Methodological Aspects for the Implementation of the Air Pesticide Control and Surveillance Network (PESTNet) of the Valencian Region (Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Pesticides
- (i)
- Recommendations concerning the application of pesticides in crops of the Valencian region by the agricultural reports of the Regional Department of Agriculture of the Valencian Government [38];
- (ii)
- The frequency of pesticide residue detection in food according to the Valencian Public Health Regional Government reports [39];
- (iii)
- The most frequently used pesticide per crop in the region, as stated by the regional agri-food cooperatives [40];
- (iv)
- According to the Spanish Ministry of Agriculture, the most widely marketed plant protection products in Spain [41];
- (v)
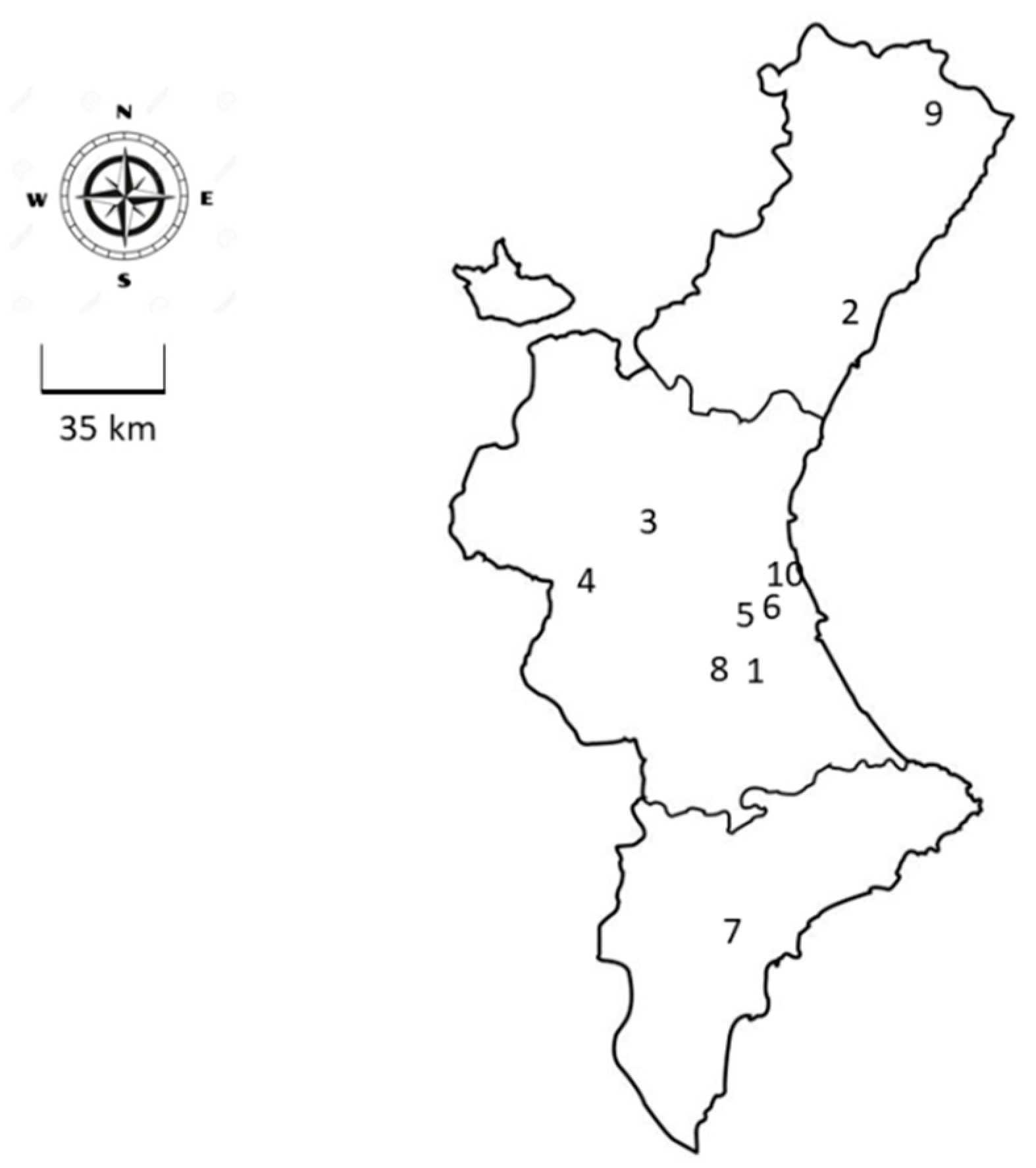
2.2. Sampling Locations for the PESTNet Network
- (i)
- The sampling sites had to be in close vicinity of the major cultivation areas in the Valencian region (citrus, stone fruit trees, persimmon, vineyards, or rice);
- (ii)
- The number of pesticides applied in these areas;
- (iii)
- The areas had to have appropriate logistic conditions such as accessible surfaces to place the samplers and connection points to the power grid;
- (iv)
- An urban site had to be included in order to assess the medium atmospheric range transport from rural areas to urban areas.
