On the Problem of Critical Electric Field of Atmospheric Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ambient Conditions
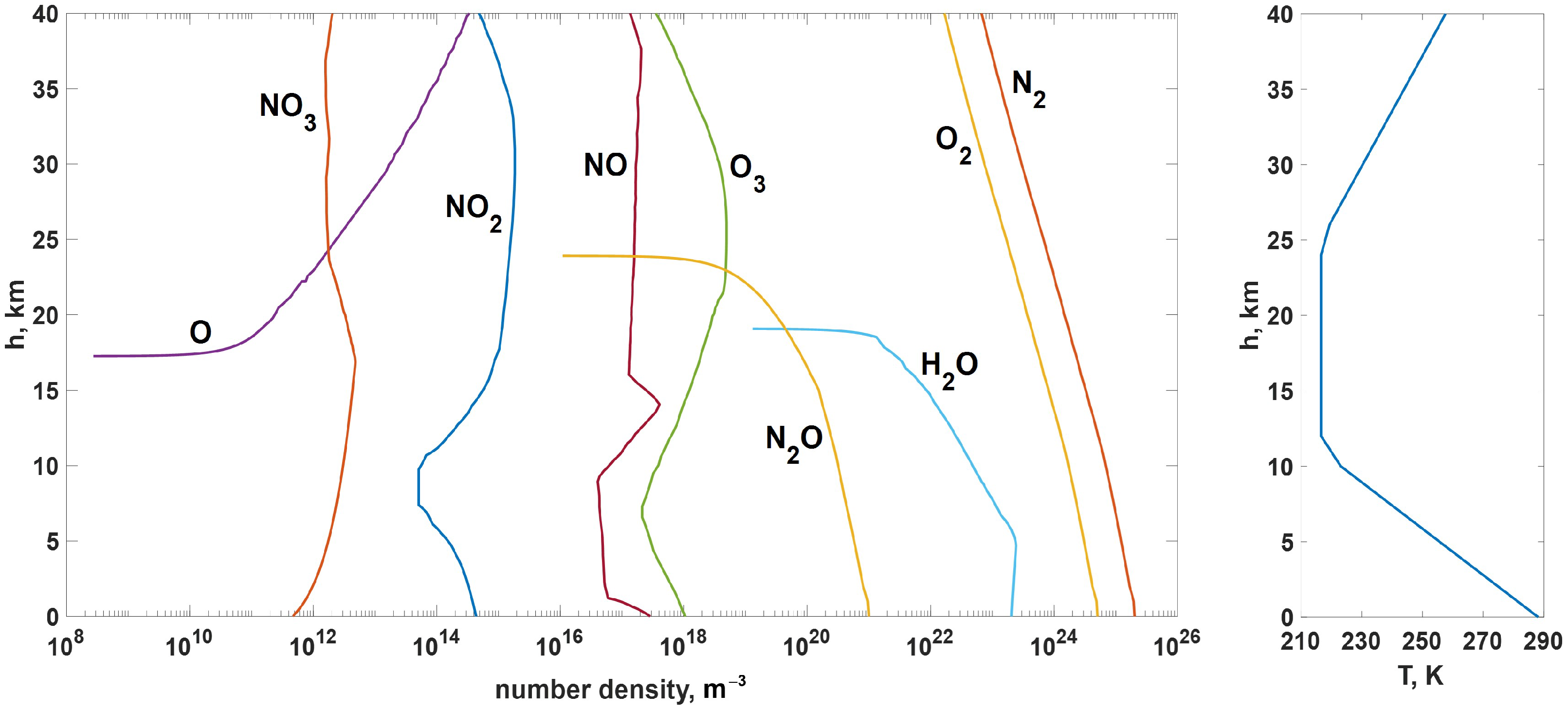
- Air temperature (T) and pressure (p) altitude distributions—Table 1 from the peer-reviewed web resource [8];
- Water vapor number density ([])—Figure 2 from the peer-reviewed web resource [8];
- Atomic oxygen number density ([])—Figure VIII-10 from [9];
- Ozone number density ([])—Figure 1.4 from [10];
- Nitric oxide (II) number density ([])—Figure 1 from [11];
- Nitric oxide (IV) number density ([])—Figure 5 from [12];
- Nitric oxide number density ([])—Figure 1(b) from [13];
- Nitric oxide (I) number density ([])—Figure 8 from [14].
2.2. Evolution Matrix
- 1.
