Bacterial Contamination in Health Care Centers: Differences between Urban and Rural Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. PHCC Characterization and Measurement of Environmental Parameters
2.2. Sampling and Characterization of Viable Bacteria
2.3. Electrostatic Dust Collector
2.4. Settled Dust Sampling
2.5. Antibiotic Susceptibility Testing
2.6. Detection of the Presence of Pseudomonas aeruginosa, Staphylococcus aureus and Legionella sp.
2.7. Statistical Analysis
3. Results
3.1. Environmental Parameters
3.2. Culturable Bacteriota
3.3. Compliance of Bacteriota with IAQ Legal Criteria
3.4. Electrostatic Dust Collector Extraction
3.5. Settled Dust Sampling
3.6. Characterization of Bacterial Isolates
3.7. Antibiotic Susceptibility
3.8. Detection of Targeted Bacteria Species
3.9. Statistical Information
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabo Verde, S.; Almeida, S.M.; Matos, J.; Guerreiro, D.; Meneses, M.; Faria, T.; Botelho, D.; Santos, M.; Viegas, C. Microbiological assessment of indoor air quality at different hospital sites. Res. Microbiol. 2015, 166, 557–563. [Google Scholar] [CrossRef]
- Ferdyn-Grygierek, J. Monitoring of indoor air parameters in large museum exhibition halls with and without air-conditioning systems. Build. Environ. 2016, 107, 113–126. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Aranha, L.; Carolino, E.; Gomes, A.Q.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in health care centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160. [Google Scholar] [CrossRef]
- Zahar, J.R.; Jolivet, S.; Adam, H.; Dananché, C.; Lizon, J.; Alfandari, S.; Boulestreau, H.; Baghdadi, N.; Bay, J.-O.; Bénéteau, A.-M.; et al. Quelles mesures pour maîtriser le risque infectieux chez les patientsimmunodéprimés? Recommandations formalisées d’experts. J. Mycol. Med. 2017, 27, 449–456. [Google Scholar] [CrossRef]
- Leung, M.; Chan, A. Control and management of hospital indoor air quality. Med. Sci. Monit. 2006, 12, 17–23. [Google Scholar]
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Burke, J.P. Infection control—A problem for patient safety. N. Engl. J. Med. 2003, 348, 651–656. [Google Scholar] [CrossRef]
- McFee, R.B. Nosocomial or hospital-acquired infections: An overview. Dis. Mon. 2009, 55, 422–438. [Google Scholar] [CrossRef]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Doerr, J.A. Mycotoxin interactions in poultry and swine. J. Anim. Sci. 1988, 66, 2351–2355. [Google Scholar] [CrossRef][Green Version]
- Kummer, V.; Thiel, W. Bioaerosols—Sources and control measures. Int. J. Hyg. Environ. Health 2008, 211, 299–307. [Google Scholar] [CrossRef]
- Dharmadhikari, A.S.; Mphahlele, M.; Stoltz, A.; Venter, K.; Mathebula, R.; Masotla, T.; Lubbe, W.; Pagano, M.; First, M.; Jensen, P.A.; et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: Impact on infectivity of air on a hospital ward. Am. J. Respir Crit. Care Med. 2012, 185, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Bloch, A.B.; Orenstein, W.; Ewing, W.M.; Spain, W.H.; Mallison, G.F.; Herrmann, K.L.; Hinman, A. Measles outbreak in a pediatric practice: Airborne transmission in an office setting. Pediatrics 1985, 75, 676–683. [Google Scholar]
- Leclair, J.; Zaia, J.; Levin, M.; Congdon, R.; Goldmann, D. Airborne transmission of chickenpox in a hospital. N. Engl. J. Med. 1980, 302, 450–453. [Google Scholar] [CrossRef]
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2003, 7, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.; Smith, C.; Lee Ddo, H.; Hirst, R.; Easton, A.; O’Callaghan, C. Evidence of respiratory syncytial virus spread by aerosol. Time to revisit infection control strategies? Am. J. Respir. Crit. Care Med. 2016, 194, 308–316. [Google Scholar] [CrossRef]
- Warfel, J.M.; Beren, J.; Merkel, T.J. Airborne transmission of Bordetella pertussis. J. Infect. Dis. 2012, 206, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Bonifait, L.; Charlebois, R.; Vimont, A.; Turgeon, N.; Veillette, M.; Longtin, Y.; Jean, J.; Duchaine, C. Detection and quantification of airborne norovirus during outbreaks in healthcare facilities. Clin. Infect. Dis. 2015, 61, 299–304. [Google Scholar] [CrossRef]