2.3. Sampling Protocols
2.4. Sample Preparation
2.5. Analysis
2.5.1. GC-MS/MS
2.5.2. LC-HRMS
2.6. Quality Assurance
2.7. Exposure and Risk Assessment
2.8. Pilot Scheme
3. Results and Discussion
3.1. Selected Pesticides to Be Evaluated
3.2. Locations Selected in the PESTnet Network
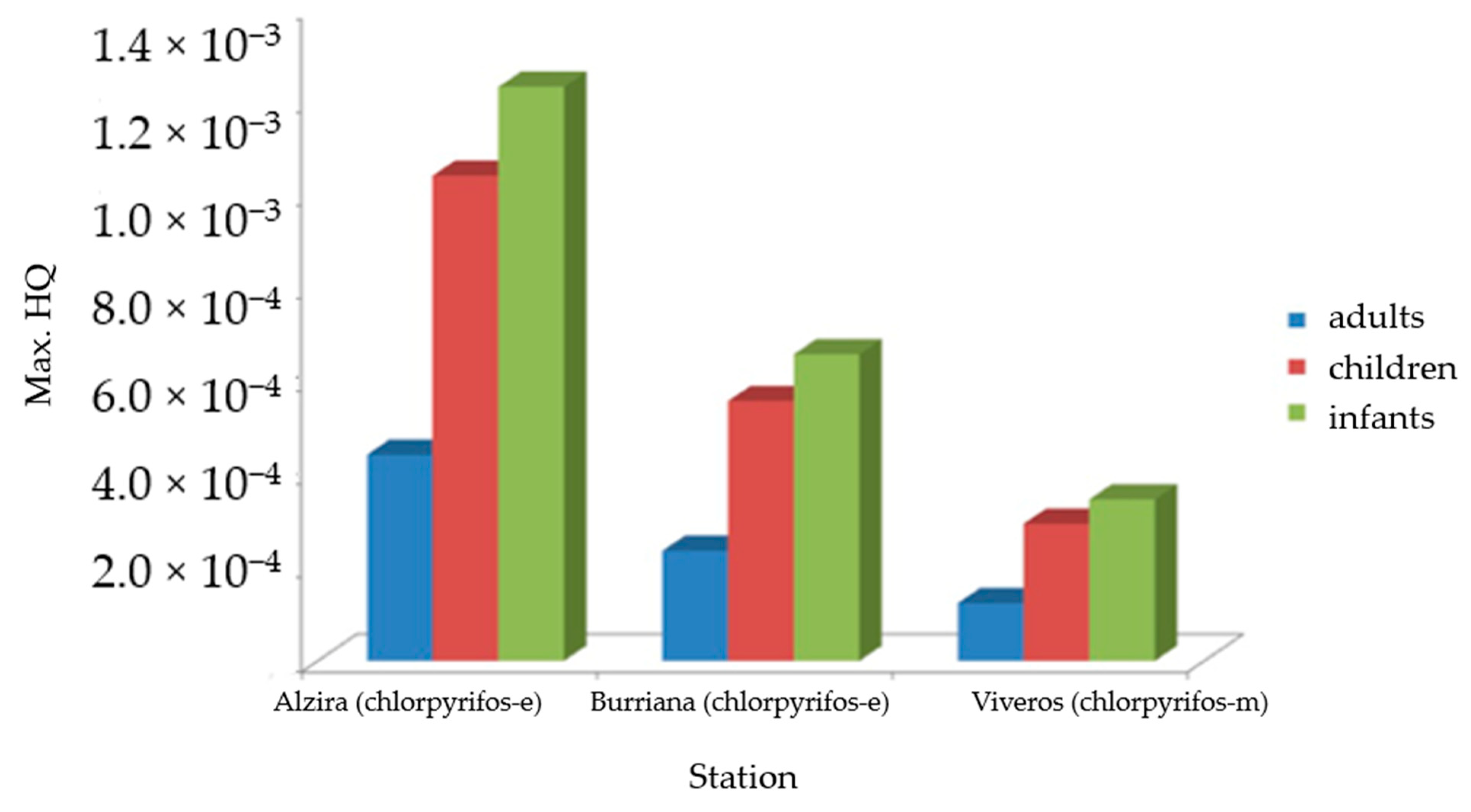
3.3. Results of the Pilot Scheme
3.4. Impact of the Pesticide Control Network
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU Pesticide Database. 2021. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN (accessed on 8 January 2021).