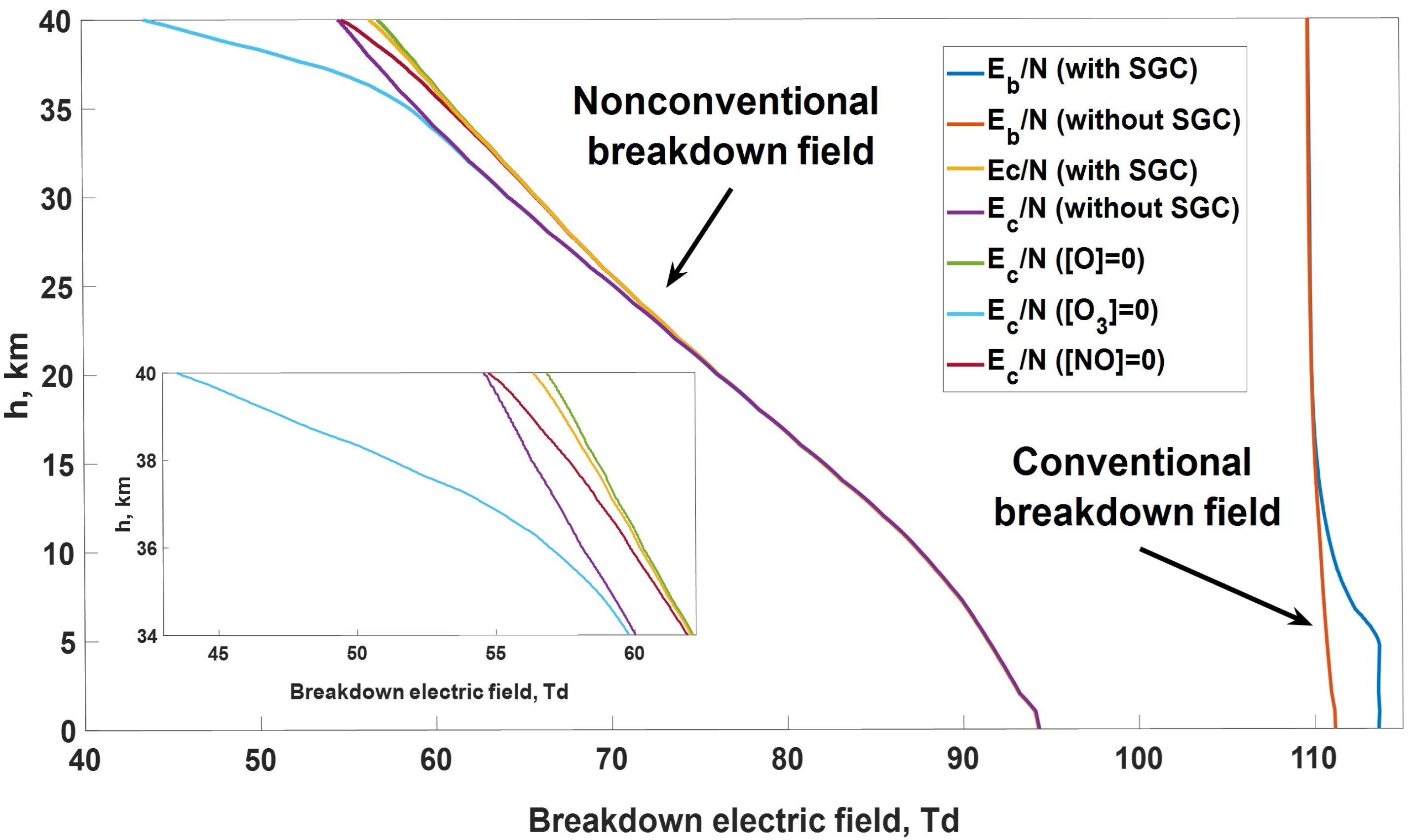
- How does the critical electric field of atmospheric air depend on altitude AMSL?
- 2.
- How does the presence of SGCs influence the critical electric field altitude profile?
- 3.
