Combined Effect of Hot Weather and Outdoor Air Pollution on Respiratory Health: Literature Review
Abstract
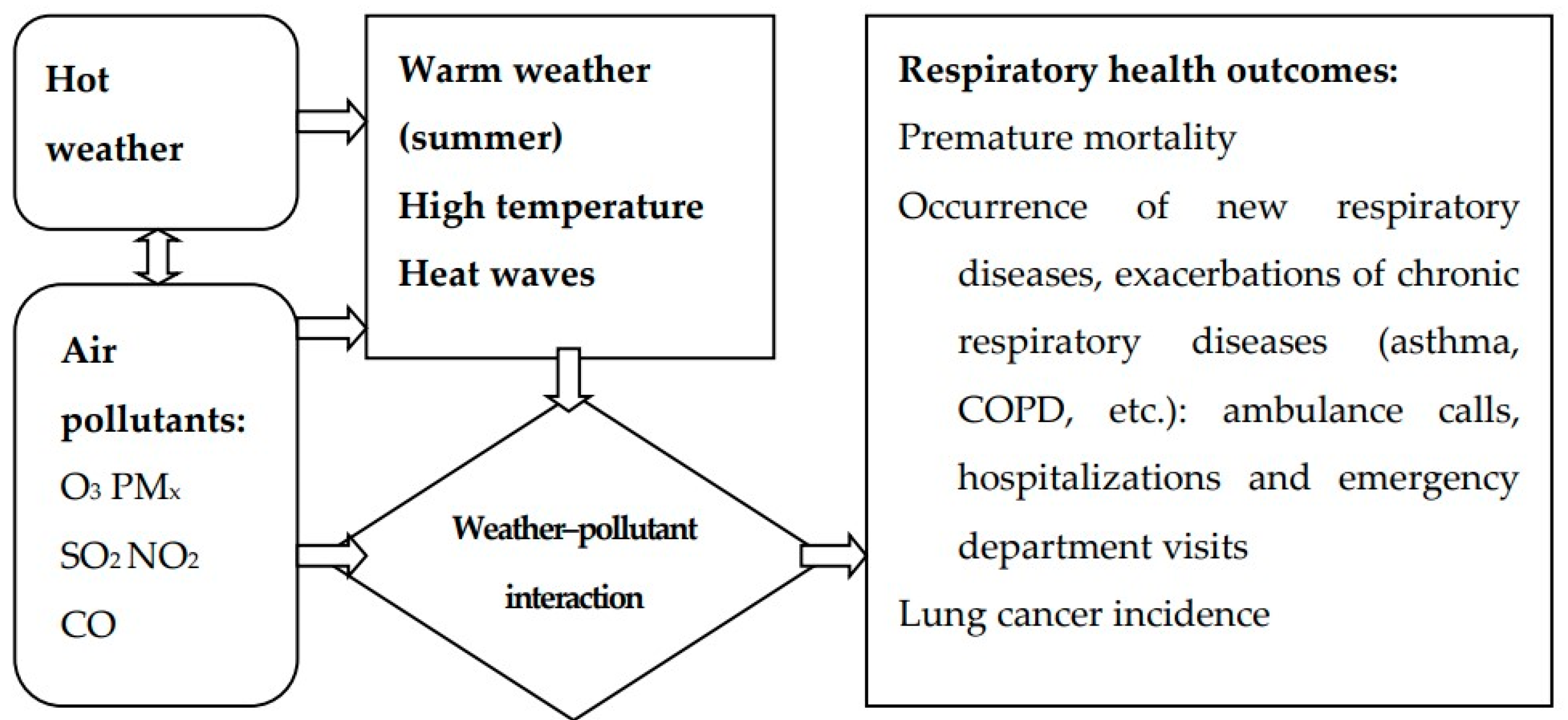
1. Introduction
2. Materials and Methods
3. Results
3.1. Heat, Air Pollution and Morbidity
3.2. Heat, Air Pollution and Mortality
3.3. Heat Wave Episodes
4. Discussion
4.1. Wildfires
4.2. Urban Heat Island Effect
4.3. Possible Biological Mechanisms
4.4. Virus and Bacteria Infections and Epidemics
4.5. Indoor Pollution
4.6. Vulnerable or Susceptible Population Groups
4.7. Confounder Effect of Multiple Environmental Stressors
4.8. Climate Change
4.9. Prevention Policy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Sario, M.; Katsouyanni, K.; Michelozzi, P. Climate change, extreme weather events, air pollution and respiratory health in Europe. Eur. Respir. J. 2013, 42, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Veremchuk, L.V.; Mineeva, E.E.; Vitkina, T.I.; Grigorieva, E.A.; Gvozdenko, T.A.; Golokhvast, K.S. The response ranges of pulmonary function and the impact criteria of weather and industrial influence on patients with asthma living in Vladivostok. J. Environ. Health Sci. Eng. 2020, 18, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Katsouyanni, K.; Analitis, A. Investigating the Synergistic Effects between Meteorological Variables and Air Pollutants: Results from the European PHEWE, EUROHEAT and CIRCE Projects. Epidemiology 2009, 20, S264. [Google Scholar] [CrossRef]
- Analitis, A.; Donato, F.D.; Scortichini, M.; Lanki, T.; Basagana, X.; Ballester, F.; Astrom, C.; Paldy, A.; Pascal, M.; Gasparrini, A.; et al. Synergistic Effects of Ambient Temperature and Air Pollution on Health in Europe: Results from the PHASE Project. Int. J. Environ. Res. Public Health 2018, 15, 1856. [Google Scholar] [CrossRef]
- Lepeule, J.; Litonjua, A.A.; Gasparrini, A.; Koutrakis, P.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Lung function association with outdoor temperature and relative humidity and its interaction with air pollution in the elderly. Environ. Res. 2018, 165, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, R.; Kan, H. The Interaction of Ambient Temperature and Air Pollution in China. In Ambient Temperature and Health in China; Springer Nature: Singapore, 2019; pp. 105–116. [Google Scholar]
- Krug, A.; Fenner, D.; Holtmann, A.; Scherer, D. Occurrence and Coupling of Heat and Ozone Events and Their Relation to Mortality Rates in Berlin, Germany, between 2000 and 2014. Atmosphere 2019, 10, 348. [Google Scholar] [CrossRef]
- Joshi, M.; Goraya, H.; Joshi, A.; Bartter, T. Climate change and respiratory diseases. Curr. Opin. Pulm. Med. 2020, 26, 119–127. [Google Scholar] [CrossRef] [PubMed]
- The Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/ (accessed on 20 May 2021).
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Al-Jabi, S.W.; Zyoud, S.H.; Sawalha, A.F. Outdoor air pollution and respiratory health: A bibliometric analysis of publications in peer-reviewed journals (1900–2017). Multidiscip. Respir. Med. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Lelieveld, J.; Barlas, C.; Giannadaki, D.; Pozzer, A. Model calculated global, regional and megacity premature mortality due to air pollution. Atmos. Chem. Phys. Discuss. 2013, 13, 7023–7037. [Google Scholar] [CrossRef]
- Karimi, B.; Shokrinezhad, B.; Samadi, S. Mortality and hospitalizations due to cardiovascular and respiratory diseases associated with air pollution in Iran: A systematic review and meta-analysis. Atmos. Environ. 2019, 198, 438–447. [Google Scholar] [CrossRef]
- Sicard, P.; Khaniabadi, Y.O.; Perez, S.; Gualtieri, M.; De Marco, A. Effect of O3, PM10 and PM2.5 on cardiovascular and respiratory diseases in cities of France, Iran and Italy. Environ. Sci. Pollut. Res. 2019, 26, 32645–32665. [Google Scholar] [CrossRef]
- Soleimani, Z.; Boloorani, A.D.; Khalifeh, R.; Teymouri, P.; Mesdaghinia, A.; Griffin, D.W. Air pollution and respiratory hospital admissions in Shiraz, Iran, 2009 to 2015. Atmos. Environ. 2019, 209, 233–239. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Revuelta, M.A.; McIntosh, G.; Pey, J.; Pérez, N.; Querol, X.; Alastuey, A. Partitioning of magnetic particles in PM10, PM2.5 and PM1 aerosols in the urban atmosphere of Barcelona (Spain). Environ. Pollut. 2014, 188, 109–117. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, C.-P.; Tang, C.-S.; Lung, S.-C.; Chuang, H.-C.; Chou, D.-W.; Chang, L.-T. Risk Assessment for People Exposed to PM2.5 and Constituents at Different Vertical Heights in an Urban Area of Taiwan. Atmosphere 2020, 11, 1145. [Google Scholar] [CrossRef]
- Nogarotto, D.C.; Pozza, S.A. A review of multivariate analysis: Is there a relationship between airborne particulate matter and meteorological variables? Environ. Monit. Assess. 2020, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abdo, N.; Khader, Y.S.; Abdelrahman, M.; Graboski-Bauer, A.; Malkawi, M.; Al-Sharif, M.; Elbetieha, A.M. Respiratory health outcomes and air pollution in the Eastern Mediterranean Region: A systematic review. Rev. Environ. Health 2016, 31, 259–280. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Newell, K.; Kartsonaki, C.; Lam, K.B.H.; Kurmi, O. Cardiorespiratory health effects of gaseous ambient air pollution exposure in low and middle income countries: A systematic review and meta-analysis. Environ. Health 2018, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Amoatey, P.; Sicard, P.; De Marco, A.; Khaniabadi, Y.O. Long-term exposure to ambient PM2.5 and impacts on health in Rome, Italy. Clin. Epidemiol. Glob. Health 2020, 8, 531–535. [Google Scholar] [CrossRef]
- Karimi, B.; Shokrinezhad, B. Air pollution and mortality among infant and children under five years: A systematic review and meta-analysis. Atmos. Pollut. Res. 2020, 11, 61–70. [Google Scholar] [CrossRef]
- Jerrett, M.; Shankardass, K.; Berhane, K.; Gauderman, W.J.; Künzli, N.; Avol, E.; Gilliland, F.; Lurmann, F.; Molitor, J.N.; Molitor, J.T.; et al. Traffic-Related Air Pollution and Asthma Onset in Children: A Prospective Cohort Study with Individual Exposure Measurement. Environ. Health Perspect. 2008, 116, 1433–1438. [Google Scholar] [CrossRef]
- Ren, C.; Tong, S. Health effects of ambient air pollution–recent research development and contemporary methodological challenges. Environ. Health 2008, 7, 56. [Google Scholar] [CrossRef]
- Hogea, S.; Tudorache, E.; Fildan, A.P.; Fira-Mladinescu, O.; Marc, M.; Oancea, C.; Stanca, P.H. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin. Respir. J. 2019, 14, 183–197. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J.; Samoli, E.; Gryparis, A.; Touloumi, G.; Peacock, J.; Anderson, R.H.; Le Tertre, A.; Bobros, J.; Celko, M.; et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ. Health Perspect. 2003, 111, 1188–1193. [Google Scholar] [CrossRef]
- Michelozzi, P.; Accetta, G.; De Sario, M.; D’Ippoliti, D.; Marino, C.; Baccini, M.; Biggeri, A.; Anderson, H.R.; Katsouyanni, K.; Ballester, F.; et al. High Temperature and Hospitalizations for Cardiovascular and Respiratory Causes in 12 European Cities. Am. J. Respir. Crit. Care Med. 2009, 179, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Zanobetti, A.; Schwartz, J.; Atkinson, R.; Le Tertre, A.; Schindler, C.; Perez, L.; Cadum, E.; Pekkanen, J.; Paldy, A.; et al. The temporal pattern of mortality responses to ambient ozone in the APHEA project. J. Epidemiol. Community Health 2009, 63, 960–966. [Google Scholar] [CrossRef]
- Nejjari, C.; Marfak, A.; Rguig, A.; Maaroufi, A.; El Marouani, I.; El Haloui, A.; El Johra, B.; Ouahabi, R.; Moulki, R.; Azami, A.I.; et al. Ambient air pollution and emergency department visits among children and adults in Casablanca, Morocco. AIMS Public Health 2021, 8, 285–302. [Google Scholar] [CrossRef]
- Fuzzi, S.; Baltensperger, U.; Carslaw, K.; Decesari, S.; Van Der Gon, H.D.; Facchini, M.C.; Fowler, D.; Koren, I.; Langford, B.; Lohmann, U.; et al. Particulate matter, air quality and climate: Lessons learned and future needs. Atmos. Chem. Phys. Discuss. 2015, 15, 8217–8299. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Vicedo-Cabrera, A.M.; Dubrow, R. Projections of Ambient Temperature- and Air Pollution-Related Mortality Burden under Combined Climate Change and Population Aging Scenarios: A Review. Curr. Environ. Health Rep. 2020, 7, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Chatkin, J.; Correa, L.; Santos, U. External Environmental Pollution as a Risk Factor for Asthma. Clin. Rev. Allergy Immunol. 2021, 1–18. [Google Scholar] [CrossRef]
- Guo, Y.; Gasparrini, A.; Li, S.; Sera, F.; Vicedo-Cabrera, A.M.; Coelho, M.; Saldiva, P.; Lavigne, E.; Tawatsupa, B.; Punnasiri, K.; et al. Quantifying excess deaths related to heatwaves under climate change scenarios: A multicountry time series modelling study. PLoS Med. 2018, 15, e1002629. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef]
