Concentrations, Size Distribution, and Community Structure Characteristics of Culturable Airborne Antibiotic-Resistant Bacteria in Xinxiang, Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. DNA Extraction, DNA Amplification, and Illumina Sequencing
2.3. Health Risk Assessment
2.4. Statistical Analysis
3. Results and Discussion
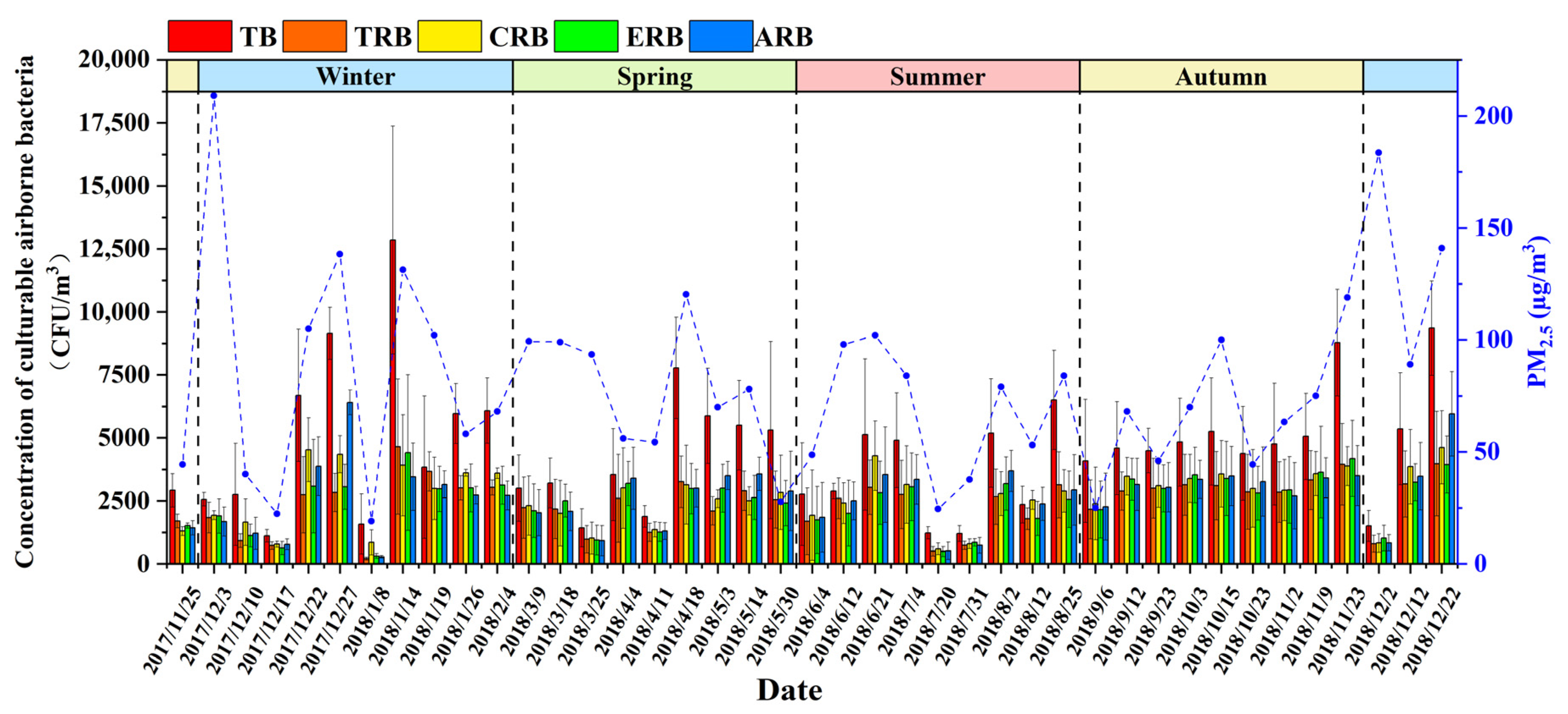
3.1. Concentrations of Airborne Bacteria and Antibiotic-Resistant Bacteria during Sampling Days
3.2. Bioaerosols Concentration and Size Distribution at Different Air Quality Levels
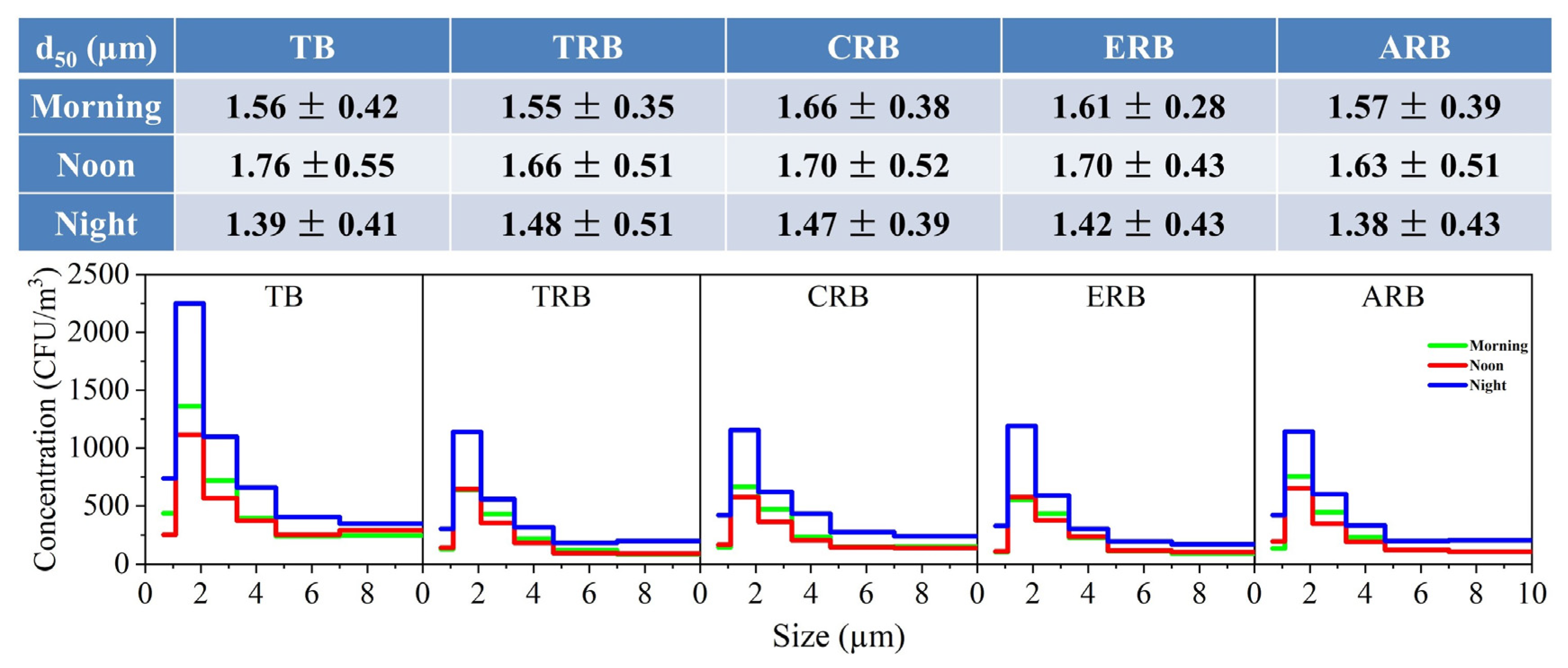
3.3. Effect of Time Factor on Bioaerosol Concentration and Particle Size Distribution
3.4. Meteorological Factors of Airborne Bacteria and Four Antibiotic-Resistant Bacteria
3.5. Community Structure of Four Antibiotic-Resistant Bacteria
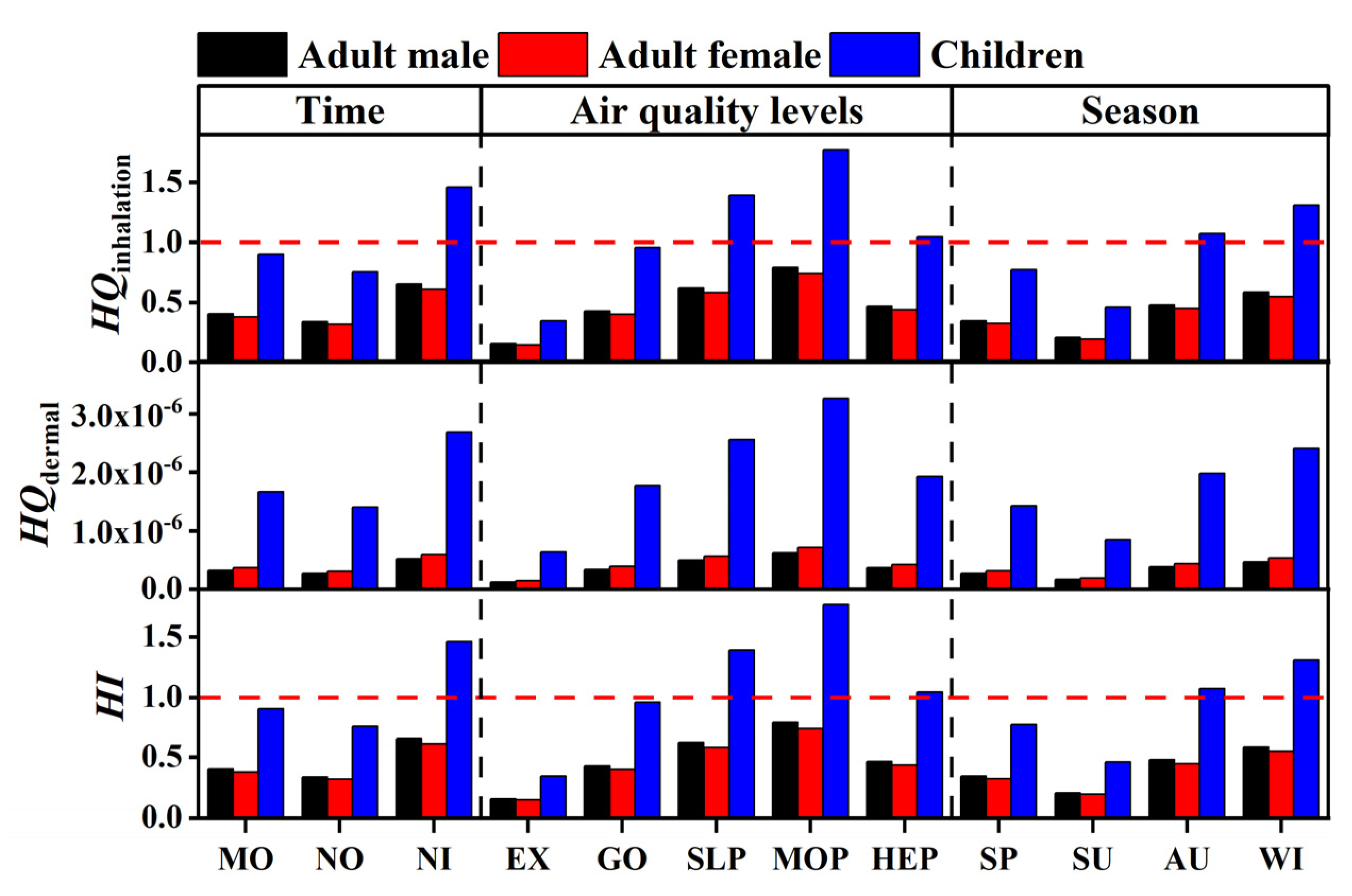
3.6. Human Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stetzenbach, L.D.; Buttner, M.P.; Cruz, P. Detection and enumeration of airborne biocontaminants. Curr. Opin. Biotechnol. 2004, 15, 170–174. [Google Scholar] [CrossRef]
