Study on Diesel Low-Nitrogen or Nitrogen-Free Combustion Performance in Constant Volume Combustion Vessels and Contributory
Abstract
:1. Introduction
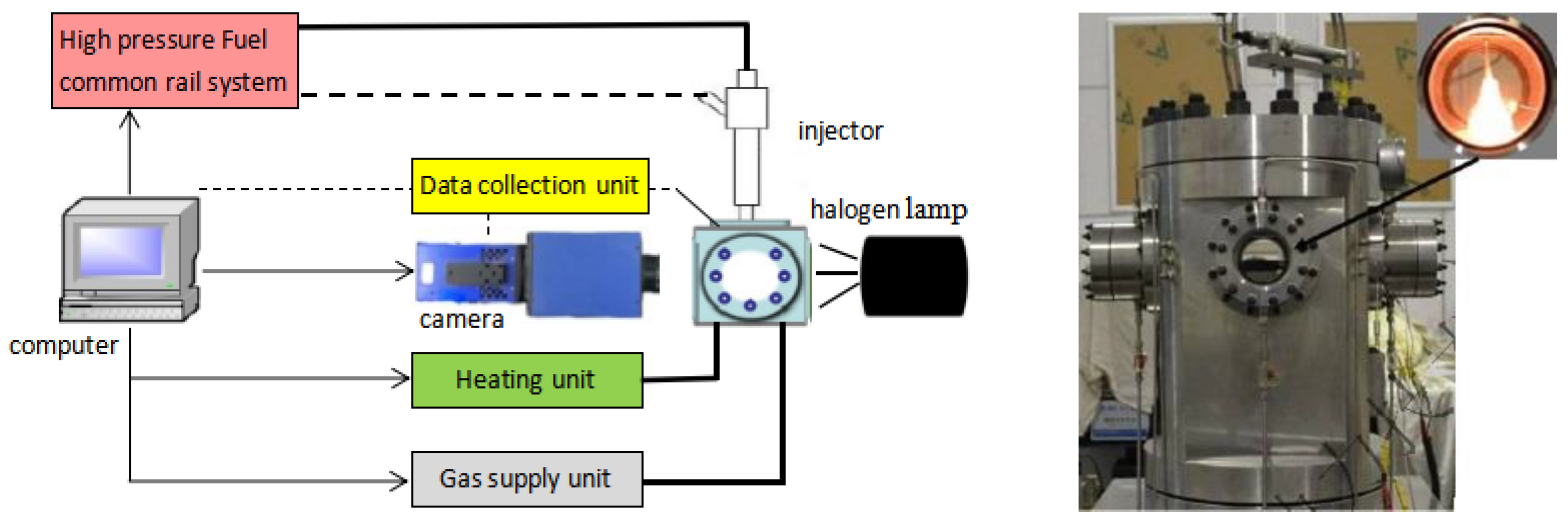
2. Experimental and Test Method
2.1. Experimental Method
2.2. Test Method
3. Results and Discussion
3.1. Low-Nitrogen Combustion
3.2. Nitrogen-Free Combustion
3.3. Factors Affecting Nitrogen-Free Combustion Performance
3.3.1. Initial Temperature
3.3.2. Injection Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, P.; Tan, Q.; Zhang, C.; Wei, L.; He, X.; Cao, E.; Jiang, K. Experimental investigation on NOx and green house gas emissions from a marine auxiliary diesel engine using ultralow sulfur light fuel. Sci. Total Environ. 2016, 572, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Fontaras, G.; Martini, G.; Manfredi, U.; Marotta, A.; Krasenbrink, A.; Maffioletti, F.; Terenghi, R.; Colombo, M. Assessment of on-road emissions of four Euro V diesel and CNG waste collection trucks for supporting air-quality improvement initiatives in the city of Milan. Sci. Total Environ. 2012, 426, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, T.; Fontaras, G.; Martini, G.; Peletto, C. A study of regulated and green house gas emissions from a prototype heavy-duty compressed natural gas engine under transient and real life conditions. Sci. Total Environ. 2016, 103, 340–355. [Google Scholar] [CrossRef]
- Koenig, D. Global and regional approaches to ship air emissions regulation: The international maritime organization and the European Union. In Regions, Institutions, and Law of the Sea; Brill|Nijhoff: Leiden, The Netherlands, 2013; pp. 317–336. [Google Scholar] [CrossRef]