- Hara, S.; Yamamoto, H.; Kawabata, A.; Azuma, T.; Ishii, S.; Okumura, N.; Ito, Y. Airborne transmission from a neonate with Netherton syndrome during an outbreak of MRSA. Pediatr. Int. 2016, 58, 518–520. [Google Scholar] [CrossRef]
- Best, E.L.; Fawley, W.N.; Parnell, P.; Wilcox, M.H. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin. Infect. Dis. 2010, 50, 1450–1457. [Google Scholar] [CrossRef]
- Roberts, K.; Smith, C.F.; Snelling, A.M.; Kerr, K.G.; Banfield, K.R.; Sleigh, P.A.; Beggs, C.B. Aerial dissemination of Clostridium difficile spores. BMC Infect. Dis. 2008, 24. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Napoli, C.; Cannova, L.; Cristina, M.L.; Deriu, M.G.; Delia, S.A.; Giuliano, A.; Guida, M.; Laganà, P.; et al. La contaminazione indoor da Legionella spp.: Risultati preliminari di una indagine multicentrica italiana [Legionella spp. contamination in indoor air: Preliminary results of an Italian multicenter study]. Epidemiol. Prev. 2014, 38, 62–65. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/legionella/wmp/overview/growth-and-spread.html (accessed on 28 March 2021).
- Herwaldt, L.A.; Marra, A.R. Legionella: A reemerging pathogen. Curr. Opin. Infect. Dis. 2018, 31, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Peacock, S.; Paterson, G. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Rolo, J.; Miragaia, M.; Turlej-Rogacka, A.; Empel, J.; Bouchami, O.; Faria, N.; Tavares, A.; Hryniewicz, W.; Fluit, A.; de Lencastre, H. High genetic diversity among community-associated Staphylococcus aureus in Europe: Results from a multicenter study. PLoS ONE 2012, 7, e34768. [Google Scholar] [CrossRef]
- Klevens, R.; Morrison, M.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.; Lynfield, R.; Dumyati, G.; Townes, J.; et al. Active bacterial core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.; Ghods, S.; Rehm, B. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Russo, A.; Croxatto, A.; Calandra, T.; Guery, B. Rational approach in the management of Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2018, 31, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Veeraraghavan, B.; Elangovan, R.; Vivekanandan, P. Antibiotic resistance and epigenetics: More to it than meets the eye. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E.; Biedroń, I. Indoor air quality and potential health risk impacts of exposure to antibiotic resistant bacteria in an office rooms in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2604. [Google Scholar] [CrossRef]
- Mbareche, H.; Morawska, L.; Duchaine, C. On the interpretation of bioaerosol exposure measurements and impacts on health. J. Air Waste Manag. Assoc. 2019, 69, 789–804. [Google Scholar] [CrossRef]
- Tanaka, D.; Fujiyoshi, S.; Maruyama, F.; Goto, M.; Koyama, S.; Kanatani, J.; Isobe, J.; Watahiki, M.; Sakatoku, A.; Kagaya, S.; et al. Size resolved characteristics of urban and suburban bacterial bioaerosols in Japan as assessed by 16S rRNA amplicon sequencing. Sci. Rep. 2020, 10, 12406. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.; Robertson, S.; Gay, H.A.; Gant, T. A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health. 2018, 221, 134–173. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Dunn, R.R.; Reich, B.J.; Pacifici, K.; Laber, E.B.; Menninger, H.L.; Morton, J.M.; Henley, J.B.; Leff, J.W.; Miller, S.L.; et al. The ecology of microscopic life in household dust. Proc. Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Carter, J. Enterically infecting viruses: Pathogenecity, transmission and significance for food and water borne infections. J. Appl. Microbiol. 2005, 98, 1354–1380. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering and Medicine. Microbiomes of the Built Environment: A Research Agenda for Indoor Microbiology, Human Health, and Buildings; The National Academies Press: Washington, DC, USA, 2017; pp. 95–131. [Google Scholar]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6. [Google Scholar] [CrossRef]
- Lax, S.; Smith, D.P.; Hampton-Marcell, J.; Owens, S.M.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S.; et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048–1052. [Google Scholar] [CrossRef]
