A Case Study of Birch Pollen and the Synergy with Environmental Factors: Relation to Asthma in Montreal, Canada
Abstract
1. Introduction
1.1. Scientific Context
1.2. Aeroallergens Concentration, Pollution, Climate and Weather: What Are the Connections?
1.3. Respiratory Health Risks under Specific Weather Conditions
1.4. Transport of Pollen from Source Areas to Urban Environment vs. Local Sources
2. Materials and Methods
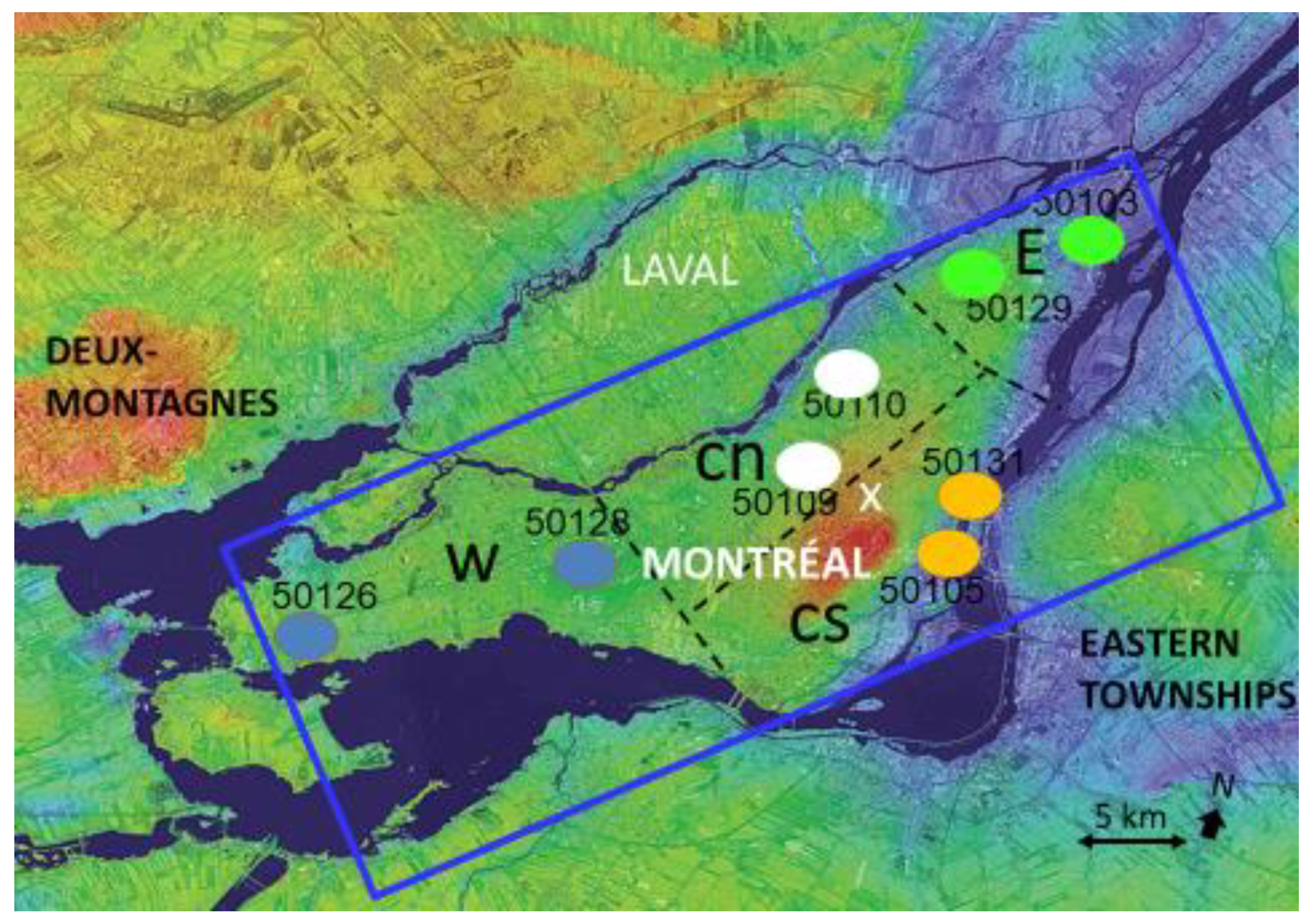
2.1. Study Region and Site for Data Analysis and Measurements
2.2. Health Data
2.3. Other Environmental Data
2.4. Pollen Measurement
2.5. Regional Modelling and Pollen Emissions