- Faber, J.; Markowska, A.; Nelissen, D.; Davidson, M.; Eyring, V.; Cionni, I.; Selstad, E.; Kågeson, P.; Lee, D.; Buhaug, Ø.; et al. Technical support for European action to reducing Greenhouse Gas Emissions from international maritime transport. Transp. Syst. Logist. 2013, 37, 1198–1209. [Google Scholar]
- Schinas, O.; Stefanakos, C.N. Selecting technologies towards compliance with MARPOL Annex VI: The perspective of operators. Emiss. Control Areas Impact Marit. Transp. 2014, 28, 28–40. [Google Scholar] [CrossRef]
- Divekar, P.S.; Chen, X.; Tjong, J.; Zheng, M. Energy efficiency impact of EGR on organizing clean combustion in diesel engines. Energy Convers. Manag. 2016, 112, 369–381. [Google Scholar] [CrossRef]
- Saravanan, S. Effect of exhaust gas recirculation (EGR) on performance and emissions of a constant speed DI diesel engine fueled with pentanol/diesel blends. Fuel 2015, 160, 217–226. [Google Scholar]
- Assanis, D.N.; Poola, R.B.; Sekar, R.; Cataldi, G.R. Study of using oxygen-enriched combustion air for locomotive. J. Eng. Gas Turbines Power 2001, 123, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Porpatham, E.; Ramesh, A.; Nagalingam, B. Experimental studies on the effects of enhancing the concentration of oxygen in the inducted charge of a biogas fueled spark ignition. Energy 2018, 142, 303–312. [Google Scholar] [CrossRef]
