Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept
Abstract
:1. Introduction
2. Materials and Methods
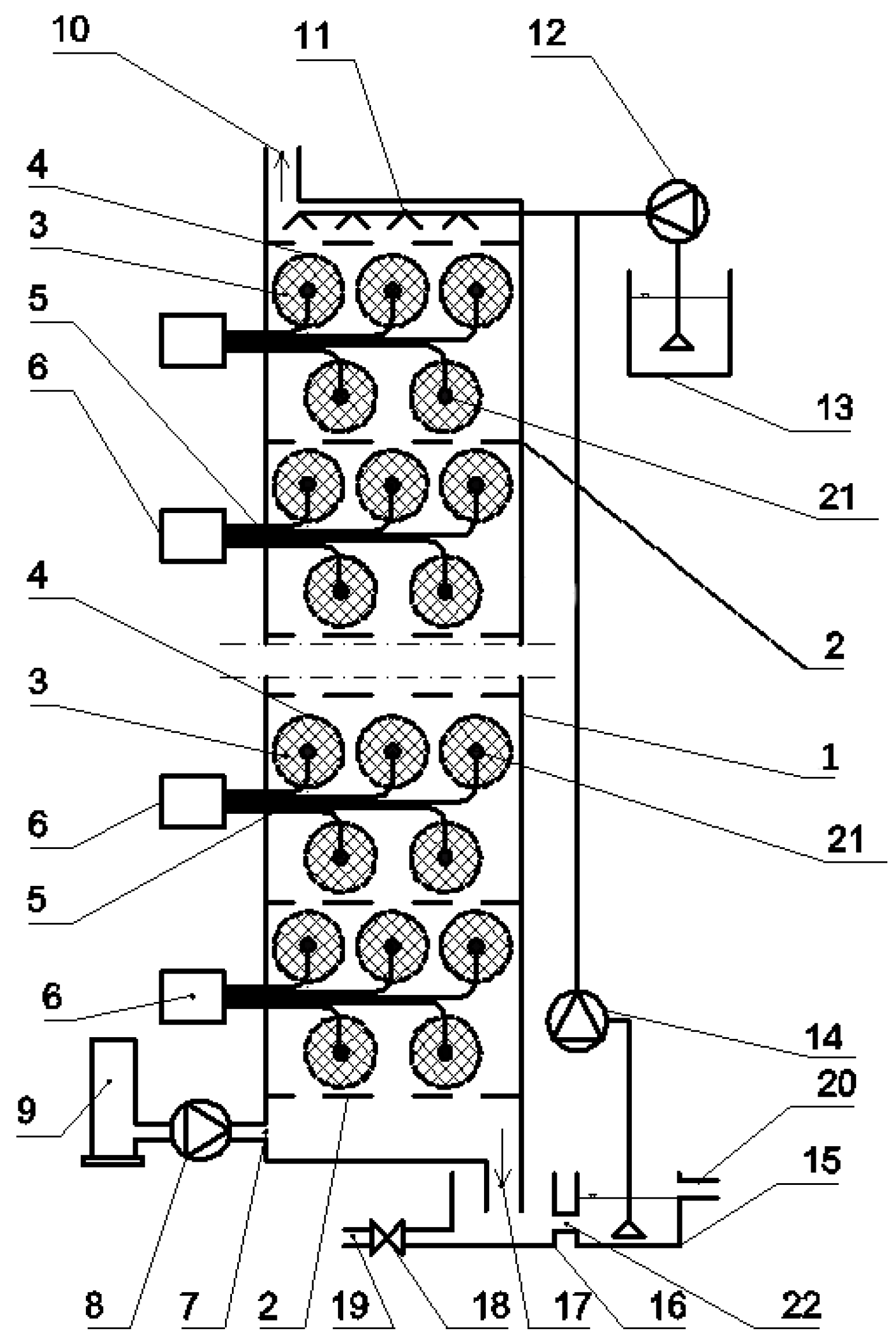
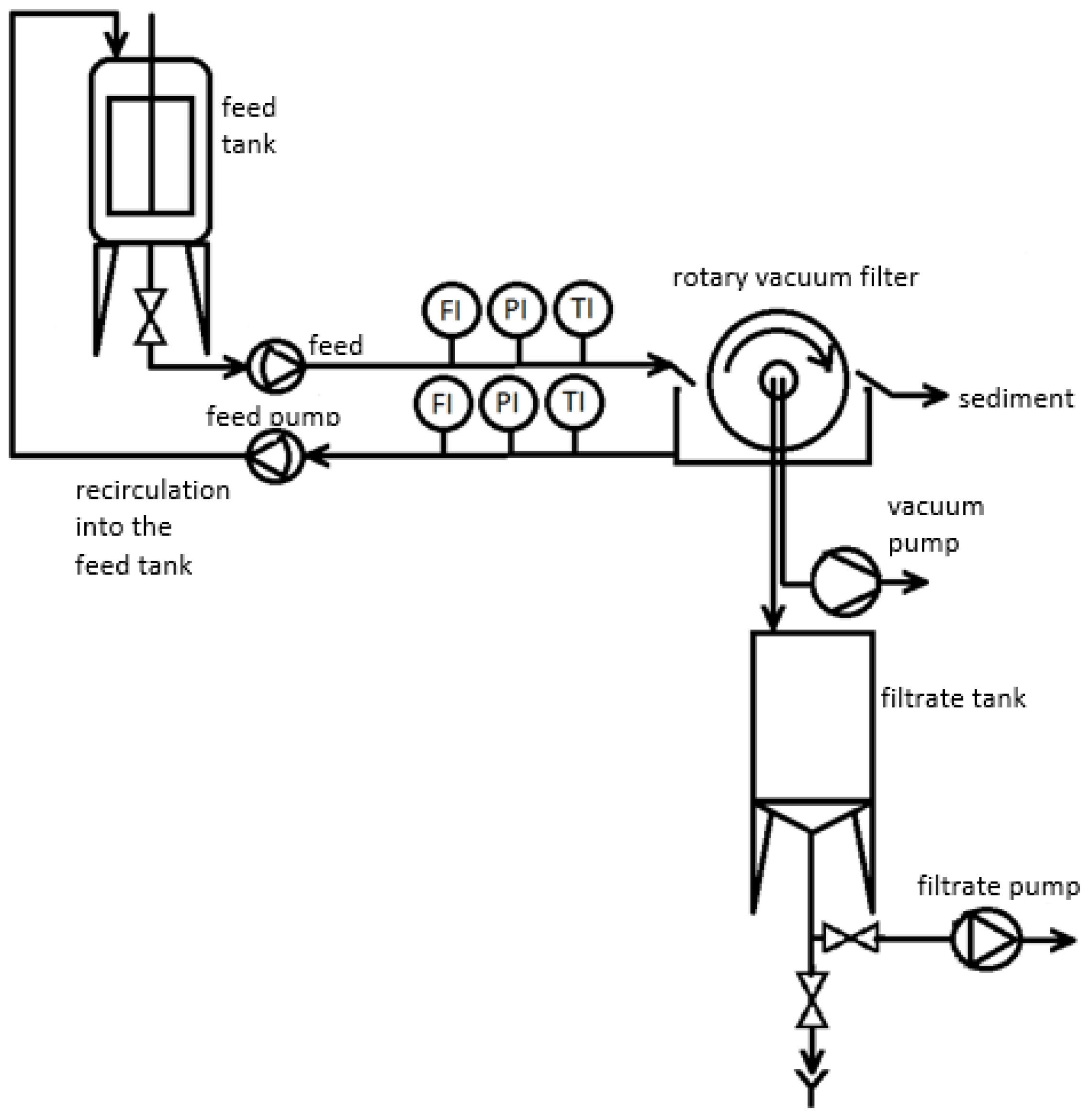
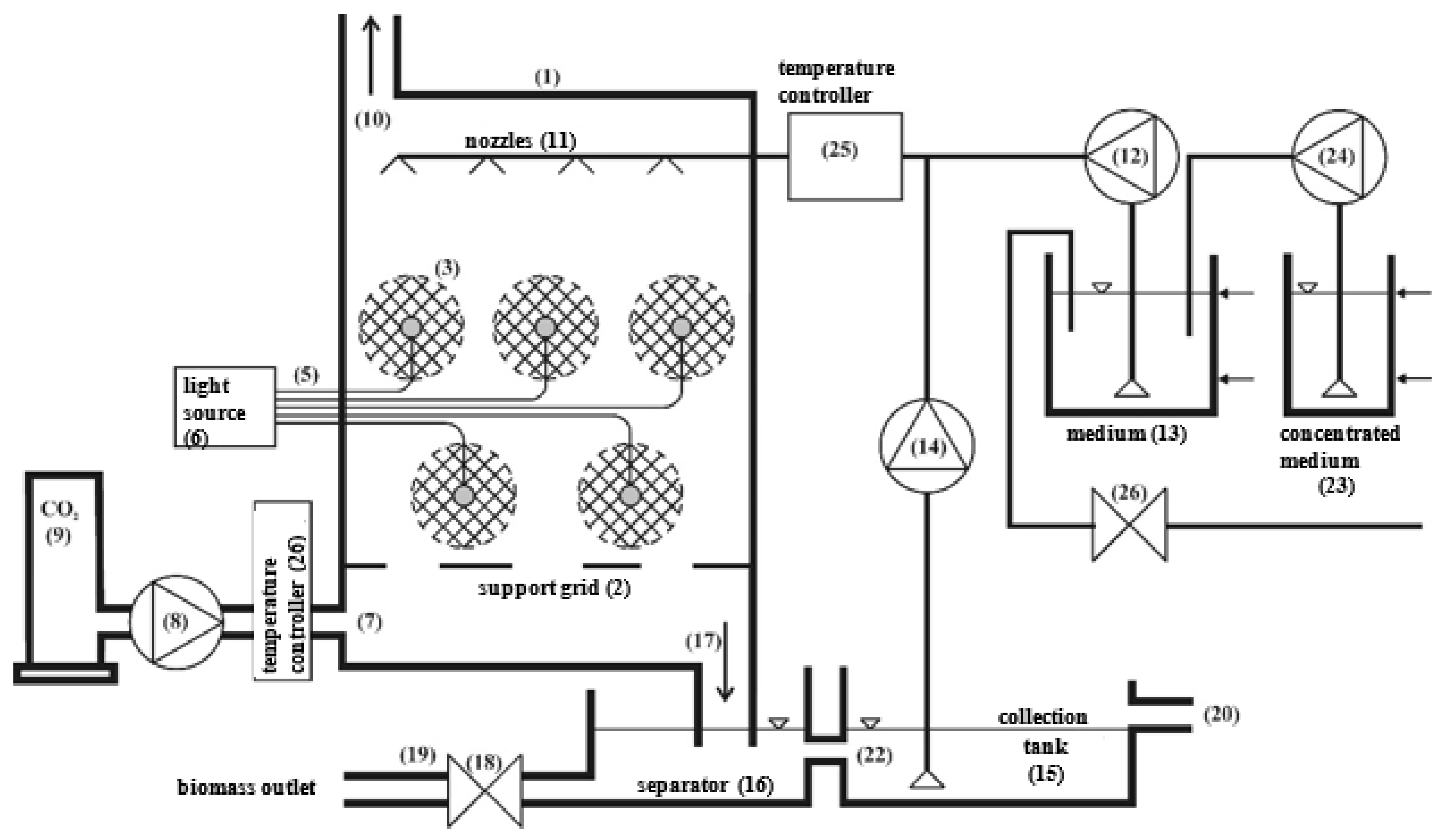
2.1. Experimental Set-Up
2.2. Immobilized Microalgal Biomass
2.3. Materials
2.4. Analytical and Statistical Methods
3. Results and Discussion
4. Technical/Technological Concept of a Large-Scale IMC-CO2PBR
4.1. Concentration of Biomass
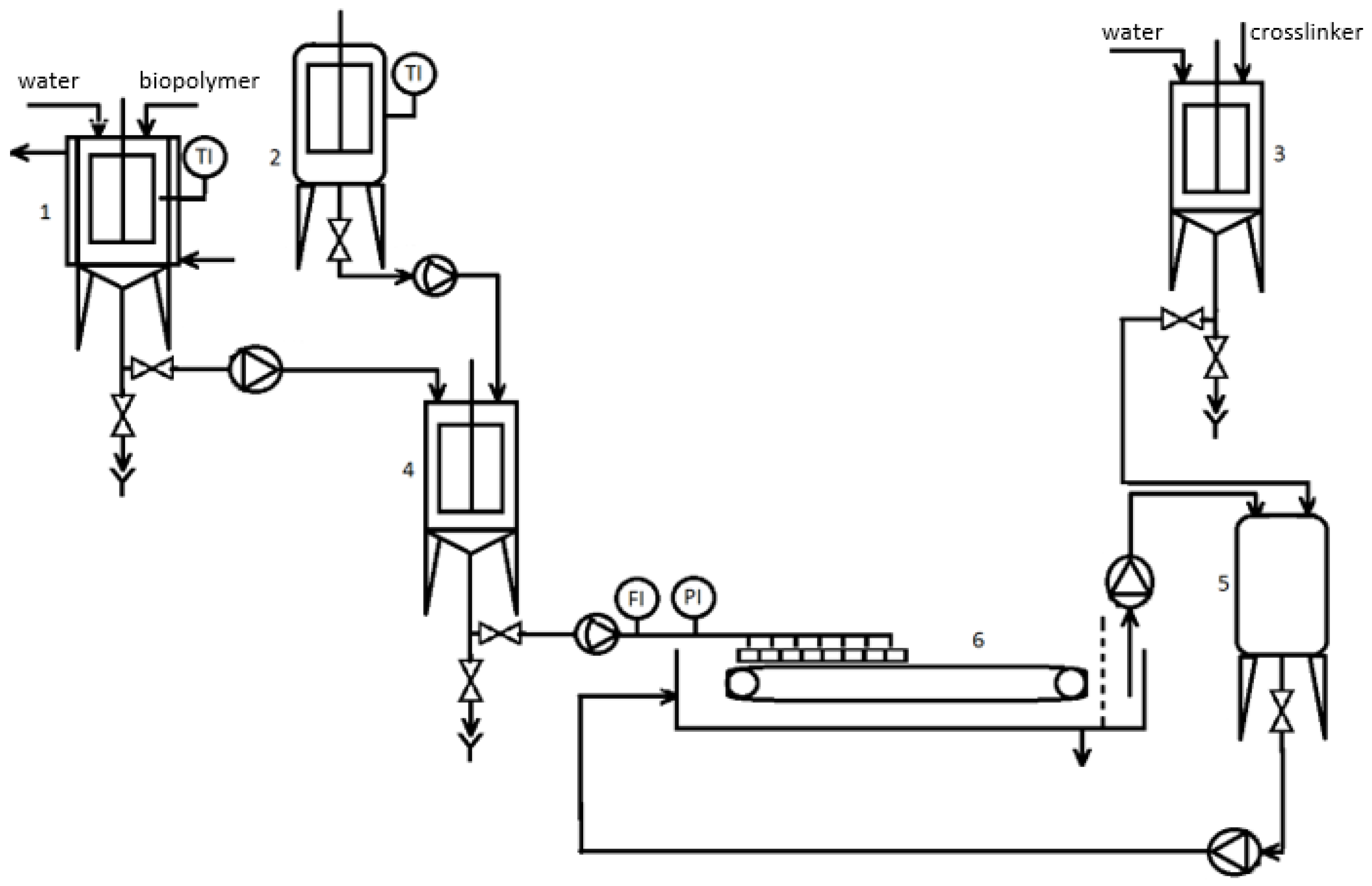
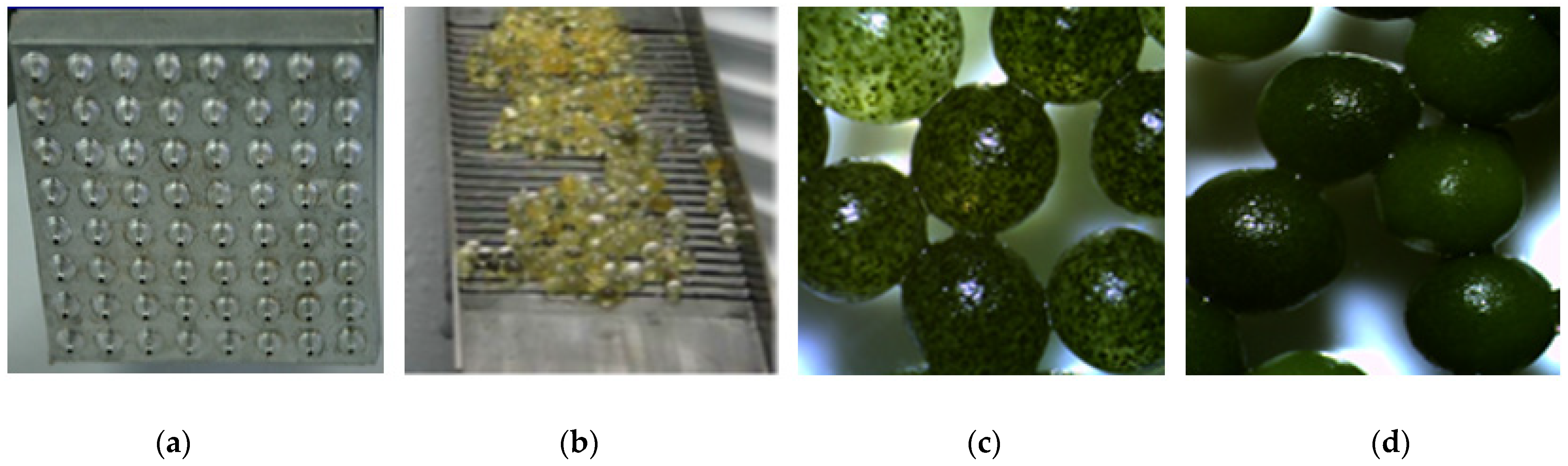
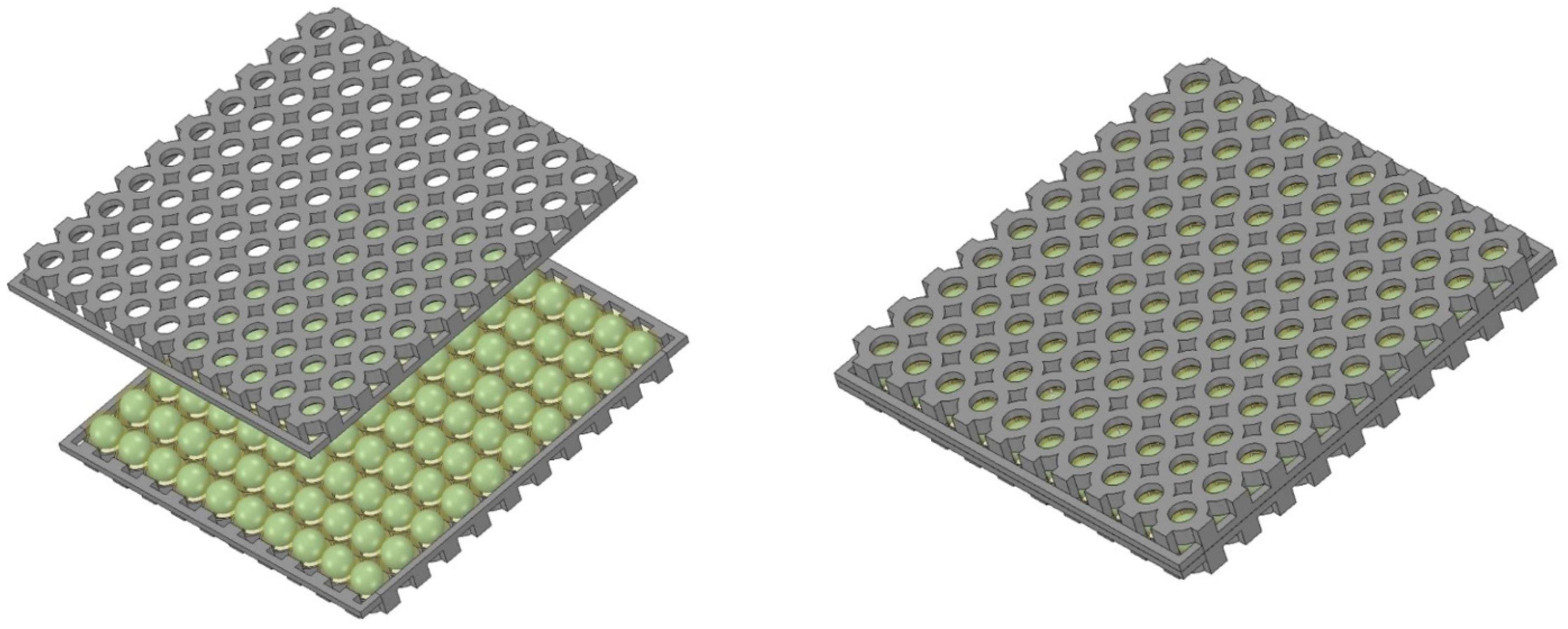
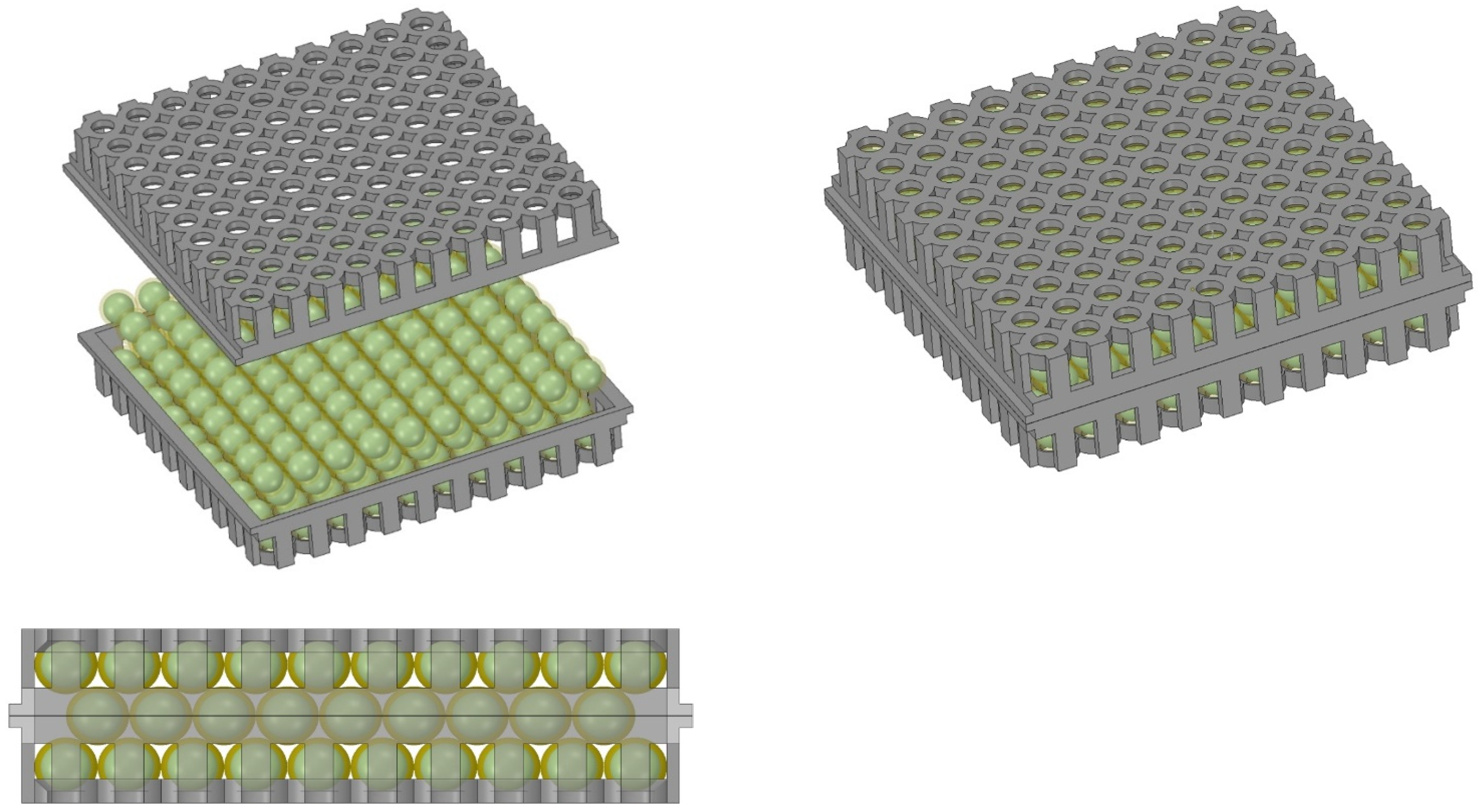
4.2. Immobilization of Biomass Using Hydrogel Encapsulation
4.3. Light Source
4.4. Size and Weight Analysis for the Photobioreactor Module
4.5. Analysis of Solar Energy Requirements
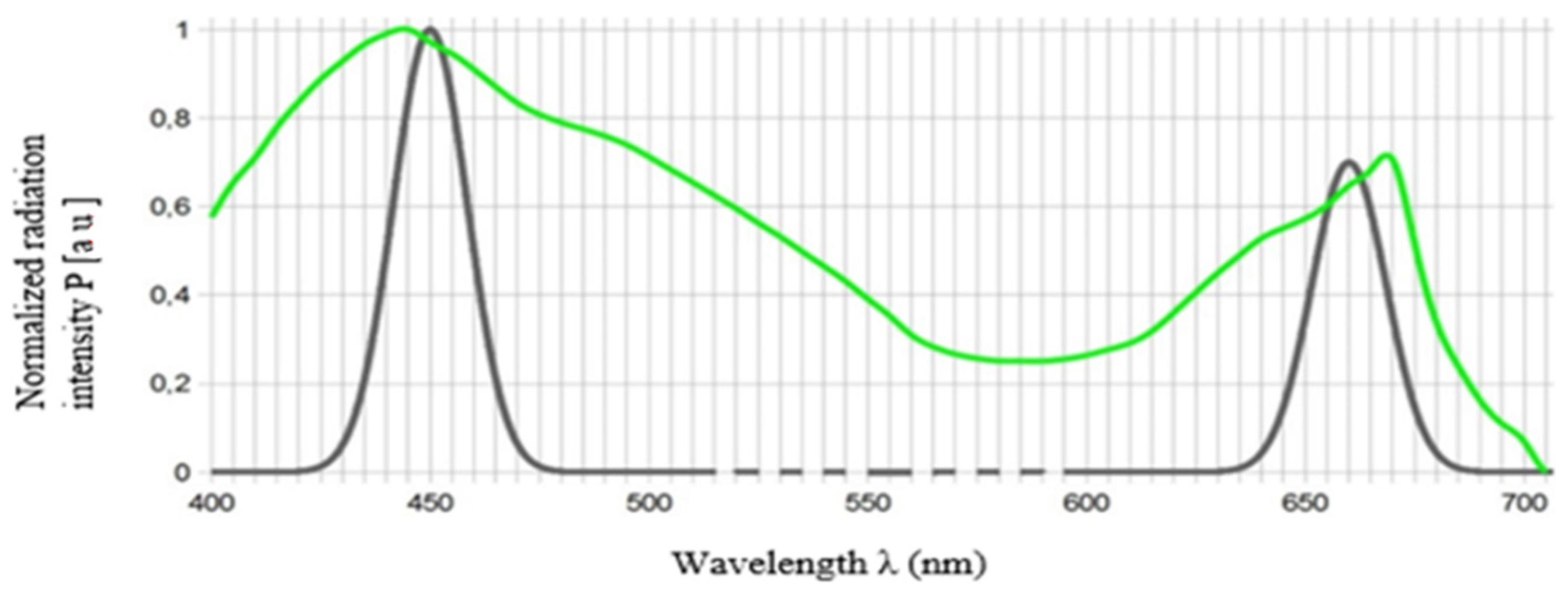
4.6. Red (660 nm) and Blue (450 nm) LED Light Requirements
4.7. Supplying Sunlight to the Capsules
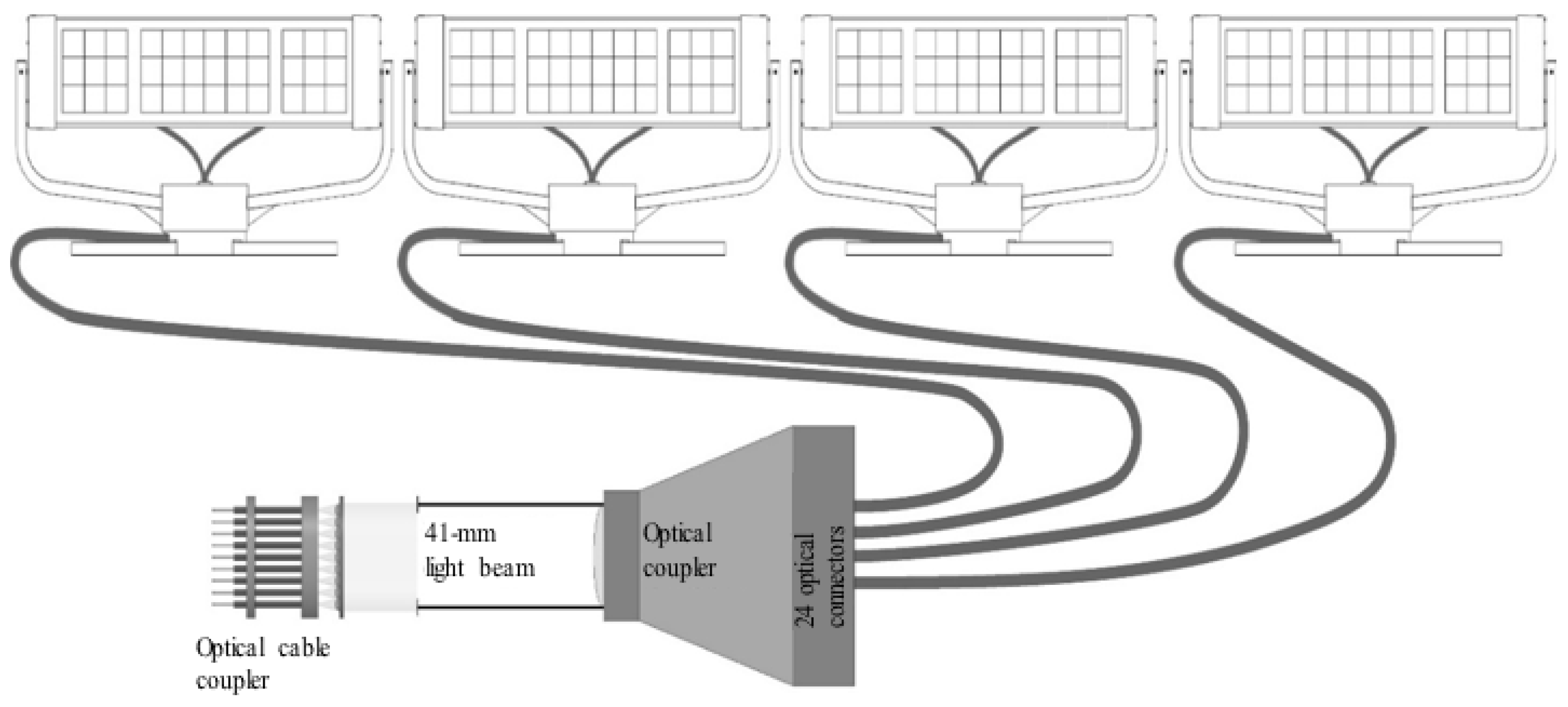
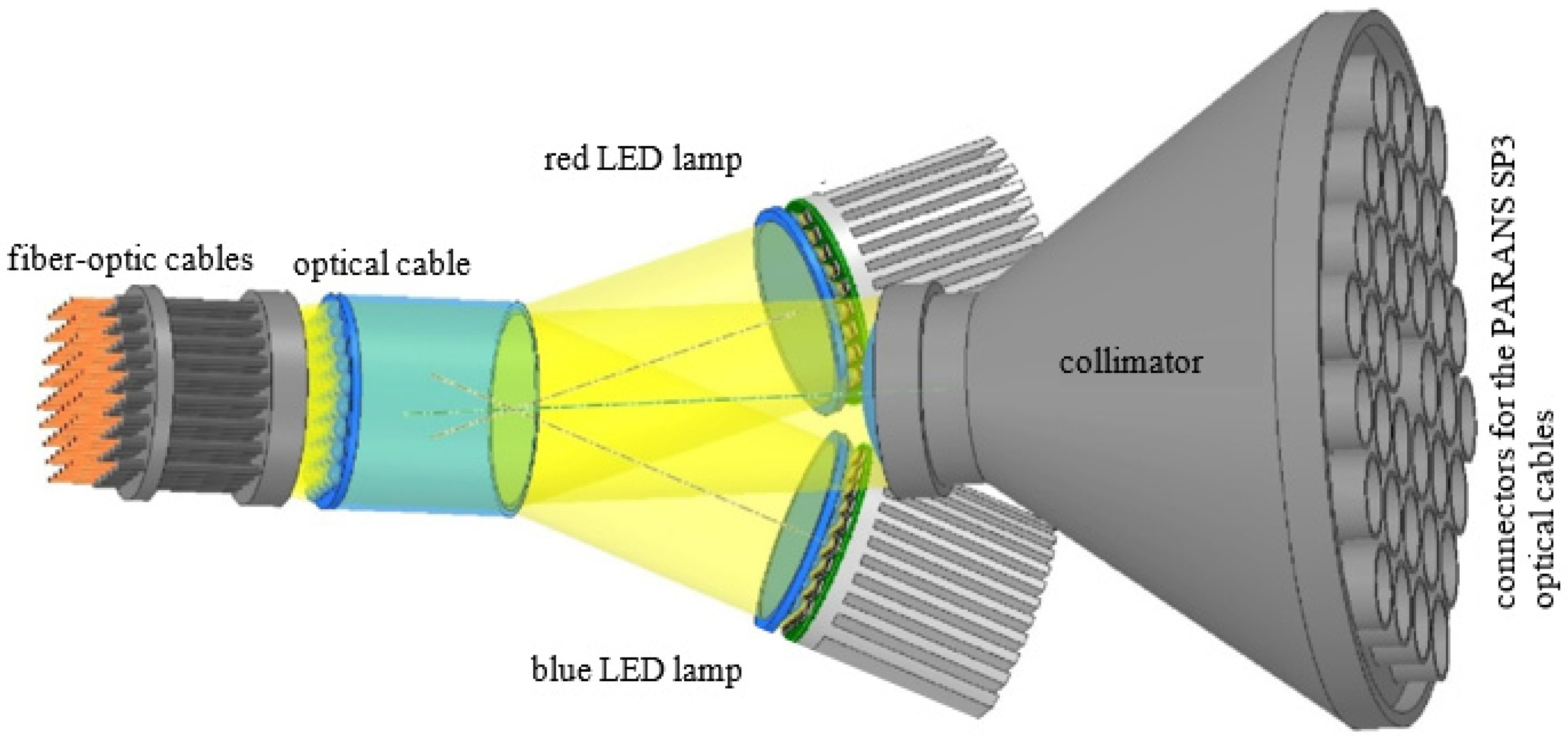
4.8. Supplying LED Light to the Capsules
4.9. Fixing the Fiber-Optic Cables within the Capsules
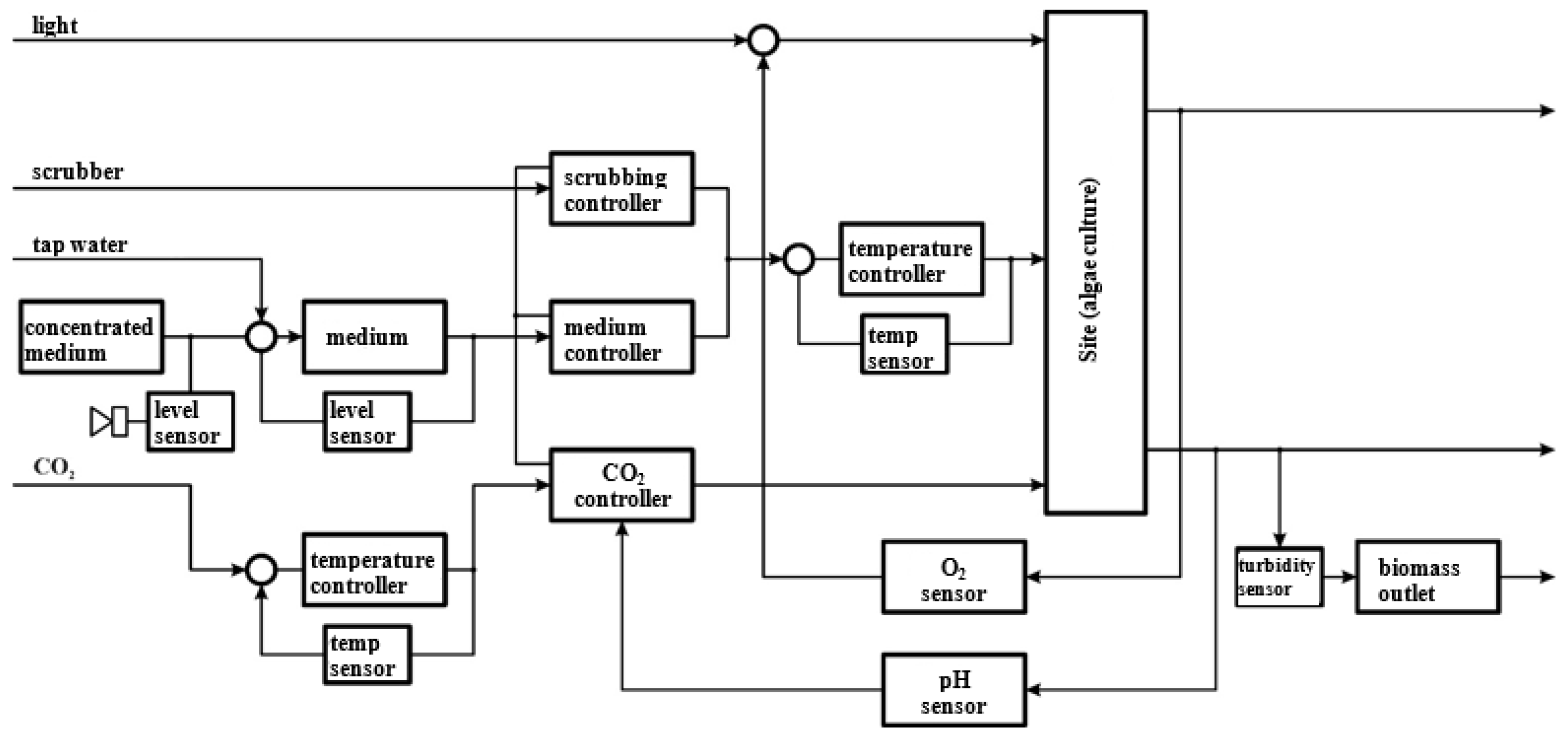
4.10. Automated Process Control Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.L.; Ma, C.Q.; Ren, Y.S.; Zhao, X.W. Do Real Output and Renewable Energy Consumption Affect CO2 Emissions? Evidence for Selected BRICS Countries. Energies 2020, 13, 960. [Google Scholar] [CrossRef] [Green Version]
- Jacob-Lopes, E.; Scoparo, C.H.G.; Queiroz, M.I.; Franco, T.T. Biotransformations of carbon dioxide in photobioreactors. Energy Convers. Manag. 2010, 51, 894–900. [Google Scholar] [CrossRef]
- Panepinto, D.; Riggio, V.A.; Zanetti, M. Analysis of the Emergent Climate Change Mitigation Technologies. Int. J. Environ. Res. Public Health 2021, 18, 6767. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.P.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Ho, S.H.; Chang, J.S.; Show, P.L. Microalgae for biofuels, wastewater treatment and environmental monitoring. Environ. Chem. Lett. 2021, 19, 2891–2904. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Show, P.L. Microalgal-bacterial consortia as future prospect in wastewater bioremediation, environmental management and bioenergy production. Indian J. Microbiol. 2021, 61, 262–269. [Google Scholar] [CrossRef]
- Aghaalipour, E.; Akbulut, A.; Güllü, G. Carbon dioxide capture with microalgae species in continuous gas-supplied closed cultivation systems. Biochem. Eng. J. 2020, 163, 107741. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.J.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Morais, K.C.; Conceicao, D.; Vargas, J.V.; Mitchell, D.A.; Mariano, A.B.; Ordonez, J.C.; GalliTersawa, L.V.; Kava, V.M. Enhanced microalgae biomass and lipid output for increased biodiesel productivity. Renew. Energy 2021, 163, 138–145. [Google Scholar] [CrossRef]