- Täubel, M.; Rintala, H.; Pitkäranta, M.; Paulin, L.; Laitinen, S.; Pekkanen, J.; Hyvärinen, A.; Nevalainen, A. The occupant as a source of house dust bacteria. J. Allergy Clin. Immunol. 2009, 124, 834–840.e47. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, S.; Cencetti, S.; Rovesti, S.; Marchesi, I.; Bargellini, A.; Borella, P. Risk factors for particulate and microbial contamination of air in operating theatres. J. Hosp. Infect. 2007, 66, 320–326. [Google Scholar] [CrossRef]
- Jung, C.; Wu, P.; Tseng, C.; Su, H. Indoor air quality varies with ventilation types and working areas in hospitals. Build. Environ. 2015, 85, 190–195. [Google Scholar] [CrossRef]
- Obbard, J.; Fang, L. Airborne concentrations of bacteria in a hospital environment in Singapore. Water Air Soil Pollut. 2003, 144, 333–341. [Google Scholar] [CrossRef]
- Qudiesat, K.; Abu-Elteen, K.; Elkarmi, A.; Hamad, M.; Abussaud, M. Assessment of airborne pathogens in healthcare settings. Afr. J. Microbiol. Res. 2009, 2, 66–76. [Google Scholar]
- Klánová, K.; Hollerová, J. Hospital indoor environment: Screening for micro-organisms and particulate matter. Indoor Built Environ. 2003, 12, 61–67. [Google Scholar] [CrossRef]
- Marchand, G.; Duchaine, C.; Lavoie, J.; Veillette, M.; Cloutier, Y. Bacteria emitted in ambient air during bronchoscopy-a risk to health care workers? Am. J. Infect. Control 2016, 44, 1634–1638. [Google Scholar] [CrossRef]
- Park, D.U.; Yeom, J.K.; Lee, W.J.; Lee, K.M. Assessment of the levels of airborne bacteria, Gram-negative bacteria, and fungi in hospital lobbies. Int. J. Environ. Res. Public Health 2013, 10, 541–555. [Google Scholar] [CrossRef]
- Tang, C.; Wan, G. Air quality monitoring of the post-operative recovery room and locations surrounding operating theaters in a medical center in Taiwan. PLoS ONE 2013, 8, e61093. [Google Scholar] [CrossRef]
- Viegas, C.; Ramos, C.; Almeida, M.; Sabino, R.; Veríssimo, C.; Rosado, L. Air fungal contamination in ten hospitals’ food units from Lisbon. WIT Trans. Ecol. Environ. 2011, 127–132. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines Global Update 2005; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2006. [Google Scholar]
- Ministério da Economia e do Emprego. Decreto-Lei No. 118/2013. Diário República 2013, 159, 4988–5005. [Google Scholar]
- Ministérios do Ambiente, Ordenamento do Território e Energia, da Saúde e da Solidariedade, Emprego e Segurança Social. Portaria No. 353-A/2013. In Diário da República; 1.a Serie—No. 235; República Portuguesa: Lisbon, Portugal, 2013. [Google Scholar]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.; Aguiar, L.; Lage, B.; Diogo Gonçalves, L.M.; Caetano, L.A.; Carolino, E.; et al. Exposure assessment in one central hospital: A multi-approach protocol to achieve an accurate risk characterization. Environ. Res. 2020, 181. [Google Scholar] [CrossRef] [PubMed]
- American National Standards Institute. ISO 18593: 2004, Microbiology of Food and Animal Feeding Stuffs, Horizontal Methods for Sampling Techniques from Surfaces Using Contact Plates and Swabs; American National Standards Institute: Washington, DC, USA, 2007. [Google Scholar]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Aspergillus spp. prevalence in different Portuguese occupational environments: What is the real scenario in high load settings? J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.; Krieg, N.; Holt, J. Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Baltimore, MD, USA, 1984. [Google Scholar]
- Viegas, C.; Monteiro, A.; Aranha Caetano, L.; Faria, T.; Carolino, E.; Viegas, S. Electrostatic Dust Cloth: A passive screening method to assess occupational exposure to organic dust in bakeries. Atmosphere 2018, 9, 64. [Google Scholar] [CrossRef]
- Viegas, C.; Santos, P.; Almeida, B.; Monteiro, A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Electrostatic dust collector: A passive screening method to assess occupational exposure to organic dust in primary health care centers. Air Qual. Atmos. Health 2019, 12, 573–583. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.C.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; et al. Settled dust assessment in clinical environment: Useful for the evaluation of a wider bioburden spectrum. Int. J. Environ. Health Res. 2019, 26, 1–19. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. Available online: http://www.eucast.org (accessed on 10 November 2020).