- Gallo, Y.; Malmborg, V.B.; Simonsson, J.; Svensson, E.; Shen, M.; Bengtsson, P.E.; Pagels, J.; Tunér, M.; Garcia, A.; Andersson, Ö. Investigation of late-cycle soot oxidation using laser extinction and in-cylinder gas sampling at varying inlet oxygen concentrations in diesel engines. Fuel 2017, 193, 308–314. [Google Scholar] [CrossRef]
- Mei, D.; Zhao, X.; Wu, H.; Qian, J. Effects of enriched oxygen and nitrogen intake on combustion process and emission features in a diesel engine. J. Energy Eng. 2016, 143, 04016062. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, L.; Chen, X.; Zhang, Q. Thermodynamic and numerical analysis of intake air humidification of a turbocharged GDI engin. Sādhanā 2018, 43, 79. [Google Scholar] [CrossRef] [Green Version]
- Şahin, Z.; Tuti, M.; Durgun, O. Experimental investigation of the effects of water adding to the intake air on the engine performance and exhaust emissions in a DI automotive diesel engine. Fuel 2014, 115, 884–895. [Google Scholar] [CrossRef]
- Mingrui, W.; Sa, N.T.; Turkson, R.F.; Jinping, L.; Guanlun, G. Water injection for higher engine performance and lower emissions. J. Energy Inst. 2017, 90, 285–299. [Google Scholar] [CrossRef]
- Boretti, A. Water injection in directly injected turbocharged spark ignition engines. Appl. Therm. Eng. 2013, 52, 62–68. [Google Scholar] [CrossRef]
- Tan, Q.; Hu, Y.; Zhang, X. Research on conservation and emission technology of marine diesel engine. Environ. Eng. 2015, 33, 76–80. [Google Scholar]