3. Results
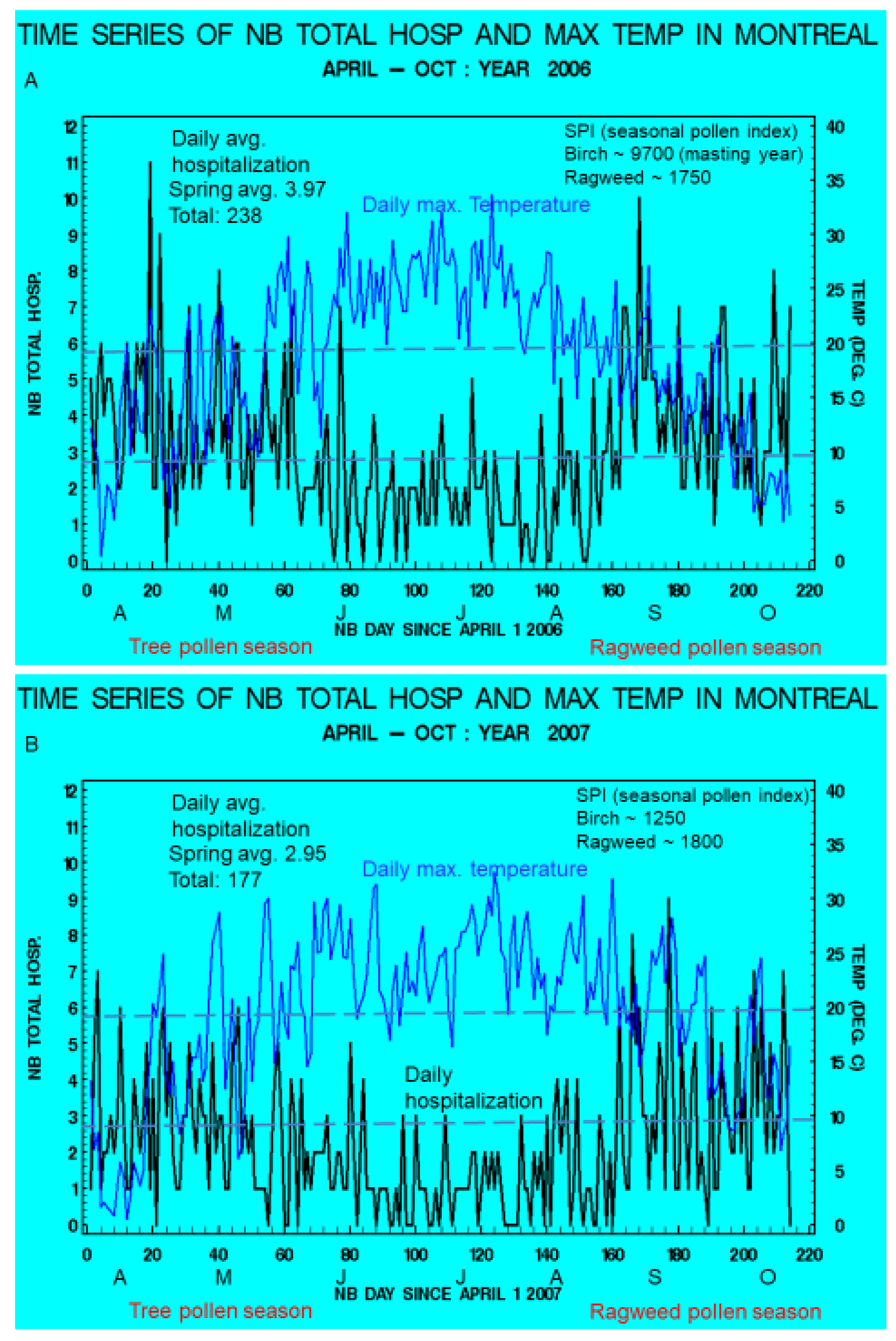
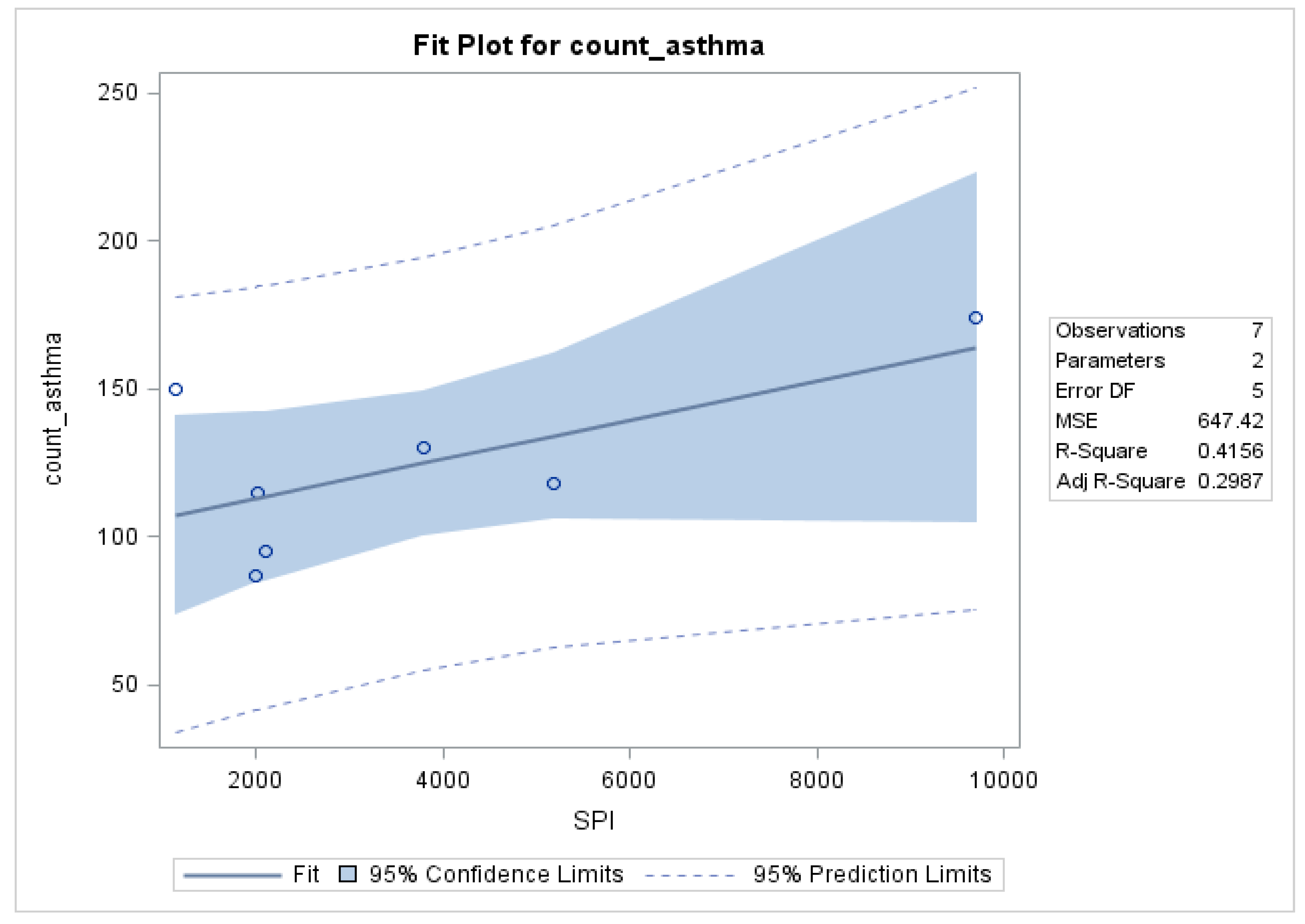
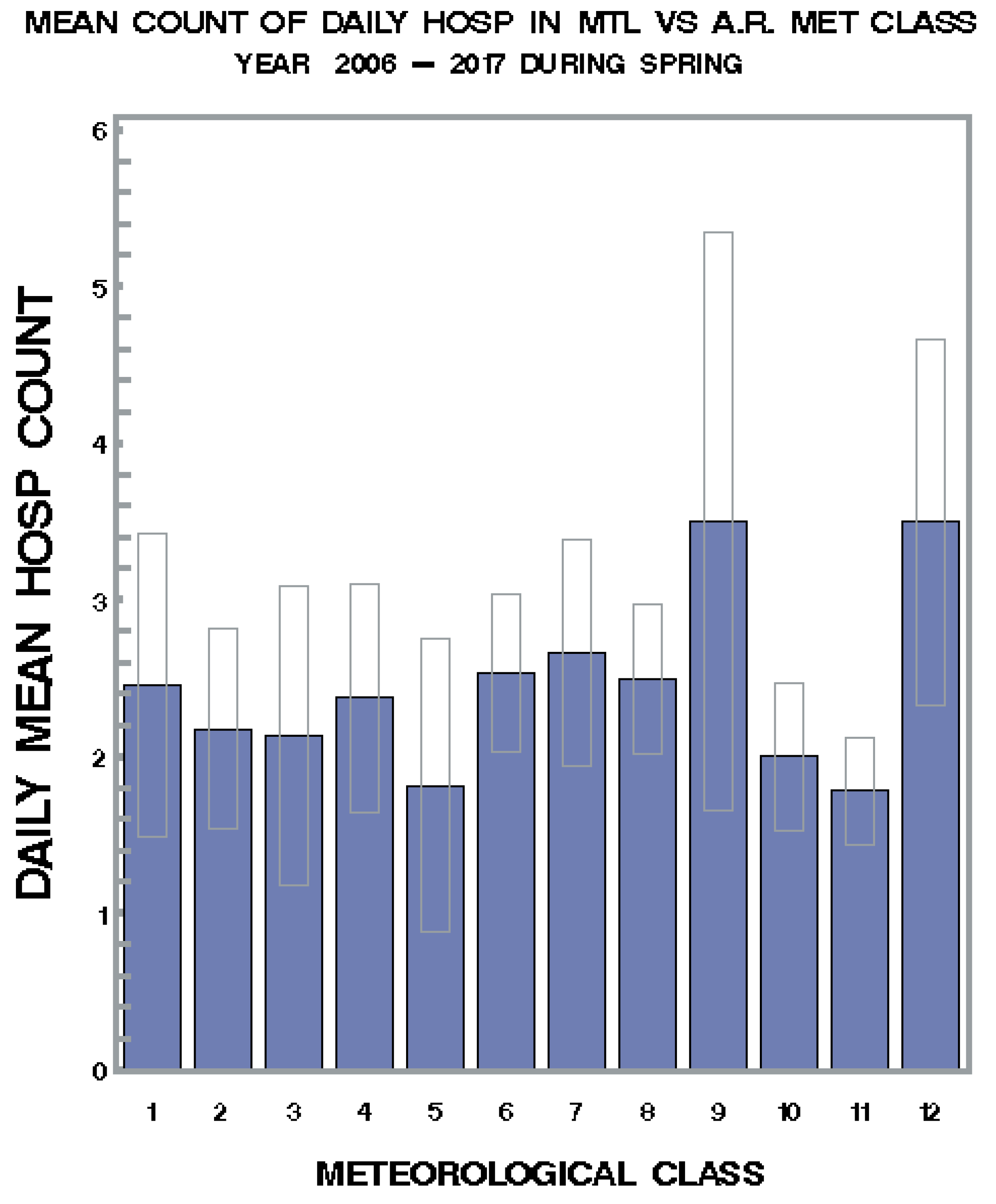
3.1. Seasonal Variations and Statistical Relationships between Daily Asthma Counts and Environmental Variables
3.2. Spatio-Temporal Coincidence of Birch Pollen and Air Pollutants
4. Discussion
5. Conclusions
- (1)
- An increase of monitoring pollen and associated allergens (subpollen and pollen fragments) is necessary in Canada to better assess the population exposure and to better understand the relation to asthma and related disease (e.g., allergic rhinitis).