- Eurostat. Eurostat Statistical. 2018. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do (accessed on 8 January 2021).
- AEPLA (Asociación Empresarial Para la Protección de las Plantas). 2015. Memoria AEPLA. 2015. Available online: http://www.aepla.es/tmp/images/publicaciones/P0017_Memoria_AEPLA_2015.pdf (accessed on 1 January 2021).
- Portal Estadístico de la Generalitat Valenciana. 2021. Available online: http://www.pegv.gva.es/va/temas/agriculturaganaderiaselviculturacazapescayacuicultura/agricultura/superficiesyproduccionesanualesdecultivo (accessed on 8 January 2021).
- Van Den Berg, F.; Kubiak, R.; Benjey, W.G.; Majewski, M.S.; Yates, S.J.; Reeves, G.L.; Smelt, J.H.; van der Linden, A.M.A. Emissions of pesticides into air. Water, Air Soil Pollut. 1999, 115, 195–218. [Google Scholar] [CrossRef]
- Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E. Occurrence of pesticides in the atmosphere in France. Agronomie 2002, 22, 35–49. [Google Scholar] [CrossRef]
- Bedos, C.; Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E.; Gabrielle, B. Mass transfer of pesticides into the atmosphere by volatilization from soils and plants: Overview. Agronomie 2002, 22, 21–33. [Google Scholar] [CrossRef]
- Coscollà, C.; Yusà, V. Pesticides and agricultural air quality. In The Quality of Air; de la Guardia, M., Armenta, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–490. [Google Scholar]
- Yao, Y.; Tuduri, L.; Harner, T. Spatial and temporal distribution of pesticide air concentrations in Canadian agricultural regions. Atmos. Environ. 2006, 40, 4339–4351. [Google Scholar] [CrossRef]
- Tuduri, L.; Harner, T.; Blanchard, P.; Li, Y.F.; Poissant, L.; Waite, D.T.; Murphy, C.; Belzer, W. A review of currently used pesticides (CUPs) in Canadian air and precipitation: Part 1: Lindane and endosulfans. Atmos. Environ. 2006, 40, 1563–1578. [Google Scholar] [CrossRef]
- Scheyer, A.; Graeff, C.; Morville, S.; Mirabel, P.; Millet, M. Analysis of some organochlorine pesticides in an urban atmosphere (Strasbourg, east of France). Chemosphere 2005, 58, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Alegria, H.; Bidlemann, T.F.; Figueroa, M.S. Organochlorine pesticides in the ambient air of Chiapas, Mexico. Environ. Pollut. 2006, 140, 483–491. [Google Scholar] [CrossRef]
- Hart, E.; Coscollà, C.; Pastor, A.; Yusà, V. GC–MS characterization of contemporary pesticides in PM10 of Valencia Region, Spain. Atmos. Environ. 2012, 62, 118–129. [Google Scholar] [CrossRef]
- Coscollà, C.; Hart, E.; Pastor, A.; Yusà, V. LC-MS characterization of contemporary pesticides in PM10 of Valencia Region, Spain. Atmos. Environ. 2013, 77, 394–403. [Google Scholar] [CrossRef]
- López, A.; Coscollà, C.; Yusà, V. Selection of sampling adsorbents and optimisation and validation of a GC-MS/MS method for airborne pesticides. Int. J. Environ. Anal. Chem. 2017, 97, 949–964. [Google Scholar] [CrossRef]
- López, A.; Coscollà, C.; Yusà, V. Evaluation of sampling adsorbents and validation of a LC-HRMS method for determination of 28 airborne pesticides. Talanta 2018, 189, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Yusà, V.; Coscollà, C.; Mellouki, W.; Pastor, A.; de la Guardia, M. Sampling and analysis of pesticides in ambient air. J. Chromatogr. A 2009, 1216, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Baraud, L.; Tessier, D.; Aaron, J.J.; Quisefit, J.P.; Pinart, J. A multi-residue method for characterization and determination of atmospheric pesticides measured at two French urban and rural sampling