- European Committee for Standardization. ISO 11731: 2017-Water Quality-Enumeration of Legionella; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Yagupsky, P.; Katz, O.; Peled, N. Antibiotic susceptibility of Kingella kingae isolates from respiratory carriers and patients with invasive infections. J. Antimicrob. Chemother. 2001, 47, 191–193. [Google Scholar] [CrossRef]
- Sudharsanam, S.; Swaminathan, S.; Ramalingam, A.; Thangavel, G.; Annamalai, R.; Steinberg, R.; Balakrishnan, K.; Srikanth, P. Characterization of indoor bioaerosols from a hospital ward in a tropical setting. Afr. Health Sci. 2012, 12, 217–225. [Google Scholar] [CrossRef]
- Wan, G.H.; Chung, F.F.; Tang, C.S. Long-term surveillance of air quality in medical center operating rooms. Am. J. Infect. Control. 2011, 39, 302–308. [Google Scholar] [CrossRef]
- Baurès, E.; Blanchard, O.; Mercier, F.; Surget, E.; le Cann, P.; Rivier, A.; Gangneux, J.P.; Florentin, A. Indoor air quality in two French hospitals: Measurement of chemical and microbiological contaminants. Sci. Total Environ. 2018, 642, 168–179. [Google Scholar] [CrossRef]
- Dascalaki, E.; Lagoudi, A.; Balaras, C.; Gaglia, A. Air quality in hospital operating rooms. Build. Environ. 2008, 43, 1945–1952. [Google Scholar] [CrossRef]
- Karwowska, E.; Miaśkiewicz-Pęska, E.; Andrzejewska-Morzuch, D. Microbiological Air Contamination in Premises of the Primary Health-care. Arch. Environ. Prot. 2013, 39, 51–59. [Google Scholar] [CrossRef]
- Sobotova, L.; Noskova, T.; Volekova, J.; Aghova, L. Practical training on nosocomial infections in a hospital environment. Indoor Built Environ. 2006, 15, 73–76. [Google Scholar] [CrossRef]
- Bernasconi, C.; Rodolfi, M.; Picco, A.M.; Grisoli, P.; Dacarro, C.; Rembges, D. Pyrogenic activity of air to characterize bioaerosol exposure in public buildings: A pilot study. Lett. Appl. Microbiol. 2010, 50, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Reponen, T. Sampling for microbial determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A., Täubel, M., Sabino, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 85–96. [Google Scholar]
- Viegas, C.; Faria, T.; Monteiro, A.; Caetano, L.A.; Carolino, E.; Quintal Gomes, A.; Viegas, S. A novel multi-approach protocol for the characterization of occupational exposure to organic dust-swine production case study. Toxics 2017, 6, 5. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Dias, M.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Faria, T.; Martins, V.; Marta Almeida, S. Assessment of children’s potential exposure to bioburden in indoor environments. Atmosphere 2020, 11, 993. [Google Scholar] [CrossRef]
- Leppänen, H.; Täubel, M.; Jayaprakash, B.; Vepsäläinen, A.; Pasanen, P.; Hyvärinen, A. Quantitative assessment of microbes from samples of indoor air and dust. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Giovannangelo, M.; Nordling, E.; Bellander, T.; Gerritsen, J.; de Jongste, J.; Smit, H.; Wichmann, H.; Wickman, M.; Brunekreef, B. Bacteria and mould components in house dust and children’s allergic sensitisation. Eur. Respir. J. 2007, 29, 1144–1153. [Google Scholar] [CrossRef]
- Viegas, C.; Dias, M.; Almeida, B.; Vicente, E.; Caetano, L.A.; Carolino, E.; Alves, C. Settleable dust and bioburden in portuguese dwellings. Microorganisms 2020, 8, 1799. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, C. Airborne microbiological characteristics in public buildings of Korea. Build. Environ. 2007, 5, 2188–2196. [Google Scholar] [CrossRef]