- Wang, F.; Chen, T.; Chang, Q.; Kao, Y.-W.; Li, J.; Chen, M.; Li, Y.; Shia, B.-C. Respiratory diseases are positively associated with PM2.5 concentrations in different areas of Taiwan. PLoS ONE 2021, 16, e0249694. [Google Scholar] [CrossRef]
- Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project: Final Technical Report; Technical Report; WHO: Geneva, Switzerland, 2013.
- Wang, C.; Feng, L.; Chen, K. The impact of ambient particulate matter on hospital outpatient visits for respiratory and circulatory system disease in an urban Chinese population. Sci. Total Environ. 2019, 666, 672–679. [Google Scholar] [CrossRef]
- Liu, J.; Yin, H.; Tang, X.; Zhu, T.; Zhang, Q.; Liu, Z.; Tang, X.; Yi, H. Transition in air pollution, disease burden and health cost in China: A comparative study of long-term and short-term exposure. Environ. Pollut. 2021, 277, 116770. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Hear. J. 2015, 36, 83–93. [Google Scholar] [CrossRef]
- Cascio, W.E. Wildland fire smoke and human health. Sci. Total Environ. 2018, 624, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Linares, C.; Ovando-Fuentealba, L.; Orellana-Donoso, S.; Gatica, S.; Klerman, F.; Mudge, S.M.; Gallardo, W.; Pinaud, J.P.; Loyola-Sepulveda, R. Source identification, apportionment and toxicity of indoor and outdoor PM2.5airborne particulates in a region characterised by wood burning. Environ. Sci. Process. Impacts 2016, 18, 575–589. [Google Scholar] [CrossRef]
- Collart, P.; Dramaix, M.; Levêque, A.; Mercier, G.; Coppieters, Y. Concentration–response curve and cumulative effects between ozone and daily mortality: An analysis in Wallonia, Belgium. Int. J. Environ. Health Res. 2018, 28, 147–158. [Google Scholar] [CrossRef]
- Wang, T.; Chiang, E.T.; Moreno-Vinasco, L.; Lang, G.D.; Pendyala, S.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; Chillrud, S.N.; Natarajan, V.; et al. Particulate Matter Disrupts Human Lung Endothelial Barrier Integrity via ROS- and p38 MAPK–Dependent Pathways. Am. J. Respir. Cell Mol. Biol. 2010, 42, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, S.; Tang, R.; Qiu, H.; Huang, Q.; Mason, T.G.; Tian, L. Major air pollutants and risk of COPD exacerbations: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Ohlwein, S.; Kappeler, R.; Joss, M.K.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef]
- Olivieri, D.; Scoditti, E. Impact of environmental factors on lung defences. Eur. Respir. Rev. 2005, 14, 51–56. [Google Scholar] [CrossRef]
- Ebi, K.L.; McGregor, G. Climate Change, Tropospheric Ozone and Particulate Matter, and Health Impacts. Environ. Health Perspect. 2008, 116, 1449–1455. [Google Scholar] [CrossRef]
- Turner, M.C.; Jerrett, M.; Pope, C.A.; Krewski, D.; Gapstur, S.M.; Diver, W.R.; Beckerman, B.S.; Marshall, J.D.; Su, J.; Crouse, D.; et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am. J. Respir. Crit. Care Med. 2016, 193, 1134–1142. [Google Scholar] [CrossRef]
- Baccini, M.; Biggeri, A.; Accetta, G.; Kosatsky, T.; Katsouyanni, K.; Analitis, A.; Anderson, H.R.; Bisanti, L.; D’Ippoliti, D.; Danova, J.; et al. Heat effects on mortality in 15 European cities. Epidemiology 2008, 19, 711–719. [Google Scholar] [CrossRef]
- D’Ippoliti, D.; Michelozzi, P.; Marino, C.; De’Donato, F.; Menne, B.; Katsouyanni, K.; Kirchmayer, U.; Analitis, A.; Medina-Ramón, M.; Paldy, A.; et al. The impact of heat waves on mortality in 9 European cities: Results from the EuroHEAT project. Environ. Health 2010, 9, 37. [Google Scholar] [CrossRef]
- Witt, C.; Schubert, J.A.; Jehn, M.; Holzgreve, A.; Liebers, U.; Endlicher, W.; Scherer, D. The Effects of Climate Change on Patients With Chronic Lung Disease. Dtsch. Aerzteblatt Online 2015, 112, 878. [Google Scholar] [CrossRef]
- Lemmetyinen, R.E.; Karjalainen, J.V.; But, A.; Renkonen, R.L.; Pekkanen, J.R.; Toppila-Salmi, S.K.; Haukka, J. Higher mortality of adults with asthma: A 15-year follow-up of a population-based cohort. Allergy 2018, 73, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Achebak, H.; Devolder, D.; Ingole, V.; Ballester, J. Reversal of the seasonality of temperature-attributable mortality from respiratory diseases in Spain. Nat. Commun. 2020, 11, 2457. [Google Scholar] [CrossRef]
- Hotz, I.C.; Hajat, S. The Effects of Temperature on Accident and Emergency Department Attendances in London: A Time-Series Regression Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1957. [Google Scholar] [CrossRef]
- Pfeifer, K.; Åström, D.O.; Martinsone, Ž.; Kaļužnaja, D.; Oudin, A. Evaluating Mortality Response Associated with Two Different Nordic Heat Warning Systems in Riga, Latvia. Int. J. Environ. Res. Public Health 2020, 17, 7719. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ye, D.; Li, N.; Bi, P.; Tong, S.; Wang, Y.; Cheng, Y.; Li, Y.; Yao, X. High temperatures and emergency department visits in 18 sites with different climatic characteristics in China: Risk assessment and attributable fraction identification. Environ. Int. 2020, 136, 105486. [Google Scholar] [CrossRef]
- Basu, R. California and Climate Changes: An Update. In Climate Change and Global Public Health; Springer: Cham, Switzerland, 2021; pp. 237–251. [Google Scholar]
- Jahn, S.; Hertig, E. Modeling and projecting health-relevant combined ozone and temperature events in present and future Central European climate. Air Qual. Atmos. Health 2021, 14, 563–580. [Google Scholar] [CrossRef]
- Weilnhammer, V.; Schmid, J.; Mittermeier, I.; Schreiber, F.; Jiang, L.; Pastuhovic, V.; Herr, C.; Heinze, S. Extreme weather events in europe and their health consequences–A systematic review. Int. J. Hyg. Environ. Health 2021, 233, 113688. [Google Scholar] [CrossRef]
- Matthies, F.; Bickler, G.; Marín, N.C. Heat-Health Action Plans: Guidance; World Health Organization: Copenhagen, Denmark, 2008. [Google Scholar]
- Watts, N.; Adger, W.N.; Agnolucci, P.; Blackstock, J.; Byass, P.; Cai, W.; Chaytor, S.; Colbourn, T.; Collins, M.; Cooper, A.; et al. Health and climate change: Policy responses to protect public health. Lancet 2015, 386, 1861–1914. [Google Scholar] [CrossRef]
- Revich, B.A.; Grigorieva, E.A. Health Risks to the Russian Population from Weather Extremes in the Beginning of the XXI Century. Part 1. Heat and Cold Waves. Issues Risk Anal. 2021, 18, 12–33. [Google Scholar] [CrossRef]
- Analitis, A.; Katsouyanni, K.; Pedeli, X.; Kirchmayer, U.; Michelozzi, P.; Menne, B. Investigating the Independent and Synergistic Effects of Heat Waves and Air Pollution on Health: The EuroHEAT Project. Epidemiology 2008, 19, S214–S215. [Google Scholar] [CrossRef]
- Schlegel, I.; Muthers, S.; Mücke, H.-G.; Matzarakis, A. Comparison of Respiratory and Ischemic Heart Mortalities and their Relationship to the Thermal Environment. Atmosphere 2020, 11, 826. [Google Scholar] [CrossRef]
- Semenza, J. Excess hospital admissions during the July 1995 heat wave in Chicago. Am. J. Prev. Med. 1999, 16, 269–277. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B.; Kovats, S.; Wilkinson, P. The effect of high temperatures on cause-specific mortality in England and Wales. Occup. Environ. Med. 2011, 69, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Harlan, S.L.; Chowell, G.; Yang, S.; Petitti, D.B.; Butler, E.J.M.; Ruddell, B.L.; Ruddell, D.M. Heat-Related Deaths in Hot Cities: Estimates of Human Tolerance to High Temperature Thresholds. Int. J. Environ. Res. Public Health 2014, 11, 3304–3326. [Google Scholar] [CrossRef]
- Hoffmann, C.; Hanisch, M.; Heinsohn, J.B.; Dostal, V.; Jehn, M.; Liebers, U.; Pankow, W.; Donaldson, G.C.; Witt, C. Increased vulnerability of COPD patient groups to urban climate in view of global warming. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3493–3501. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, L.; Chen, K. Burden of cause-specific mortality attributable to heat and cold: A multicity time-series study in Jiangsu Province, China. Environ. Int. 2020, 144, 105994. [Google Scholar] [CrossRef]
- Stafoggia, M.; Forastiere, F.; Agostini, D.; Caranci, N.; De’Donato, F.; DeMaria, M.; Michelozzi, P.; Miglio, R.; Rognoni, M.; Russo, A.; et al. Factors affecting in-hospital heat-related mortality: A multi-city case-crossover analysis. J. Epidemiol. Community Health 2008, 62, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Breitner, S.; Wolf, K.; Devlin, R.B.; Diaz-Sanchez, D.; Peters, A.; Schneider, A. Short-term effects of air temperature on mortality and effect modification by air pollution in three cities of Bavaria, Germany: A time-series analysis. Sci. Total Environ. 2014, 485-486, 49–61. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Lu, X.; Li, Y. A differential Alamouti coding scheme for OFDM based asynchronous cooperative systems. In Proceedings of the 2011 IEEE 3rd International Conference on Communication Software and Networks, Xi’an, China, 27–29 May 2011; pp. 51–56. [Google Scholar] [CrossRef]