- Zhang, F.; Kabeya, H.; Kitagawa, R.; Hirotsu, T.; Yamashita, M.; Otsuki, T. An exploratory research of PVC-Chlorella composite material (PCCM) as effective utilization of Chlorella biologically fixing CO2. J. Mater. Sci. 2000, 35, 2603–2609. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Rumin, J.; Nicolau, E.; Gonçalves de Oliveira Junior, R.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Fermentative ethanol production from Madhuca indica flowers using immobilized yeast cells coupled with solar driven direct contact membrane distillation with commercial hydrophobic membranes. Energy Convers. Manag. 2019, 181, 593–607. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: A review of membrane-integrated green approach. Sci. Total Environ. 2020, 698, 134169. [Google Scholar] [CrossRef]
- Emparan, Q.; Jye, Y.S.; Danquah, M.K.; Harun, R. Cultivation of Nannochloropsis sp. microalgae in palm oil mill effluent (POME) media for phycoremediation and biomass production: Effect of microalgae cells with and without beads. J. Water Process Eng. 2020, 33, 101043. [Google Scholar] [CrossRef]
- Jimenez-Perez, M.V.; Sánchez-Castillo, P.; Romera, O.; Fernández-Moreno, D.; Pérez-Martínez, C. Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzym. Microb. Technol. 2004, 34, 392–398. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Cheirsilp, B.; Boonsawang, P.; Prasertsan, P. Immobilized oleaginous microalgae as effective two-phase purify unit for biogas and anaerobic digester effluent coupling with lipid production. Bioresour. Technol. 2019, 281, 149–157. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Soo, C.-L.; Chen, C.-A.; Bojo, O.; Hii, Y.-S. Feasibility of marine microalgae immobilization in alginate bead for marine water treatment: Bead stability, cell growth, and ammonia removal. Int. J. Polym. Sci. 2017, 2017, 6951212. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-May, B.; del Pilar Sánchez-Saavedra, M.; Lizardi, J.; Voltolina, D. Growth of Synechococcus sp. immobilized in chitosan with different times of contact with NaOH. J. Appl. Phycol. 2007, 19, 181–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pane, L.; Feletti, M.; Bertino, C.; Carli, A. Viability of the marine microalga Tetraselmis suecica grown free and immobilized in alginate beads. Aquac. Int. 1998, 6, 411–420. [Google Scholar] [CrossRef]
- Barati, B.; Zeng, K.; Baeyens, J.; Wang, S.; Addy, M.; Gan, S.-Y.; El-Fatah Abomohra, A. Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass Bioenergy 2021, 145, 105927. [Google Scholar] [CrossRef]
- Kim, P.; Otim, O. Optimizing a municipal wastewater-based Chlorella vulgaris photobioreactor for sequestering atmospheric CO2. South. Calif. Acad. Sci. 2019, 118, 42–57. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; González-López, C.V.; Fernández Sevilla, J.M.F.; Grima, E.M. Conversion of CO2 into biomass by microalgae: How realistic a contribution may it be to significant CO2 removal? Appl. Microbiol. Biotechnol. 2012, 96, 577–586. [Google Scholar] [CrossRef]