- Rajasekar, E.; Murugesan, A.; Subramanian, R. Review of NOx reduction technologies in CI engines fuelled with oxygenated biomass fuels. Renew. Sustain. Energy Rev. 2010, 14, 2113–2121. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Li, W.; Shu, G.; Xu, B.; Shen, Y. Influence of EGR and oxygen-enriched air on diesel engine NO-Smoke emission and combustion characteristic. Appl. Energy 2013, 107, 304–314. [Google Scholar] [CrossRef]
- Zaidaoui, H.; Boushaki, T.; Koched, A.; Sautet, J.C.; Sarh, B.; Gökalp, I. Experimental study of EGR dilution and O2 enrichment effects on turbulent non-premixed Swirling flames. Combust. Sci. Technol. 2021, 193, 280–289. [Google Scholar] [CrossRef]
- Varuvel, E.G.; Sonthalia, A.; Subramanian, T.; Aloui, F. NOx-smoke trade-off characteristics of minor vegetable oil blends synergy with oxygenate in a commercial CI engine. Environ. Sci. Pollut. Res. 2018, 25, 35715–35724. [Google Scholar] [CrossRef]
- Dempsey, A.B.; Curran, S.J.; Wagner, R.M. A perspective on the range of gasoline compression ignition combustion strategies for high engine efficiency and low NOx andsoot emissions: Effects of in-cylinder fuel stratification. Int. J. Engine Res. 2016, 17, 897–917. [Google Scholar] [CrossRef]
- Machač, P.; Baraj, E. A simplified simulation of the reaction mechanism of NOx formation and non-catalytic reduciton. Combust. Sci. Technol. 2018, 190, 967–982. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, H.; Zhong, X.; Wang, Z.; Hu, H.; Qiu, J. Modeling of NOx conversion during combustion under high CO2 concentration using detailed chemical kinetics. Fuel Process. Technol. 2011, 92, 939–945. [Google Scholar] [CrossRef]