- Basu, R.; Samet, J.M. Relation between elevated ambient temperature and mortality: A review of the epidemiologic evidence. Epidemiol. Rev. 2002, 24, 190–202. [Google Scholar] [CrossRef]
- Chen, F.; Fan, Z.; Qiao, Z.; Cui, Y.; Zhang, M.; Zhao, X.; Li, X. Does temperature modify the effect of PM10 on mortality? A systematic review and meta-analysis. Environ. Pollut. 2017, 224, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Analitis, A.; Michelozzi, P.; D’Ippoliti, D.; De’Donato, F.; Menne, B.; Matthies, F.; Atkinson, R.; Iñiguez, C.; Basagaña, X.; Schneider, A.; et al. Effects of Heat Waves on Mortality. Epidemiology 2014, 25, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kaercher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Fiore, A.M.; Naik, V.; Spracklen, D.V.; Steiner, A.; Unger, N.; Prather, M.; Bergmann, D.; Cameron-Smith, P.J.; Cionni, I.; Collins, W.J.; et al. Global air quality and climate. Chem. Soc. Rev. 2012, 41, 6663–6683. [Google Scholar] [CrossRef]
- Kalisa, E.; Fadlallah, S.; Amani, M.; Nahayo, L.; Habiyaremye, G. Temperature and air pollution relationship during heatwaves in Birmingham, UK. Sustain. Cities Soc. 2018, 43, 111–120. [Google Scholar] [CrossRef]
- Anderson, G.B.; Krall, J.R.; Peng, R.D.; Bell, M.L. Is the Relation between Ozone and Mortality Confounded by Chemical Components of Particulate Matter? Analysis of 7 Components in 57 US Communities. Am. J. Epidemiol. 2012, 176, 726–732. [Google Scholar] [CrossRef]
- Meehl, G.A.; Tebaldi, C.; Tilmes, S.; Lamarque, J.-F.; Bates, S.; Pendergrass, A.; Lombardozzi, D. Future heat waves and surface ozone. Environ. Res. Lett. 2018, 13, 064004. [Google Scholar] [CrossRef]
- Jhun, I.; Fann, N.; Zanobetti, A.; Hubbell, B. Effect modification of ozone-related mortality risks by temperature in 97 US cities. Environ. Int. 2014, 73, 128–134. [Google Scholar] [CrossRef]
- Cheng, Y.; Kan, H. Effect of the Interaction between Outdoor Air Pollution and Extreme Temperature on Daily Mortality in Shanghai, China. J. Epidemiol. 2012, 22, 28–36. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, M.; Lv, J.; Shi, T.; Liu, P.; Wu, Y.; Feng, W.; He, W.; Guo, P. Interactions between ambient air pollutants and temperature on emergency department visits: Analysis of varying-coefficient model in Guangzhou, China. Sci. Total Environ. 2019, 668, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Lim, Y.-H.; Honda, Y.; Guo, Y.-L.L.; Hashizume, M.; Bell, M.; Chen, B.-Y.; Kim, H. Mortality Related to Extreme Temperature for 15 Cities in Northeast Asia. Epidemiology 2015, 26, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.-H.; Wang, G.-S.; Guo, Y.-L.; Chang, S.-C.; Wan, G.-H. Urban air pollution and meteorological factors affect emergency department visits of elderly patients with chronic obstructive pulmonary disease in Taiwan. Environ. Pollut. 2017, 224, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Lim, Y.-H.; Kim, H. Temperature modifies the association between particulate air pollution and mortality: A multi-city study in South Korea. Sci. Total Environ. 2015, 524–525, 376–383. [Google Scholar] [CrossRef]
- Leung, S.Y.; Lau, S.Y.F.; Kwok, K.L.; Mohammad, K.N.; Chan, P.K.S.; Chong, K.C. Short-term association among meteorological variation, outdoor air pollution and acute bronchiolitis in children in a subtropical setting. Thorax 2021, 76, 360–369. [Google Scholar] [CrossRef]
- Li, G.; Zhou, M.; Cai, Y.; Zhang, Y.; Pan, X. Does temperature enhance acute mortality effects of ambient particle pollution in Tianjin City, China. Sci. Total Environ. 2011, 409, 1811–1817. [Google Scholar] [CrossRef]
- Li, G.; Jiang, L.; Zhang, Y.; Cai, Y.; Pan, X.; Zhou, M. The Impact of Ambient Particle Pollution during Extreme-Temperature Days in Guangzhou City, China. Asia Pac. J. Public Health 2014, 26, 614–621. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Cheng, C.-P.; Kim, H.; Wang, Y.-C. Risk of ambulance services associated with ambient temperature, fine particulate and its constituents. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Zhao, Z.; Duan, X.; Xu, X.; Kan, H. Temperature modifies the acute effect of particulate air pollution on mortality in eight Chinese cities. Sci. Total Environ. 2012, 435–436, 215–221. [Google Scholar] [CrossRef]
- Park, A.K.; Hong, Y.C.; Kim, H. Effect of changes in season and temperature on mortality associated with air pollution in Seoul, Korea. J. Epidemiol. Community Health 2010, 65, 368–375. [Google Scholar] [CrossRef]
- Qian, Z.; He, Q.; Lin, H.-M.; Kong, L.; Bentley, C.M.; Liu, W.; Zhou, D. High Temperatures Enhanced Acute Mortality Effects of Ambient Particle Pollution in the “Oven” City of Wuhan, China. Environ. Health Perspect. 2008, 116, 1172–1178. [Google Scholar] [CrossRef]
- Qin, R.X.; Xiao, C.; Zhu, Y.; Li, J.; Yang, J.; Gu, S.; Xia, J.; Su, B.; Liu, Q.; Woodward, A. The interactive effects between high temperature and air pollution on mortality: A time-series analysis in Hefei, China. Sci. Total Environ. 2017, 575, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jiang, L.; Wang, S.; Tian, J.; Yang, K.; Wang, X.; Guan, H.; Zhang, N. The impact of main air pollutants on respiratory emergency department visits and the modification effects of temperature in Beijing, China. Environ. Sci. Pollut. Res. 2021, 28, 6990–7000. [Google Scholar] [CrossRef]
- Tian, L.; Liang, F.; Guo, Q.; Chen, S.; Xiao, S.; Wu, Z.; Jin, X.; Pan, X. The effects of interaction between particulate matter and temperature on mortality in Beijing, China. Environ. Sci. Process. Impacts 2018, 20, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Lin, Y.-K.; Chen, Y.-J.; Hung, S.-C.; Zafirah, Y.; Sung, F.-C. Ambulance Services Associated with Extreme Temperatures and Fine Particles in a Subtropical Island. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Sung, F.-C.; Chen, Y.-J.; Cheng, C.-P.; Lin, Y.-K. Effects of extreme temperatures, fine particles and ozone on hourly ambulance dispatches. Sci. Total Environ. 2020, 765, 142706. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, F.; Guo, M.; Fan, W.; Ji, W.; Dong, Z. Associations between PM1 exposure and daily emergency department visits in 19 hospitals, Beijing. Sci. Total Environ. 2021, 755, 142507. [Google Scholar] [CrossRef]
- Ye, F.; Piver, W.T.; Ando, M.; Portier, C. Effects of temperature and air pollutants on cardiovascular and respiratory diseases for males and females older than 65 years of age in Tokyo, July and August 1980-1995. Environ. Health Perspect. 2001, 109, 355–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Fan, X.; Ye, X. Temperature modulation of the health effects of particulate matter in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 10857–10866. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Falq, G.; Wagner, V.; Chatignoux, E.; Corso, M.; Blanchard, M.; Host, S.; Pascal, L.; Larrieu, S. Short-term impacts of particulate matter (PM10, PM10–2.5, PM2.5) on mortality in nine French cities. Atmos. Environ. 2014, 95, 175–184. [Google Scholar] [CrossRef]
- Pattenden, S.; Armstrong, B.; Milojevic, A.; Heal, M.; Chalabi, Z.; Doherty, R.; Barratt, B.; Kovats, S.; Wilkinson, P. Ozone, heat and mortality: Acute effects in 15 British conurbations. Occup. Environ. Med. 2010, 67, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, D.; Revich, B.; Bellander, T.; Bedada, G.B.; Bottai, M.; Kharkova, T.; Kvasha, E.; Lezina, E.; Lind, T.; Semutnikova, E.; et al. Mortality Related to Air Pollution with the Moscow Heat Wave and Wildfire of 2010. Epidemiology 2014, 25, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Schwartz, J.; Forastiere, F.; Perucci, C.A. Does Temperature Modify the Association between Air Pollution and Mortality? A Multicity Case-Crossover Analysis in Italy. Am. J. Epidemiol. 2008, 167, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.G.; Bell, M.L. Weather-Related Mortality. Epidemiology 2009, 20, 205–213. [Google Scholar] [CrossRef]
- Byers, N.; Ritchey, M.; Vaidyanathan, A.; Brandt, A.J.; Yip, F. Short-term effects of ambient air pollutants on asthma-related emergency department visits in Indianapolis, Indiana, 2007–2011. J. Asthma 2015, 53, 245–252. [Google Scholar] [CrossRef]
- Hanna, A.F.; Yeatts, K.B.; Xiu, A.; Zhu, Z.; Smith, R.L.; Davis, N.N.; Talgo, K.D.; Arora, G.; Robinson, P.J.; Meng, Q.; et al. Associations between ozone and morbidity using the Spatial Synoptic Classification system. Environ. Health 2011, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Mohr, L.B.; Luo, S.; Mathias, E.; Tobing, R.; Homan, S.; Sterling, D. Influence of Season and Temperature on the Relationship of Elemental Carbon Air Pollution to Pediatric Asthma Emergency Room Visits. J. Asthma 2008, 45, 936–943. [Google Scholar] [CrossRef]