- Azimi, F.; Naddafi, K.; Nabizadeh, R.; Hassanvand, M.S.; Alimohammadi, M.; Afhami, S.; Musavi, S.N. Fungal air quality in hospital rooms: A case study in Tehran, Iran. J. Environ. Health Sci. Eng. 2013, 11, 30. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in chemical and biological methods to identify microorganisms-from past to present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Zeeshan, M.; Hashmi, I.; Zahid, U.B.M. Microbial quality assessment of indoor air in a large hospital building during winter and spring seasons. Build. Environ. 2018, 135, 68–73. [Google Scholar] [CrossRef]
- Mirhoseini, S.; Didehdar, M.; Akbari, M.; Moradzadeh, R.; Jarishidi, R.; Torabi, S. Indoor exposure to airborne bacteria and fungi in sensitive wards of an academic pediatric hospital. Aerobiologia 2020, 36, 225–232. [Google Scholar] [CrossRef]
- Sudharsanam, S.; Srikanth, P.; Sheela, M.; Steinberg, R. Study of the indoor air quality in hospital in South Chennai, India—Microbial profile. Indoor Built Environ. 2008, 5, 435–441. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Samarghandie, M.; Ghiasian, S.; Alikhani, M.; Roshanaie, G. Evaluation of bioaerosols in five educational hospitals wards air in Hamedan, during 2011–2012. Jundishapur J. Microbiol. 2013, 6. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, Y.S.; Kim, D. Distribution characteristics of airborne bacteria and fungi in the general hospitals of Korea. Ind. Health 2010, 48, 236–243. [Google Scholar] [CrossRef]
- Würtz, E.T.; Bønløkke, J.H.; Urth, T.R.; Larsen, J.; Islam, M.Z.; Sigsgaard, T.; Schlünssen, V.; Skou, T.; Madsen, A.M.; Feld, L.; et al. No apparent transmission of livestock-associated methicillin-resistant Staphylococcus aureus CC398 in a survey of staff at a regional Danish hospital. Antimicrob. Resist. Infect. Control 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Selebonis, A.; Nena, E.; Panopoulou, M.; Kontogiorgis, C.; Bezirtzoglou, E.; Constantinidis, T. Air contamination in different departments of a tertiary hospital. Assessment of microbial load and of antimicrobial susceptibility. Biomedicines 2020, 8, 163. [Google Scholar] [CrossRef]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Mahy, B.W.J.; Meulen, V.T.; Borriello, P.S.; Murray, P.R.; Guido, F. Topley and Wilson’s Microbiology and Microbial Infections: Bacteriology-I, 10th ed.; American Society for Microbiology Press: Washington, DC, USA, 2005; pp. 185–194. [Google Scholar]
- Bonetta, S.; Bonetta, S.; Mosso, S.; Sampò, S.; Carraro, E. Assessment of microbiological indoor air quality in an Italian office building equipped with an HVAC system. Environ. Monit. Assess. 2010, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Kozajda, A.; Jeżak, K.; Kapsa, A. Airborne Staphylococcus aureus in different environments—A review. Environ. Sci. Pollut. Res. 2019, 26, 34741–34753. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Mwatondo, A.; Alimi, Y.; Varma, J.; Del Rio Vilas, V. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: A review. Int. J. Infect. Dis. 2021, 103, 469–477. [Google Scholar] [CrossRef]
- Robertson, C.E.; Baumgartner, L.K.; Harris, J.K.; Peterson, K.L.; Stevens, M.J.; Frank, D.N.; Pace, N.R. Culture-independent analysis of aerosol microbiology in a metropolitan subway system. Appl. Environ. Microbiol. 2013, 79, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Watanabe, S.; Baba, T.; Yuzawa, H.; Ito, T.; Morimoto, Y.; Kuroda, M.; Cui, L.; Takahashi, M.; Ankai, A.; et al. Whole genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J. Bacteriol. 2005, 187, 7292–7308. [Google Scholar] [CrossRef] [PubMed]
- Castle, S. Ticarcillin in Pharm: The Comprehensive Pharmacology Reference. Enna, S.J., David, B., Eds.; Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/ticarcillin (accessed on 28 February 2021).