sites. Anal. Bional. Chem. 2003, 377, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Sun, L. Trace level determination of selected organophosphorus pesticides and their degradation products in environmental air samples by liquid chromatography-positive ion electrospray tandem mass spectrometry. J. Environ. Sci. Health B 2008, 43, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.M.; Omar, D.; Mohamad, R.; Wahed, S.; Rahman, M.A. Airborne paraquat measurement and its exposure to spray operators in treated field environment. Int. J. Agric. Biol. 2010, 12, 679–684. [Google Scholar]
- Armstrong, J.L.; Dills, R.L.; Yu, J.; Yost, M.G.; Fenske, R.A. A sensitive LC-MS/MS method for measurement of organophosphorus pesticides and their oxygen analogs in air sampling matrices. J. Environ. Sci. Health B 2014, 49, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hengel, M.; Lee, P. Community air monitoring for pesticides-part 2: Multiresidue determination of pesticides in air by gas chromatography, gas chromatography-mass spectrometry, and liquid chromatography-mass spectrometry. Environ. Monit. Assess 2014, 186, 1343–1353. [Google Scholar] [CrossRef]
- Degrendele, C.; Okonski, K.; Melymuk, L.; Landlová, L.; Kukucka, P.; Audy, O.; Kohoutek, J.; Cupr, P.; Klánová, J. Pesticides in the atmosphere: A comparison of gas-particle partitioning and particle size distribution of legacy and current-use pesticides. Atmos. Chem. Phys. 2016, 16, 1531–1544. [Google Scholar] [CrossRef]
- Raina, R.; Smith, E. Determination of Azole Fungicides in Atmospheric Samples Collected in the Canadian Prairies by LC/MS/MS. J. AOAC 2012, 95, 1350–1356. [Google Scholar] [CrossRef]
- Coscollà, C.; León, N.; Pastor, A.; Yusà, V. Combined target and post-run target strategy for a comprehensive analysis of pesticides in ambient air using Liquid Chromatography—Orbitrap High Resolution Mass Spectrometry. J. Chromatogr. A 2014, 1368, 132–142. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; Ferrer, C.; Malato, O.; Agüera, A.; Fernández-Alba, A.R. Liquid chromatography-high resolution mass spectrometry for pesticide residue analysis in fruit and vegetables: Screening and quantitative studies. J. Chromatogr. A 2013, 1287, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Polgár, L.; García-Reyes, J.F.; Fodor, P.; Gyepes, A.; Dernovics, D.; Abrankó, L.; Gilbert-López, B.; Molina-Diaz, A. Retrospective screening of relevant pesticide metabolites in food using liquid chromatography high resolution mass spectrometry and accurate-mass databases of parent molecules and diagnostic fragment ions. J. Chromatogr. A 2012, 1249, 83–91. [Google Scholar] [CrossRef]
- López, A.; Coscollà, C.; Yusà, V.; Armenta, S.; de la Guardia, M.; Esteve-Turrillas, F.A. Comprehensive analysis of airborne pesticides using hard cap espresso extraction-liquid chromatography-high-resolution mass spectrometry. J. Chromatogr. A 2017, 1506, 27–36. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Yusà, V.; Millet, M.; Coscollà, C. Retrospective screening of pesticide metabolites in ambient air using liquid chromatography coupled to high-resolution mass spectrometry. Talanta 2016, 150, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; Yusà, V.; Martí, P.; Pastor, A. Analysis of currently used pesticides in fine airborne particulate matter (PM 2.5) by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1200, 100–107. [Google Scholar] [CrossRef]