- Vanos, J.K.; Hebbern, C.; Cakmak, S. Risk assessment for cardiovascular and respiratory mortality due to air pollution and synoptic meteorology in 10 Canadian cities. Environ. Pollut. 2014, 185, 322–332. [Google Scholar] [CrossRef]
- Hales, S.; Salmond, C.; Town, G.I.; Kjellstrom, T.; Woodward, A. Daily mortality in relation to weather and air pollution in Christchurch, New Zealand. Aust. N. Z. J. Public Health 2000, 24, 89–91. [Google Scholar] [CrossRef]
- Pinheiro, S.D.L.L.D.A.; Saldiva, P.H.N.; Schwartz, J.; Zanobetti, A. Isolated and synergistic effects of PM10 and average temperature on cardiovascular and respiratory mortality. Rev. Saúde Pública 2014, 48, 881–888. [Google Scholar] [CrossRef]
- Steadman, R.G. A universal scale of apparent temperature. J. Clim. Appl. Meteor. 1984, 23, 1674–1687. [Google Scholar] [CrossRef]
- Kalkstein, L.S.; Valimont, K.M. An evaluation of summer discomfort in the United States using a relative climatological in-dex. Am. Meteorol. Soc. 1986, 67, 842–848. [Google Scholar] [CrossRef]
- Sun, Q.; Miao, C.; Hanel, M.; Borthwick, A.G.; Duan, Q.; Ji, D.; Li, H. Global heat stress on health, wildfires, and agricultural crops under different levels of climate warming. Environ. Int. 2019, 128, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Li, B.; Pilot, E.; Chen, R.; Wang, B.; Yang, J. Effect modification of the short-term effects of air pollution on morbidity by season: A systematic review and meta-analysis. Sci. Total Environ. 2020, 716, 136985. [Google Scholar] [CrossRef]
- Meehl, G.A. More Intense, More Frequent, and Longer Lasting Heat Waves in the 21st Century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Stedman, J.R. The predicted number of air pollution related deaths in the UK during the August 2003 heatwave. Atmos. Environ. 2004, 38, 1087–1090. [Google Scholar] [CrossRef]
- Thabethe, N.D.L.; Voyi, K.; Wichmann, J. Association between ambient air pollution and cause-specific mortality in Cape Town, Durban, and Johannesburg, South Africa: Any susceptible groups? Environ. Sci. Pollut. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Burke, M.; Driscoll, A.; Heft-Neal, S.; Xue, J.; Burney, J.; Wara, M. The changing risk and burden of wildfire in the United States. Proc. Natl. Acad. Sci. USA 2021, 118, 2011048118. [Google Scholar] [CrossRef]
- Sorensen, C.; House, J.A.; O’Dell, K.; Brey, S.J.; Ford, B.; Pierce, J.R.; Fischer, E.V.; Lemery, J.; Crooks, J.L. Associations Between Wildfire-Related PM 2.5 and Intensive Care Unit Admissions in the United States, 2006–2015. GeoHealth 2021, 5, e2021GH000385. [Google Scholar] [CrossRef]
- Grumm, R.H. The Central European and Russian Heat Event of July–August 2010. Bull. Am. Meteorol. Soc. 2011, 92, 1285–1296. [Google Scholar] [CrossRef]
- Xu, R.; Yu, P.; Abramson, M.J.; Johnston, F.H.; Samet, J.M.; Bell, M.L.; Haines, A.; Ebi, K.L.; Li, S.; Guo, Y. Wildfires, Global Climate Change, and Human Health. N. Engl. J. Med. 2020, 383, 2173–2181. [Google Scholar] [CrossRef]
- Henderson, S.B.; Brauer, M.; Macnab, Y.C.; Kennedy, S.M. Three Measures of Forest Fire Smoke Exposure and Their Associations with Respiratory and Cardiovascular Health Outcomes in a Population-Based Cohort. Environ. Health Perspect. 2011, 119, 1266–1271. [Google Scholar] [CrossRef]
- Finlay, S.E.; Moffat, A.; Gazzard, R.; Baker, D.; Murray, V. Health Impacts of Wildfires. PLoS Curr. 2012, 4, e4f959951cce2c. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.S.; Rice, M.B. Lungs in a Warming World Climate Change and Respiratory Health. Chest 2013, 143, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Faustini, A.; Alessandrini, E.R.; Pey, J.; Perez, N.; Samoli, E.; Querol, X.; Cadum, E.; Perrino, C.; Ostro, B.; Ranzi, A.; et al. Short-term effects of particulate matter on mortality during forest fires in Southern Europe: Results of the MED-PARTICLES Project. Occup. Environ. Med. 2015, 72, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.J.; Bowman, D.M.J.S.; Price, O.F.; Henderson, S.B.; Johnston, F.H. A transdisciplinary approach to understanding the health effects of wildfire and prescribed fire smoke regimes. Environ. Res. Lett. 2016, 11, 125009. [Google Scholar] [CrossRef]
- Black, C.; Tesfaigzi, Y.; Bassein, J.A.; Miller, L.A. Wildfire smoke exposure and human health: Significant gaps in research for a growing public health issue. Environ. Toxicol. Pharmacol. 2017, 55, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.W.; Ford, B.; Lassman, W.; Pfister, G.; Vaidyanathan, A.; Fischer, E.; Volckens, J.; Pierce, J.; Magzamen, S. Comparison of wildfire smoke estimation methods and associations with cardiopulmonary-related hospital admissions. GeoHealth 2017, 1, 122–136. [Google Scholar] [CrossRef]
- Lipner, E.M.; O’Dell, K.; Brey, S.J.; Ford, B.; Pierce, J.R.; Fischer, E.V.; Crooks, J.L. The Associations between Clinical Respiratory Outcomes and Ambient Wildfire Smoke Exposure among Pediatric Asthma Patients at National Jewish Health, 2012–2015. GeoHealth 2019, 3, 146–159. [Google Scholar] [CrossRef]
- Karanasiou, A.; Alastuey, A.; Amato, F.; Renzi, M.; Stafoggia, M.; Tobias, A.; Reche, C.; Forastiere, F.; Gumy, S.; Mudu, P.; et al. Short-term health effects from outdoor exposure to biomass burning emissions: A review. Sci. Total Environ. 2021, 781, 146739. [Google Scholar] [CrossRef]
- Revich, B.; Avaliani, S.; Simons, G. Air pollution and public health in a megalopolis: A case study of Moscow. Econ. Reg. 2016, 12, 1069–1078. [Google Scholar] [CrossRef]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke Health Effects: A Review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Salonen, R.O.; Hälinen, A.I.; Penttinen, P.; Pennanen, A.; Sillanpää, M.; Sandell, E.; Hillamo, R.; Hirvonen, M.-R. In vitro inflammatory and cytotoxic effects of size-segregated particulate samples collected during long-range transport of wildfire smoke to Helsinki. Toxicol. Appl. Pharmacol. 2006, 215, 341–353. [Google Scholar] [CrossRef]
- Xu, Y.; Dadvand, P.; Barrera-Gómez, J.; Sartini, C.; Marí-Dell’Olmo, M.; Borrell, C.; Medina-Ramón, M.; Sunyer, J.; Basagaña, X. Differences on the effect of heat waves on mortality by sociodemographic and urban landscape characteristics. J. Epidemiol. Community Health 2013, 67, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sabrin, S.; Karimi, M.; Nazari, R. Developing Vulnerability Index to Quantify Urban Heat Islands Effects Coupled with Air Pollution: A Case Study of Camden, NJ. ISPRS Int. J. Geo-Inf. 2020, 9, 349. [Google Scholar] [CrossRef]
- Lai, L.-W.; Cheng, W.-L. Air quality influenced by urban heat island coupled with synoptic weather patterns. Sci. Total Environ. 2009, 407, 2724–2733. [Google Scholar] [CrossRef]
- Ulpiani, G. On the linkage between urban heat island and urban pollution island: Three-decade literature review towards a conceptual framework. Sci. Total Environ. 2021, 751, 141727. [Google Scholar] [CrossRef]
- Sera, F.; Armstrong, B.; Tobias, A.; Vicedo-Cabrera, A.M.; Åström, C.; Bell, M.L.; Chen, B.-Y.; Coelho, M.D.S.Z.S.; Correa, P.M.; Cruz, J.C.; et al. How urban characteristics affect vulnerability to heat and cold: A multi-country analysis. Int. J. Epidemiol. 2019, 48, 1101–1112. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.Y.; Lee, H.; Kim, H. Predicted Future Mortality Attributed to Increases in Temperature and PM10 Concentration under Representative Concentration Pathway Scenarios. Int. J. Environ. Res. Public Health 2020, 17, 2600. [Google Scholar] [CrossRef]
- Gordon, C.J. Role of environmental stress in the physiological response to chemical toxicants. Environ. Res. 2003, 92, 1–7. [Google Scholar] [CrossRef]
- Leon, L.R. Thermoregulatory responses to environmental toxicants: The interaction of thermal stress and toxicant exposure. Toxicol. Appl. Pharmacol. 2008, 233, 146–161. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Guo, C.; Chen, P.-Y.; Ou, C.-Q.; Guo, Y. Particulate matter modifies the magnitude and time course of the non-linear temperature-mortality association. Environ. Pollut. 2015, 196, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Carvalho, V.; Oliveira, T.; Sousa, C. Excess mortality and morbidity during the July 2006 heat wave in Porto, Portugal. Int. J. Biometeorol. 2013, 57, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.L.; Alessandrini, F.; Beck-Speier, I.; Hofer, T.P.J.; Diabaté, S.; Bitterle, E.; Stöger, T.; Jakob, T.; Behrendt, H.; Horsch, M.; et al. Health Effects of Ambient Particulate Matter—Biological Mechanisms and Inflammatory Responses to In Vitro and In Vivo Particle Exposures. Inhal. Toxicol. 2008, 20, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Health Protection Agency. Public Health Adaptation Strategies to Extreme Weather Events (PHASE). Available online: http://www.phaseclimatehealth.eu/ (accessed on 20 May 2021).