- (2)
- The use of satellite data to evaluate in near real-time phenology parameters (which are critical input to pollen emission simulation) should be further developed.
- (3)
- (4)
- Developing a machine-learning algorithm to predict pollen/allergen occurrence is desirable.
- (5)
- Predicting pollen concentration using land-use regression models only without a regional transport model is not adequate, at least in the case of birch pollen in Canada.
- (6)
- A strong regional variation of overlap between birch pollen and air pollution is revealed by GEM-MACH simulation within most zones of higher health risk in Canada (Windsor area, Toronto-Montreal-Quebec corridor and Sudbury regions). Better characterization of this variability is needed to better understand the link between asthma and environmental factors.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Weather Types Used and Most Likely Associated Clouds and Weather
| Weather Types Impacting Montreal | Clouds and Weather in Montreal | Nb. Cases Frequency (%) (18 UTC) | |
|---|---|---|---|
| 1 | Back of the high pressure located on the eastern US seabord | Sunny with temperature much above normal (i.e., heat wave). | 11 (4.5%) |
| 2 | Flat pressure gradient | Calm winds. Temperature are often above normal. | 10 (4.1%) |
| 3 | Warm sector | Temperature above normal (unstable situation) with southerly winds. | 18 (7.4%) |
| 4 | Cold front passage | Sudden changes of wind (speed and direction) and temperatures turning colder. | 21 (8.6%) |
| 5 | Behind cold front | Northwest winds and colder and below normal. | 5 (2.1%) |
| 6 | Approaching high pressure | Sunny, windy and cool. | 19 (7.8%) |
| 7 | Ridge of high pressure | Sunny skies, dry and windy. | 19 (7.8%) |
| 8 | Low pressure | Clouds and steady light rain. | 34 (14.0%) |
| 9 | Occluded front | Unstable, clouds and showers, turbulence. | 10 (4.1%) |
| 10 | Approaching low | Increasing cloudiness and winds. | 13 (5.3%) |
| 11 | Surface through | Windy, cloudy and unstable, risk of showers. | 29 (11.9%) |
| 12 | Warm front | Cloudy, unstable, showers, increasing temperature and humidity. | 13 (5.3%) |
| 13 | Unclassified/missing data | Variable | 41(16.9%) |
References
- Crighton, E.J.; Feng, J.; Gershon, A.; Guan, J.; To, T. A spatial analysis of asthma prevalence in Ontario. Can. J. Public Health 2012, 103, 384–389. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Report from the Canadian Chronic Disease Surveillance System: Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Canada; Public Health Agency of Canada: Ottawa, ON, Canada, 2018; ISBN 978-0-660-09274-4.
- Sierra-Heredia, C.; North, M.; Brook, J.; Daly, C.; Ellis, A.K.; Henderson, D.; Henderson, S.B.; Lavigne, É.; Takaro, T.K. Aeroallergens in Canada: Distribution, public health impacts, and opportunities for prevention. Int. J. Res. Public Health 2018, 15, 1577. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.M.; Sbihi, H.; Dai, D.L.Y.; Mamun, A.; Rasali, D.; Rose, C.; Marra, F.; Boutin, R.C.T.; Petersen, C.; Stiemsma, L.T.; et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020, 8, 1094–1105. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; the ISACC Phase Three Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multi-country cross-sectional survey. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226. [Google Scholar] [CrossRef]
- Anandan, C.; Nurmatov, U.; van Schayck, O.C.P.; Sheik, A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy 2010, 65, 152–167. [Google Scholar] [CrossRef]
- Beasly, R.; Semprini, A.; Mitchell, E.A. Risk factors for asthma: Is prevention possible? Lancet 2015, 386, 1075–1085. [Google Scholar] [CrossRef]
- D’Amato, G.; Holgate, S.T.; Pawankar, R.; Ledford, D.K.; Cecchi, L.; Al-Ahmad, M.; Al-Enezl, F.; Al-Muhsen, S.; Ansotegui, I.; Baena-Cagnani, C.E.; et al. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ. J. 2015, 8, 25. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; D’Amato, M.; Cecchi, L.; Liccardi, G.; Molino, A.; Vatrella, A.; Sanduzzi, A.; Maesano, C.; Annesi-Maesano, I. Thunderstorm-related asthma: What happens and why. Clin. Exp. Allergy 2015, 46, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Abatzoglou, J.T.; Anderegg, L.D.L.; Bielory, L.; Kinney, P.L.; Ziska, L. Anthropogenic climate change is worsening North American pollen season. Proc. Natl. Acad. Sci. USA 2021, 118, e2013284118. [Google Scholar] [CrossRef] [PubMed]
- ANSES. État des Connaissances sur l’Impact Sanitaire lié à l’Exposition de la Population Générale aux Pollens Présents dans l’Air Ambient; Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail; Avis de l’ANSES: Buenos Aires, Argentina, 2014; Saisine 2011-SA-0151. [Google Scholar]
- Keith, P.K.; Desrosiers, M.; Laister, T.; Schellenberg, R.R.; Waserman, S. The burden of allergic rhinitis (AR) in Canada: Perspectives of physicians and patients. Allergy Asthma Clin. Immunol. 2012, 8, 7. [Google Scholar] [CrossRef]
- Saxon, A.; Diaz-Sanchez, D. Air pollution and allergy: You are what you breathe. Nat. Immunol. 2005, 6, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Traidl-Hoffmann, C.; Jakob, T.; Behrendt, H. Determinants of allergenicity. J. Allergy Clin. Immunol. 2009, 123, 558–566. [Google Scholar] [CrossRef]
- Behrendt, H.; Becker, W.M.; Fritzsche, C.; Sliwa-Tomczok, W.; Tomczok, J.; Friedrichs, K.H.; Ring, J. Air pollution and allergy: Experimental studies on modulation of allergen release from pollen by air pollutants. Int. Arch. Allergy Immunol. 1997, 113, 69–74. [Google Scholar] [CrossRef]
- Behrendt, H.; Krämer, U.; Schäfer, T.; Kasche, A.; Eberlein-König, B.; Darsow, U.; Ring, J. Allergotoxicology—A research concept to study the role of environmental pollutants in allergy. ACI Int. 2001, 13, 122–128. [Google Scholar]
- Schäppi, G.F.; Suphioglu, C.; Taylor, P.E.; Knox, R.B. Concentrations of the major birch tree allergen Bet v 1 in pollen and respirable fine particles in the atmosphere. J. Allergy Clin. Immunol. 1997, 100, 656–661. [Google Scholar] [CrossRef]
- Behrendt, H.; Becker, W.M. Localization, release and bioavailability of pollen allergens: The influence of environmental factors. Curr. Opin. Immunol. 2001, 13, 709–715. [Google Scholar] [CrossRef]