- Yi, B.; Zhang, L.; Mao, Z.; Huang, F.; Zheng, C. Effect of the particle size on combustion characteristics of pulverized coal in an O2/CO2 atomosphere. Fuel Process. Technol. 2014, 128, 17–27. [Google Scholar] [CrossRef]
- Hecht, E.S.; Shaddix, C.R.; Geier, M.; Molina, A.; Haynes, B.S. Effect of CO2 and steam gasification reactions on the oxy-combustion of pulverized coal char. Combust. Flame 2012, 159, 3437–3447. [Google Scholar] [CrossRef]
- Ma, L.; Guo, A.; Fang, Q.; Wang, T.; Zhang, C.; Chen, G. Combustion interactions of blended coals in an O2/CO2 mixture in a droptube furnace: Experimental investigation and numerical simulation. Appl. Therm. Eng. 2018, 145, 184–200. [Google Scholar] [CrossRef]
- Maffei, T.; Khatami, R.; Pierucci, S.; Faravelli, T.; Ranzi, E.; Levendis, Y.A. Experimental and modeling study single coal particle combustion in O2/N2 and oxy-fuel (O2/CO2) atmospheres. Combust. Flame 2013, 160, 2559–2572. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, C.; Ding, H.; Wang, Z.; Wang, D. Numerical simulation on combustion processes of a diesel engine under O2/CO2 atmosphere. HKIE Trans. 2013, 20, 157–163. [Google Scholar] [CrossRef]
- Wang, Z.; Zuo, C.; Chen, H.; Ding, H.; Wang, D. Emission characteristics of ZS195 diesel engine under O2/CO2 combustion condition. J. Hefei Univ. Technol. 2013, 36, 257–260. [Google Scholar]
- Liu, Y.; Zhao, T. Analysis of combustion characteristics of diesel oil in O2/CO2 environment. J. Beijing Univ. Civ. Eng. Archit. 2016, 32, 121–126. [Google Scholar]