- Ruan, T.; Gu, Q.; Kou, Y.R.; Lee, L.-Y. Hyperthermia increases sensitivity of pulmonary C-fibre afferents in rats. J. Physiol. 2005, 565, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Gu, Q.; Hu, H.-Z.; Gao, N.; Zhu, M.X.; Lee, L.-Y. Thermal sensitivity of isolated vagal pulmonary sensory neurons: Role of transient receptor potential vanilloid receptors. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R541–R550. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.L.; Marini, J.J. Effect of heat delivery and extraction on airway conductance in normal and in asthmatic subjects. Am. Rev. Respir. Dis. 1985, 131, 357–361. [Google Scholar] [CrossRef]
- Hayes, D., Jr.; Collins, P.B.; Khosravi, M.; Lin, R.-L.; Lee, L.-Y. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: Role of cholinergic reflex. Am. J. Respir. Crit. Care Med. 2012, 185, 1190–1196. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Hashizume, M.; Goggins, W.B.; Luo, C. A systematic review on lagged associations in climate–health studies. Int. J. Epidemiol. 2021, dyaa286. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Armstrong, B.G.; Gouveia, N.; Wilkinson, P. Mortality Displacement of Heat-Related Deaths. Epidemiology 2005, 16, 613–620. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B. The Impact of Heat Waves on Mortality. Epidemiology 2011, 22, 68–73. [Google Scholar] [CrossRef]
- Iñiguez, C.; Royé, D.; Tobías, A. Contrasting patterns of temperature related mortality and hospitalization by cardiovascular and respiratory diseases in 52 Spanish cities. Environ. Res. 2021, 192, 110191. [Google Scholar] [CrossRef] [PubMed]
- Zafeiratou, S.; Samoli, E.; Dimakopoulou, K.; Rodopoulou, S.; Analitis, A.; Gasparrini, A.; Stafoggia, M.; Donato, F.D.; Rao, S.; Monteiro, A.; et al. A systematic review on the association between total and cardiopulmonary mortality/morbidity or cardiovascular risk factors with long-term exposure to increased or decreased ambient temperature. Sci. Total Environ. 2021, 772, 145383. [Google Scholar] [CrossRef]
- Tzampoglou, P.; Loukidis, D. Investigation of the Importance of Climatic Factors in COVID-19 Worldwide Intensity. Int. J. Environ. Res. Public Health 2020, 17, 7730. [Google Scholar] [CrossRef] [PubMed]
- Bourdrel, T.; Annesi-Maesano, I.; Alahmad, B.; Maesano, C.N.; Bind, M.-A. The impact of outdoor air pollution on COVID-19: A review of evidence from in vitro, animal, and human studies. Eur. Respir. Rev. 2021, 30, 200242. [Google Scholar] [CrossRef]
- Coccia, M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020, 729, 138474. [Google Scholar] [CrossRef] [PubMed]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free. Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Stone, B.; Mallen, E.; Rajput, M.; Broadbent, A.; Krayenhoff, E.S.; Augenbroe, G.; Georgescu, M. Climate change and infrastructure risk: Indoor heat exposure during a concurrent heat wave and blackout event in Phoenix, Arizona. Urban Clim. 2021, 36, 100787. [Google Scholar] [CrossRef]
- Hansel, N.N.; McCormack, M.C.; Belli, A.J.; Matsui, E.C.; Peng, R.D.; Aloe, C.; Paulin, L.; Williams, D.L.; Diette, G.B.; Breysse, P.N. In-Home Air Pollution Is Linked to Respiratory Morbidity in Former Smokers with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 1085–1090. [Google Scholar] [CrossRef]
- Gayle, A.V.; Quint, J.K.; Fuertes, E.I. Understanding the relationships between environmental factors and exacerbations of COPD. Expert Rev. Respir. Med. 2021, 15, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Katsouyanni, K.; Trichopoulos, D.; Zavitsanos, X.; Touloumi, G. THE 1987 ATHENS HEATWAVE. Lancet 1988, 332, 573. [Google Scholar] [CrossRef]
- Chen, S.; Wu, S. Deep learning for identifying environmental risk factors of acute respiratory diseases in Beijing, China: Implications for population with different age and gender. Int. J. Environ. Health Res. 2020, 30, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, D.; Liu, X.; Zhu, J.; Wang, F.; Li, B.; Li, L. Effects of PM2.5 exposure on reproductive system and its mechanisms. Chemosphere 2021, 264, 128436. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Gogna, P.; Maquiling, A.; Parajuli, R.P.; Haque, L.; Burr, B. Comparison of hospitalization and mortality associated with short-term exposure to ambient ozone and PM2.5 in Canada. Chemosphere 2021, 265, 128683. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J.; Gold, D. Are there sensitive subgroups for the effects of airborne particles? Environ. Health Perspect. 2000, 108, 841–845. [Google Scholar] [CrossRef]
- Ko, F.W.S.; Tam, W.; Wong, T.W.; Lai, C.K.W.; Wong, G.W.K.; Leung, T.-F.; Ng, S.; Hui, D.S.C. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clin. Exp. Allergy 2007, 37, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.; Hattis, D. Assessing Cumulative Health Risks from Exposure to Environmental Mixtures—Three Fundamental Questions. Environ. Health Perspect. 2007, 115, 825–832. [Google Scholar] [CrossRef]
- Sexton, K. Cumulative Risk Assessment: An Overview of Methodological Approaches for Evaluating Combined Health Effects from Exposure to Multiple Environmental Stressors. Int. J. Environ. Res. Public Health 2012, 9, 370–390. [Google Scholar] [CrossRef]
- Al Ahad, M.A.; Sullivan, F.; Demšar, U.; Melhem, M.; Kulu, H. The effect of air-pollution and weather exposure on mortality and hospital admission and implications for further research: A systematic scoping review. PLoS ONE 2020, 15, e0241415. [Google Scholar] [CrossRef]
- Leitte, A.M.; Petrescu, C.; Franck, U.; Richter, M.; Suciu, O.; Ionovici, R.; Herbarth, O.; Schlink, U. Respiratory health, effects of ambient air pollution and its modification by air humidity in Drobeta-Turnu Severin, Romania. Sci. Total Environ. 2009, 407, 4004–4011. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; McGregor, G.R.; Enfield, K.B. Humidity: A review and primer on atmospheric moisture and human health. Environ. Res. 2016, 144, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Katsouyanni, K.; Touloumi, G.; Samoli, E.; Gryparis, A.; Le Tertre, A.; Monopolis, Y.; Rossi, G.; Zmirou, D.; Ballester, F.; Boumghar, A.; et al. Confounding and Effect Modification in the Short-Term Effects of Ambient Particles on Total Mortality: Results from 29 European Cities within the APHEA2 Project. Epidemiology 2001, 12, 521–531. [Google Scholar] [CrossRef]
- Yitshak-Sade, M.; Bobb, J.F.; Schwartz, J.D.; Kloog, I.; Zanobetti, A. The association between short and long-term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short-term exposures. Sci. Total Environ. 2018, 639, 868–875. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef]
- Johnston, F.H.; Borchers-Arriagada, N.; Morgan, G.G.; Jalaludin, B.; Palmer, A.J.; Williamson, G.J.; Bowman, D.M.J.S. Unprecedented health costs of smoke-related PM2.5 from the 2019–20 Australian megafires. Nat. Sustain. 2021, 4, 42–47. [Google Scholar] [CrossRef]
- Viegi, G.; Maio, S.; Fasola, S.; Baldacci, S. Global Burden of Chronic Respiratory Diseases. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 171–177. [Google Scholar] [CrossRef]
- McMichael, A.J.; Lindgren, E. Climate change: Present and future risks to health, and necessary responses. J. Intern. Med. 2011, 270, 401–413. [Google Scholar] [CrossRef]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, J.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2021, 42, 293–315. [Google Scholar] [CrossRef]
- Rai, M.; Breitner, S.; Wolf, K.; Peters, A.; Schneider, A.; Chen, K. Impact of climate and population change on temperature-related mortality burden in Bavaria, Germany. Environ. Res. Lett. 2019, 14, 124080. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nat. Cell Biol. 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Glick, S.; Gehrig, R.; Eeftens, M. Multi-decade changes in pollen season onset, duration, and intensity: A concern for public health? Sci. Total Environ. 2021, 781, 146382. [Google Scholar] [CrossRef]
- Fann, N.; Alman, B.; Broome, R.A.; Morgan, G.; Johnston, F.H.; Pouliot, G.; Rappold, A.G. The health impacts and economic value of wildland fire episodes in the U.S.: 2008–2012. Sci. Total Environ. 2018, 610–611, 802–809. [Google Scholar] [CrossRef]
- Li, L.; Du, T.; Zhang, C. The Impact of Air Pollution on Healthcare Expenditure for Respiratory Diseases: Evidence from the People’s Republic of China. Health Policy Politi sante 2020, 13, 1723–1738. [Google Scholar] [CrossRef]
- Bikomeye, J.; Rublee, C.; Beyer, K. Positive Externalities of Climate Change Mitigation and Adaptation for Human Health: A Review and Conceptual Framework for Public Health Research. Int. J. Environ. Res. Public Health 2021, 18, 2481. [Google Scholar] [CrossRef]
- Pascal, M.; Wagner, V.; Alari, A.; Corso, M.; Le Tertre, A. Extreme heat and acute air pollution episodes: A need for joint public health warnings? Atmos. Environ. 2021, 249, 118249. [Google Scholar] [CrossRef]
- Chen, B.; Xie, M.; Feng, Q.; Li, Z.; Chu, L.; Liu, Q. Heat risk of residents in different types of communities from urban heat-exposed areas. Sci. Total Environ. 2021, 768, 145052. [Google Scholar] [CrossRef]
- Ehsan, S.; Abbas, F.; Ibrahim, M.; Ahmad, B.; Farooque, A. Thermal Discomfort Levels, Building Design Concepts, and Some Heat Mitigation Strategies in Low-Income Communities of a South Asian City. Int. J. Environ. Res. Public Health 2021, 18, 2535. [Google Scholar] [CrossRef]
- Yang, H.; Yoon, H. Revealing an Integrative Mechanism of Cognition, Emotion, and Heat-Protective Action of Older Adults. Sustainability 2021, 13, 3534. [Google Scholar] [CrossRef]
- Lou, J.; Ban, J.; Zhang, T.; Wang, P.; Wu, Y.; Huang, L.; Li, T.; Bi, J. An intervention study of the rural elderly for improving exposure, risk perception and behavioral responses under high temperature. Environ. Res. Lett. 2021, 16, 055029. [Google Scholar] [CrossRef]
- Public Health England. Heatwave Plan for England; Public Health England: London, UK. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/888668/Heatwave_plan_for_England_2020.pdf (accessed on 20 May 2021).