- Nasaj, M.; Saeidi, Z.; Asghari, B.; Roshanaei, G.; Arabestani, M.R. Identification of hemolysin encoding genes and their association with antimicrobial resistance pattern among clinical isolates of coagulase-negative Staphylococci. BMC Res. Notes 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ripa, L.; Feßler, A.T.; Hanke, D.; Sanz, S.; Olarte, C.; Mama, O.M.; Eichhorn, I.; Schwarz, S.; Torres, C. Coagulase-negative staphylococci carrying cfr and PVL genes, and MRSA/MSSA-CC398 in the swine farm environment. Vet. Microbiol. 2000, 243, 108631. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.; Zanichelli, V.; Grayson, M.; Twyman, A.; Abbas, M.; Pires, D.; Allegranzi, B.; Harbarth, S. Control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in healthcare facilities: A systematic review and reanalysis of quasi-experimental studies. Clin. Infect. Dis. 2019, 68, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R. Norma No. 24/2017, de 15 de Novembro; Direção Geral da Saúde e Instituto Nacional de Saúde Dr. Ricardo Jorge. Available online: http://www.aenfermagemeasleis.pt/2017/11/16/norma-dgs-insa-prevencao-e-controlo-ambiental-da-bacteria-legionella-em-unidades-de-saude/ (accessed on 28 February 2021).
- Hambraeus, A.; Bengtsson, S.; Laurell, G. Bacterial contamination in a modern operating suite. 3. Importance of floor contamination as a source of airborne bacteria. J. Hyg. 1978, 80, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bouillard, L.; Michel, O.; Dramaix, M.; Devleeschouwer, M. Bacterial contamination of indoor air, surfaces, and settled dust, and related dust endotoxin concentrations in healthy office buildings. Ann. Agric. Environ. Med. 2005, 12, 187–192. [Google Scholar]
- Institute of Medicine (US) Committee on Damp Indoor Spaces and Health. Damp Indoor Spaces and Health; National Academies Press: Washington, DC, USA, 2004; pp. 90–115. Available online: www.ncbi.nlm.nih.gov/books/NBK215643/ (accessed on 28 February 2021). [CrossRef]
