.; Fan, M.; Huang, G.; Zhu, M.; Zhang, M.; Liu, Y.; Weng, Y. Benzo[a]pyrene injures BMP2-induced osteogenic differentiation of mesenchymal stem cells through AhR reducing BMPRII. Ecotoxicol. Environ. Saf. 2020, 203, 110930. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, L.-Y.; Meng, L.; Pittman, E.N.; Trinidad, D.A.; Hubbard, K.L.; Etheredge, A.; Del Valle-Pinero, A.Y.; Zamoiski, R.; van Bemmel, D.M.; et al. Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the U.S. Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Environ. Int. 2019, 123, 201–208. [Google Scholar] [CrossRef]
- Idowu, O.; Semple, K.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Kao, T.-W.; Wang, C.-C.; Chen, Y.-J.; Wu, C.-J.; Lai, C.-H.; Chen, W.-L. Exposure to polycyclic aromatic hydrocarbons and risk of disability among an elderly population. Environ. Sci. Pollut. Res. 2019, 26, 10719–10726. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.; Heather, C.S. Diarrhoea in adults (acute). BMJ Clin. Évid. 2011, 2011. [Google Scholar]
- Defois, C.; Ratel, J.; Denis, S.; Batut, B.; Beugnot, R.; Peyretaillade, E.; Engel, E.; Peyret, P. Environmental Pollutant Benzo[a]Pyrene Impacts the Volatile Metabolome and Transcriptome of the Human Gut Microbiota. Front. Microbiol. 2017, 8, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defois, C.; Ratel, J.; Garrait, G.; Denis, S.; Le Goff, O.; Talvas, J.; Mosoni, P.; Engel, E.; Peyret, P. Food Chemicals Disrupt Human Gut Microbiota Activity and Impact Intestinal Homeostasis as Revealed by In Vitro Systems. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tsiaoussis, J.; Antoniou, M.N.; Koliarakis, I.; Mesnage, R.; Vardavas, C.I.; Izotov, B.N.; Psaroulaki, A.; Tsatsakis, A. Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol. Lett. 2019, 312, 72–97. [Google Scholar] [CrossRef] [PubMed]
- Ribière, C.; Peyret, P.; Parisot, N.; Darcha, C.; Déchelotte, P.J.; Barnich, N.; Peyretaillade, E.; Boucher, D. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci. Rep. 2016, 6, 31027. [Google Scholar] [CrossRef]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V.; Niaz, M.S.; Ramesh, A. Polycyclic Aromatic Hydrocarbons and Digestive Tract Cancers: A Perspective. J. Environ. Sci. Health Part C 2011, 29, 324–357. [Google Scholar] [CrossRef] [Green Version]