- Jaenicke, R. Abundance of cellular material and proteins in the atmosphere. Science 2005, 308, 73. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Pratt, K.A.; Demott, P.J.; French, J.R.; Wang, Z.; Westphal, D.L.; Heymsfield, A.J.; Twohy, C.H.; Prenni, A.J.; Prather, K.A. In situ detection of biological particles in cloud ice-crystals. Nat. Geosci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Vaïtilingom, M.; Deguillaume, L.; Vinatier, V.; Sancelme, M.; Amato, P.; Chaumerliac, N.; Delort, A.M. Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds. Proc. Natl. Acad. Sci. USA 2013, 110, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.J.; Qi, J.H.; Shao, C.C.; Zhong, X.; Gao, D.M.; Cao, W.W.; Gao, J.W.; Bai, R.; Long, G.Y.; Chu, C.C. Concentration and size distribution of total airborne microbes in hazy and foggy weather. Sci. Total Environ. 2016, 541, 1011–1018. [Google Scholar] [CrossRef]
- Riggs, D.W.; Yeager, R.A.; Bhatnagar, A. Defining the human envirome: An omics approach for assessing the environmental risk of cardiovascular disease. Circ. Res. 2018, 122, 1259–1275. [Google Scholar] [CrossRef]
- Roy, R.; Jan, R.; Joshi, U.; Bhor, R.; Pai, K.; Satsangi, P.G. Characterization, pro-inflammatory response and cytotoxic profile of bioaerosols from urban and rural residential settings in Pune, India. Environ. Pollut. 2020, 264, 114698. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 11, 6772–6782. [Google Scholar] [CrossRef]
- Mao, Y.; Ding, P.; Wang, Y.; Ding, C.; Wu, L.; Zheng, P.; Zhang, X.; Li, X.; Wang, L.; Sun, Z. Comparison of culturable antibiotic-resistant bacteria in polluted and non-polluted air in Beijing, China. Environ. Int. 2019, 131, 104936. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, J.; Zhu, Y.G.; Chen, Q.L.; Shen, F.; Wu, Y.; Xu, S.; Fan, H.; Da, G.; Huang, R.J.; et al. Global Survey of Antibiotic Resistance Genes in Air. Environ. Sci. Technol. 2018, 52, 10975–10984. [Google Scholar] [CrossRef] [Green Version]
- Seifert, H.; Strate, A.; Schulze, A.; Pulverer, G. Bacteremia due to Acinetobacter Species Other than Acinetobacter baumannii. Infection 1994, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, X.; Bao, Y.; Ma, R.; Zhou, Y. Antibiotic resistance and OXA-type carbapenemases-encoding genes in airborne Acinetobacter baumannii isolated from burn wards. Burns 2014, 40, 295–299. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Talepour, N.; Hassanvand, M.S.; Abbasi-Montazeri, E.; Latifi, S.M.; Fard, N.J.H. Spatio-temporal variations of airborne bacteria from the municipal wastewater treatment plant: A case study in Ahvaz, Iran. J. Environ. Health Sci. Eng. 2020, 18, 423–432. [Google Scholar] [CrossRef]
- Fan, C.; Li, Y.; Liu, P.; Mu, F.; Xie, Z.; Lu, R.; Qi, Y.; Wang, B.; Jin, C. Characteristics of airborne opportunistic pathogenic bacteria during autumn and winter in Xi’an, China. Sci. Total Environ. 2019, 672, 834–845. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, H.; Zhang, H.; Zhang, X.; Zhou, M.; Lou, L.; Zheng, P.; Xi, C.; Hu, B. Temporal discrepancy of airborne total bacteria and pathogenic bacteria between day and night. Environ. Res. 2020, 186, 109540. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; Wang, W.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef]
- French, G.L. Clinical impact and relevance of antibiotic resistance. Adv. Drug Deliv. Rev. 2005, 57, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- China MEE. Ambient Air Quality Standards (GB 3095-2012); Ministry of Ecology and Environment of The People’s Republic of China: Beijing, China, 2012.