- Degirmenci, K.; Desouza, K.C.; Fieuw, W.; Watson, R.T.; Yigitcanlar, T. Understanding policy and technology responses in mitigating urban heat islands: A literature review and directions for future research. Sustain. Cities Soc. 2021, 70, 102873. [Google Scholar] [CrossRef]
- Jandaghian, Z.; Akbari, H. Increasing urban albedo to reduce heat-related mortality in Toronto and Montreal, Canada. Energy Build. 2021, 237, 110697. [Google Scholar] [CrossRef]
- Semeraro, T.; Scarano, A.; Buccolieri, R.; Santino, A.; Aarrevaara, E. Planning of Urban Green Spaces: An Ecological Perspective on Human Benefits. Land 2021, 10, 105. [Google Scholar] [CrossRef]
- McGregor, G.R.; Bessemoulin, P.; Ebi, K.L.; Menne, B. Heatwaves and health: Guidance on Warning-System Development: World Meteorological Organization. 2015. Available online: http://www.who.int/globalchange/publications/WMO_WHO_Heat_Health_Guidance_2015.pdf (accessed on 20 May 2021).
- Abu-Rayash, A.; Dincer, I. Development of integrated sustainability performance indicators for better management of smart cities. Sustain. Cities Soc. 2021, 67, 102704. [Google Scholar] [CrossRef]
- Shamsuzzoha, A.; Nieminen, J.; Piya, S.; Rutledge, K. Smart city for sustainable environment: A comparison of participatory strategies from Helsinki, Singapore and London. Cities 2021, 114, 103194. [Google Scholar] [CrossRef]
- Glagolev, V.A.; Zubareva, A.M.; Grigorieva, E.A. Grassfire forecast at agricultural lands in Jewish Autonomous Region. Reg. Probl. 2018, 21, 93–97. [Google Scholar] [CrossRef]
| Study | Study Area | Study Period | Weather Parameter 1 | Hot Weather Description 2 | Air Pollutant 3 | Health Indicator 4 | Exposure and Confounders 5 | Statistical Methods and Study Design 6 | Results |
|---|---|---|---|---|---|---|---|---|---|
| Analitis et al. [79] | Europe: Athens, Barcelona, Budapest, London, Milan, Munich, Paris, Rome, Valencia | Summer months, 1990–2004 | 3-h T, Td, V, P | HW: period of at least 2 days with ATmax > 90th percentile of the monthly city-specific distribution, or a period of at least 2 days with Tmin > 90th percentile of the minimum monthly distribution and ATmax > the median monthly value; intensity and duration of HW | PM10, O₃, NO₂, SO2, CO High pollution >75th percentile of the overall pollutant distribution | MRC (ICD9: 460–519) | HW—main exposure, adjusted for AP, confounders: P, V, calendar month, DOW, holiday, time trend | An interaction term between HW and each single pollutant; random effects meta-analysis for summarizing the city-specific results; time-series analysis | MRC increase during HW 54% higher on high O3 days compared with low, among people age 75–84 years; significant positive interaction of HW and PM10 for MRC in Mediterranean cities (65–74 years); HW effect on MRC is larger than on other causes of death, although the effect modification was less evident for MRC |
| Analitis et al. [5] | Europe: Athens, Barcelona, Budapest, Helsinki, London, Paris, Rome, Stockholm, Valencia | 2004–2010 | 1-h T, Td, RH, V, D, P | HW: (1) period of at least 2 days with AT > the 90th percentile of the monthly distribution; or (2) period of at least 2 days with Tmin > the 90th percentile of its respective distribution and AT > median monthly value | PM10, O₃, NO₂ High pollution >75th percentile of the city-specific pollutant distribution | MRC (ICD9: 460–519; ICD10: group J), age groups: 15–64, 65–74, >75 years and all ages | HW—main exposure, adjusted for pollutants, P, V, calendar month, day of the week, holiday, time trend | GEE modeling approach; Poisson regression for city-specific analysis; random effects meta-analysis to combine city-specific results; time-series of HW days | Synergistic effect between hot temperature and O3, PM10, and NO2 on MRC No evidence for synergy for any of the pollutants and health endpoints analyzed during heat wave days 7 |
| Anderson and Bell [110] | USA: 107 urban communities | 1987–2000 | Daily Tmean, Tmax, Tmin, Td | HW, 6 types: periods of 2 or more or 4 or more days of continuous AT more than 98.5th, 99th, or 99.5th percentile of the community’s temperature distribution | PM10 (lag 0–1), O3 | MRC (ICD9 codes 480–486, 490–497, or 507), age (<65, 65–74, ≥75 years) | T—main exposure, adjusted for pollutants; confounders: day of the week, Td; income, unemployment, education, public transportation, race, urbanicity, population | Bayesian hierarchical model used for community-specific estimates of absolute and relative heat and HW effects | Association between HW and MRC, although estimates are uncertain; somewhat higher estimates for respiratory, compared with total deaths. Heat effects slightly lowered when models included O3 and PM10 |
| Baccini et al. [53] | Europe: Athens, Barcelona, Ljubljana, Milan, Rome, Turin, Valencia (Mediterranean), Budapest, Dublin, Helsinki, London, Paris, Prague, Stockholm, Zurich (north-continental) | 1990–2000 | 3-h T, Td, V, P | High temperature: percent change in mortality associated with a 1 °C increase in ATmax above the city-specific threshold, defined as AT with the minimum mortality rate (lag 0–3) | NO2 (lag 0–1) | MRC (ICD9: 460–519), age (15–64, 65–74, 75+ years) | AT—main exposure, adjusted for AP; confounders: holidays, DOW, day of calendar month, long-term time trend, P (lag 0–1), V | GEE, combined in a Bayesian random effects meta-analysis; distributed lag models for studying the delayed effect of exposure; time-varying coefficient models are used to check the assumption of a constant heat effect | Higher associations between heat and MRC, with estimated percent changes equal to 6.7 (2.4 to 11.3) and 6.1 (2.6 to 11.1) for Mediterranean and north continental cities, respectively; heat effect particularly large in the elderly (75+): 8.1% for the Mediterranean region and of 6.6% for the north-continental region; adjustment for AP changed the MRC increase with 1 °C rise in ATmax from 6.2% to 5.5% in Athens to a negligible effect in Stockholm |
| Breitner et al. [75] | Germany: Munich, Nuremberg, Augsburg | 1990–2006 | Daily T, RH, P | High temperature: increase from the 90th (20.0 °C) to the 99th percentile (24.8 °C) of 2-day Tmean (lag 0–3) | PM10, O3 | MRC (ICD9: 460–519; ICD10: J00-J99) | T—main exposure, adjusted for AP; confounders: long-term trend/seasonality, calendar effects, DOW, influenza, RH, P | Poisson regression models combined with distributed lag non-linear models; time-series analysis | High 2 day and 15 day Tmean and consistent MRC increases; 85+ most susceptible to heat effects; some effect modification by O3 but not for PM10 |
| Byers et al. [111] | USA: Indianapolis | 2007–2011 | T, RH, P | Warm season (April–September) | PM2.5, O3, SO2 | Daily asthma-related EDV; ≥5 years old | AP—main exposure, adjusted for T; confounders: year, month, DOW, holidays; smoothing function of time | Poisson GLM to estimate the association between AP and asthma ED; RR for risk estimation, using interquartile range in single pollutant models, accounting for age group and season | O3 and SO2 increases associated with increased asthma morbidity |
| Chen and Kan [86] | China: Shanghai | January 2001–December 2004 | T, RH | High temperature: T > 85th percentile of distribution | PM10, O3, NO2, SO2 | MRC (ICD9: 460–519; ICD10: J00–J99) | AP—main exposure | GAM; time-series analysis | No significant interaction between air pollution and extremely high temperature for MRC |
| Chen et al. [87] | China: Guangzhou | January 2014–December 2017 | T, RH | High temperature: T > 75% quartile, or 4th DTL defined as 27.4–31.1 °C | CO, PM2.5, SO2, NO2, O3 | REDV | DTL—main exposure, adjusted for AP; confounders: DOW, holiday, RH | Quasi-Poisson varying coefficient regression models | Significant adverse effect on REDV of interactions between SO2 and the 4th DTL (27.4–31.1 °C) |
| Chung et al. [88] | Taiwan: Taipei, Taichung, Kaohsiung; South Korea: Seoul, Incheon, Daejeon, Daegu, Gwangju, Busan; Japan: Sapporo, Sendai, Tokyo, Nagoya, Osaka, Kitakyush | Taiwan (1994– 2007), South Korea (1992–2010), Japan (1972–2009) | Daily Tmean, Tmax, Tmin, RH, P | High temperature: T > 99th percentile; comparing the 90th and 99th percentiles of Tmean percentiles specific to each city | O3, PM10 | MRC (ICD8 460–519; ICD9 460–519; ICD10 J00–J99), age (<65, 65–75, >75 years) | T—main exposure, adjusted for AP; confounders: DOW, influenza, RH, P | Generalized Poisson semiparametric regression model; city-specific effect estimates combined to generate an overall estimate for each country using Bayesian hierarchical modeling | Heat effects greater for cities in Korea and Japan; in all countries, heat effect increases with age and is higher for cardiorespiratory mortality than for non–cardiorespiratory mortality |