- Esser, C. The Aryl Hydrocarbon Receptor in Immunity: Tools and Potential. Methods Mol. Biol. 2016, 1371, 239–257. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, I.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor and colitis. Semin. Immunopathol. 2013, 35, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Romanoff, L.C.; Lewin, M.D.; Porter, E.N.; A Trinidad, D.; Needham, L.L.; Patterson, D.G.; Sjödin, A. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J. Expo. Sci. Environ. Epidemiol. 2009, 20, 526–535. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number | Mean (S.D.) or Percentage |
|---|---|---|
| Age | 10,537 | 32.60 (24.91) |
| Male | 5225 | 32.24 (24.94) or 49.6% |
| Young (<65 year old) | 4526 | 86.6% |
| Elderly (≥65 year old) | 699 | 13.4% |
| Female | 5312 | 32.95 (24.87) or 50.4% |
| Young (<65 year old) | 4581 | 86.2% |
| Elderly (≥65 year old) | 731 | 13.8% |
| Laboratory DataCreatinine | 6860 | 0.88 (0.45) |
| ALT | 6859 | 24.89 (17.84) |
| Glucose | 6860 | 99.07 (35.17) |
| Race | ||
| Mexican American | 2384 | 22.6% |
| Other Hispanic | 1133 | 10.8% |
| Non-Hispanic White | 4420 | 41.9% |
| Non-Hispanic Black | 1957 | 18.6% |
| Other Race—Including Multi-Racial | 643 | 6.1% |
| BMI | ||
| Male | 4673 | 25.52 (7.12) |
| Female | 4739 | 26.32 (8.28) |
| Congestive Heart Failure | ||
| Yes | 174 | 2.8% |
| No | 6025 | 96.9% |
| Coronary Heart Disease | ||
| Yes | 254 | 4.1% |
| No | 5936 | 95.5% |
| Myocardial Infarction History | ||
| Yes | 261 | 4.2% |
| No | 5940 | 95.5% |
| Thyroid Problem | ||
| Yes | 608 | 9.8% |
| No | 5597 | 90.0% |
| Smoking History | ||
| Yes | 2866 | 46.1% |
| No | 3352 | 53.9% |
| Variables | Number | Mean (S.D.) (ng/L) |
|---|---|---|
| 1-hydroxynaphthalene | 2747 | 21.61 (328.26) |
| 2-hydroxynaphthalene | 2747 | 7.34 (9.47) |
| 3-hydroxyfluorene | 2744 | 0.26 (0.50) |
| 2-hydroxyfluorene | 2747 | 0.55 (0.95) |
| 3-hydroxyphenanthrene | 2746 | 0.12 (0.18) |
| 1-hydroxyphenanthrene | 2746 | 0.20 (0.26) |
| 2-hydroxyphenanthrene | 2744 | 0.10 (0.14) |
| 1-hydroxypyrene | 2746 | 0.25 (0.47) |
| 9-hydroxyfluorene | 2747 | 0.54 (1.62) |
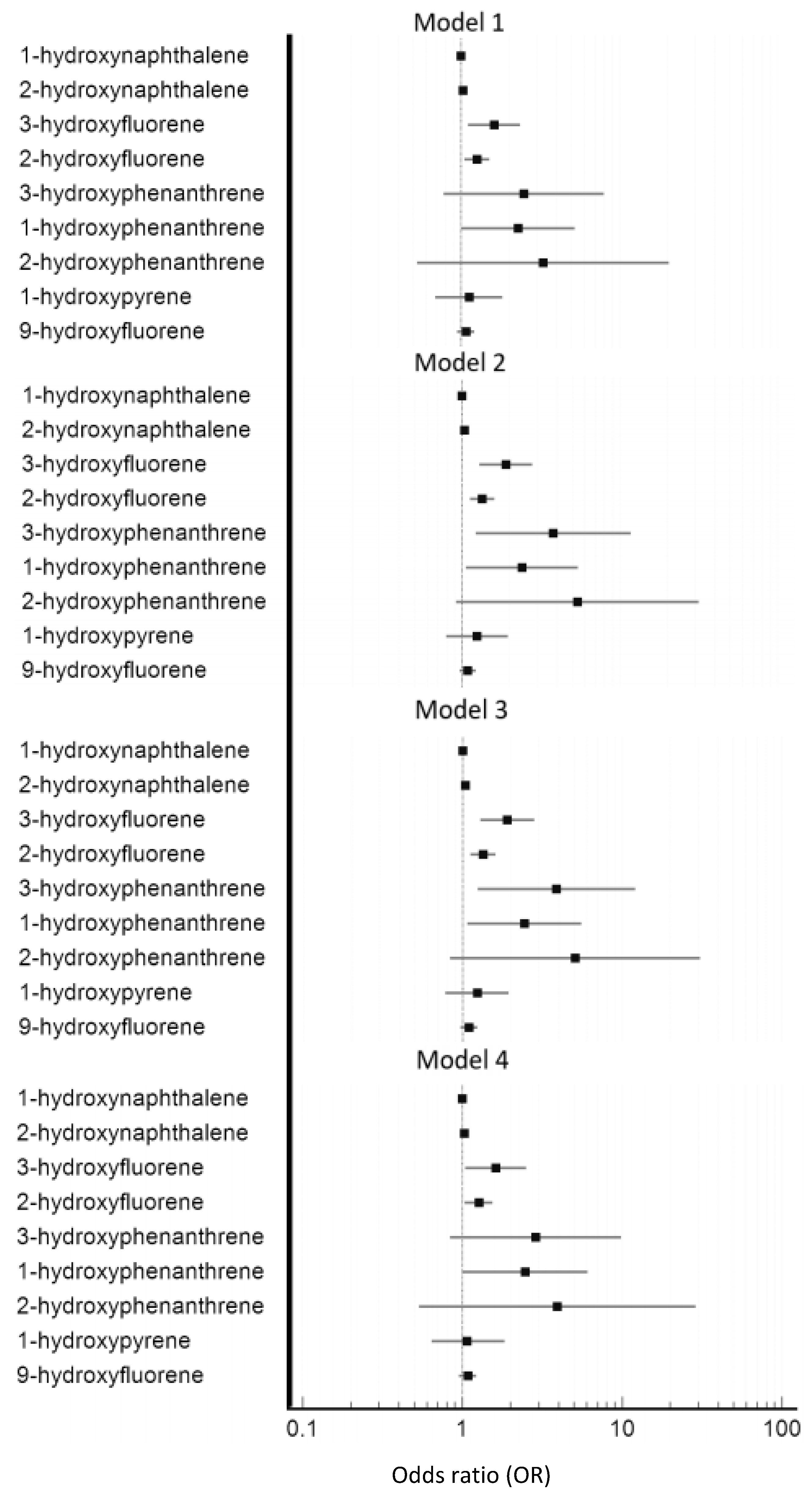
| Variables | Model 1 | p Value | Model 2 | p Value | Model 3 | p Value | Model 4 | p Value |
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||||
| 1-hydroxynaphthalene | 1.000 (0.999, 1.001) | 0.866 | 1.000 (0.999, 1.001) | 0.932 | 1.000 (0.999, 1.001) | 0.982 | 1.000 (0.999, 1.001) | 0.973 |