- China MEE. Technical Regulation on Ambient Air Quality Index (on Trial) (hj 633-2012); Ministry of Ecology and Environment of The People’s Republic of China: Beijing, China, 2012.
- Zhang, S.; Han, B.; Gu, J.; Wang, C.; Wang, P.; Ma, Y.; Cao, J.; He, Z. Fate of antibiotic resistant cultivable heterotrophic bacteria and antibiotic resistance genes in wastewater treatment processes. Chemosphere 2015, 135, 138–145. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.; Guo, Y.; Tian, T. Influence of chicken manure fertilization on antibiotic-resistant bacteria in soil and the endophytic bacteria of pakchoi. Int. J. Environ. Res. Public Health 2016, 13, 662. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, M.; Yang, W.; Fang, Y.; Wang, G.; Hou, F. A seventeen-year observation of the antimicrobial susceptibility of clinical Campylobacter jejuni and the molecular mechanisms of erythromycin-resistant isolates in Beijing, China. Int. J. Infect. Dis. 2016, 42, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, H.; Qiu, X.; Zhang, Y.; Wang, H. Dispersion and risk assessment of bacterial aerosols emitted from rotating-brush aerator during summer in a wastewater treatment plant of Xi’an, China. Aerosol Air Qual. Res. 2013, 13, 1807–1814. [Google Scholar] [CrossRef]
- Chen, H.; Carter, K.E. Modeling potential occupational inhalation exposures and associated risks of toxic organics from chemical storage tanks used in hydraulic fracturing using AERMOD. Environ. Pollut. 2017, 224, 300–309. [Google Scholar] [CrossRef]
- Eeftens, M.; Tsai, M.Y.; Ampe, C.; Anwander, B.; Beelen, R.; Bellander, T. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PM coarse concentrations between and within 20 European study areas and the relationship with NO2—Results of the ESCAPE project. Atmos. Environ. 2012, 62, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Li, Y.; Li, W.; Xie, Z.; Fan, C.; Liu, P.; Deng, S. Bacterial community structure in atmospheric particulate matters of different sizes during the haze days in Xi’an, China. Sci. Total Environ. 2018, 637, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Jia, R.; Qiu, T.; Han, M.; Song, Y.; Wang, X. Seasonal size distribution of airborne culturable bacteria and fungi and preliminary estimation of their deposition in human lungs during non-haze and haze days. Atmos. Environ. 2015, 118, 203–210. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. The structure and diversity of human, animal and environmental resistomes. Microbiome 2016, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fu, H.; Wang, W.; Liu, J.; Meng, Q.; Wang, W. Characteristics of bacterial and fungal aerosols during the autumn haze days in Xi’an, China. Atmos. Environ. 2015, 122, 439–447. [Google Scholar] [CrossRef]
- Xie, Z.; Li, Y.; Lu, R.; Li, W.; Fan, C.; Liu, P.; Wang, J.; Wang, W. Characteristics of total airborne microbes at various air quality levels. J. Aerosol Sci. 2018, 116, 57–65. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef]
- Wei, M.; Liu, H.; Chen, J.; Xu, C.; Li, J.; Xu, P.; Sun, Z. Effects of aerosol pollution on PM2.5-associated bacteria in typical inland and coastal cities of northern China during the winter heating season. Environ. Pollut. 2020, 262, 114188. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, H.; Yao, X.; Zhou, M.; Wang, J.; He, Z.; Zhang, H.; Lou, L.; Mao, W.; et al. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ. Pollut. 2018, 233, 483–493. [Google Scholar] [CrossRef]