- Behrendt, H.; Majd, A.; Chehregani, A.; Moin, M.; Gholami, M.; Kohno, S.; Nabe, T.; Shariatzade, M.A. The effects of air pollution on structures, proteins and allergenicity of pollen grains. Aerobiologia 2004, 20, 111–118. [Google Scholar]
- Reinmuth-Selzle, K.; Kampf, C.J.; Lucas, K.; Lang-Yona, N.; Frohlich-Nowoisky, J.; Shiraiwa, M.; Lakey, P.S.; Lai, S.; Liu, F.; Kunert, A.T.; et al. Air Pollution and Climate Change Effects on Allergies in the Anthropocene: Abundance, Interaction, and Modification of Allergens and Adjuvants. Environ. Sci. Technol. 2017, 51, 4119–4141. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A. The effects of climate change on the birch pollen season in Denmark. Aerobiologia 2002, 18, 253–265. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Emberlin, J.; Laaidi, M.; Detandt, M. Changement climatique et évolution du contenu pollinique de l’air dans sept pays européens: Exemple du bouleau. Rev. Fr. D’allergologie Et D’immunologie Clin. 2007, 47, 57–63. [Google Scholar] [CrossRef]
- Robichaud, A.; Comtois, P. Statistical modelling, forecasting and time series analysis of birch phenology in Montreal, Canada. Aerobiologia 2017, 33, 529–554. [Google Scholar] [CrossRef]
- Frey, T.; Gassner, E. Climate change and its impact on birch pollen quantities and the start of the pollen season: An example from Switzerland for the period 1969–2006. Int. J. Biometeorol. 2008, 52, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Makra, L.; Harry, S.K.; Bruffaerts, N.; Hendrickx, M.; Coates, F.; Saarto, A.; Thibaudon, M.; Oliver, G.; Damialis, A.; et al. Temperature-related changes in airborne allergenic pollenabundance and seasonality across the northern hemisphere: A retrospective data analysis. Lancet Planet Health 2019, 3, e124–e131. [Google Scholar] [CrossRef]
- Ahlholm, J.U.; Helander, M.L.; Savolainen, J. Genetic and environmental factors affecting the allergenicity of birch pollen (Betula pubescens ssp. Czerepanobii (Orl) Hämet-Ahti). Clin. Exp. Allergy 1998, 28, 1384–1388. [Google Scholar] [CrossRef]
- Taylor, P.E.; Flagan, R.C.; Valenta, R.; Glovsky, M.M. Release of allergens as respirable aerosols: A link between grass pollen and asthma. J. Allergy Clin. Immunol. 2002, 109, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.E.; Flagan, R.C.; Miguel, A.G.; Valenta, R.; Glovsky, M. Birch pollen rupture and the release of aerosols of respirable allergens. Clin. Exp. Allergy 2004, 34, 1591–1596. [Google Scholar] [CrossRef]
- Miguel, A.G.; Taylor, P.E.; House, J.; Glovsky, M.M.; Flagan, R.C. Meteorological influences on respirable fragment release from Chinese Elm pollen. Aerosol Sci. Technol. 2006, 40, 690–696. [Google Scholar] [CrossRef]
- Sénéchal, H.; Visez, N.; Charpin, D.; Shahali, Y.; Peltre, G.; Biolley, J.P.; Lhuissier, F.; Couderc, R.; Yamada, O.; Malrat-Domenge, A.; et al. A review of the effects of major atmospheric pollutants on pollen grains, pollen content and allergenicity. Sci. World J. 2015, 2015, 940243. [Google Scholar] [CrossRef] [PubMed]
- Sedghy, F.; Varasteh, A.-R.; Sankia, M.; Moghadam, M. Interaction between air pollutants and pollen grains: The role on the rising trend in allergy. Rep. Biochem. Mol. Biol. 2018, 6, 219–224. [Google Scholar] [PubMed]
- Peltre, G. Inter-relationship between allergenic pollens and air pollution. Allerg. Immunol. 1998, 30, 324–326. [Google Scholar]
- Chassard, G.; Choël, M.; Gosselin, S.; Vorng, H.; Petitprez, D.; Shahali, Y. Kinetic of NO2 uptake by Phleum pratense pollen: Chemical and allergenic implications. Environ. Pollut. 2015, 196, 107–113. [Google Scholar] [CrossRef]
- Okuyama, Y.; Matsumoto, K.; Okochi, H.; Igawa, M. Adsorption of air pollutants on the grain surface of Japanese cedar pollen. Atmos. Env. 2007, 41, 253–260. [Google Scholar] [CrossRef]
- Rantio-Lehtimäki, A.; Viander, M.; Koivikko, A. Airborne birch pollen antigens in different particle sizes. Clin. Exp. Allergy 1994, 24, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Puc, M.; Kotrych, D.; Lipiec, A.; Rapiejko, P.; Siergiejko, G. Birch pollen grains without cytoplasmic content in the air of Szczecin and Bialystok. Alergoprofil 2016, 12, 101–105. [Google Scholar]
- Laaidi, M.; Chinet, T.; Aegerter, P. Allergies au pollen, pollution et climat: Revue de la littérature. Rev. Fr. D’allergologie 2011, 51, 622–628. (In French) [Google Scholar] [CrossRef]
- Nomak, E.; Johansen, B.V.; Lovik, M. Detection of allergens adsorbed to ambient particles collected in four European cities. Toxicol. Lett. 2006, 165, 71–78. [Google Scholar]
- Ormstad, H.; Johansen, B.V.; Gaarder, P.I. Airborne house dust particles and diesel exhaust particles as allergen carriers. Clin. Exp. Allergy 1998, 28, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kawanishi, S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res.Commun. 2004, 316, 123–128. [Google Scholar] [CrossRef]
- Ghosh, S.; Janocha, A.J.; Aronica, M.A.; Swaidani, S.; Comhair, S.A.; Xu, W.; Zheng, L.; Kaveti, S.; Kinter, M.; Hazen, S.L.; et al. Nitrotyrosineproteome survey in asthma identifies oxidative mechanism of catalase inactivation. J. Immunol. 2006, 176, 5587–5597. [Google Scholar] [CrossRef]
- Ischiropoulos, H. Protein tyrosine nitration-An update. Arch. Biochem. Biophys. 2009, 484, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gruijthuijsen, Y.K.; Grieshuber, I.; Stocklinger, A.; Tischler, U.; Fehrenbach, T.; Weller, M.G.; Vogel, L.; Vieths, S.; Pöschl, U.; Duschl, A. Nitration enhances the allergenic potential of proteins. Int. Arch. Allergy Immunol. 2006, 141, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.B.; Bush, R.K. It’s blowing in the wind: New insights into thunderstorm-related asthma. J. Allergy Clin. Immuno. 2007, 120, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.E.; Jonsson, H. Thunderstorm asthma. Curr. Allergy Asthma Rep. 2004, 4, 409–413. [Google Scholar] [CrossRef]
- Robichaud, A.; Comtois, P. Environmental factors and asthma hospitalization in Montreal, Canada, during spring 2006–2008: A synergy perspective. Air Qual. Atmos. Health 2019, 12, 1495–1509. [Google Scholar] [CrossRef]