- Krzemieniewski, M.; Szwarc, D.; Zieliński, M.; Kupczyk, K.; Rokicka, M.; Dębowski, M. The possibility of using carbon dioxide from biogas in the production of microalgae biomass. Maint. Probl. 2016, 1, 5–15. [Google Scholar]
- Zaporozhets, A. Development of software for fuel combustion control system based on frequency regulator. CEUR Workshop Proc. 2019, 2387, 223–230. [Google Scholar]
- Packer, M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 2009, 37, 3428–3437. [Google Scholar] [CrossRef]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating algae for CO2 capture and accumulation and simultaneous production of biomass for biodiesel production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Huang, T.T.; Lin, C.J.; Ong, S.C.; Chen, C.D.; Chang, J.S.; Lin, C.S. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. Cultures. Bioresour. Technol. 2011, 102, 9135–9142. [Google Scholar] [CrossRef]
- Hende, S.V.D.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae: (bio) chemical interactions leading to biotechnological opportunities. Biotech. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Shen, Y.; Zhang, J.; Wang, C.-H.; Ge, T.S.; Tong, Y.W. A comparative life cycle assessment on four waste-to-energy scenarios for food waste generated in eateries. Appl. Energy 2018, 225, 1143–1157. [Google Scholar] [CrossRef]
- Kandimalla, P.; Vatte, P.; Bandaru, C.S.R. Phycoremediation of automobile exhaust gases using green microalgae. Environ. Dev. Sustain. 2021, 23, 6301–6322. [Google Scholar] [CrossRef]
- Lee, C.M.; Chen, P.C.; Wang, C.C.; Tung, Y.C. Photohydrogen production using purple nonsulfur bacteria with hydrogen fermentation reactor effluent. Int. J. Hydrogen Energy 2002, 27, 1309–1313. [Google Scholar] [CrossRef]
- Lam, M.K.; TeongLee, K.; Rahman, M.A. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control. 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Kumar, K.; Banerjee, D.; Das, D. Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana. Bioresour. Technol. 2014, 152, 225–233. [Google Scholar] [CrossRef]
- Kvamsdal, H.M.; Haugen, G.; Svendsen, H.F. Flue-gas cooling in post combustion capture plants. Chem. Eng. Res. Des. 2011, 89, 1544–1552. [Google Scholar] [CrossRef]
- Lill, J.O.; Salovius-Lauren, S.; Harju, L.; Rajander, J.; Saarela, K.-E.; Lindroos, A. Temporal changes in elemental composition in decomposing filamentous algae (Cladophora glomerata and Pilayella littoralis) determined with PIXE and PIGE. Sci. Total Environ. 2012, 414, 646–652. [Google Scholar] [CrossRef]