- Yang, T.; Han, Y.P.; Li, L.; Liu, J.X. Microbial Properties of Different Size Aerosols at Human Average Respiratory Height During Fog-haze Days. Environ. Sci. 2019, 40, 1680–1687. [Google Scholar] [CrossRef]
- Gong, J.; Qi, J.; Beibei, E.; Yin, Y.; Gao, D. Concentration, viability and size distribution of bacteria in atmospheric bioaerosols under different types of pollution. Environ. Pollut. 2020, 257, 113485. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, F.; Zhang, X.X.; Li, K.; Li, C.; Ye, L.; Li, M. Metagenomic profiling of ARGs in airborne particulate matters during a severe smog event. Sci. Total Environ. 2018, 615, 1332–1340. [Google Scholar] [CrossRef]
- Fang, Z.; Yao, W.; Lou, X.; Hao, C.; Gong, C.; Ouyang, Z. Profile and characteristics of culturable airborne bacteria in Hangzhou, southeast of China. Aerosol Air Qual. Res. 2016, 16, 1690–1700. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Qiu, T.; Jia, R.; Han, M.; Song, Y.; Wang, X. Concentration and size distribution of viable bioaerosols during non-haze and haze days in Beijing. Environ. Sci. Pollut. Res. 2015, 22, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Ulevičius, V.; Pečiulyte, D.; Mordas, G.; Lugauskas, A. Field study on changes in viability of airborne fungal propagules exposed to solar radiation. J. Aerosol Sci. 2000, 31, 961–962. [Google Scholar] [CrossRef]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface. 2009, 6, S737–S746. [Google Scholar] [CrossRef] [Green Version]
- Hwang, G.B.; Jung, J.H.; Jeong, T.G.; Lee, B.U. Effect of hybrid UV-thermal energy stimuli on inactivation of S. epidermidis and B. subtilis bacterial bioaerosols. Sci. Total Environ. 2010, 408, 5903–5909. [Google Scholar] [CrossRef]
- Li, Y.; Lu, R.; Li, W.; Xie, Z.; Song, Y. Concentrations and size distributions of viable bioaerosols under various weather conditions in a typical semi-arid city of Northwest China. J. Aerosol Sci. 2017, 106, 83–92. [Google Scholar] [CrossRef]
- Thomas, R.J.; Webber, D.; Sellors, W.; Collinge, A.; Frost, A.; Stagg, A.J.; Bailey, S.C.; Jayasekera, P.N.; Taylor, R.R.; Eley, S.; et al. Characterization and deposition of respirable large- and small-particle bioaerosols. Appl. Environ. Microbiol. 2008, 74, 6437–6443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Sagona, J.A.; Secondo, L.E.; Mainelis, G. Comparison of Two Models to Estimate Deposition of Fungi and Bacteria in the Human Respiratory Tract. Atmosphere 2020, 11, 561. [Google Scholar] [CrossRef]
- Yan, X.; Qiu, D.; Zheng, S.; Yang, J.; Sun, H.; Wei, Y.; Han, J.; Sun, J.; Su, X. Distribution characteristics and noncarcinogenic risk assessment of culturable airborne bacteria and fungi during winter in Xinxiang, China. Environ. Sci. Pollut. Res. 2019, 26, 36698–36709. [Google Scholar] [CrossRef]
- Hwang, S.H.; Park, J.B. Comparison of culturable airborne bacteria and related environmental factors at underground subway stations between 2006 and 2013. Atmos. Environ. 2014, 84, 289–293. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, A.; Lu, P.; Fang, Z.; Jiang, A.; Li, H.; Lu, Y. Study on the sterilization effects of bacteria by the treatment of ozone. J. Biol. 1999, 16, 19–20. [Google Scholar] [CrossRef]
- Cox, C.S.; Hood, A.M.; Baxter, J. Method for Comparing Concentrations of the Open-Air Factor. Appl. Microbiol. 1973, 26, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.S. Stability of airborne microbes and allergens. In Bioaerosols Handbook; Cox, C.S., Wathes, C.M., Eds.; Lewis Publishers: New York, NY, USA, 1995; pp. 77–99. [Google Scholar]