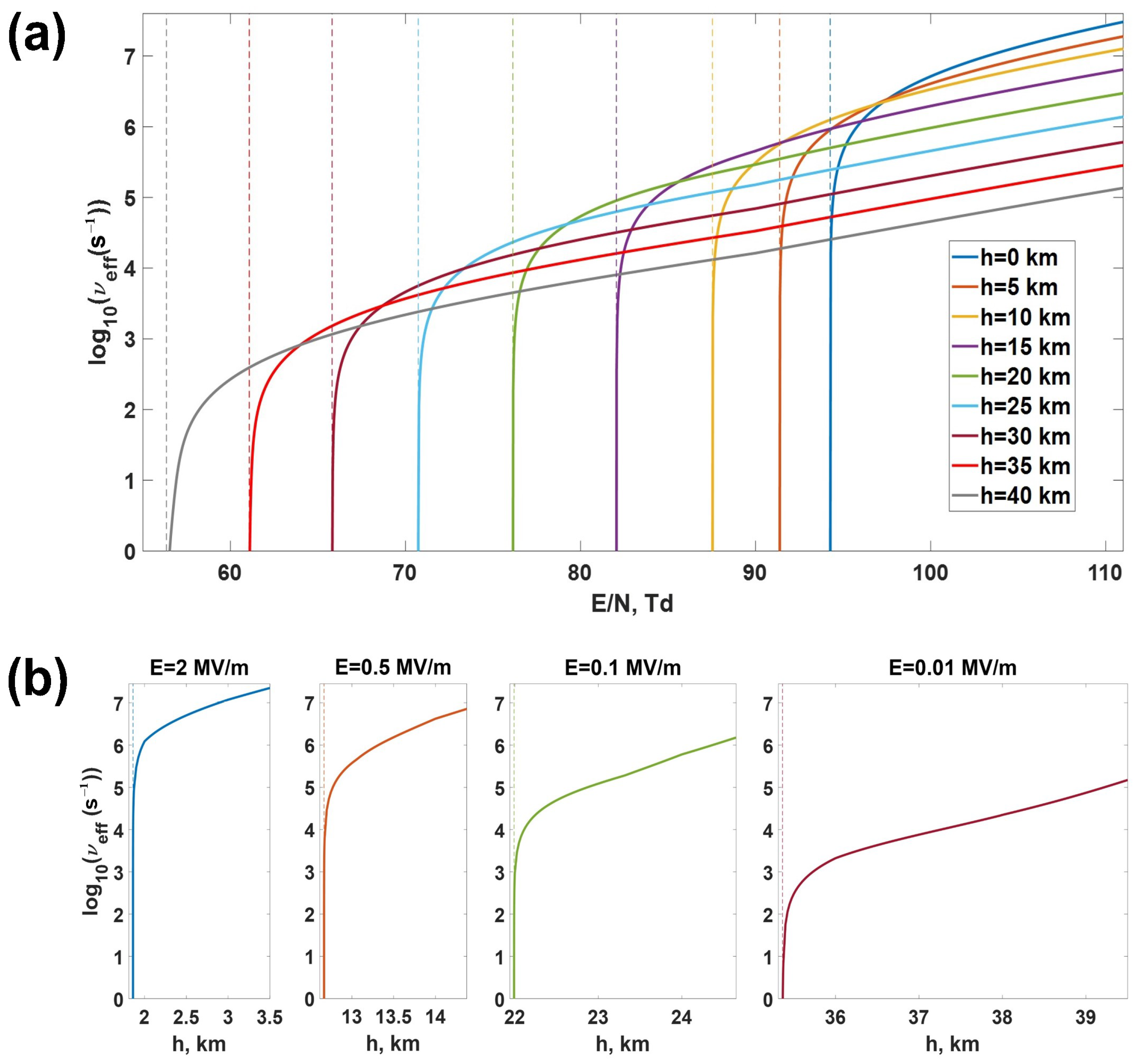
- How does the effective ionization frequency depend on electric field and altitude AMSL?
- 4.