| Ding et al. [89] | Taiwan: Taipei, New Taipei | 2000–2013 | Daily T, RH, P | High temperature: T > 27.9 °C | PM2.5, O3, SO2, CO, NO2 | REDV COPD-associated, including: chronic bronchitis (ICD9 491), emphysema (492), chronic, airway obstruction (496); >40 years old (40–64, 65–79, >80) | AP—main exposure, adjusted for T, RH, P | Conditional logistic regression models with ORs and 95% CIs; case-crossover study design | PM2.5 and O3 have significantly greater impact on elderly COPD-associated EDV on hot days; greatest effect in the days with T > 27.9 °C (OR = 1.037 (95% CI 1.001–1.074) |
| Guo et al. [37] | China: 345 counties (districts) | 2014–2015 | Annual and monthly T, RH | High temperature: T > 75% percentile of distribution | PM1 | Lung cancer incidence (ICD10 C33–34) | T and RH—main exposure, adjusted for AP, confounders (smoking, education, economic status, occupation, employment, urban, population size) | Multivariable linear regression model for stratified and combined datasets | Strong association between PM1 and the incidence rate of male lung cancer with high T |
| Hales et al. [115] | New Zealand: Christchurch | June 1988–December 1993 | Tmax | High temperature: Tmax > 3rd quartile (20.5 °C) | PM10 | MRC (ICD9 460–519) | AP—main exposure, adjusted for Tmax, controlling for season | GLM; interaction terms; time-series analysis | Increase of 1 °C on the day of death associated with a 3% MRC increase; increase in PM10 (lag 0–1)—4% MRC increase |
| Hanna et al. [112] | USA: 5 cities in North Carolina (Asheville, Charlotte, Greensboro, Raleigh, Wilmington) | 1996–2004 | Daily Tmax, Td, V, D, P, CC, R | Weather types: DT (hot and dry), MT (hot and humid) | O3 (lag 0–5) | HA for asthma (ICD9 493.x) | AP—main exposure, adjusted for DOW, seasonality, long-term trend, Td | GLM; time-series analysis | Increased asthma hospitalizations during episodes with high O3 levels associated with DT and MT air masses |
| Kim et al. [90] | South Korea: seven cities | 2000–2009 | Daily T, RH, P | High temperature: T > 95–99th percentile of distribution | PM10 | MRC (ICD10 J00–J99) | AP—main exposure, adjusted for T; confounding factors: seasonal variation, DOW, RH, P | GAM; stratification; time-series analysis | Strong harmful effects from PM10 with the highest temperature range (>99th percentile) in men, with a very high temperature range (95–99th percentile) in women |
| Leung et al. [91] | Hong Kong | January 2008–December 2017 | Daily T, RH, R, V | High temperature: high T and AT | PM10, NO2, SO2, O3 | 29,688 acute bronchiolitis-related HA (ICD9: 466.1 487.0, 487.1, 487.8), children ≤2 years old | T and AP—main exposure, adjusted for DOW, holiday | Quasi-Poisson GAM in conjunction with DLNMs; time-series analysis | Increased adjusted RR of acute bronchiolitis-related hospitalisation among children is associated with high T and exposure to NO2 and PM10 at different lag times |
| Li et al. [92] | China: Tianjin | 2007–2009 | Daily T, RH | High temperature: T > 20 °C | PM10, NO2, SO2 | MRC: respiratory (ICD8 J00–99), cardiopulmonary (J00–99), age groups <65, ≥65 | T—main exposure, adjusted for AP; confounders: days of calendar time, DOW, RH, holiday | Poisson GAM model natural logarithm of the expected daily death counts; time-series analysis | 10 μg/m3 increment of PM10 on T high for MRC (0.74% (95% CI: −0.33, 1.82), lag 0–1); PM10 effects on T high stronger on older (≥65 years) |
| Li et al. [93] | China: Guangzhou | 2005–2009 | Daily T, RH | High temperature: T > 95th percentile of distribution | PM10, NO2, SO2 | MRC (ICD9: J00–99) | T—main exposure, adjusted for AP; confounders: days of calendar time, DOW, RH, holiday | GAM; time-series analysis | 10 µg/m3 increase in PM10 concentrations (lag 0–1) on high temperature days for MRC (6.09% (95% CI: 2.42, 9.89)) |
| Lin et al. [94] | Taiwan: Kaohsiung | 2006–2010 | Daily T, RH, V | High temperature: T > 99th percentile of distribution | PM2.5, EC, NOx, SOx | Daily AC of cases diagnosed with respiratory distress | T—main exposure, adjusted for AP, and vice-versa; confounders: time trend, seasonality, DOW, influenza, holiday, RH, V | Quasi-Poisson function and DLNM; time-series analysis | Significant association with T, PM2.5, and concentrations of constituents |
| Ma et al. [73] | China: Jiangsu, 11 cities | 2014–2017 | Daily T, RH, R, P, S | High temperature: T > 97.5th percentile of distribution | PM10, PM2.5, SO2, NO2, CO, O3 | MRC (ICD10 J00–J99), including COPD (J41–J44) | T—main exposure, adjusted for AP | 1: DLNM combined with generalized linear model (GLM); 2: univariate random-effect meta-analysis for construction the overall cumulative exposure-response curves for all cities | Excess mortality for COPD (4.6% (95% CI: 2.83%–5.85%)) 20.58% (95% CI: 13.97%–24.96%) at cold exposure |
| Meng et al. [95] | China: Guangzhou, Hangzhou, Shanghai, Shenyang, Suzhou, Taiyuan, Tianjin, Wuhan | 2001–2008 | Daily T, RH | High temperature: T > 95th percentile of the distribution | PM10 (lag 0–1) | MRC (ICD10 J00–J98) | PM10—main exposure, adjusted for T; confounders: DOW, RH | Poisson GAM; time-series analysis | 10 μg/m3 increment in PM10 during T high: increase for MRC (1.79% (95% CI: 0.75–2.83)); higher effect for southern cities |
| Michelozzi et al. [30] | Europe: Barcelona, Budapest, Dublin, Ljubljana, London, Milan, Paris, Rome, Stockholm, Turin, Valencia, Zurich | 1990–2001 | T, Td | High temperature: ATmax > 90th percentile of the distribution | NO2 as an indicator of traffic-related pollution | REDV (ICD9 460–519); age groups: all ages, 65–74, 75+ | T—main exposure, adjusted for NO2 (lag 0–1); confounders: holidays, DOW, calendar month, P (lag 0–3), V | Semiparametric approach, which includes penalized cubic regression splines; time-series analysis | 1 °C increase in ATmax gives increase in REDV by +4.5% (95% CI: 1.9–7.3) and +3.1% (95% CI: 0.8–5.5) in the 75+ age group in Mediterranean and North-Continental cities |
| Mohr et al. [113] | USA: St. Louis | 1 June 2001–31 May 2003 | Tmax | Warm season: median seasonal T (86.5 °F = 30.3 °C) | EC, O3, SO2, NOx | EDV for asthma (ICD9 J493), children aged 2–17 years | AP—main exposure, controlling for season (T), weekend, DOW, exposure, allergens | Poisson generalized estimating equations using a 1-day lag between exposure and ED visit; time-series analysis | 0.10 μg/m3 increase in EC gives 9.45% increase in asthma ED visits among 11–17-year-olds (95% CI = 1.02, 1.17); risk increased with increasing temperatur |
| Park et al. [96] | South Korea: Seoul | June 1999–December 2007 | 1-hourly T, RH | High temperature: T ≥ 90th, 95th percentile of distribution | PM10,CO, NO2, O3, SO2 (lag 0–1) | MRC (ICD10 J00–J99) | AP—main exposure, adjusted for T; confounders: long-term and seasonal trends, DOW, holiday, RH, influenza | GLM with natural cubic splines; temperature-stratified model; time-series analysis | O3 effect stronger in summer; 85+ especially vulnerable to AP during extremely high T |
| Pascal et al. [106] | France: Bordeaux, Le Havre, Lille, Lyon, Marseille, Paris, Rouen, Strasbourg, Toulouse | 2000–2006 (August 2003 excluded due to extreme HW) | T | High temperature: T > 97.5th percentile | PM10, PM10–2.5, PM2.5, O3 | MRC (ICD10 J00–J99) | AP—main exposure, adjusted for T; confounders: long-term and seasonal trends, DOW, influenza, holiday | GAM; stratification; time-series analysis | Statistically non-significant results for MRC |
| Pattenden et al. [107] | England and Wales: 15 conurbations | May–September, 1993–2003 | Daily T, RH | High temperature: ‘Hot days’—2-day T > 95th percentile whole-year distribution | O3, PM10 | MRC (ICD9 4600–5199, ICD-10 ‘J’) | AP—main exposure, adjusted for T; confounders: DOW, holiday, seasonal and long-term time trends; time series analysis | Poisson regression GLM; time-series analysis | Independent association of heat with MRC, mean rate ratio 1.139 (95% CI: 1.079–1.202); adjusted for O3 mean interaction rate ratio 1.008 (95% CI: 0.992–1.023); high heat and O3 effect for aged <75 |