- Zhao, T.; Liu, Y.; He, X. Simulation and experiment of auto-ingnition characteristics for diesel fuel in O2/CO2 atmosphere. Trans. CSICE 2017, 35, 415–422. [Google Scholar]
- Tan, Q.; Hu, Y.; Zhang, X.; Zhang, H. A study on the combustion and emission performance of diesel engines under different proportions of O2 & N2 & CO2. Appl. Therm. Eng. 2016, 108, 508–515. [Google Scholar]
- Tan, Q.; Hu, Y.; Zhang, X.; Zhang, H. A Study on the combustion performance of diesel engines with O2 and CO2 Suction. J. Chem. 2016, 2016, 1258314. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, C.; Liu, Y. Experiment Investigation on combustion characteristics of gasoline in O2/CO2 atmosphere. Sci. Technol. Eng. 2018, 18, 191–196. [Google Scholar]
- Yao, L. Visualization Study on Constant Volume Combustion of Gasoline in Nitrogen Free Environment; Jilin University: Changchun, China, 2018; pp. 10–80. [Google Scholar]
- Hu, Z.; Somers, L.M.; Davies, T.; McDougall, A.; Cracknell, R.F. A study of liquid fuel injection and combustion in a constant volume vessel at diesel engine conditions. Fuel 2013, 107, 63–73. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, S.; Yang, J.; Meng, H. Numerical study of geometric effects on burning velocity measurement by constant volume method. Combust. Sci. Technol. 2020, 192, 1475–1492. [Google Scholar] [CrossRef]
- Aghdam, E.A. Wall effect on determination of laminar burning velocity in a constant volume bomb using a quasi-dimensional model. Appl. Math. Model. 2014, 38, 5811–5821. [Google Scholar] [CrossRef]