- Aráujo, S.C.; Garcia, V.M.T. Growth biochemical composition of the diatom Chaetoceros cf. Wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 2005, 46, 405–412. [Google Scholar] [CrossRef]
- Chang, E.H.; Yang, S.S. Some characteristics of microalgae isolated in Taiwan for biofixation of carbon dioxide. Bot. Bull. Acad. Sin. 2003, 44, 43–52. [Google Scholar]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Show, P.L.; Tan, I.S.; Lee, K.T. Cultivation of Chlorella vulgaris using sequential-flow bubble column photobioreactor: A stress-inducing strategy for lipid accumulation and carbon dioxide fixation. J. CO2 Util. 2020, 41, 101226. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, X.; Martin, G.J.O.; Kentish, S.E. Critical review of strategies for CO2 delivery to large-scale microalgae cultures. Chin. J. Chem. Eng. 2018, 26, 2219–2228. [Google Scholar] [CrossRef]
- Cheng, J.; Miao, Y.; Guo, W.; Song, Y.; Tian, J.; Zhou, J. Reduced generation time and size of carbon dioxide bubbles in a volute aerator for improving Spirulina sp. growth. Bioresour. Technol. 2018, 270, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Martin, G.J.; Wu, Y.; Kentish, S.E. The use of monoethanolamine and potassium glycinate solvents for CO2 delivery to microalgae through a polymeric membrane system. Biochem. Eng. J. 2017, 128, 126–133. [Google Scholar] [CrossRef]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Chlamydomonas reinhardii optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brilman, W.; Alba, L.G.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents for microalgae cultivation. Biomass Bioenerg. 2013, 53, 39–47. [Google Scholar] [CrossRef]
- Vargas-Estrada, L.; Torres-Arellano, S.; Longoria, A.; Arias, D.M.; Okoye, P.U.; Sebastian, P.J. Role of nanoparticles on microalgal cultivation: A review. Fuel 2020, 280, 228598. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, K. Effects of CO2 concentrations on the freshwatermicroalgae, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J. Appl. Phycol. 2003, 15, 379–389. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Fan, L.H.; Zhang, Y.T.; Zhang, L.; Chen, H.L. Evaluation of a membrane-sparged helical tubular photobioreactor for carbon dioxide biofixation by Chlorella vulgaris. J. Membr. Sci. 2008, 325, 336–345. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Z.; Ye, Q.; Zhou, J.; Cen, K. Improving CO2 fixation with microalgae by bubble breakage in raceway ponds with up–down chute baffles. Bioresour. Technol. 2016, 201, 174–181. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Continuous hydrogen production by immobilized Enterobacter cloacae IIT-BT 08 using lignocellulosic materials as solid matrices. Enzym. Microb. Technol. 2001, 29, 280–287. [Google Scholar] [CrossRef]