- Rosas, I.; McCartney, H.A.; Payne, R.W.; Calderón, C.; Lacey, J.; Chapela, R.; Ruiz-Velazco, S. Analysis of the relationship between environmental factors (aeroallergens, air pollution and weather) and asthma emergency admissions to a hospital in Mexico City. Allergy 1998, 53, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hebbern, C.; Cakmak, S. Synoptic weather types and aeroallergens modify the effect of air pollution on hospitalization for asthma hospitalizations in Canadian cities. Environ. Pollut. 2015, 204, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Sheridan, S.C.; Lin, S.S. Relating weather types to asthma-related hospital admissions in New-York State. EcoHealth 2012, 9, 427–439. [Google Scholar] [CrossRef]
- Mireku, N.; Wang, Y.; Ager, J.; Reddy, R.C.; Baptist, A.P. Changes in weather and the effects on pediatric asthma exacerbations. Ann. Allergy Asthma Immunol. 2009, 103, 220–224. [Google Scholar] [CrossRef]
- Mandrioli, P.; Negrini, M.G.; Cesari, G.; Morgan, G. Evidence for long range transport of biological and anthropogenic aerosol particles in the atmosphere. Grana 1984, 23, 43–53. [Google Scholar] [CrossRef]
- Robichaud, A.; Comtois, P. Numerical modelling of birch pollen dispersion in Canada. Environ. Res. 2021, 194, 110554. [Google Scholar] [CrossRef] [PubMed]
- Gregory, H.P. Distribution of airborne pollen and spores and their long-distance transport. Pure Appl. Geophys. 1978, 116, 309–315. [Google Scholar] [CrossRef]
- Mandrioli, P.; Negrini, M.G.; Zanotti, A.L. Airborne pollen from the Yugoslavian coast to the Po Valley (Italy). Grana 1982, 21, 121–128. [Google Scholar] [CrossRef]
- Comtois, P. Pollen dispersal and long-distance transport: The case of thermophilic pollen in subarctic Canada. Aerobiologia 1996, 13, 37–42. [Google Scholar] [CrossRef]
- Rogers, C.A.; Levetin, E. Evidence of long-distance transport of mountain cedar pollen in Tulsa, Oklahoma. Int. J. Biometeorol. 1998, 50, 392–402. [Google Scholar] [CrossRef]
- Rousseau, D.D.; Duzer, D.; Cambon, G.; Jolly, D.; Poulsen, U.; Ferrier, J.; Schevin, P.; Gros, R. Long distance transport of pollen to Greenland. Geophys. Res. Lett. 2003, 30, 1765. [Google Scholar] [CrossRef]
- Smith, M.; Skjoth, C.A.; Myszkowska, D.; Uruska, A.; Puc, M.; Stach, A.; Balwierz, Z.; Chlopek, K.; Piotrowska, K.; Kasprzyk, I.; et al. Long-range transport of Ambrosia pollen to Poland. Agric. For. Meteorol. 2008, 148, 1402–1411. [Google Scholar] [CrossRef]
- Sofiev, M.; Siljamo, P.; Ranta, H.; Rantio-Lehtimäki, A. Towards numerical forecasting of long-range air transport of birch pollen: Theoretical considerations and a feasibility study. Int. J. Biometeorol. 2006, 50, 392–402. [Google Scholar] [CrossRef]
- Mahura, A.G.; Korsholm, U.; Baklanov, S.; Rasmussen, A. Elevated birch pollen episodes in Denmark: Contributions from remote sources. Aerobiologia 2007, 23, 171–179. [Google Scholar] [CrossRef]
- 63. Siljamo, P.; Sofiev, M.; Severova, E.; Ranta, H.; Kukkonen, J.; Polevova, S.; Kubin, E.; Minin, A.A. Sources, impact and exchange of early-spring birch pollen in the Moscow region and Finland. Aerobiologia 2008, 24, 211–230. [Google Scholar] [CrossRef]
- Siljamo, P. Numerical Modelling of Birch Pollen Emissions and Dispersion on Regional and Continental Scales. Ph.D. Thesis, Finnish Meteorological Institute Contributions No. 99. FMI-CONT-99, Helsinki, Finland, 2013. [Google Scholar]
- Wallin, J.E.; Segerstrom, U.; Rosenhall, L.; Bergmann, E.; Hjelmroos, M. Allergic symptoms caused by long-distance transported birch pollen. Grana 1991, 30, 265–268. [Google Scholar] [CrossRef]
- Laaidi, K.; Laaidi, M.; Besancenot, J.P. Pollens, pollinoses et météorologie. Météorologie 1997, 20, 41–55. (In French) [Google Scholar] [CrossRef]
- de Weger, L.A.; Bergmann, K.C.; Rantio-Lehtimäki, A.; Dahl, A.; Buters, J.; Déchamp, J.; Belmonte, J. Impact of pollen. In Allergenic Pollen, A Review of the Production, Release, Distribution and Health Impacts; Sofiev, M., Bergmann, K.C., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2013; 256p, ISBN1 978-94-007-4880-4e. ISBN2 978-94-94-007-4881-1. [Google Scholar] [CrossRef]
- Ribeiro, H.; Costa, C.; Abreu, I.; da Silva, J.C.E. Effect of O3 and NO2 atmospheric pollutants on Platanus acerifolia pollen: Immunochemical and spectroscopic analysis. Sci. Total Environ. 2017, 599–600, 291–297. [Google Scholar] [CrossRef]
- Hong, Q.; Zhou, S.; Zhao, H.; Peng, J.; Li, Y.; Shang, Y.; Wu, M.; Zhang, W.; Lu, S.; Li, S.; et al. Allergenicity of recombinant Humulus japonicus pollen allergen 1 after combined exposure to ozone and nitrogen dioxide. Environ. Pollut. 2018, 234, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, K.R.; Kinney, P.L.; Robinson, G.S.; Sheehan, D.; Kheirbek, I.; Matte, T.D.; Lovasi, G.S. Levels and determinants of tree pollen in New York City. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sofiev, M.; Bergmann, K.C. Allergenic Pollen. A Review of the Production, Release, Distribution and Health Impacts; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; ISBN1 978-94-007-4880-4e. ISBN2 978-94-94-007-4881-1. [Google Scholar]
- Dales, R.E.; Cakmak, S.; Judek, S.; Dann, T.; Coates, F.; Brook, J.R.; Burnett, R.T. Influence of outdoor aeroallergens on hospitalization for asthma in Canada. Environ. Occup. Disord. 2004, 113, 303–306. [Google Scholar] [CrossRef]
- Dales, R.E.; Cakmak, S.; Judek, S.; Coates, F. Tree Pollen and Hospitalization for Asthma in urban Canada. Int. Arch. Allergy Immunol. 2008, 146, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Guérin, B. Pollen et Allergies; Édition Allerbio: Varennes-en-Argonne, France, 1993. [Google Scholar]
- Hasnain, S.M. Influence of meteorological factors on the air spora. Grana 1993, 32, 184–188. [Google Scholar] [CrossRef]
- Barnes, C.; Pacheco, F.; Landuyt, J.; Hu, F.; Portnoy, J. Hourly variation of airborne ragweed pollen in Kansas City. Ann. Allergy Asthma Immunol. 2001, 86, 166–171. [Google Scholar] [CrossRef]
- 77. Chathurika, M.R.; Metwali, N.; Jayarathne, T.; Kettler, J.; Huang, Y.; Thorne, P.S.; O’Shaughnessy, P.T.; Stone, E.A. Influence of rain on the abundance of bioaerosols in fine and coarse particles. Atmos. Chem. Phys. 2017, 17, 2459–2475. [Google Scholar] [CrossRef]
- Laaidi, M. Regional variations in the pollen season of Betula in Burgundy: Two models for predicting the start of the pollination. Aerobiologia 2001, 17, 247–254. [Google Scholar] [CrossRef]
- Climate: Montreal—Climate graph, Temperature graph, Climate table. Climate-Data.org. Archived. Retrieved 24 May 2021. Available online: https://en.climate-data.org/north-america/canada/quebec/montreal-3704/ (accessed on 17 June 2021).