- Alghamdi, M.A.; Shamy, M.; Redal, M.A.; Khoder, M.; Awad, A.H.; Elserougy, S. Microorganisms associated particulate matter: A preliminary study. Sci. Total Environ. 2014, 479, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Chen, Z.; Mao, D.; Luo, Y. Significant higher airborne antibiotic resistance genes and the associated inhalation risk in the indoor than the outdoor. Environ. Pollut. 2021, 268, 115620. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Yao, M. Microbial emission levels and diversities from different land use types. Environ. Int. 2020, 143, 105988. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y. Characteristics of antibiotic resistance of airborne Staphylococcus isolated from metro stations. Int. J. Environ. Res. Public Health 2013, 10, 2412–2426. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.Y.; Yang, X.R.; Zhu, Y.G.; Hong, Y.W.; Su, J.Q. Spatial and seasonal variation of the airborne microbiome in a rapidly developing city of China. Sci. Total Environ. 2019, 665, 61–68. [Google Scholar] [CrossRef]
- Ruiz-Gil, T.; Acuña, J.J.; Fujiyoshi, S.; Tanaka, D.; Noda, J.; Maruyama, F.; Jorquera, M.A. Airborne bacterial communities of outdoor environments and their associated influencing factors. Environ. Int. 2020, 145, 106156. [Google Scholar] [CrossRef]
- Gao, X.L.; Shao, M.F.; Wang, Q.; Wang, L.T.; Fang, W.Y.; Ouyang, F.; Li, J. Airborne microbial communities in the atmospheric environment of urban hospitals in China. J. Hazard. Mater. 2018, 349, 10–17. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Q.; Fu, X.; Zheng, L.; Dong, J.; Wang, J.; Guo, S. Feedback of airborne bacterial consortia to haze pollution with different PM2.5 levels in typical mountainous terrain of Jinan, China. Sci. Total Environ. 2019, 695, 133912. [Google Scholar] [CrossRef]
- de Paiva-Santos, W.; de Sousa, V.S.; Giambiagi-deMarval, M. Occurrence of virulence-associated genes among Staphylococcus saprophyticus isolated from different sources. Microb. Pathog. 2018, 119, 9–11. [Google Scholar] [CrossRef]
- Landrum, M.L.; Neumann, C.; Cook, C.; Chukwuma, U.; Ellis, M.W.; Hospenthal, D.R.; Murray, C.K. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US Military Health System, 2005–2010. JAMA J. Am. Med. Assoc. 2012, 308, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Rohlwink, U.K.; Figaji, A.; Wilkinson, K.A.; Horswell, S.; Sesay, A.K.; Deffur, A.; Enslin, N.; Solomons, R.; Van Toorn, R.; Eley, B.; et al. Tuberculous meningitis in children is characterized by compartmentalized immune responses and neural excitotoxicity. Nat. Commun. 2019, 10, 3767. [Google Scholar] [CrossRef]
- Nudel, K.; Zhao, X.; Basu, S.; Dong, X.; Hoffmann, M.; Feldgarden, M.; Allard, M.; Klompas, M.; Bry, L. Genomics of Corynebacterium striatum, an emerging multidrug-resistant pathogen of immunocompromised patients. Clin. Microbiol. Infect. 2018, 24, 1016.e7–1016.e13. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Han, Y.; Liu, J.; Li, L. Aerosols from a wastewater treatment plant using oxidation ditch process: Characteristics, source apportionment, and exposure risks. Environ. Pollut. 2019, 250, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, K.; Yang, T.; Zhang, M.; Li, L. Bioaerosols emission and exposure risk of a wastewater treatment plant with A2O treatment process. Ecotoxicol. Environ. Saf. 2019, 169, 161–168. [Google Scholar] [CrossRef]