| Pinheiro et al. [116] | Brazil: Sao Paulo | 1998–2008 | Daily T, Tmax, RHmin | High temperature: T > temperature of the minimum mortality | PM10 | MRC (ICD10-X), >60 years old | T—main exposure, adjusted for AP; AP—main exposure, adjusted for T; controlled for seasonality, DOW, holidays | (1) Case-crossover approach with different types of case-control matching for isolated effects; (2) GAM, bidirectional case-crossover analysis matched by period, time-series analysis; (3) conditional logistic regression models to compare results (1) and (2) | 10 μg/m3 increase in PM10 for MRC (RR = 1.60% (95% CI: 0.74–2.46)); higher RR for MRC at high T and PM10 = 60 μg/m3 |
| Qian et al. [97] | China: Wuhan | 1 July 2000–30 June 2004 | T, RH | High temperature: T > 95th percentile distribution (31.7 °C) | PM10, SO2, NO2, O3 | MRC (ICD9 460–519; ICD10 J00–J98) | AP—main exposure, adjusted for T; confounders: DOW, time trend, RH | GAM; stratification; time series analysis | Synergistic effects of PM10 and T high: 10 μg/m3 increase in PM10 (lag 0–1) during T high gives increase in MRC 1.15% (CI: 3.54% to 6.07%) |
| Qin et al. [98] | China: Hefei | January2008–December 2014 | Daily T, RH, P | High temperature: T > 95th percentile distribution (30.25 °C) | PM10, SO2, NO2 | MRC (ICD10 J00–J98) stratified by gender, age (<75, 75–84, 85+) | AP—main exposure, adjusted for t; confounders: holiday, RH, P, age, gender, and educational levels | Quasi-Poisson regression GAM model with natural cubic splines; time series analysis | 10 μg/m3 at high temperatures was 7.18 (95% CI: 2.44 to 12.13) for PM10, 28.23 (95% CI: 8.25 to 48.61) for SO2, and 25.58 (95% CI: 3.66 to 47.99) for NO2 |
| Shaposhnikov et al. [108] | Russia: Moscow | 6 June–18 August 2010 | Daily T, RH | HW: T > 97th percentile of the year-round distribution, duration >5 days | PM10, O3 | MRC (ICD10 J00–J98) | T—main exposure, adjusted for AP; confounders: day number, DOW, RH | GLM; identity link and Gaussian errors; data for 2006–2010 used as a corresponding period; time series analysis | Added deaths due to interaction between HW and AP, elevated risks for MRC RR = 2.05 (95% CI: 1.80–2.39) |
| Song et al. [99] | China: Beijing | January 2009–December 2012 | Daily T, RH, P, V, S | High temperature: moderately hot (50th–75th), hot > 75th percentile of distribution | PM10, NO2, and SO2, API | REDV (ICD10 J00–J99) | AP—main exposure, adjusted for T | Quasi-Poisson GAM; bivariate response surface model and stratification model; time series analysis | No statistical significance of API effect on REDV on moderately hot days and hot days |
| Staffogia et al. [74] | Italian cities | 1997–2004 | Daily T, RH, P | High temperature: city-specific AT = 20–30 °C | PM10 (lag 0–1), O3 (lag 0) | MRC, in-hospital chronic pulmonary diseases (ICD9 490–505), aged 65+ | T—main exposure, adjusted for AP; confounders: DOW, RH, P, decrease in population during summer, holidays, influenza epidemics | Case-crossover approach; random-effects meta-analysis; pooled OR of dying on a day with AT = 30 °C compared to a day with AT = 20 °C | High mortality for patients with chronic pulmonary diseases OR = 2.48 (95% CI: 1.50–4.09) |
| Staffogia et al. [109] | Italian cities | 1997–2004 | Daily T, Td, P | High temperature: city-specific AT = 50th–75th percentiles and >75th percentile distributions | PM10 (lag 0–1) | MRC (ICD9 460–519), aged 35+ | AP—main exposure, adjusted for AT(lag 0–1); confounders: DOW, RH, P, decrease in population during summer, holidays, influenza | Case-crossover approach to study the association between PM10/AT and MRC; time-stratified approach to select control days | Higher PM10 effects on mortality during T high (lag 0–1 for PM10 and T): 10 μg/m3 variation in PM10: MRC, RR = 2.54% (95% CI: 1.31–3.78) |
| Tian et al. [100] | China: Beijing | January 2006–December 2009 | Daily T, RH | High temperature: T > 15.9 °C | PM10, NO2, SO2 | MRC (ICD10: J00–J99), gender, age (<65 years and ≥65 years) | AP—main exposure, adjusted for T; confounders: RH, long-term trend, DOW, holidays, influenza epidemics | GAM; T-stratified parametric model | Strong adverse effects of PM10 on MRC at T high: 0.45% (95% CI: 0.13–0.78); stronger effect for elderly |
| Vanos et al. [114] | Canada: 10 cities | 1981–1999 | 4 hourly T, Td, V, R | Weather types: DT (hot and dry), MT (hot and humid) | CO, NO2, SO2, O3 | MRC (ICD9 460–519) | AP—main exposure | GLM stratified by season and each of six distinctive synoptic weather types; time-series analysis | Combined effect of weather and AP is greatest during DT and MT air masses in summer |
| Wang et al. [41] | China: Nanjing | 2013–2016 | Daily T, RH | Warm season, AT | PM2.5, PM10, SO2, NO2, CO, O3 | REDV | AT—main exposure, adjusted for AP (lag 0–7); confounders: RH, time trends | Quasi-Poisson regression, GAM; DLNM to evaluate the cumulative and delayed effects of T on health; ER = percent increase with 95% CI in REDV per 10 μg/m3 increase in AP concentration; time-series analysis | Warm season: 10 μg/m3 increase in PM2.5 and PM10 concentration on the current day of exposure (lag 0), increase in RESD ER = 0.35% (95% CI: 0.20–0.50); more serious impact than in cold season |
| Wang et al. [101] | Taiwan: 15 cities and counties (without Taipei) | 2006–2014 | Hourly T, Tmax, Tmin, RH, V, P | High temperature: T > 99th percentile of distribution relative to 25 °C (T with the lowest AC frequency) | PM10, PM2.5 | AC for respiratory distress | T—main exposure, adjusted for AP; confounders: time trend, V, RH, DOW, holiday, pneumonia and influenza | DLNM with a quasi-Poisson function | Exposure to 99th percentile of PM2.5 and T control: significant for respiratory distress events; more likely to occur in low temperatures |
| Wang et al. [102] | China: 18 sites | 2014–2017 | Daily T, RH, P | High temperature:T during summer (June to August) | PM2.5 (lag 0–2), O3, (lag 0–2), PM10, CO, NO2, SO2 | REDV (ICD10: J00–J9), divided by gender, age–groups (<17, 18–44, 45–59, 60–74, ≥75) | T—main exposure, adjusted for AP; confounders: RH, P, DOW, long-term trend | Quasi-Poisson GAM for each study site; time series analysis | High T exposure increased REDVs risks; patients >18 more vulnerable to T high |
| Wang et al. [103] | China: Beijing | January 2016–December 2017 | Daily T, RH | Warm season: May to October | PM1, PM2.5, PM10, NO2, SO2, CO, O3 | REDV (ICD10: J00–J9), divided by gender, age–groups (<25, 25–65, ≥65) | AP—main exposure, adjusted for T; confounders: RH, DOW, long-term trend | GLMs; time series analysis | Stronger associations between PM exposure and EDVs during warm season |
| Ye et al. [104] | Japan: Tokyo | 1980–1995, July and August | Tmax, RH | High temperature:Tmax during summer | NO2, O3, SO2, CO, PM10 (lag 1–4) | REDV (ICD9: acute bronchitis 466, pneumonia 486, chronic bronchitis 491, asthma 493), gender, 65+ | AP; Tmax; confounders: annual trends, gender | GLMs; time series analysis | PM10 and NO2 are associated with daily REDV; greater for males than for females, except for angina and acute bronchitis |
| Zhang et al. [105] | China: Beijing | January 2009–December 2011 | T, RH | High temperature: T > 28 °C | PM10, PM2.5 (lag 0–1) | REDV (ICD10: J00–J99) | AP—main exposure, adjusted for T; confounders: calendar day, DOW, holidays, RH, influenza | Three GAMs, including a bivariate response surface model, a non-stratification parametric model, and a stratification parametric model | Increase in REDVs per 10 μg/m3 increase in PM2.5 and PM10 at T high (>28 °C): 0.35% for PM2.5, 0.08% for PM10; elderly (age ≥ 65) and women are more vulnerable to PM at high temperatures |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorieva, E.; Lukyanets, A. Combined Effect of Hot Weather and Outdoor Air Pollution on Respiratory Health: Literature Review. Atmosphere 2021, 12, 790. https://doi.org/10.3390/atmos12060790
Grigorieva E, Lukyanets A. Combined Effect of Hot Weather and Outdoor Air Pollution on Respiratory Health: Literature Review. Atmosphere. 2021; 12(6):790. https://doi.org/10.3390/atmos12060790
Chicago/Turabian StyleGrigorieva, Elena, and Artem Lukyanets. 2021. "Combined Effect of Hot Weather and Outdoor Air Pollution on Respiratory Health: Literature Review" Atmosphere 12, no. 6: 790. https://doi.org/10.3390/atmos12060790
APA StyleGrigorieva, E., & Lukyanets, A. (2021). Combined Effect of Hot Weather and Outdoor Air Pollution on Respiratory Health: Literature Review. Atmosphere, 12(6), 790. https://doi.org/10.3390/atmos12060790