- 80. Stieb, D.; Burnett, M.; Smith-Dorion, R.T.M.; Brion, O.; Shin, H.H.; Economou, V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. J. Air Waste Manag. Assoc. 2008, 58, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Kinnee, E.; Geron, C.; Pierce, T. United States Land-Use Inventory for estimating Biogenic Ozone Precursor Emissions. Ecol. Appl. 1997, 7, 46–58. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Mandrioli, P.; Comtois, P.; Levizzani, V. Methods in Aerobiology; Pitagora Editrice: Bologna, Italy, 1998; ISBN 8-371-1043-X. [Google Scholar]
- Institut National de Santé Publique du Québec (INSPQ). États des Connaissances sur le Pollen et les Allergies. Les Assises pour une Gestion Efficace; 2013; ISBN 978-2-550-68403-9. Available online: http://www.inspq.qc.ca (accessed on 17 June 2021).
- Côté, J.; Gravel, S.; Méthot, A.; Patoine, A.; Roch, M.; Staniforth, A.N. The operational CMC-MRB Global Environmental Multiscale (GEM) model. Part I: Design considerations and formulation. Mon. Weather Rev. 1998, 126, 1373–1395. [Google Scholar] [CrossRef]
- Côté, J.; Desmarais, J.G.; Gravel, S.; Méthot, A.; Patoine, A.; Roch, M.; Staniforth, A. The operational CMC–MRB Global Environmental Multiscale (GEM) model. Part II: Results. Mon. Weather Rev. 1998, 126, 1397–1418. [Google Scholar] [CrossRef]
- Moran, M.D.; Chen, J.; Ménard, S.; Pavlovic, R.; Landry, H.; Beaulieu, P.A.; Gilbert, S.; Makar, P.A.; Gong, W.; Stroud, C.; et al. Two years of operational AQ forecasting with GEM-MACH15: A look back and look ahead. In Proceedings of the 10th annual CMAS Conference, Chapel Hill, NC, USA, 24–26 October 2011. [Google Scholar]
- Moran, M.D.; Ménard, S.; Pavlovic, R.; Anselmo, D.; Antonopoulos, S.; Robichaud, A.; Gravel, S.; Makar, P.A.; Gong, W.; Stroud, C.; et al. Recent Advances in Canada’s National Operational Air Quality Forecasting System. In Proceedings of the 32nd NATO-SPS ITM, Utrecht, NL, USA, 7–11 May 2012. [Google Scholar]
- Comtois, P.; Gagnon, L. Concentration pollinique et fréquence des symptômes de pollinose: Une méthode pour déterminer les seuils cliniques. Rev. Fr. Allergol. 1998, 28, 279. [Google Scholar] [CrossRef]
- Garty, B.Z.; Kosman, E.; Ganor, E.; Berger, V.; Garty, L.; Wietzen, T.; Waisman, Y.; Mimouni, M.; Waisl, Y. Emergency room visits of asthmatic children, relation to air pollution, weather, and airborne allergens. Ann. Allergy Asthma Immunol. 1998, 81, 563–570. [Google Scholar] [CrossRef]
- Schwartz, J. Facts Not Fear on Air Pollution. NCPA Policy Report. 2006. Available online: https://www.georgiapolicy.org/issue/facts-not-fear-on-air-pollution/ (accessed on 12 May 2021).
- Robichaud, A.; Ménard, R. Multi-year objective analyses of warm season ground-level ozone and PM2.5 over North America using real-time observations and Canadian operational air quality models. Atmos. Chem. Phys. 2014, 13, 13967–14035. [Google Scholar] [CrossRef]
- Robichaud, A.; Ménard, R.; Zaitseva, Y.; Anselmo, D. Multi-pollutant objective analyses and mapping of AQHI over North America. Air Qual. Atmos. Health 2016, 97, 743–759. [Google Scholar] [CrossRef]
- Lajoie, P.; Laberge, A.; Lebel, G.; Boulet, L.P.; Demers, M.; Mercier, P.; Gagnon, M.F. Cartography of emergency department visits for asthma—Targeting high-mortality populations. Can. Respir. J. 2004, 11, 427–433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ville de Montréal. Bilan de la Qualité de l’Air. 2018. Available online: https://ville.montreal.qc.ca/pls/portal/docs/page/enviro_fr/media/documents/vdm_bilanrsqa_2018_vf.pdf (accessed on 17 June 2021).
- Lajoie, P.; Lebel, G.; Leduc, R.; Boulet, L.P.; Bourbeau, J.; Bernard, P.M. Étude sur la Relation Entre les Visites à l’Urgence des Hôpitaux pour l’Asthme, la Qualité de l’Air et les Conditions Météorologiques pour la Communauté Urbaine de Québec (1990–1991): Rapport Final 1992; QEN/QA94-2/6. Envirodoq EN940068; Département de Santé Communautaire du Centre Hospitalier de l’Université Laval, Ministère de l’Environnement et de la Faune, Gouvernement du Québec: Québec, ON, Canada, 1994.
- Goldstein, I.F.; Currie, B. Seasonal patterns of asthma: A clue to etiology. Environ. Res. 1984, 33, 201–215. [Google Scholar] [CrossRef]
- DeGuire, P.; Cao, B.; Wisnieski, L.; Strane, D.; Wahl, R.; Lyon-Callo, S.; Garcia, E. Detroit: The Current Status of Asthma. Michigan Department of Health and Human Services Bureau of Disease Control, Prevention and Epidemiology. 2016. Available online: https://www.michigan.gov/documents/mdhhs/Detroit-AsthmaBurden_516668_7.pdf (accessed on 12 May 2021).
- NRDC (Natural Resources Defence Council). Sneezing and Wheezing: How Climate Change Could Increase Ragweed Allergies, Air Pollution, and Asthma; NRDC: New York, NY, USA, 2015. [Google Scholar]
- AAFA Asthma Capital. 2014. Available online: https://www.aafa.org/media/2426/aafa-2014-asthma-capitals-report.pdf (accessed on 12 May 2021).