- Coscollà, C.; Yusà, V.; Beser, M.I.; Pastor, A. Multi-residue analysis of 30 currently used pesticides in fine airborne particulate matter (PM 2.5) by microwave-assisted extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8817–8827. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; Castillo, M.; Pastor, A.; Yusà, V. Determination of 40 currently used pesticides in airborne particulate matter (PM 10) by microwave-assisted extraction and gas chromatography coupled to triple quadrupole mass spectrometry. Anal. Chim. Acta 2011, 693, 72–81. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, M. Multimedia transport and risk assessment of organophosphate pesticides and a case study in the northern San Joaquin Valley of California. Chemosphere 2009, 75, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Yusà, V.; Coscollà, C.; Millet, M. New screening approach for risk assessment of pesticides in ambient air. Atmos. Environ. 2014, 96, 322–330. [Google Scholar] [CrossRef]
- López, A.; Yusà, V.; Muñoz, A.; Vera, T.; Borràs, E.; Ródenas, M.; Coscollà, C. Risk assessment of airborne pesticides in a Mediterranean region of Spain. Sci. Total Environ. 2017, 574, 724–734. [Google Scholar] [CrossRef]
- Coscollà, C.; López, A.; Yahyaoui, A.; Colin, P.; Robin, C.; Poinsignon, Q.; Yusà, V. Human exposure and risk assessment to airborne pesticides in a rural French community. Sci. Total Environ. 2017, 584–585, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Fédération des Associations de Surveillance de la Qualité de l´Air de France. 2020. Available online: http://www.atmo-france.org/fr/index.php?/20171128564/pesticides-dans-l-air-les-aasqa-se-mobilisent-pour-ameliorer-les-connaissances/id-menu-305.html (accessed on 22 December 2020).
- Conselleria d’Agricultura. Available online: http://www.agroambient.gva.es/es/boletin-de-avisos (accessed on 16 June 2019).
- Conselleria de Sanitat Universal i Salut Publica, Directorate-General for Public Health. Plan de Control de la Cadena Alimentaria de la Comunitat Valenciana/Plan de Seguridad Alimentaria. 2018. Available online: http://www.sp.san.gva.es/DgspPortal/docs/PLAN_CO_CADENA_ALIMENTARIA2018.pdf (accessed on 18 June 2019).
- Cooperatives Agroalimentaries de la Comunitat Valenciana. Available online: http://www.cooperativesagroalimentariescv.com/ (accessed on 17 June 2019).
- Ministerio de Agricultura. Available online: https://www.mapa.gob.es/images/es/informe_datos_utilizacion_eupf13_tcm30-122265.pdf (accessed on 7 January 2021).
- European Commission, Directorate General Health and Consumer Protection, Brussels, Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. SANTE/12682/2019 (2019). Available online: https://www.eurlpesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 21 December 2020).
- USEPA. Exposure Factors Handbook. U.S. Environmental Protection Agency. EPA/600/P-95/002/F- a-c. 1997. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=12464 (accessed on 20 April 2021).
- WHO (World Health Organization). Principle for the Assessment of Risks to Human Health from Exposure to Chemicals-Environmental Health Criteria 210. 1999. Available online: https://apps.who.int/iris/handle/10665/42156 (accessed on 20 April 2021).
- USEPA. 1989; Risk Assessment Guidance for Superfund (RAGS) (Part A). EPA/540/1-89/002. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 20 April 2021).
- USEPA. Region 9 Preliminary Remediation Goals. Memorandum from Standford Smucker, Ph.D.; Regional Toxicologist. 2004. Available online: http://www.epa.gov/region09/waste/sfund/prg/index.html (accessed on 22 July 2019).