- Chen, B.; Feng, L.; Wang, Y.; Ma, T.; Liu, H.; Geng, C.; Yao, M. Spray and flame characteristics of wall-impinging diesel fuel spray at different wall temperatures and ambient pressures in a constant volume combustion vessel. Fuel 2019, 235, 416–425. [Google Scholar] [CrossRef]
- Akram, M.; Minaev, S.; Kumar, S. Investigations on the formation of Planar flames in Mesoscale divergent channels and prediction of burning velocity at high temperatures. Combust. Sci. Technol. 2013, 185, 645–660. [Google Scholar] [CrossRef]
- Oren, D.C.; Wahiduzzaman, S.; Ferguson, C.R. A diesel combustion bomb:proof of concept. SAE Trans. 1984, 93, 945–960. [Google Scholar]
- Saeed, K.; Stone, C.R. Measurements of the laminar burning velocity for mixtures of ethanol and air from a constant-volume vessel using a multizone model. Combust. Flame 2004, 139, 152–166. [Google Scholar] [CrossRef]
- Wei, H.; Gao, D.; Zhou, L.; Feng, D.; Chen, C.; Pei, Z. Experimental analysis on spray development of 2-methylfuran-gasoline blends using multi-hole DI injector. Fuel 2016, 164, 245–253. [Google Scholar] [CrossRef]

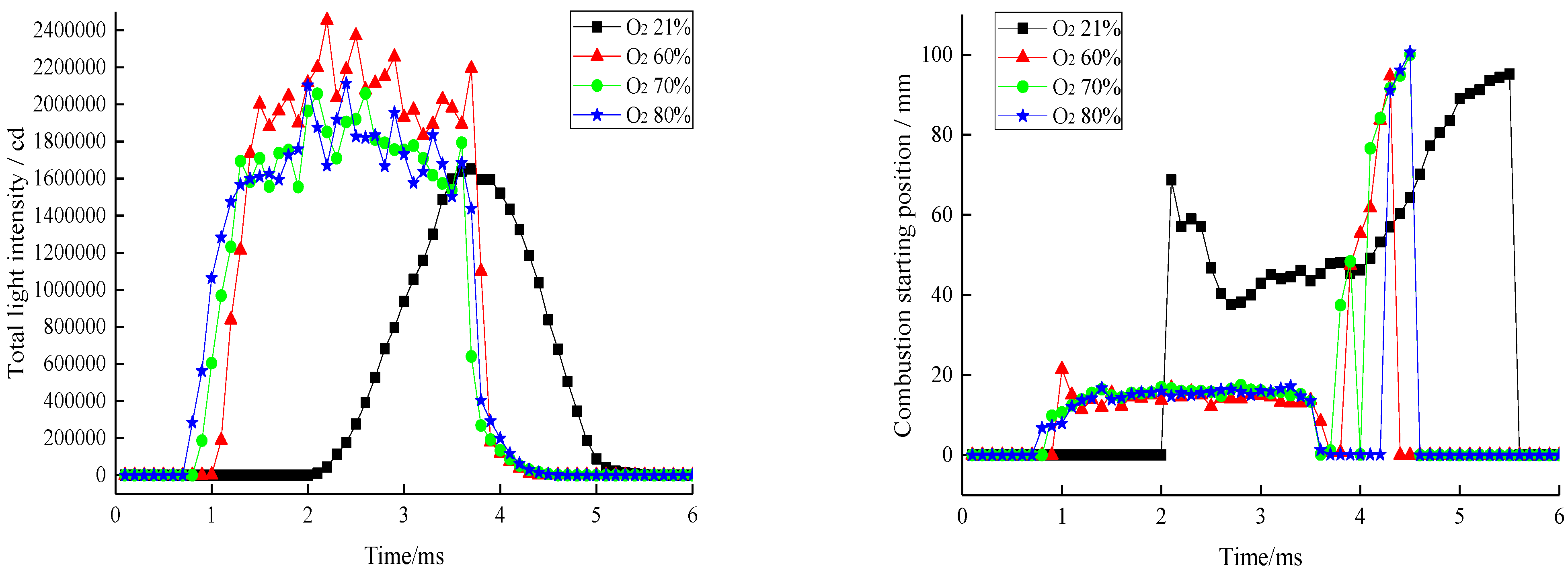
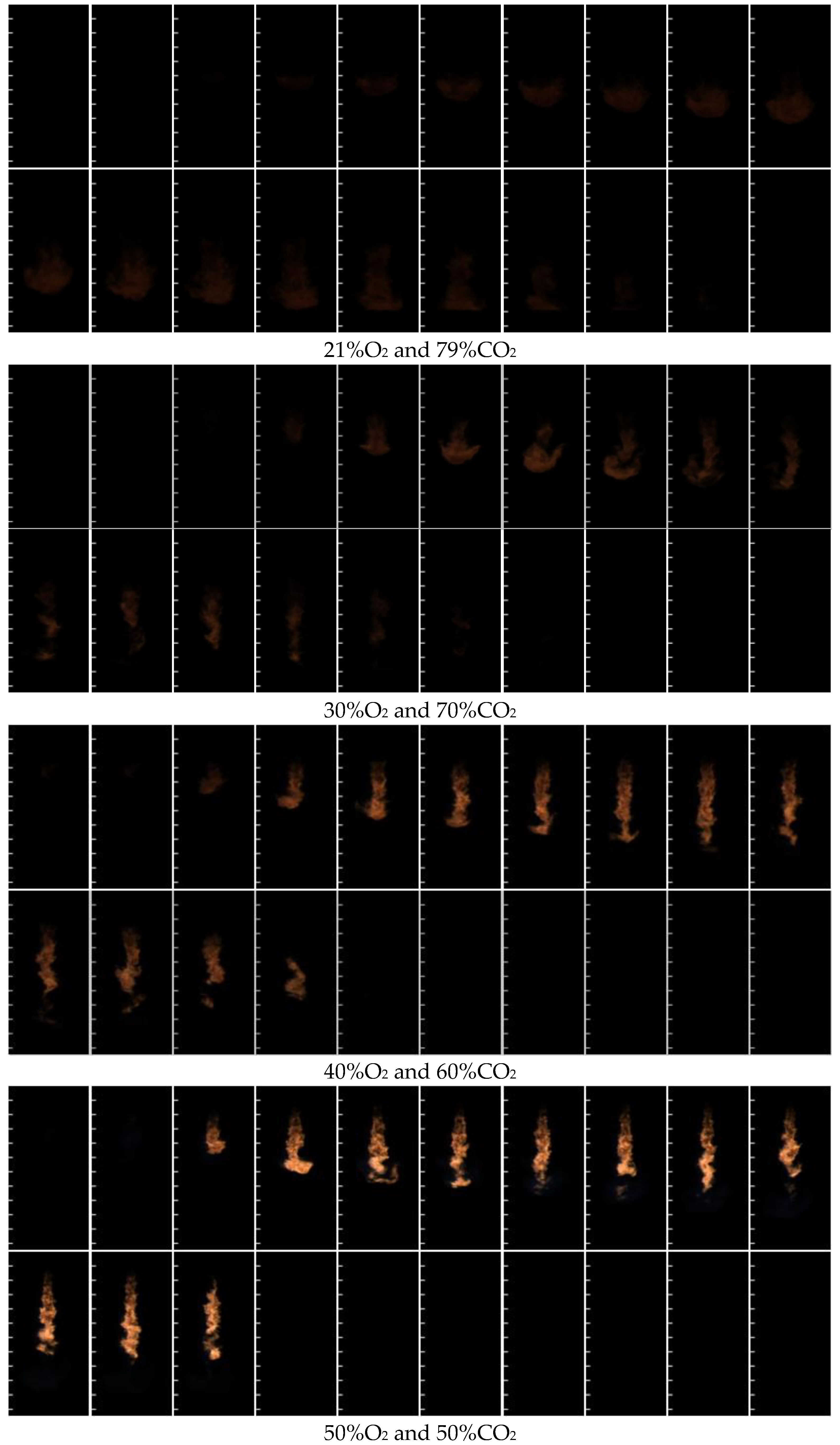
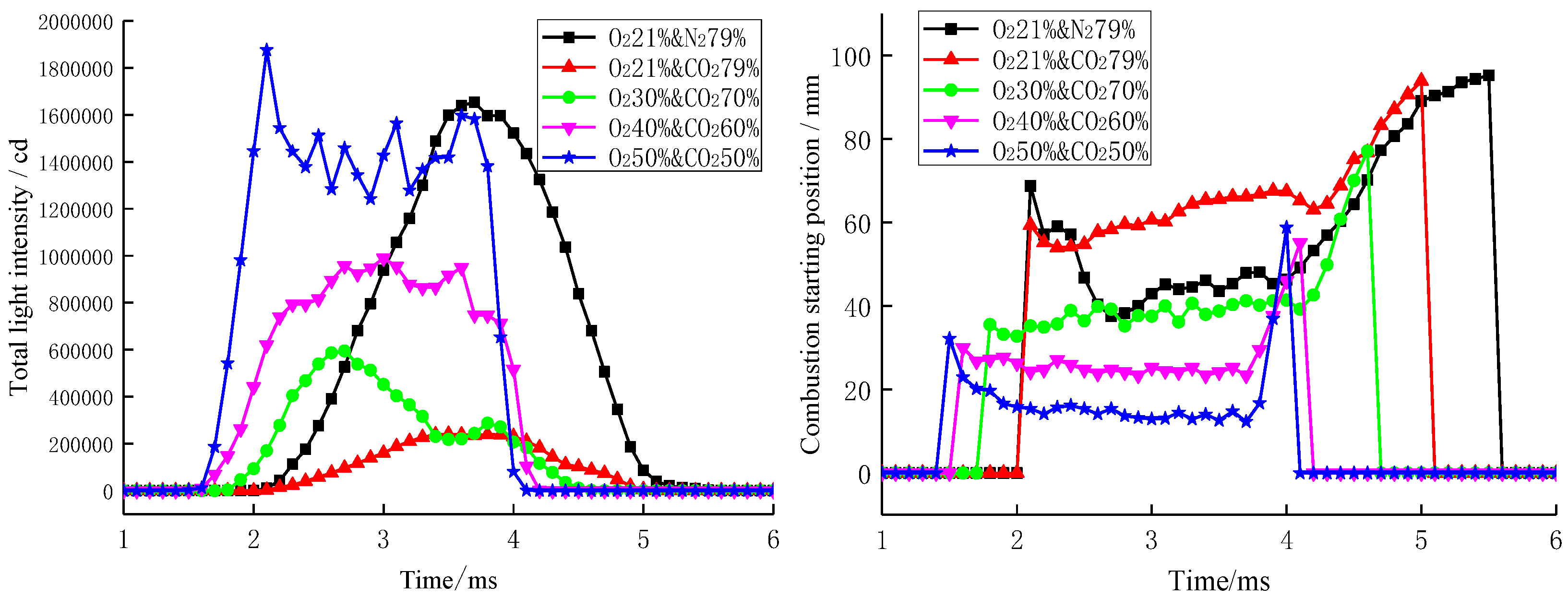

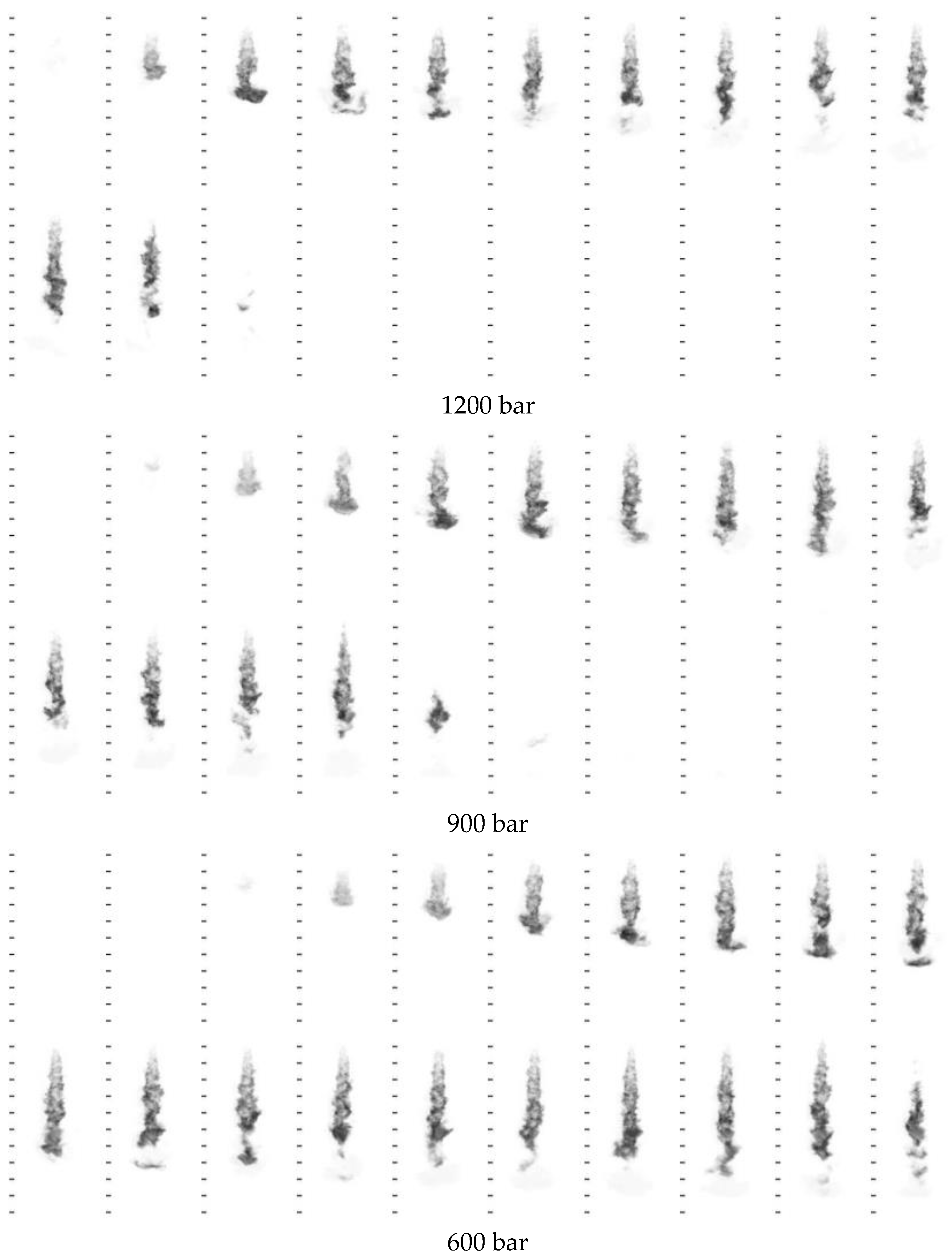
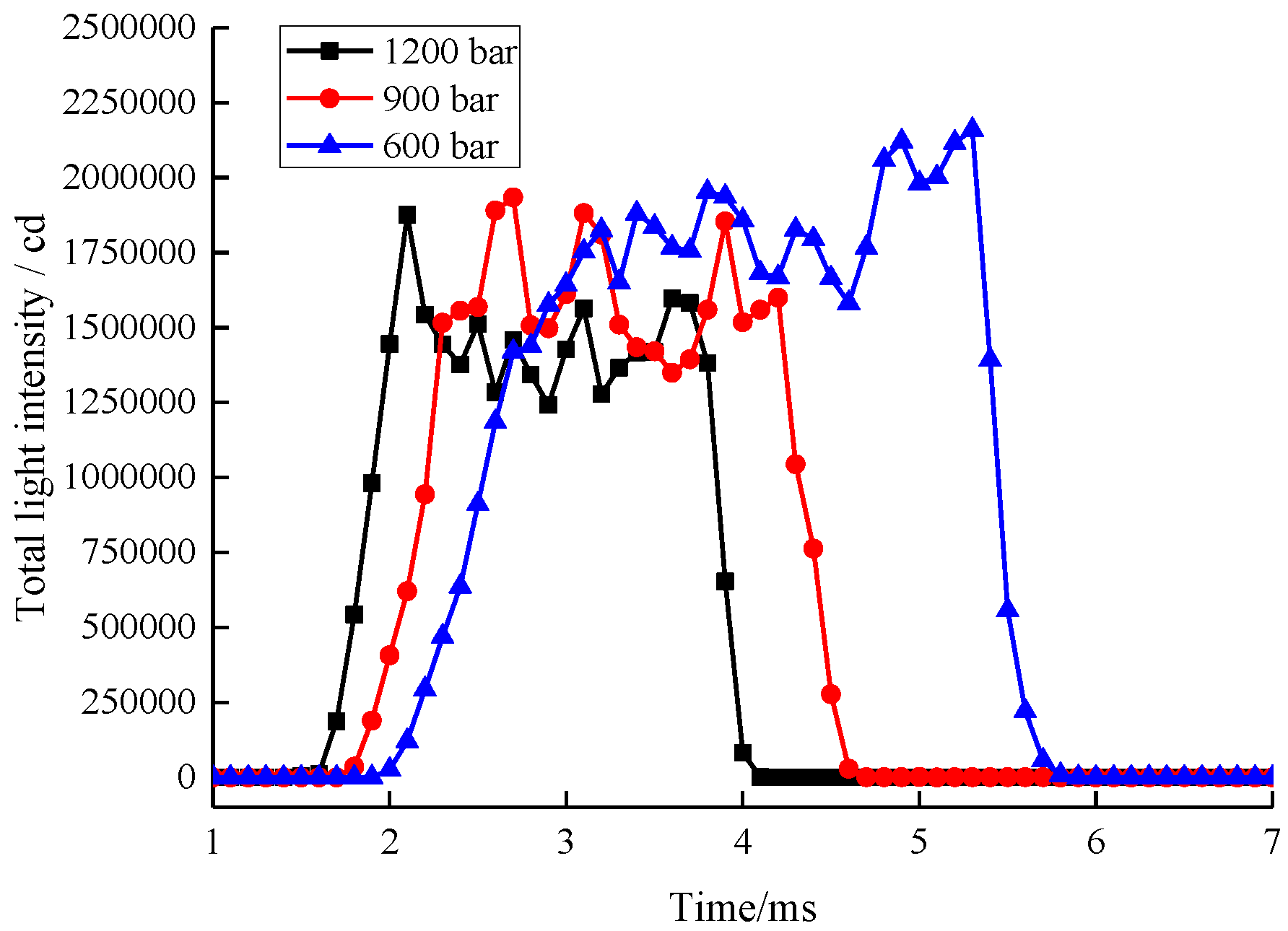
| Parameter Types | 21%O2 and 79% N2 | 60%O2 and 40% N2 | 70%O2 and 30% N2 | 80%O2 and 20% N2 |
|---|---|---|---|---|
| Ignition delay period/ms | 2.07 | 1.06 | 1.03 | 0.98 |