- Ho, S.; Chen, C.; Lee, D.; Chang, J. Perspectives on microalgal CO2-emission mitigation systems—a review. Biotech. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Eguiheneuf, F.; Ekhan, A.; Tran, L.-S.P. Genetic Engineering: A Promising Tool to Engender Physiological, Biochemical, and Molecular Stress Resilience in Green Microalgae. Front. Plant Sci. 2016, 7, 400. [Google Scholar] [CrossRef] [Green Version]
- Shokravi, Z.; Shokravi, H.; Chyuan, O.H.; Lau, W.J.; Koloor, S.S.R.; Petrů, M.; Ismail, A.F. Improving ‘Lipid Productivity’ in Microalgae by Bilateral Enhancement of Biomass and Lipid Contents: A Review. Sustainability 2020, 12, 9083. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, J.; Wu, T.; Chen, F. High-value biomass from microalgae production platforms: Strategies and progress based on carbon metabolism and energy conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Yang, J.; Cui, M.; Zhang, W.; Zhao, J. Comparative experiments of two novel tubular photobioreactors with an inner aerated tube for microalgal cultivation: Enhanced mass transfer and improved biomass yield. Algal Res. 2021, 58, 102364. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Krzemieniewski, M.; Dudek, M.; Grala, A. Microalgae cultivation methods. Pol. J. Nat. Sci. 2012, 27, 151–164. [Google Scholar]
- Huang, B.; Shan, Y.; Yi, T.; Tang, T.; Wei, W.; Quinn, N.W.T. Study on high-CO2 tolerant Scenedesmus sp. and its mechanism via comparative transcriptomic analysis. J. CO2 Util. 2020, 42, 101331. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Grala, A.; Dudek, M. Algae biomass as an alternative substrate in biogas production technologies—review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Sung, K.D.; Lee, J.S.; Shin, C.S.; Park, S.C.; Choi, M.J. CO2 fixation by Chlorella sp. KR-1 and its cultural characteristics. Bioresour. Technol. 1999, 68, 269–273. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Keffer, J.E.; Kleinheinz, G.T. Use of Chlorella vulgar is for CO2 mitigation in a photobioreactor. J. Ind. Microbiol. Biotechnol. 2002, 29, 275–280. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Tsai, M.T.; Kao, C.Y.; Ong, S.C.; Lin, C.S. The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng. Life Sci. 2009, 9, 254–260. [Google Scholar] [CrossRef]
- Stuart, C.; Hessami, M.A. A study of methods of carbon dioxide capture and sequestration–the sustainability of a photosynthetic bioreactor approach. Energy Convers. Manag. 2005, 46, 403–420. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Scoparo, C.H.G.; Franco, T.T. Rates of CO2 removal by Aphanothece microscopica Nageli in tubular photobioreactors. Chem. Eng. Process. 2008, 47, 1371–1379. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Thawechai, T.; Prasertsan, P. Immobilized oleaginous microalgae for production of lipid and phytoremediation of secondary effluent from palm oil mill in fluidized bed photobioreactor. Bioresour. Technol. 