- USEPA. RAGS Volume I: Human Health Evaluation Manual. Supplemental Guidance “Standard Default Exposure Factors”. OSWER No. 9285.6-03. 1991. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100L3HP.PDF?Dockey=P100L3HP.PDF (accessed on 20 April 2021).
- EPA; IRIS. Environmental Protection Agency. United States. Integrated Risk Information System, IRIS. 2012. Available online: http://cfpub.epa.gov/ncea/iris/index (accessed on 22 July 2019).
- USEPA. Registration Eligibility Decision for Terbuthylazine; U.S. Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- USEPA. Tebuconazole—Pesticide Tolerance Petition 3/97; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- USEPA. EPA’s Preliminary Risk Assessment on Diazinon; U.S. Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- USEPA. Registration Eligibility Decision (RED) for Carbendazim; U.S. Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Gunier, R.; Harnly, M.; Reynolds, P.; Hertz, A.; Von Behren, J. Agricultural pesticide use in California: Pesticide prioritization, use densities, and population distributions for a Childhood Cancer Study. Environ. Health Perspect. 2001, 109, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; McLaughlin, R.; Harnly, M.; Gunier, R.; Kreutzer, R. Community exposures to airborne agricultural pesticides in California: Ranking of inhalation risks. Environ. Health Perspect. 2002, 110, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
| Pesticide | Status | Pesticide Type | Substance Group | Analysis |
|---|---|---|---|---|
| Acetamiprid | In | Insecticide | Neonicotinoid | LC |
| Aldrin | Out | Insecticide | Organochlorine | GC |
| Alpha-endosulfan | Out | Insecticide/Acaricide | Organochlorine | GC |
| Azoxystrobin | In | Fungicide | Strobilurin | LC |
| Benalaxyl-M | In | Fungicide | Phenylamide | LC |
| Bentazone | In | Herbicide | Benzothiazinone | LC |
| Beta-endosulfan | Out | Insecticide/Acaricide | Organochlorine | GC |
| Bifenthrin | Out | Insecticide/Acaricide | Pyrethroid | GC |
| Bitertanol | Out | Fungicide | Triazole | LC |
| Boscalid | In | Fungicide | Carboxamide | LC |
| Buprofezin | In | Insecticide/Acaricide | Unclassified | LC |
| Carbendazim | Out | Fungicide/Metabolite | Benzimidazole | LC |
| Carbofuran | Out | Insecticide/Nematicide/Acaricide/Metabolite | Carbamate | LC |
| Chlorpropham | Out | Herbicide/Plant Growth Regulator | Carbamate | GC |
| Chlorpyrifos-e | Out | Insecticide | Organophosphate | GC |
| Chlorpyrifos-m | Out | Insecticide/Acaricide | Organophosphate | GC |
| Cyfluthrin | Out | Insecticide | Pyrethroid | GC |
| Cymoxanil | In | Fungicide | Cyanoacetamide oxime | LC |
| Cypermethrin | In | Insecticide/Veterinary substance | Pyrethroid | GC |
| Cyproconazole | In | Fungicide | Triazole | LC |
| Cyprodinil | In | Fungicide | Anilinopyrimidine | LC |
| Deltamethrin | In | Insecticide/Metabolite/Veterinary substance | Pyrethroid | GC |
| Diazinon | Out | Insecticide/Acaricide/Repellent/Veterinary substance | Organophosphate | GC |
| Dieldrin | Out | Insecticide/Metabolite | Chlorinated hydrocarbon | GC |