- Horak, B.; Dutkiewicz, J.; Solarz, K. Microflora and acarofauna of bed dust from homes in Upper Silesia, Poland. Ann. Allergy Asthma Immunol. 1996, 76, 41–50. [Google Scholar] [CrossRef]
- Frankel, M.; Timm, M.; Hansen, E.W.; Madsen, A.M. Comparison of sampling methods for the assessment of indoor microbial exposure. Indoor Air 2012, 22, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.E.; Sorenson, W.G.; Lewis, D.M.; Olenchock, S.A. Microbiological analyses and inflammatory effects of settled dusts from rice and hay. Biomed. Environ. Sci. 1990, 3, 353–363. [Google Scholar] [PubMed]
- Moscato, U.; Borghini, A.; Teleman, A. HVAC management in health facilities. In Indoor Air Quality in Healthcare Facilities; Capolongo, S., Settimo, G., Gola, M., Eds.; Springer: Berlin, Germany, 2017; pp. 95–106. [Google Scholar] [CrossRef]
- Eber, M.R.; Shardell, M.; Schweizer, M.L.; Laxminarayan, R.; Perencevich, E.N. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS ONE 2011, 6, e25298. [Google Scholar] [CrossRef] [PubMed]
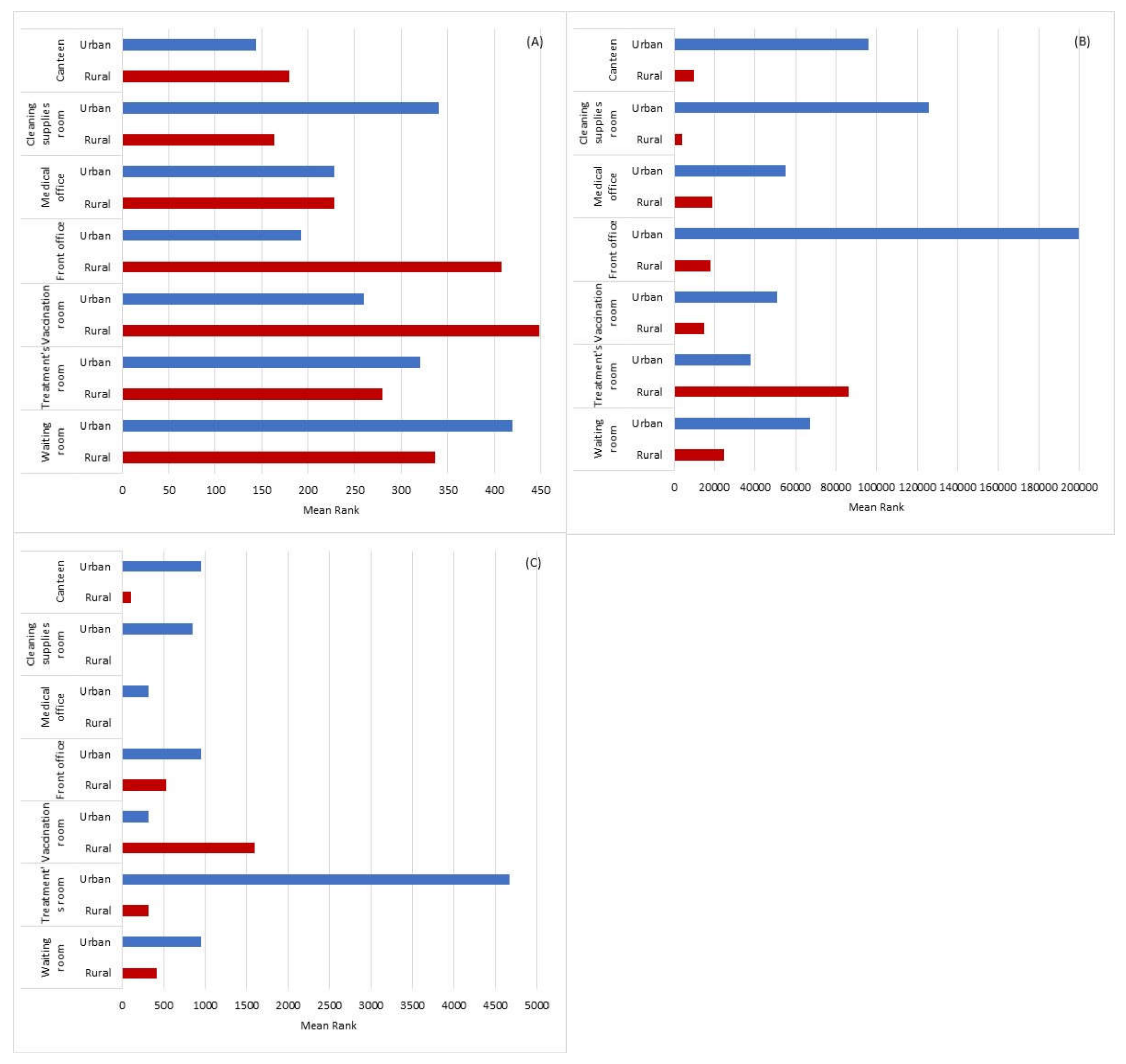
| PHCC | Air | Surface | EDC | HVAC Filters # | Settled Dust | Sampled Areas |
|---|---|---|---|---|---|---|
| urban | 7 | 7 | 7 | 1 | 1 | vaccination and treatments rooms, medical office and waiting room, front office, cleaning supplies room and canteen |
| rural | 7 | 7 | 7 | 1 | 1 |
| Environmental Parameters Measured | ||||||
|---|---|---|---|---|---|---|
| Sampled Areas | PHCC | Carbon Dioxide (ppm) | Temperature (°C) | Relative Humidity (%) | |||
| Rural | Urban | Rural | Urban | Rural | Urban | |
| Waiting room | 1130.01 | 731.89 | 21.44 | 25.65 | 62.75 | 57.03 |
| Treatments room | 1120.87 | 762.06 | 22.09 | 23.25 | 59.95 | 60.45 |
| Vaccination room | 1442.24 | 746.92 | 22.07 | 24.63 | 61.45 | 56.45 |
| Front office | 1170.16 | 658.46 | 22.63 | 24.16 | 58.45 | 59.18 |