- Gladding, T.L.; Rolph, C.A.; Gwyther, C.L.; Kinnersley, R.; Walsh, K.; Tyrrel, S. Concentration and composition of bioaerosol emissions from intensive farms: Pig and poultry livestock. J. Environ. Manag. 2020, 272, 111052. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.D.; Shakya, K.M.; Bilinski, T.; Wilson, J.W.; Ravi, S.; Choi, C.S. Variation of near surface atmosphere microbial communities at an urban and a suburban site in Philadelphia, PA, USA. Sci. Total Environ. 2020, 724, 138353. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, D.; Li, B.; Sun, S.; Yabo, S.D.; Geng, J.; Ma, L.; Qi, H. Exploring the disparity of inhalable bacterial communities and antibiotic resistance genes between hazy days and non-hazy days in a cold megacity in Northeast China. J. Hazard. Mater. 2020, 398, 122984. [Google Scholar] [CrossRef]
- Farokhi, A.; Heederik, D.; Smit, L.A.M. Respiratory health effects of exposure to low levels of airborne endotoxin—A systematic review. Environ. Health. 2018, 17, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Xiong, Y.; Shi, C.; Liu, C.; Li, H.; Qian, H.; Sun, Z.; Qin, C. Characteristics of airborne bacterial communities in indoor and outdoor environments during continuous haze events in Beijing: Implications for health care. Environ. Int. 2020, 139, 105721. [Google Scholar] [CrossRef] [PubMed]



| Stage | Aerodynamic Diameter |
|---|---|
| Ⅰ | >7.0 μm |
| Ⅱ | 4.7–7.0 μm |
| Ⅲ | 3.3–4.7 μm |
| Ⅳ | 2.1–3.3 μm |
| Ⅴ | 1.1–2.1 μm |
| Ⅵ | 0.65–1.1 μm |
| AQI | PM2.5 | PM10 | SO2 | NO2 | O3 | T (°C) | RH (%) | WS (m/s) | |
|---|---|---|---|---|---|---|---|---|---|
| TB | 0.335 ** | 0.347 ** | 0.382 ** | 0.352 ** | 0.634 ** | −0.392 ** | 0.319 * | 0.086 | −0.477 ** |
| TRB | 0.372 ** | 0.389 ** | 0.434 ** | 0.406 ** | 0.677 ** | −0.336 ** | −0.184 | 0.070 | −0.430 * |
| CRB | 0.334 ** | 0.347 ** | 0.373 ** | 0.373 ** | 0.663 ** | −0.379 ** | 0.294 * | 0.035 | −0.374 * |
| ERB | 0.376 ** | 0.394 ** | 0.419 ** | 0.366 ** | 0.648 ** | −0.307 * | −0.147 | 0.069 | −0.404 * |
| ARB | 0.384 ** | 0.396 ** | 0.428 ** | 0.418 ** | 0.677 ** | −0.367 ** | −0.234 | 0.086 | −0.392 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Ma, J.; Ren, J.; Cui, M.; Chen, X.; Qiu, D.; Lei, M.; Li, T.; Guo, L.; Chen, C.; et al. Concentrations, Size Distribution, and Community Structure Characteristics of Culturable Airborne Antibiotic-Resistant Bacteria in Xinxiang, Central China. Atmosphere 2021, 12, 1077. https://doi.org/10.3390/atmos12081077
Yan X, Ma J, Ren J, Cui M, Chen X, Qiu D, Lei M, Li T, Guo L, Chen C, et al. Concentrations, Size Distribution, and Community Structure Characteristics of Culturable Airborne Antibiotic-Resistant Bacteria in Xinxiang, Central China. Atmosphere. 2021; 12(8):1077. https://doi.org/10.3390/atmos12081077
Chicago/Turabian StyleYan, Xu, Jiahui Ma, Jingyuan Ren, Mengjia Cui, Xinqing Chen, Dezhi Qiu, Miao Lei, Tianning Li, Li Guo, Chun Chen, and et al. 2021. "Concentrations, Size Distribution, and Community Structure Characteristics of Culturable Airborne Antibiotic-Resistant Bacteria in Xinxiang, Central China" Atmosphere 12, no. 8: 1077. https://doi.org/10.3390/atmos12081077
APA StyleYan, X., Ma, J., Ren, J., Cui, M., Chen, X., Qiu, D., Lei, M., Li, T., Guo, L., Chen, C., & Han, Y. (2021). Concentrations, Size Distribution, and Community Structure Characteristics of Culturable Airborne Antibiotic-Resistant Bacteria in Xinxiang, Central China. Atmosphere, 12(8), 1077. https://doi.org/10.3390/atmos12081077