2017, 241, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dasgupta, C.N.; Nayak, B.; Lindblad, P.; Das, D. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour. Technol. 2011, 102, 4945–4953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Chiu, T.-W.; Lin, H.-Y.; Chen, C.-D.; Chang, J.-S.; Lin, C.-S. Utilization of Carbon Dioxide in Industrial Flue Gases for the Cultivation of Microalgal Chlorella sp. Bioresour. Technol. 2014, 166, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: Possibilities and challenges. Biotechnol. Adv. 2013, 31, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.C.M.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Simões, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renew. Sustain. Energy Rev. 2012, 16, 3043–3053. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, J.; Yang, Z.; Zhou, J. Heavy metal control in microalgae cultivation with power plant flue gas entering into raceway pond. Environ. Sci. Pollut. Res. 2020, 27, 37357–37362. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Rahimpour, H.R.; Ghasemi, Y.; Zoughi, S.; Rahimpour, M.R. A review of carbon capture and sequestration in Iran: Microalgal biofixation potential in Iran. Renew. Sustain. Energy Rev. 2014, 35, 73–100. [Google Scholar] [CrossRef]
- Douskova, J.; Doucha, J.; Livansky, K.; Machat, J. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Apply Microbiol. Biotechnol. 2009, 82, 179–185. [Google Scholar] [CrossRef]
- Morel, A. Available, usable, and stored radiant energy in relation to marine photosynthesis. Deep. Sea Res. 1978, 25, 673–688. [Google Scholar] [CrossRef]
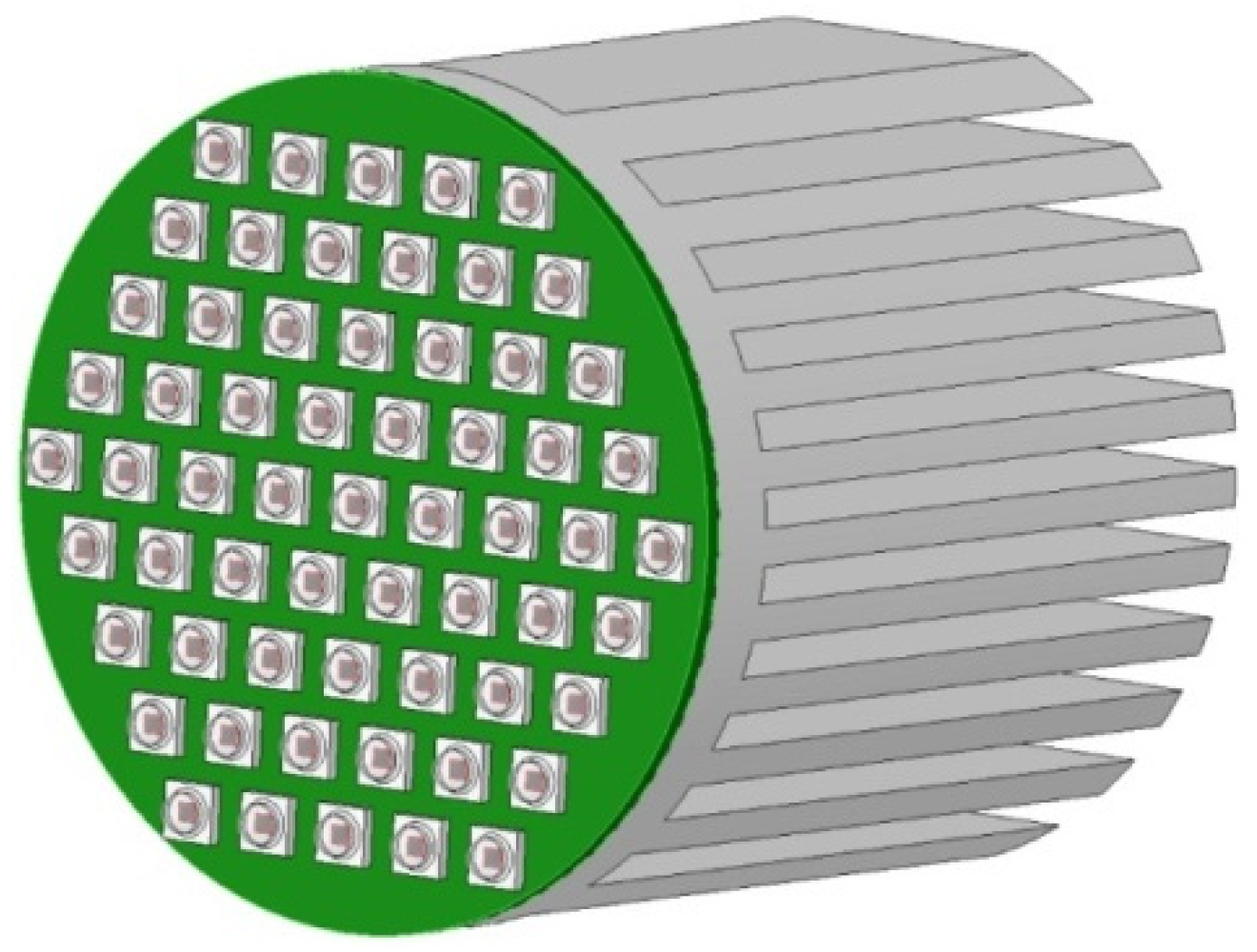
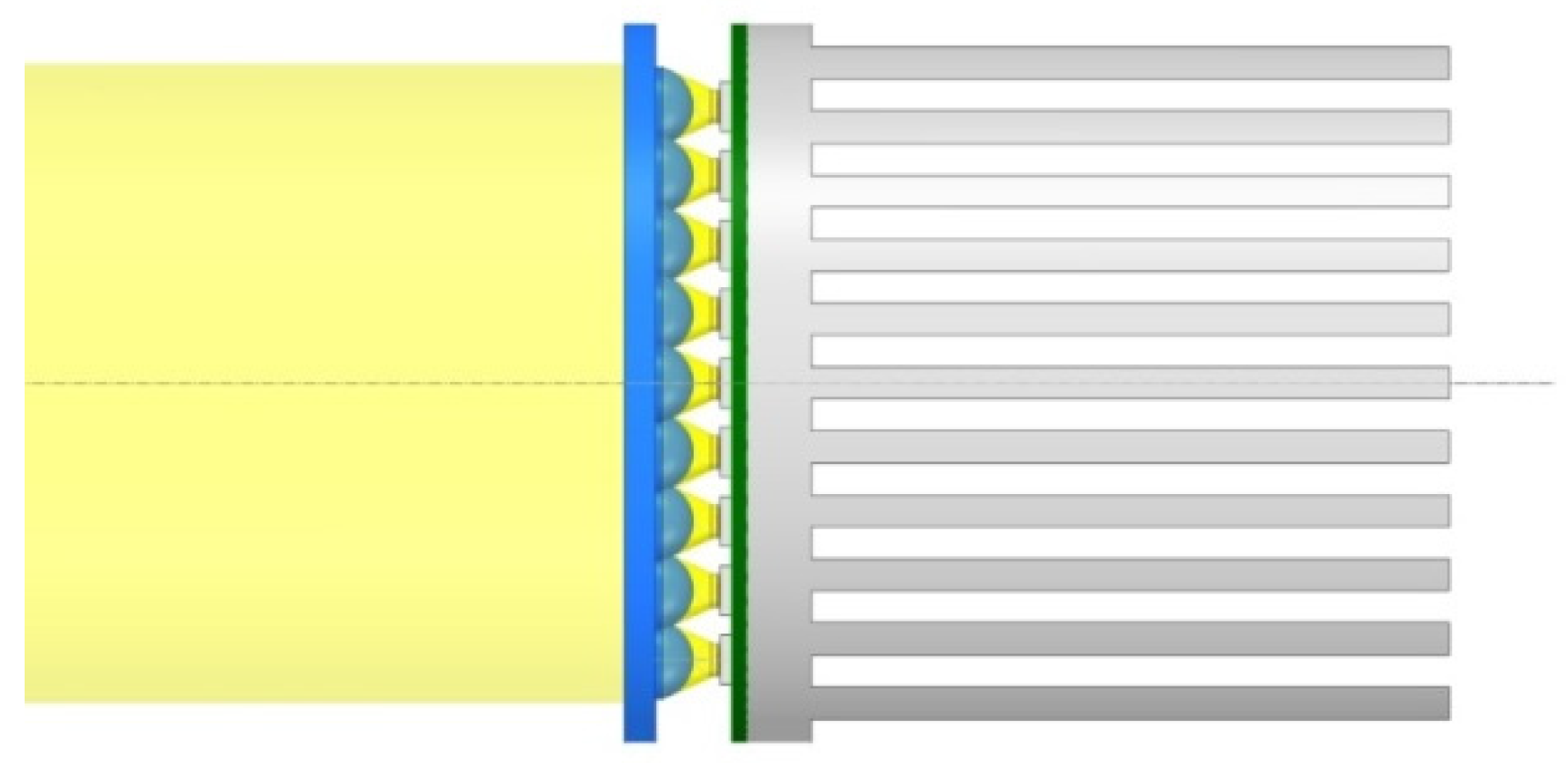
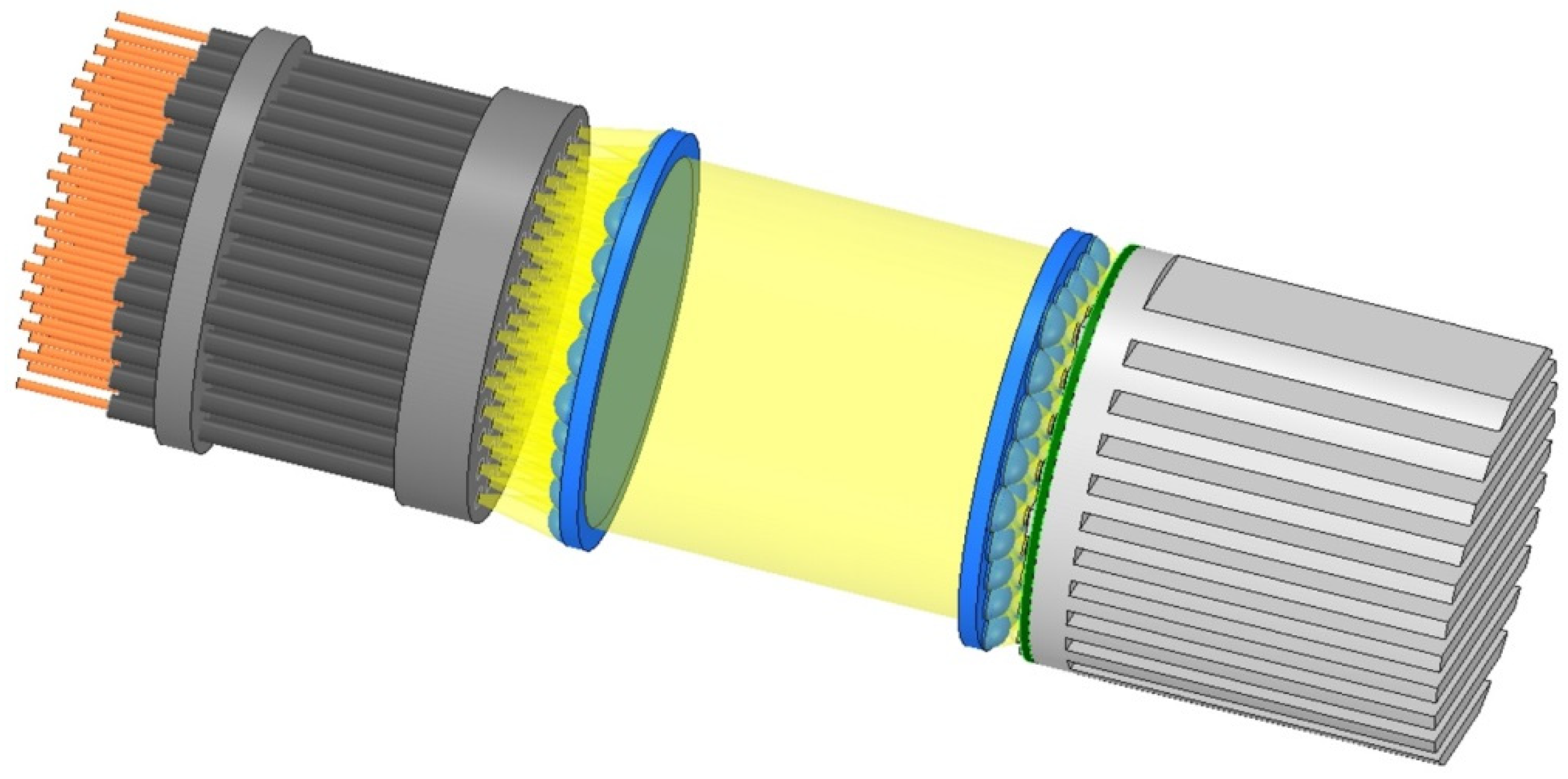
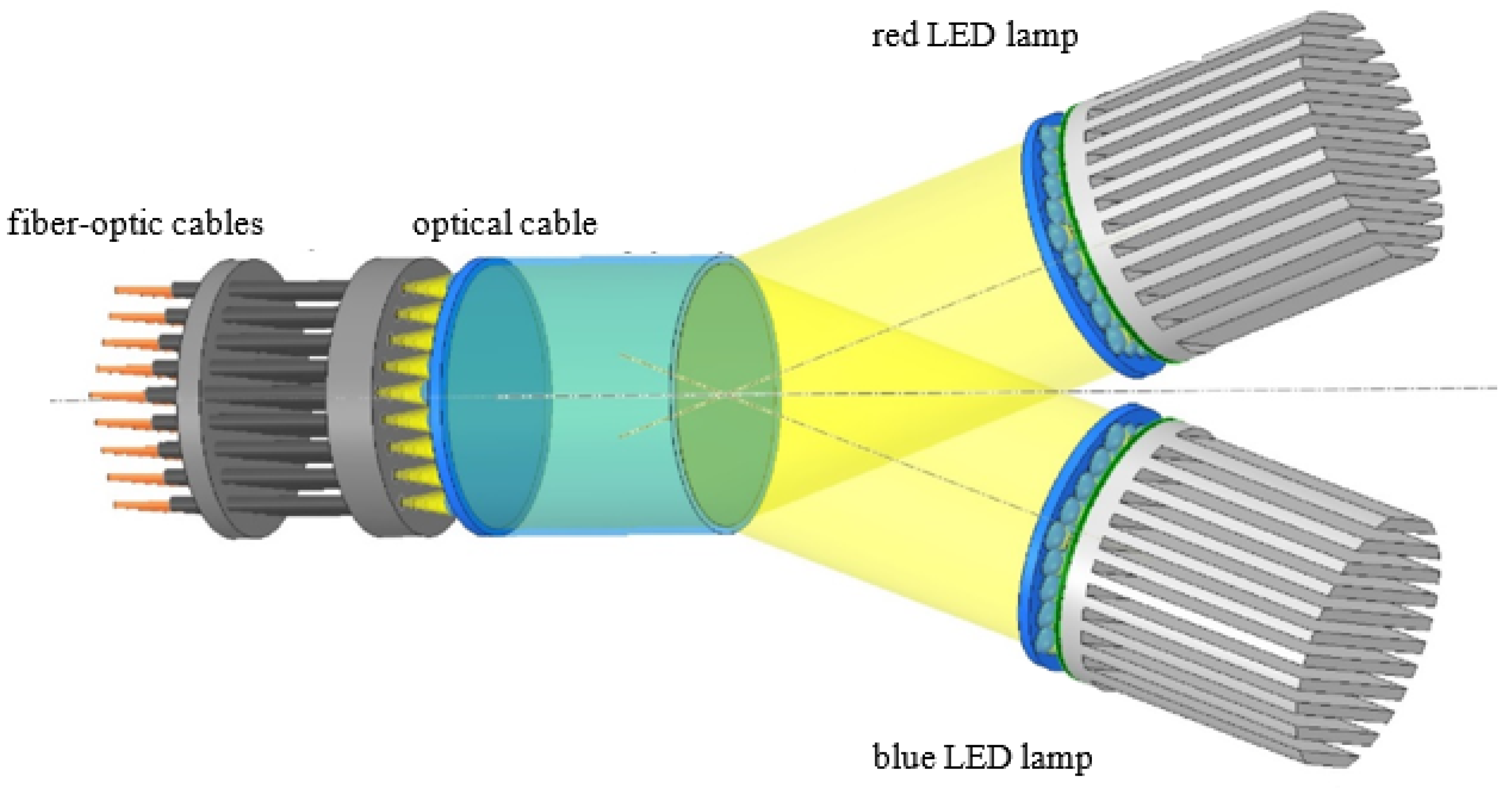


| Variant | Gas Volume Flux (dm3/h) | % CO2 by Volume (% Vol.) | CO2 Volume Flux (dm3/h) | CO2 Mass Flow Rate (g/h) | CO2 Mass Flow Rate (g/Day) |
|---|---|---|---|---|---|
| 1 | 25 | 25 | 6.25 | 12.3 | 294.5 |
| 2 | 50 | 12.5 | 24.6 | 589.1 | |
| 3 | 100 | 25 | 49.2 | 1178.2 | |
| 4 | 150 | 37.5 | 73.6 | 1767.3 | |
| 5 | 200 | 50 | 98.2 | 2356.4 |
| Variant | Microalgal Biomass Growth (g DM/Day) | CO2 Removal (g/Day) | Per-Unit CO2 Removal (gCO2/g DM) | CO2 Removal (g CO2/Day) | CO2 Removal Efficiency (%) | % CO2 by Volume (% v/v) |
|---|---|---|---|---|---|---|
| 1 | 21.4 | 98.256 | 4.59 | 117.4 | 39.9 | 15.0 |
| 2 | 27.6 | 116.844 | 4.23 | 136.0 | 23.1 | 19.2 |
| 3 | 29.1 | 125.415 | 4.31 | 144.6 | 12.3 | 21.9 |
| 4 | 32.3 | 121.041 | 3.75 | 140.2 | 7.9 | 23.0 |
| 5 | 31.9 | 119.855 | 3.76 | 139.1 | 5.9 | 23.5 |
| Variant | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 0.008176 | 0.006290 | 0.001940 | 0.002077 | |
| 2 | 0.008176 | 0.078290 | 0.014430 | 0.019011 | |
| 3 | 0.006290 | 0.078290 | 0.009663 | 0.012441 | |
| 4 | 0.001940 | 0.014430 | 0.009663 | 0.978262 | |