| 2-hydroxynaphthalene | 1.029 (1.007, 1.052) | 0.009 | 1.036 (1.013, 1.060) | 0.002 | 1.036 (1.013, 1.060) | 0.003 | 1.030 (1.005, 1.056) | 0.020 |
| 3-hydroxyfluorene | 1.618 (1.107, 2.365) | 0.013 | 1.900 (1.290, 2.798) | 0.001 | 1.904 (1.287, 2.818) | 0.001 | 1.621 (1.045, 2.515) | 0.031 |
| 2-hydroxyfluorene | 1.263 (1.055, 1.511) | 0.011 | 1.343 (1.122, 1.608) | 0.001 | 1.344 (1.121, 1.611) | 0.001 | 1.266 (1.034, 1.549) | 0.022 |
| 3-hydroxyphenanthrene | 2.486 (0.775, 7.971) | 0.126 | 3.791 (1.227, 11.714) | 0.021 | 3.874 (1.238, 12.122) | 0.020 | 2.876 (0.840, 9.848) | 0.093 |
| 1-hydroxyphenanthrene | 2.285 (1.002, 5.211) | 0.049 | 2.402 (1.059, 5.449) | 0.036 | 2.433 (1.067, 5.548) | 0.035 | 2.468 (1.004, 6.068) | 0.049 |
| 2-hydroxyphenanthrene | 3.301 (0.530, 20.557) | 0.201 | 5.401 (0.918, 31.774) | 0.062 | 5.065 (0.831, 30.885) | 0.079 | 3.935 (0.536, 28.900) | 0.178 |
| 1-hydroxypyrene | 1.123 (0.691, 1.824) | 0.641 | 1.243 (0.792, 1.951) | 0.344 | 1.231 (0.780, 1.943) | 0.371 | 1.068 (0.643, 1.837) | 0.757 |
| 9-hydroxyfluorene | 1.074 (0.947, 1.218) | 0.268 | 1.088 (0.965, 1.226) | 0.170 | 1.095 (0.970, 1.236) | 0.143 | 1.080 (0.950, 1.227) | 0.238 |
| Variables | Gender | Model 1 | p Value | Model 2 | p Value | Model 3 | p Value | Model 4 | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||||||
| 1-hydroxynaphthalene | Male Female | 1.001 (1.000, 1.003) 1.000 (0.998, 1.002) | 0.073 0.805 | 1.001 (1.000, 1.003) 1.000 (0.998, 1.002) | 0.067 0.816 | 1.002 (1.000, 1.004) 1.000 (0.997, 1.002) | 0.023 0.764 | 1.002 (1.000, 1.004) 1.000 (0.997, 1.002) | 0.021 0.800 |
| 2-hydroxynaphthalene | Male Female | 1.003 (0.944, 1.066) 1.042 (1.014, 1.071) | 0.921 0.003 | 1.003 (0.943, 1.066) 1.052 (1.023, 1.083) | 0.935 0.000 | 1.002 (0.943, 1.064) 1.052 (1.022, 1.083) | 0.948 0.001 | 0.990 (0.919, 1.066) 1.044 (1.012, 1.078) | 0.793 0.007 |
| 3-hydroxyfluorene | Male Female | 0.559 (0.086, 3.631) 2.088 (1.363, 3.198) | 0.542 0.001 | 0.527 (0.077, 3.588) 2.497 (1.606, 3.881) | 0.513 0.000 | 0.498 (0.073, 3.407) 2.479 (1.584, 3.880) | 0.477 0.000 | 0.223 (0.019, 2.564) 2.063 (1.256, 3.388) | 0.228 0.004 |
| 2-hydroxyfluorene | Male Female | 0.912 (0.437, 1.903) 1.436 (1.155, 1.786) | 0.807 0.001 | 0.899 (0.424, 1.908) 1.540 (1.227, 1.933) | 0.782 0.000 | 0.887 (0.420, 1.873) 1.530 (1.217, 1.922) | 0.753 0.000 | 0.678 (0.252, 1.823) 1.418 (1.112, 1.809) | 0.442 0.005 |