- Crighton, E.J.; Ragetlie, R.; Luo, J.; To, T.; Gershon, A. A Spatial analysis of COPD prevalence, incidence, mortality and health service use in Ontario. Health Rep. 2015, 26, 10–18. [Google Scholar] [PubMed]
- Cooper, O.R.; Parrish, D.D.; Stohl, A.; Trainer, M.; Nédélec, P.; Thouret, V.; Cammas, J.P.; Oltmans, S.J.; Johnson, B.J.; Tarasick, D.; et al. Increasing springtime ozone mixing ratios in the free troposphere over western North America. Nat. Lett. 2010, 463, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Svartengren, M.; Strand, V.; Bylin, G.; Jarup, L.; Pershagen, G. Short-term exposure to air pollution in a road tunnel enhances the asthmatic response to allergen. Eur. Respir. J. 2000, 15, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Helbig, N.; Vogel, B.; Vogel, H.; Fiedler, F. Numerical modelling of pollen dispersion on the regional scale. Aerobiologia 2004, 3, 3–19. [Google Scholar] [CrossRef]
- Andersen, T.B. A model to predict the beginning of the pollen season. Grana 1991, 30, 269–275. [Google Scholar] [CrossRef]
- García-Mozo, H.; Chine, I.; Aira, M.J. Regional phenological models for forecasting the start and peak of the Quercus pollen season in Spain. Agric. For. Meteorol. 2008, 148, 372–380. [Google Scholar] [CrossRef]
- Comtois, P.; Fernández-Gonzales, D.; Valencia-Barrera, R.; Sánchez, J.; Fraile, R.; Rodier, S. Pollen content study of the lower atmosphere in Léon (Spain) by use of a tethered balloon. Aerobiologia 2000, 16, 187–191. [Google Scholar] [CrossRef]
- Hjort, J.; Hugg, T.T.; Antikainen, H.; Rusanen, J.; Sofiev, M.; Kukkonen, J.; Jaakkola, M.S.; Jaakkola, J.J. Fine-Scale Exposure to Allergenic Pollen in the Urban Environment: Evaluation of Land Use Regression Approach. Environ. Health Perspect. 2016, 124, 619–626. [Google Scholar] [CrossRef]
- AAPCEH (American Academy of Pediatrics Committee on Environmental Health); Etzel, R.A. (Eds.) Pediatric Environmental Health, 2nd ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2003. [Google Scholar]

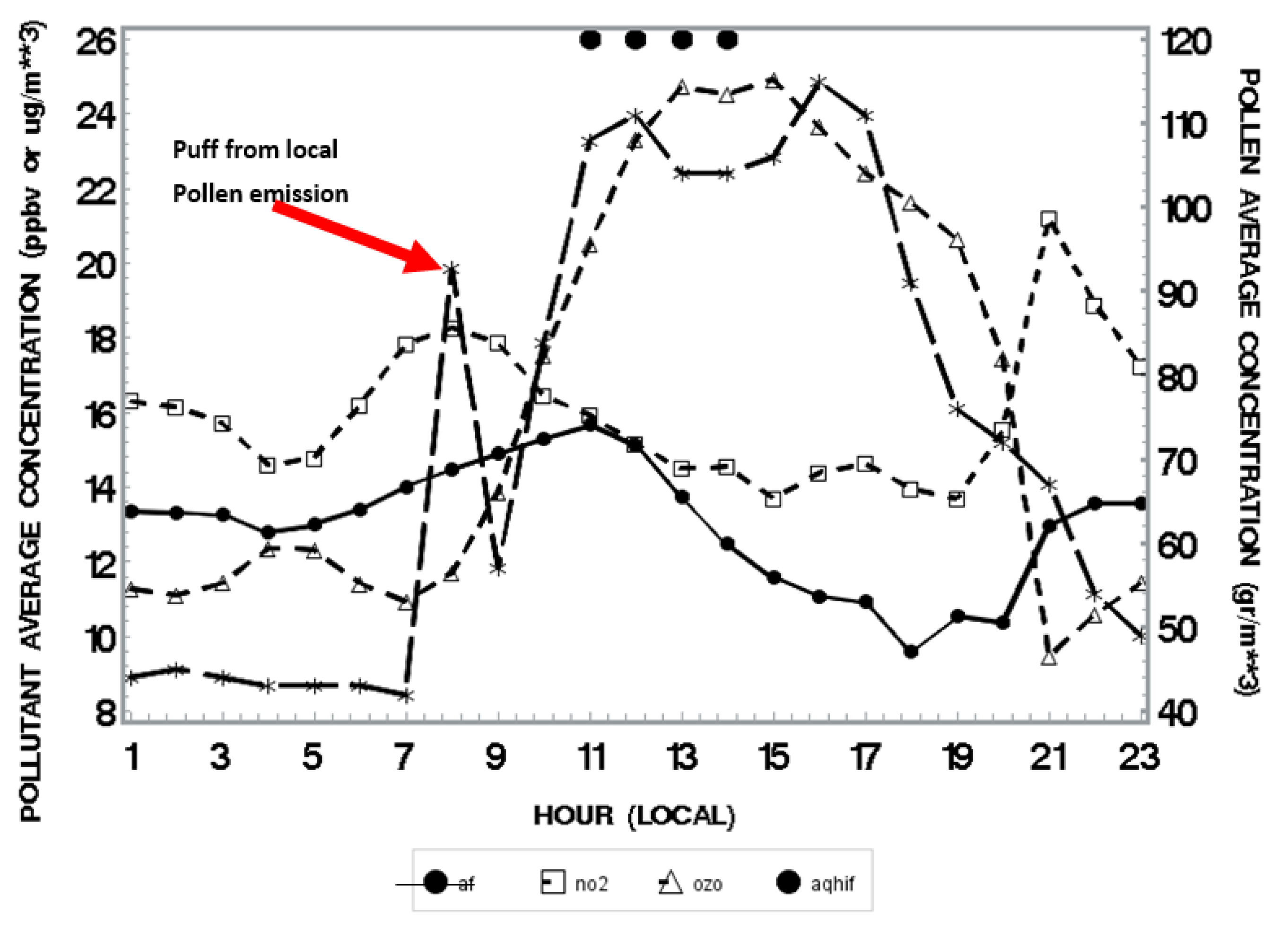
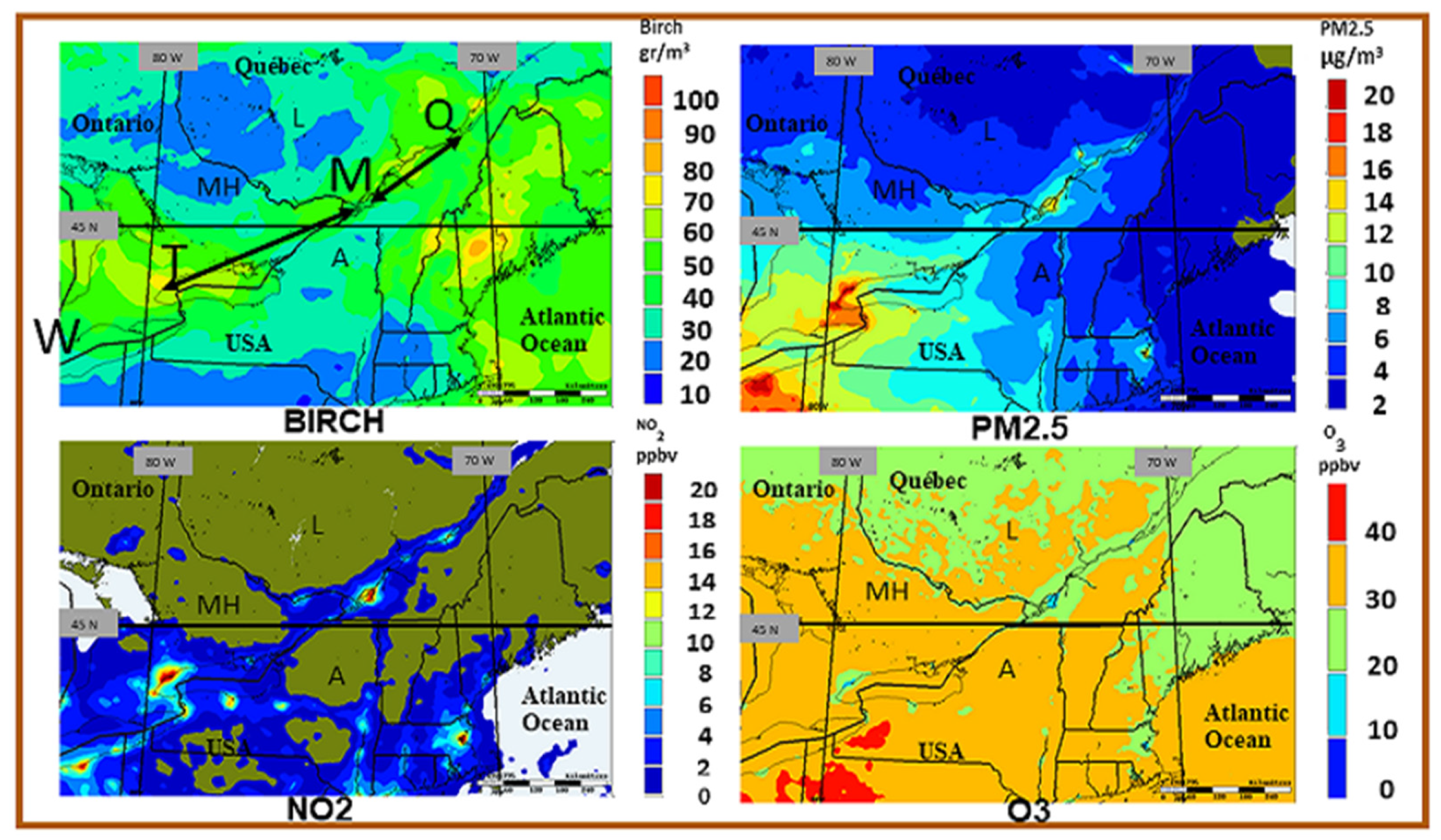
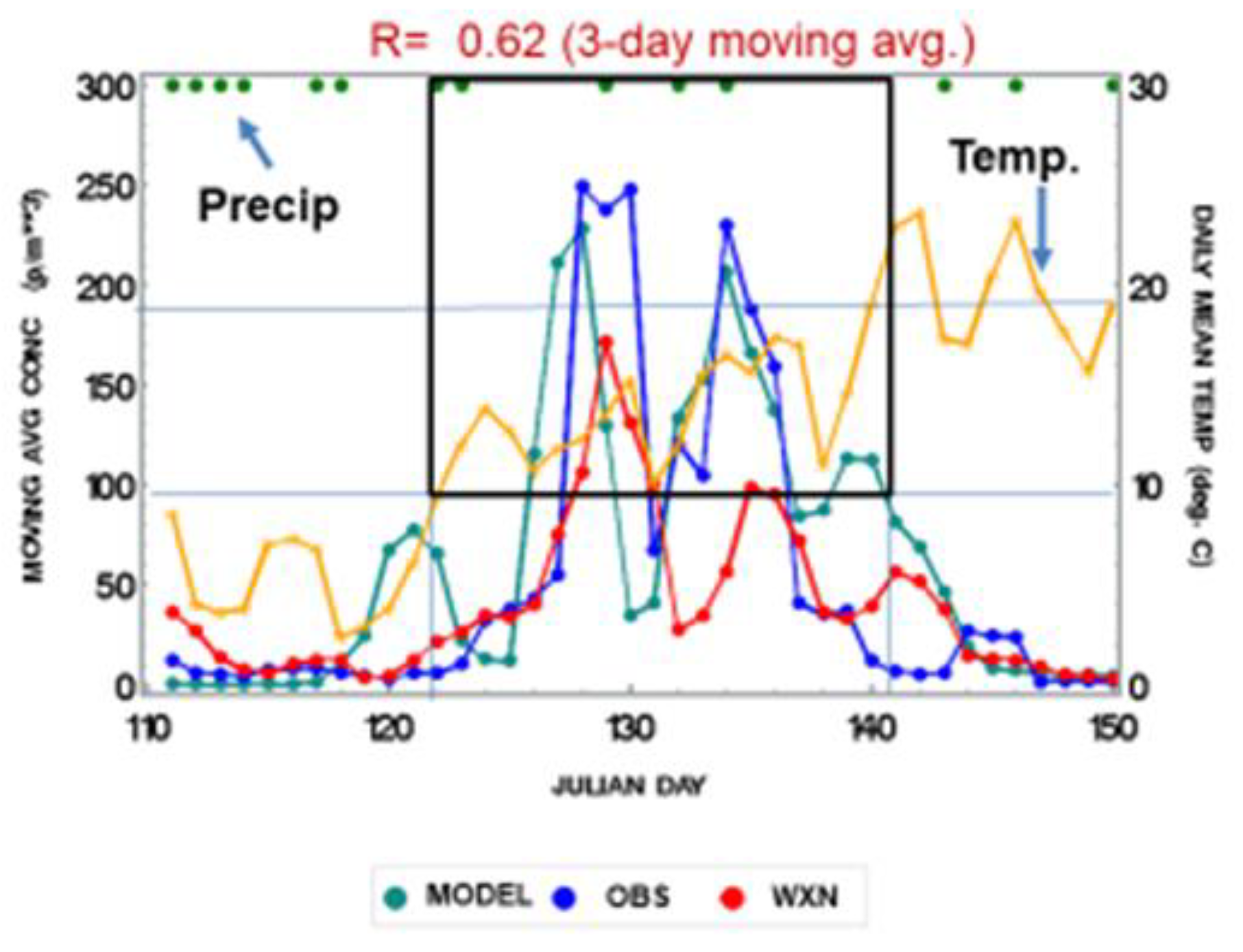
| Weather Element | Impact | Result | Reference |
|---|---|---|---|
| Continuous light rain | Likely washes out most bioaerosols | Likely low asthma | [25,75,76] |
| Intermittent rain with dry cycles | Likely washes out large aerosols but may increase small bioaerosols due to pollen rupture | Small bioaerosols penetrate deeper into lungs and increase asthma risk | [29,30,31,77] |