| 5 | 0.002077 | 0.019011 | 0.012441 | 0.978262 |
| Diameter Capsule (mm) | Capsule Load | |||||
|---|---|---|---|---|---|---|
| 5000 | 10,000 | 15,000 | 20,000 | 30,000 | 40,000 | |
| Weight (kg) | ||||||
| 5 | 0.4 | 0.7 | 1.1 | 1.4 | 2.2 | 2.9 |
| 10 | 2.9 | 5.8 | 8.6 | 11.5 | 17.3 | 23.0 |
| 15 | 9.7 | 19.4 | 29.2 | 38.9 | 58.3 | 77.8 |
| 20 | 23.0 | 46.1 | 69.1 | 92.2 | 138.2 | 184.3 |
| 25 | 45.0 | 90.0 | 135.0 | 180.0 | 270.0 | 360.0 |
| 30 | 77.8 | 155.5 | 233.3 | 311.0 | 466.5 | 622.0 |
| 35 | 123.5 | 246.9 | 370.4 | 493.9 | 740.8 | 987.8 |
| 40 | 184.3 | 368.6 | 552.9 | 737.2 | 1105.8 | 1474.5 |
| 45 | 262.4 | 524.8 | 787.3 | 1049.7 | 1574.5 | 2099.4 |
| Diameter Capsule (mm) | Capsule Load | |||||
|---|---|---|---|---|---|---|
| 5000 50 × 100 | 10,000 100 × 100 | 15,000 100 × 150 | 20,000 100 × 200 | 30,000 150 × 200 | 40,000 200 × 200 | |
| Panel Dimensions (mm) | ||||||
| 5 | 250 × 500 | 500 × 500 | 500 × 750 | 500 × 1000 | 750 × 1000 | 1000 × 1000 |
| 10 | 500 × 1000 | 1000 × 1000 | 1000 × 1500 | 1000 × 2000 | 1500 × 2000 | 2000 × 2000 |
| 15 | 750 × 1500 | 1500 × 1500 | 1500 × 2250 | 1500 × 3000 | 2250 × 3000 | 3000 × 3000 |
| 20 | 1000 × 2000 | 2000 × 2000 | 2000 × 3000 | 2000 × 4000 | 3000 × 4000 | 4000 × 4000 |
| 25 | 1250 × 2500 | 2500 × 2500 | 2500 × 3750 | 2500 × 5000 | 3750 × 5000 | 5000 × 5000 |
| 30 | 1500 × 3000 | 3000 × 3000 | 3000 × 4500 | 3000 × 6000 | 4500 × 6000 | 6000 × 6000 |
| 35 | 1750 × 3500 | 3500 × 3500 | 3500 × 5250 | 3500 × 7000 | 5250 × 7000 | 7000 × 7000 |
| 40 | 2000 × 4000 | 4000 × 4000 | 4000 × 6000 | 4000 × 8000 | 6000 × 8000 | 8000 × 8000 |
| 45 | 2250 × 4500 | 4500 × 4500 | 4500 × 6750 | 4500 × 9000 | 6750 × 9000 | 9000 × 9000 |
| Diameter of the Capsule | Transverse Sectional Area | Solar Energy Requirement | ||||||
|---|---|---|---|---|---|---|---|---|
| Light Energy Per Capsule | Energy for 5000 Capsules | Energy for 10,000 Capsules | Energy for 15,000 Capsules | Energy for 20,000 Capsules | Energy for 30,000 Capsules | Energy for 40,000 Capsules | ||
| (mm) | (mm2) | (Ws) | ||||||
| 5 | 19.635 | 8.25 × 10−4 | 4.12 | 8.25 | 12.37 | 16.49 | 24.74 | 32.99 |
| 10 | 78.540 | 3.30 × 10−3 | 16.49 | 32.99 | 49.48 | 65.97 | 98.96 | 131.95 |
| 15 | 176.715 | 7.42 × 10−3 | 37.11 | 74.22 | 111.33 | 148.44 | 222.66 | 296.88 |
| 20 | 314.159 | 1.32 × 10−2 | 65.97 | 131.95 | 197.92 | 263.89 | 395.84 | 527.79 |
| 25 | 490.874 | 2.06 × 10−2 | 103.08 | 206.17 | 309.25 | 412.33 | 618.50 | 824.67 |
| 30 | 706.858 | 2.97 × 10−2 | 148.44 | 296.88 | 445.32 | 593.76 | 890.64 | 1187.52 |
| 35 | 962.113 | 4.04 × 10−2 | 202.04 | 404.09 | 606.13 | 808.17 | 1212.26 | 1616.35 |
| 40 | 1256.637 | 5.28 × 10−2 | 263.89 | 527.79 | 791.68 | 1055.58 | 1583.36 | 2111.15 |
| 45 | 1590.431 | 6.68 × 10−2 | 333.99 | 667.98 | 1001.97 | 1335.96 | 2003.94 | 2671.92 |