| Difenoconazole | In | Fungicide | Triazole | LC |
| Dimethoate | Out | Insecticide/Acaricide/Metabolite | Organophosphate | LC |
| Diphenylamine | Out | Plant growth regulator/Fungicide/Insecticide | Amine | GC |
| Diuron | Out | Herbicide | Phenylamide | LC |
| Endosulfan-sulfate | Out | Metabolite | Unclassified | GC |
| Ethoprophos | In | Insecticide/Nematicide | Organophosphate | GC |
| Fenbuconazole | In | Fungicide | Triazole | LC |
| Fenhexamid | In | Fungicide | Hydroxyanilide | LC |
| Fenitrothion | Out | Insecticide | Organophosphate | GC |
| Fipronil | Out | Insecticide/Veterinary substance | Phenylpyrazole | GC |
| Fluazifop | Out | Metabolite | Unclassified | LC |
| Fludioxonil | In | Fungicide | Phenylpyrrole | GC |
| Fluquinconazole | In | Fungicide | Triazole | LC |
| Fluroxypyr | In | Herbicide | Pyridine compound | LC |
| Flusilazole | Out | Fungicide | Triazole | LC |
| Folpet | In | Fungicide | Phthalimide | GC |
| Imazalil | In | Fungicide/Veterinary substance | Imidazole | LC |
| Imidacloprid | Out | Insecticide/Veterinary substance | Neonicotinoid | LC |
| Iprodione | Out | Fungicide | Dicarboxamide | GC |
| Iprovalicarb | In | Fungicide | Carbamate | LC |
| Kresoxim-m | In | Fungicide/Bactericide | Strobilurin | GC |
| Lambda-cyhalothrin | In | Insecticide | Pyrethroid | GC |
| Lindane | Out | Insecticide/Acaricide/Veterinary substance | Organochlorine | GC |
| Malathion | In | Insecticide/Acaricide/Veterinary substance | Organophosphate | GC |
| Metalaxyl-M | In | Fungicide | Phenylamide | LC |
| Methidathion | Out | Insecticide/Acaricide | Organophosphate | LC |
| Molinate | Out | Herbicide | Thiocarbamate | LC |
| Myclobutanil | In | Fungicide | Triazole | LC |
| Omethoate | Out | Insecticide/Acaricide/Metabolite | Organophosphate | LC |
| Oxyfluorfen | In | Herbicide | Diphenyl ether | LC |
| Penconazole | In | Fungicide | Triazole | GC |
| Penoxsulam | In | Herbicide | Triazopyrimidine | LC |
| Permethrin | Out | Insecticide, Veterinary substance | Pyrethroid | GC |
| Pirimicarb | In | Insecticide | Carbamate | LC |
| Pirimicarb-desmethyl | In | Fungicide | Imidazole | LC |
| Prochloraz | In | Fungicide | Imidazole | LC |
| Propargite | Out | Acaricide | Sulphite ester | GC |
| Pyrimethanil | In | Fungicide | Anilinopyrimidine | LC |
| Pyriproxifen | In | Insecticide/Veterinary substance/ Insect growth regulator | Unclassified | LC |
| Pyroquilone | Out | Fungicide | Unclassified | LC |
| Quinoxyfen | Out | Fungicide | Quinoline | GC |
| Spirotetramat | In | Insecticide | Tetramic acid | LC |
| Tebuconazole | In | Fungicide/Plant growth regulator | Triazole | LC |
| Tebufenpyrad | In | Acaricide | Pyrazolium | LC |
| Terbuthylazine | In | Herbicide/Microbiocide/Algicide | Triazine | LC |
| Terbuthylazine-desethyl | In | Metabolite | Unclassified | LC |
| Terbuthylzine-2-OH | In | Metabolite | Unclassified | LC |
| Thiabendazole | In | Fungicide/Veterinary substance | Benzimidazole | LC |
| Thiamethoxam | In | Insecticide | Neonicotinoid | LC |
| Thiophanate-methyl | Out | Fungicide | Benzimidazole | LC |
| Tolclofos-methyl | In | Fungicide | Chlorophenyl | GC |