| 3-hydroxyphenanthrene | Male Female | 0.050 (0.000, 66.735) 6.439 (1.756, 23.611) | 0.414 0.005 | 0.034 (0.000, 64.445) 9.212 (2.474, 34.298) | 0.379 0.001 | 0.030 (0.000, 62.866) 9.125 (2.456, 33.905) | 0.370 0.001 | 0.005 (0.000, 20.243) 6.532(1.632, 26.147) | 0.212 0.008 |
| 1-hydroxyphenanthrene | Male Female | 0.002 (0.000, 7.263) 3.347(1.389, 8.069) | 0.137 0.007 | 0.002 (0.000, 6.523) 3.643 (1.457, 9.106) | 0.128 0.006 | 0.002 (0.000, 6.867) 3.878 (1.537, 9.783) | 0.133 0.004 | 0.000 (0.000, 3.374) 3.946 (1.543, 10.093) | 0.091 0.004 |
| 2-hydroxyphenanthrene | Male Female | 0.005 (0.000, 164.563) 12.472 (1.754, 88.711) | 0.316 0.012 | 0.003 (0.000, 152.784) 16.957(2.339, 122.941) | 0.295 0.005 | 0.004 (0.000, 186.132) 15.391 (2.093, 113.168) | 0.315 0.007 | 0.000 (0.000, 34.855) 11.728 (1.441, 95.487) | 0.176 0.021 |
| 1-hydroxypyrene | Male Female | 0.163 (0.002, 14.771) 1.260 (0.791, 2.008) | 0.430 0.330 | 0.136 (0.001, 14.311) 1.474 (0.935, 2.323) | 0.401 0.095 | 0.138 (0.001, 12.722) 1.445 (0.910, 2.293) | 0.390 0.118 | 0.032 (0.000, 5.620) 1.262 (0.742, 2.146) | 0.191 0.390 |
| 9-hydroxyfluorene | Male Female | 0.533 (0.101, 2.824) 1.140 (0.977, 1.331) | 0.459 0.095 | 0.496 (0.087, 2.809) 1.179 (1.012, 1.373) | 0.428 0.035 | 0.494 (0.084, 2.913) 1.185 (1.016, 1.383) | 0.436 0.031 | 0.335 (0.049, 2.299) 1.172 (0.998, 1.377) | 0.266 0.053 |
| Variables | BMI | Model 1 | p Value | Model 2 | p Value | Model 3 | p Value | Model 4 | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||||||
| 1-hydroxynaphthalene | <30 >30 | 1.000 (0.998, 1.002) 1.000 (0.999, 1.001) | 0.985 0.908 | 1.000 (0.998, 1.002) 1.000 (0.999, 1.001) | 0.975 0.967 | 1.000 (0.998, 1.002) 1.000 (0.999, 1.001) | 0.978 0.967 | 1.000 (0.997, 1.002) 1.000 (0.999, 1.001) | 0.880 0.940 |
| 2-hydroxynaphthalene | <30 >30 | 1.044 (1.015, 1.073) 1.009 (0.968, 1.052) | 0.002 0.666 | 1.050 (1.020, 1.080) 1.011 (0.963, 1.061) | 0.001 0.666 | 1.050 (1.020, 1.081) 1.013 (0.965, 1.063) | 0.001 0.606 | 1.040 (1.007, 1.073) 1.008 (0.955, 1.063) | 0.017 0.781 |
| 3-hydroxyfluorene | <30 >30 | 2.057 (1.347, 3.143) 0.724 (0.162, 3.239) | 0.001 0.673 | 2.287 (1.484, 3.524) 0.937 (0.231, 3.802) | 0.000 0.927 | 2.312 (1.490, 3.587) 0.927 (0.220, 3.901) | 0.000 0.918 | 1.850 (1.138, 3.006) 0.701 (0.133, 3.689) | 0.013 0.675 |