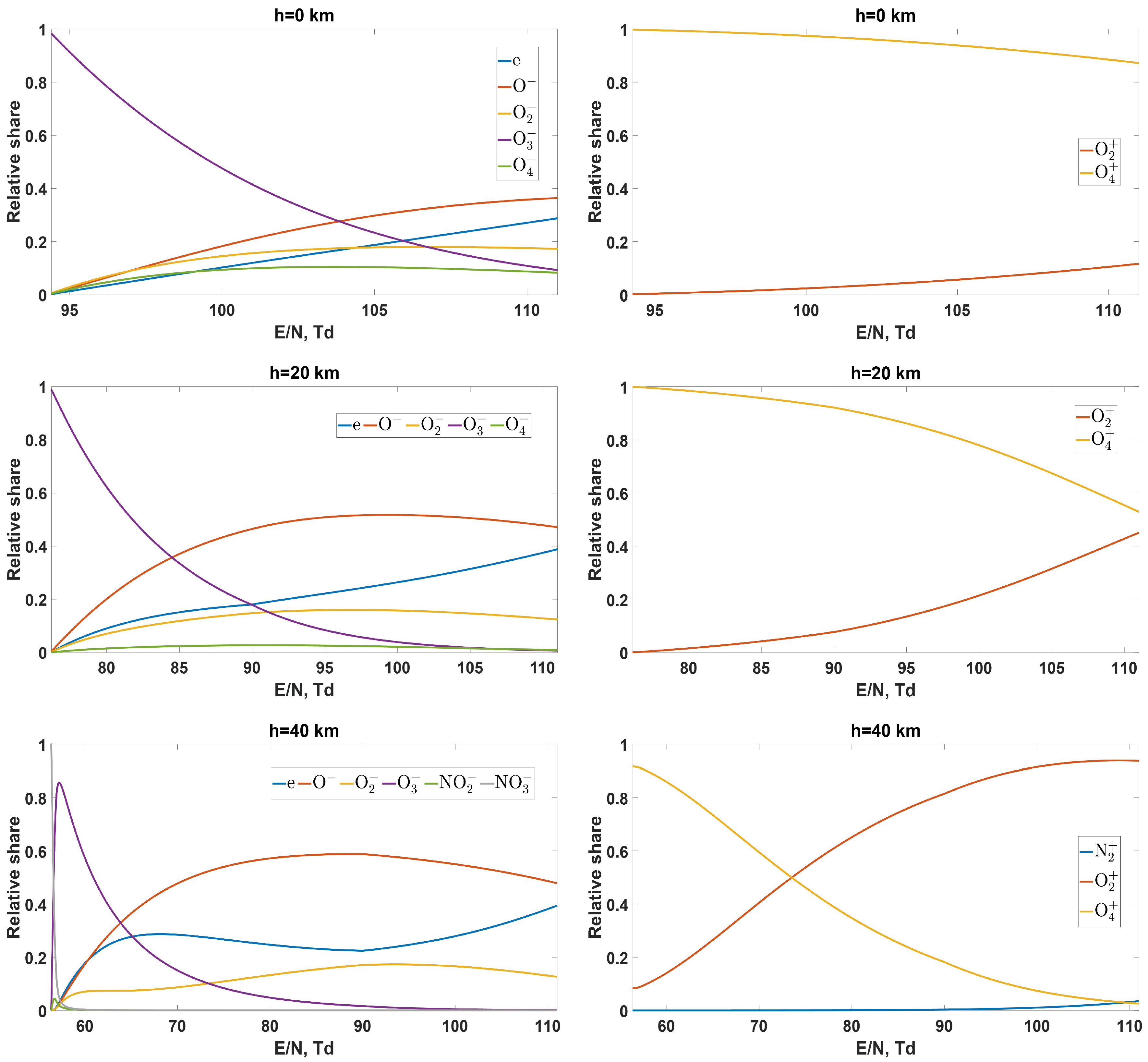
- How does the composition of charged particles (electrons and ions) vary with both electric field and altitude AMSL?
- 5.
- What is the ratio of detachment frequency to ionization frequency at different electric fields and altitudes AMSL?
3. Results
4. Discussion
5. Conclusions
- 1.
- The critical electric field of atmospheric air, at which the multiplication of charged particles begins, is significantly smaller than the conventional value, mostly due to electrons’ detachment from negative ions. The gap between conventional and nonconventional thresholds increases with increasing altitude AMSL from 15% at the ground level to 50% at the height of 40 km.
- 2.
- The presence of SGCs does not significantly influence the critical electric field.
- 3.
- Close to the critical threshold, the effective ionization frequency is a sharp function of the reduced electric field. The rate of its growth decreases with increasing altitude AMSL which partially compensates for the critical electric field reduction.
- 4.