| Triadimefon | Out | Fungicide, Metabolite | Triazole | GC |
| Trifluralin | Out | Herbicide | Dinitroaniline | GC |
| Vinclozolin | Out | Fungicide | Oxazole | GC |
| Pesticide | Frequency of Detection (%) a | Frequency of Quantification (%) b | Average (pg m−3) c | Range (pg m−3) | LOQ (pg m−3) (P/G) |
|---|---|---|---|---|---|
| Acetamiprid | 83 | 6 | 18.52 | <LOQ-18.52 | 6.5/12.9 |
| Alpha-endosulfan | 11 | 11 | 74.35 | 61.98–86.72 | 10.0/16.1 |
| Azoxystrobin | 100 | 6 | 31.15 | <LOQ-31.15 | 6.5/12.9 |
| Beta-endosulfan | 6 | 6 | 91.33 | - | 10.0/16.1 |
| Boscalid | 56 | 6 | 14.35 | <LOQ-14.35 | 6.5/12.9 |
| Carbendazim | 67 | - | - | <LOQ | 6.5/12.9 |
| Chlorpropham | 6 | - | - | - | 6.6/129.3 |
| Chlorpyrifos-e | 39 | 33 | 726.58 | <LOQ-1553.58 | 6.5/129.3 |
| Chlorpyrifos-m | 100 | 100 | 1783.33 | 115.15–4372.96 | 6.5/16.1 |
| Cyproconazole | 67 | 33 | 20.88 | <LOQ-32.20 | 6.5/51.8 |
| Dimethoate | 89 | - | - | <LOQ | 6.5/12.9 |
| Diuron | 33 | - | - | <LOQ | 6.5/12.9 |
| Endosulfan-sulfate | 6 | 6 | 326.30 | 326.30 | 10.0/129.3 |
| Imidacloprid | 94 | - | - | <LOQ | 6.5/12.9 |
| Iprovalicarb | 61 | - | - | <LOQ | 6.5/12.9 |
| Kresoxim-m | 6 | 6 | 127.39 | 127.39 | 6.5/64.3 |
| Lambda-cyhalothrin | 22 | 22 | 186.42 | 80.60–492.26 | 6.5/16.1 |
| Metalaxyl-M | 100 | 28 | 27.48 | <LOQ-44.16 | 6.5/12.9 |
| Myclobutanil | 22 | - | - | <LOQ | 6.5/12.9 |
| Omethoate | 94 | 33 | 25.78 | <LOQ-37.37 | 2.6/12.9 |
| Permethrin | 28 | 28 | 147.11 | 52.75–324.00 | 6.5/16.1 |
| Prochloraz | 6 | - | - | - | 13.0/51.8 |
| Pyrimethanil | 72 | 6 | 106.47 | <LOQ-106.47 | 6.5/12.9 |
| Pyriproxifen | 61 | - | - | <LOQ | 6.5/12.9 |
| Spirotetramat | 67 | 33 | 66.90 | <LOQ-232.35 | 6.5/12.9 |
| Tebuconazole | 67 | - | - | <LOQ | 6.5/12.9 |
| Terbuthylazine-2-OH | 11 | 11 | 19.79 | 19.52–20.06 | 6.5/12.9 |
| Thiabendazole | 28 | - | - | <LOQ | 75.0/12.9 |
| Trifluralin | 61 | 61 | 216.59 | 63.60–444.32 | 6.5/16.1 |
| Vinclozolin | 11 | 11 | 33.68 | 33.18–34.19 | 6.5/16.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, A.; Ruiz, P.; Yusà, V.; Coscollà, C. Methodological Aspects for the Implementation of the Air Pesticide Control and Surveillance Network (PESTNet) of the Valencian Region (Spain). Atmosphere 2021, 12, 542. https://doi.org/10.3390/atmos12050542
López A, Ruiz P, Yusà V, Coscollà C. Methodological Aspects for the Implementation of the Air Pesticide Control and Surveillance Network (PESTNet) of the Valencian Region (Spain). Atmosphere. 2021; 12(5):542. https://doi.org/10.3390/atmos12050542
Chicago/Turabian StyleLópez, Antonio, Pablo Ruiz, Vicent Yusà, and Clara Coscollà. 2021. "Methodological Aspects for the Implementation of the Air Pesticide Control and Surveillance Network (PESTNet) of the Valencian Region (Spain)" Atmosphere 12, no. 5: 542. https://doi.org/10.3390/atmos12050542
APA StyleLópez, A., Ruiz, P., Yusà, V., & Coscollà, C. (2021). Methodological Aspects for the Implementation of the Air Pesticide Control and Surveillance Network (PESTNet) of the Valencian Region (Spain). Atmosphere, 12(5), 542. https://doi.org/10.3390/atmos12050542