| 2-hydroxyfluorene | <30 >30 | 1.478 (1.160, 1.884) 1.071 (0.739, 1.552) | 0.002 0.717 | 1.525 (1.198, 1.941) 1.164 (0.798, 1.698) | 0.001 0.430 | 1.532 (1.198, 1.958) 1.175 (0.801, 1.724) | 0.001 0.409 | 1.354 (1.034, 1.773) 1.137 (0.719, 1.799) | 0.028 0.582 |
| 3-hydroxyphenanthrene | <30 >30 | 4.362 (1.198, 15.879) 0.621 (0.019, 20.003) | 0.025 0.788 | 5.585 (1.558, 20.024) 1.416 (0.069, 29.182) | 0.008 0.822 | 5.590 (1.544, 20.038) 1.468 (0.067, 32.369) | 0.009 0.808 | 3.831 (0.957, 15.326) 1.202 (0.040, 36.117) | 0.058 0.916 |
| 1-hydroxyphenanthrene | <30 >30 | 3.324 (1.238, 8.923) 1.179 (0.197, 7.062) | 0.017 0.857 | 3.596 (1.322, 9.783) 1.262 (0.259, 6.138) | 0.012 0.773 | 3.586 (1.312, 9.800) 1.307 (0.267, 6.411) | 0.013 0.741 | 3.939 (1.307, 11.873) 1.259 (0.213, 7.429) | 0.015 0.800 |
| 2-hydroxyphenanthrene | <30 >30 | 7.527 (0.902, 62.825) 0.712 (0.016, 31.838) | 0.062 0.861 | 9.706 (1.203, 78.275) 1.703 (0.050, 58.408) | 0.033 0.768 | 9.322 (1.123, 77.395) 1.657 (0.046, 59.678) | 0.039 0.782 | 6.529 (0.598, 71.223) 1.267 (0.023, 69.837) | 0.124 0.908 |
| 1-hydroxypyrene | <30 >30 | 1.400 (0.859, 2.281) 0.214 (0.009, 5.082) | 0.177 0.340 | 1.481 (0.925, 2.373) 0.283 (0.013, 6.248) | 0.102 0.424 | 1.448 (0.897, 2.338) 0.317 (0.014, 7.089) | 0.130 0.469 | 1.248 (0.726, 2.147) 0.259 (0.009, 7.075) | 0.423 0.423 |
| 9-hydroxyfluorene | <30 >30 | 1.089 (0.964, 1.230) 0.855 (0.368, 1.985) | 0.172 0.715 | 1.091 (0.967, 1.231) 0.977 (0.527, 1.810) | 0.157 0.940 | 1.099 (0.972, 1.241) 0.999 (0.542, 1.841) | 0.131 0.997 | 1.072 (0.943, 1.219) 1.011 (0.514, 1.988) | 0.287 0.974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Fang, W.-H.; Wang, C.-C.; Lai, C.-H.; Chen, W.-L. Association between Polycyclic Aromatic Hydrocarbon Exposure and Diarrhea in Adults. Atmosphere 2021, 12, 919. https://doi.org/10.3390/atmos12070919
Wu C-C, Fang W-H, Wang C-C, Lai C-H, Chen W-L. Association between Polycyclic Aromatic Hydrocarbon Exposure and Diarrhea in Adults. Atmosphere. 2021; 12(7):919. https://doi.org/10.3390/atmos12070919
Chicago/Turabian StyleWu, Chia-Che, Wen-Hui Fang, Chung-Ching Wang, Ching-Huang Lai, and Wei-Liang Chen. 2021. "Association between Polycyclic Aromatic Hydrocarbon Exposure and Diarrhea in Adults" Atmosphere 12, no. 7: 919. https://doi.org/10.3390/atmos12070919
APA StyleWu, C.-C., Fang, W.-H., Wang, C.-C., Lai, C.-H., & Chen, W.-L. (2021). Association between Polycyclic Aromatic Hydrocarbon Exposure and Diarrhea in Adults. Atmosphere, 12(7), 919. https://doi.org/10.3390/atmos12070919