| Clouds and fog | Nitration of pollen grain is activated in water droplets | Increased risk of pollen-pollution synergy | [21,35] |
| Ridge of high pressure with winds | Optimum weather for pollen emission. Wind is the necessary condition for mechanical vibration to drive pollen grains out of the anther and be regionally transported | Airborne pollen load is maximum and allergy is up likely due to pollutant-pollen synergy | [48,54,76] |
| Thunderstorm | Pollen rupture increasing drastically the number of sub-pollen particles of smaller size | Sudden increase of asthma emergency visit and hospitalization | [9,10,46] |
| Active fronts | Pollen-pollution-weather synergy | Decrease of pollen counts but increase of asthma hospitalization (suggesting pollen rupture) | [48,77] |
| Air masses | Synergy between pollen with environmental factors | Asthma more likely in certain air masses | [50,51,78] |
| Fronts, occlusion/trowal and high pressure | Pollution trapped in convergence zone and high pressure systems. | Higher asthma counts | [48,53] |
| A | Lag 0 | Lag 1 | Lag 2 |
|---|---|---|---|
| [Birch] | 0.15 | 0.25 | −0.12 * |
| Ln [Birch] | 0.23 | 0.26 | NS |
| Ozone | NS | 0.15 | NS |
| PM2.5 | −0.13 * | NS | NS |
| Temperature | −0.12 | −0.13 | −0.17 |
| AQHI | NS | NS | NS |
| B | Lag 0 | Lag 1 | Lag 2 |
| [Birch] | NS | 0.28 * (West) | NS |
| Ln [Birch] | 0.40 (ALL SECTORS) | 0.41 (West) | NS |
| Ozone | NS | 0.41 (Center North) | NS |
| PM2.5 | NS | 0.39 (Center South) | NS |
| Temperature | NS | NS | −0.29 (Center South) |
| AQHI | 0.37 (Center North) | 0.53 (Center North) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robichaud, A. A Case Study of Birch Pollen and the Synergy with Environmental Factors: Relation to Asthma in Montreal, Canada. Atmosphere 2021, 12, 789. https://doi.org/10.3390/atmos12060789
Robichaud A. A Case Study of Birch Pollen and the Synergy with Environmental Factors: Relation to Asthma in Montreal, Canada. Atmosphere. 2021; 12(6):789. https://doi.org/10.3390/atmos12060789
Chicago/Turabian StyleRobichaud, Alain. 2021. "A Case Study of Birch Pollen and the Synergy with Environmental Factors: Relation to Asthma in Montreal, Canada" Atmosphere 12, no. 6: 789. https://doi.org/10.3390/atmos12060789
APA StyleRobichaud, A. (2021). A Case Study of Birch Pollen and the Synergy with Environmental Factors: Relation to Asthma in Montreal, Canada. Atmosphere, 12(6), 789. https://doi.org/10.3390/atmos12060789