- Above the critical electric field, ionized air contains some amount of free electrons. Their relative share in “community” of negatively charged particles, which can be expressed via the ratio of effective detachment frequency to the attachment frequency, generally increases with increasing reduced electric field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMSL | Above mean sea level |
| SGC | Small gas component |
Appendix A. Model Reactions
| Reaction | Frequency, s | Rate Constant k, ms or ms | Study |
|---|---|---|---|
| Impact ionization | |||
| (1a) | [19] | ||
| (1b) | [19] | ||
| Electron attachment to neutrals | |||
| (2a) | [19] | ||
| (2b) | [17] | ||
| (2c) | [17] | ||
| (2d) | [17] | ||
| (2e) | [17] | ||
| (2f) | [17] | ||
| (2g) | [17] | ||
| (2h) | the same as in (2b) | [17] | |
| (2i) | [17] | ||
| (2g) | [17] | ||
| (2k) | [17] | ||
| (2l) | [20] | ||
| Electron detachment from negative ions | |||
| (3a) | [2] | ||
| (3b) | [17] | ||
| (3c) | [2] | ||
| (3d) | [17] | ||
| (3e) | [17] | ||
| (3f) | [17] | ||
| (3g) | [17] | ||
| (3h) | [17] | ||
| Ion-ion conversion without nitrogen oxides | |||
| (4a) | [2] | ||
| (4b) | [2] | ||
| (4c) | [17] | ||
| (4d) | [17] | ||
| (4e) | [17] | ||
| (4f) | [17] | ||
| (4g) | [17] | ||
| (4h) | [17] | ||
| (4i) | [17] | ||
| (4j) | [17] | ||
| (4k) | [17] | ||
| (4l) | [17] | ||
| (4m) | [17] | ||
| (4n) | [21] | ||
| (4o) | [17] | ||
| (4p) | [17] | ||
| (4q) | [17] | ||
| (4r) | [17] | ||
| (4s) | [17] | ||
| (4t) | – | [17] | |
| (4u) | [17] | ||
| (4v) | [21] | ||
| (4w) | [17] | ||
| (4x) | [17] | ||
| (4y) | [21] | ||
| Ion-ion conversion involving nitrogen oxides | |||
| (5a) | [17] | ||
| (5b) | [17] | ||
| (5c) | [17] | ||
| (5d) | [17] | ||
| (5e) | [17] | ||
| (5f) | [17] | ||
| (5g) | [17] | ||
| (5h) | [17] | ||
| (5i) | [17] | ||
| (5j) | [17] | ||
| (5k) | [17] | ||
| (5l) | [17] | ||
| (5m) | [17] | ||
| (5n) | [17] | ||
| (5o) | [17] | ||
| (5p) | [17] | ||
| (5q) | [17] | ||
| (5r) | [17] | ||
| (5s) | [17] | ||
| (5t) | [22] | ||
| (5u) | [17] | ||
| (5v) | [22] | ||
| (5w) | [22] | ||
| (5x) | [22] | ||
| (5y) | [22] | ||
Appendix B. Evolution Matrix Components
References
- Raizer, Y.P. Gas Discharge Physics; Springer: Berlin, Germany, 1991; 449p. [Google Scholar]
- Pancheshnyi, S. Effective ionization rate in nitrogen-oxygen mixtures. J. Phys. D Appl. Phys. 2013, 46, 155201. [Google Scholar] [CrossRef]
- Da Silva, C.L.; Pasko, V.P. Dynamics of streamer-to-leader transition at reduced air densities and its implications for propagation of lightning leaders and gigantic jets. J. Geophys. Res. Atmos. 2013, 118, 13561–13590. [Google Scholar] [CrossRef]
- Aleksandrov, A.F.; Bychkov, V.L.; Grachev, L.P.; Esakov, I.I.; Lomteva, A.Y. Air ionization in a near-critical electric field. Tech. Phys. 2006, 51, 330–335. [Google Scholar] [CrossRef]
- Chachereau, A.; Pancheshnyi, S. Calculation of the effective ionization rate in air by considering electron detachment from negative ions. IEEE Trans. Plasma Sci. 2014, 42, 3328–3338. [Google Scholar] [CrossRef] [Green Version]
- Luque, A.; Gordillo-Vázquez, F. Mesospheric electric breakdown and delayed sprite ignition caused by electron detachment. Nat. Geosci. 2012, 5, 22–25. [Google Scholar] [CrossRef]
- Iudin, D.I.; Rakov, V.A.; Syssoev, A.A.; Bulatov, A.A.; Hayakawa, M. Formation of decimeter-scale, long-lived elevated ionic conductivity regions in thunderclouds. NPJ Clim. Atmos. Sci. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Thermopedia. Atmosphere (Physical Properties of). 2021. Available online: https://www.thermopedia.com/content/570/ (accessed on 12 August 2021).