| Diameter of the Capsule | Transverse Sectional Area | Red Light Energy Requirement | ||||||
|---|---|---|---|---|---|---|---|---|
| Light Energy Per Capsule | Energy for 5000 Capsules | Energy for 10,000 Capsules | Energy for 15,000 Capsules | Energy for 20,000 Capsules | Energy for 30,000 Capsules | Energy for 40,000 Capsules | ||
| (mm) | (mm2) | (Ws) | ||||||
| 5 | 19.635 | 1.75 × 10−4 | 0.87 | 1.75 | 2.62 | 3.50 | 5.24 | 6.99 |
| 10 | 78.540 | 6.99 × 10−4 | 3.50 | 6.99 | 10.49 | 13.98 | 20.97 | 27.96 |
| 15 | 176.715 | 1.57 × 10−3 | 7.86 | 15.73 | 23.59 | 31.46 | 47.18 | 62.91 |
| 20 | 314.159 | 2.80 × 10−3 | 13.98 | 27.96 | 41.94 | 55.92 | 83.88 | 111.84 |
| 25 | 490.874 | 4.37 × 10−3 | 21.84 | 43.69 | 65.53 | 87.38 | 131.06 | 174.75 |
| 30 | 706.858 | 6.29 × 10−3 | 31.46 | 62.91 | 94.37 | 125.82 | 188.73 | 251.64 |
| 35 | 962.113 | 8.56 × 10−3 | 42.81 | 85.63 | 128.44 | 171.26 | 256.88 | 342.51 |
| 40 | 1256.637 | 1.12 × 10−2 | 55.92 | 111.84 | 167.76 | 223.68 | 335.52 | 447.36 |
| 45 | 1590.431 | 1.42 × 10−2 | 70.77 | 141.55 | 212.32 | 283.10 | 424.65 | 566.19 |
| Diameterof the Capsule | Transverse Sectional Area | Blue Light Energy Requirement | ||||||
|---|---|---|---|---|---|---|---|---|
| Light Energy Per Capsule | Energy for 5000 Capsules | Energy for 10,000 Capsules | Energy for 15,000 Capsules | Energy for 20,000 Capsules | Energy for 30,000 Capsules | Energy for 40,000 Capsules | ||
| (mm) | (mm2) | (Ws) | ||||||
| 5 | 19.635 | 2.77 × 10−4 | 1.38 | 2.77 | 4.15 | 5.54 | 8.31 | 11.07 |
| 10 | 78.540 | 1.11 × 10−3 | 5.54 | 11.07 | 16.61 | 22.15 | 33.22 | 44.30 |
| 15 | 176.715 | 2.49 × 10−3 | 12.46 | 24.92 | 37.38 | 49.83 | 74.75 | 99.67 |
| 20 | 314.159 | 4.43 × 10−3 | 22.15 | 44.30 | 66.44 | 88.59 | 132.89 | 177.19 |
| 25 | 490.874 | 6.92 × 10−3 | 34.61 | 69.21 | 103.82 | 138.43 | 207.64 | 276.85 |
| 30 | 706.858 | 9.97 × 10−3 | 49.83 | 99.67 | 149.50 | 199.33 | 299.00 | 398.67 |
| 35 | 962.113 | 1.36 × 10−2 | 67.83 | 135.66 | 203.49 | 271.32 | 406.97 | 542.63 |
| 40 | 1256.637 | 1.77 × 10−2 | 88.59 | 177.19 | 265.78 | 354.37 | 531.56 | 708.74 |
| 45 | 1590.431 | 2.24 × 10−2 | 112.13 | 224.25 | 336.38 | 448.50 | 672.75 | 897.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. https://doi.org/10.3390/atmos12081031
Dębowski M, Krzemieniewski M, Zieliński M, Kazimierowicz J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere. 2021; 12(8):1031. https://doi.org/10.3390/atmos12081031
Chicago/Turabian StyleDębowski, Marcin, Mirosław Krzemieniewski, Marcin Zieliński, and Joanna Kazimierowicz. 2021. "Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept" Atmosphere 12, no. 8: 1031. https://doi.org/10.3390/atmos12081031
APA StyleDębowski, M., Krzemieniewski, M., Zieliński, M., & Kazimierowicz, J. (2021). Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere, 12(8), 1031. https://doi.org/10.3390/atmos12081031