| Standard deviation of ignition delay | 1.57 | 1.17 | 1.7 | 1.75 |
| Combustion duration/ms | 3.3 | 3.4 | 3.5 | 3.6 |
| Standard deviation of combustion duration | 1.45 | 1.39 | 0.5 | 0.83 |
| Parameter Types | 21%O2 and 79% N2 | 21%O2 and 79% CO2 | 30%O2 and 70% CO2 | 40%O2 and 60% CO2 | 50%O2 and 50% CO2 |
|---|---|---|---|---|---|
| Ignition delay period/ms | 2.07 | 2.01 | 1.59 | 1.46 | 1.18 |
| Standard deviation of ignition delay | 1.57 | 1.38 | 1.66 | 1.65 | 0.41 |
| Combustion duration/ms | 3.3 | 3.2 | 2.9 | 2.7 | 2.7 |
| Standard deviation of combustion duration | 1.45 | 2.57 | 0.25 | 1.49 | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Q.; Hu, Y.; Tan, Z. Study on Diesel Low-Nitrogen or Nitrogen-Free Combustion Performance in Constant Volume Combustion Vessels and Contributory. Atmosphere 2021, 12, 923. https://doi.org/10.3390/atmos12070923
Tan Q, Hu Y, Tan Z. Study on Diesel Low-Nitrogen or Nitrogen-Free Combustion Performance in Constant Volume Combustion Vessels and Contributory. Atmosphere. 2021; 12(7):923. https://doi.org/10.3390/atmos12070923
Chicago/Turabian StyleTan, Qinming, Yihuai Hu, and Zhiwen Tan. 2021. "Study on Diesel Low-Nitrogen or Nitrogen-Free Combustion Performance in Constant Volume Combustion Vessels and Contributory" Atmosphere 12, no. 7: 923. https://doi.org/10.3390/atmos12070923
APA StyleTan, Q., Hu, Y., & Tan, Z. (2021). Study on Diesel Low-Nitrogen or Nitrogen-Free Combustion Performance in Constant Volume Combustion Vessels and Contributory. Atmosphere, 12(7), 923. https://doi.org/10.3390/atmos12070923