- Okabe, H. Photochemistry of Small Molecules; John Wihely & Sons Inc.: Hoboken, NJ, USA, 1978; 413p. [Google Scholar]
- Andrews, D.G. An Introduction to Atmospheric Physics; Cambridge University Press: New York, NY, USA, 2010; 237p. [Google Scholar]
- Zuev, V.Y.; Komarov, V.S.; Lomakina, N.Y.; Mikhailov, S.A. On the optimal representation of altitude profiles of CO2 and atmospheric trace gases. Dokl. Akad. Nauk SSSR 1985, 280, 1086–1090. (In Russian) [Google Scholar]
- Melo, S.M.L.; Farahani, E.; Strong, K.; Bassford, M.R.; Preston, K.E.; McLinden, C.A. NO2 vertical profiles retrieved from ground-based measurements during spring 1999 in the Canadian Arctic. Adv. Space Res. 2004, 34, 786–792. [Google Scholar] [CrossRef]
- Renard, J.B.; Chipperfield, M.P.; Berthet, G.; Goffinont-Taupin, F.; Robert, C.; Chartier, M.; Roscoe, H.; Feng, W.; Rivière, E.; Pirre, M. NO3 Vertical profile measurements from remote sensing balloon-borne spectrometers and comparison with model calculations. J. Atmos. Chem. 2005, 51, 65–78. [Google Scholar] [CrossRef]
- Tsai, T.R.; Rose, R.A.; Weidmann, D.; Wysocki, G. Atmospheric vertical profiles of O3, N2O, CH4, CCl2F2, and H2O retrieved from external-cavity quantum-cascade laser heterodyne radiometer measurements. Appl. Opt. 2012, 51, 8779–8792. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, J.R.; Uman, M.A. The physics of lightning. Phys. Rep. 2014, 534, 147–241. [Google Scholar] [CrossRef]
- Rakov, V.A.; Uman, M.A. Lightning: Physics and Effects; Cambridge University Press: New York, NY, USA, 2003; 687p. [Google Scholar]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207–220. [Google Scholar] [CrossRef]
- Benilov, M.S.; Naidis, G.V. Modelling of low-current discharges in atmospheric-pressure air taking account of non-equilibrium effects. J. Phys. D Appl. Phys. 2003, 36, 1834–1841. [Google Scholar] [CrossRef] [Green Version]
- Aleskandrov, N.L.; Bazelyan, A.E.; Bazelyan, E.M.; Kochetov, I.V. Modeling of long streamers in gases at atmospheric pressure. Plasma Phys. Rep. 1995, 21, 57–69. [Google Scholar]
- Huertas, M.L.; Fontan, J.; Gonzalez, J. Evolution times of tropospheric negative ions. Atmos. Environ. 1978, 12, 2351–2362. [Google Scholar] [CrossRef]
- Sieck, L.W.; Heron, J.T.; Green, D.S. Chemical kinetics database and predictive schemes for humid air plasma chemistry. Part I: Positive ion–molecule reactions. Plasma Chem. Plasma Process. 2000, 20, 235–258. [Google Scholar] [CrossRef]
- Popov, N.A. Evolution of the negative ion composition in the afterglow of a streamer discharge in air. Plasma Phys. Rep. 2010, 36, 812–818. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syssoev, A.; Iudin, D.; Iudin, F.; Klimashov, V.; Emelyanov, A. On the Problem of Critical Electric Field of Atmospheric Air. Atmosphere 2021, 12, 1046. https://doi.org/10.3390/atmos12081046
Syssoev A, Iudin D, Iudin F, Klimashov V, Emelyanov A. On the Problem of Critical Electric Field of Atmospheric Air. Atmosphere. 2021; 12(8):1046. https://doi.org/10.3390/atmos12081046
Chicago/Turabian StyleSyssoev, Artem, Dmitry Iudin, Fedor Iudin, Vitaly Klimashov, and Alexey Emelyanov. 2021. "On the Problem of Critical Electric Field of Atmospheric Air" Atmosphere 12, no. 8: 1046. https://doi.org/10.3390/atmos12081046
APA StyleSyssoev, A., Iudin, D., Iudin, F., Klimashov, V., & Emelyanov, A. (2021). On the Problem of Critical Electric Field of Atmospheric Air. Atmosphere, 12(8), 1046. https://doi.org/10.3390/atmos12081046