| Medical office | 1232.73 | 787.74 | 21.85 | 25.38 | 61.16 | 56.54 |
| Cleaning supplies room | 724.06 | 844.44 | 21.57 | 25.24 | 58.38 | 59.18 |
| Canteen | 905.67 | 638.15 | 22.11 | 24.90 | 59.21 | 57.42 |
| Outdoor | 398.48 | 418.43 | 18.57 | 24.70 | 60.24 | 61.04 |
| Bacterial Phenotype/Genus | Total Rural | Total Urban |
|---|---|---|
| Brevibacterium (Gp B) | 1% | |
| Hafnia alvei | 1% | |
| Kingella kingae | 2% | |
| Kytococcus sedentarius | 7% | 22% |
| Macrococcus caseolyticus | 6% | |
| Micrococcus sp. | 21% | 32% |
| Moraxella catarrhalis | 15% | 15% |
| Neisseria gonorrhoeae | 1% | |
| Neisseria havescen | 0% | |
| Neisseria meningitidis | 2% | |
| Neisseria sicca/subflara | 2% | |
| Oligella ureolytica | 1% | |
| Oligella urethralis | 13% | |
| Providencia alcalifaciens | 3% | |
| Psychrobacter phenylpyruvicus | 1% | 2% |
| Staphylococcus aureus | 1% | |
| Staphylococcus auricularis | 1% | |
| Staphylococcus capitis subs. capitis | 5% | |
| Staphylococcus cohnii ssp cohnii | 1% | 1% |
| Staphylococcus equorum | 1% | |
| Staphylococcus haemolyticus | 1% | |
| Staphylococcus hominis hominis | 5% | |
| Staphylococcus simulans | 0.20% | |
| Staphylococcus uralyticus | 10% | |
| Staphylococcus warneri | 2% | |
| Yersinia pseudotuberculosis | 3% | |
| Others | 10% | 11% |
| Bacterial Phenotype/Genus | No. Isolates (%) | Antibiotics | |||||
|---|---|---|---|---|---|---|---|
| ATM | CTX | GEN | IPM | TIC | Observations | ||
| Gram-positive cocci | |||||||
| Staphylococcus capitis subs. capitis | 15 (2.8) | NA | S | S | S | R | Urban PHCC, waiting room |
| Staphylococcus cohnii ss cohnii | 6 (1.2) | NA | S | S | S | R | Urban PHCC, outdoor |
| 5 (0.9) | NA | S | S | S | R | Rural PHCC, medical office | |
| Staphylococcus equorum | 7 (1.3) | NA | S | S | S | R | Rural PHCC, treatment room |
| Staphylococcus haemolyticus | 4 (0.7) | NA | S | S | S | R | Rural PHCC, cleaning supplies room |
| Staphylococcus hominis hominis | 29 (5.4) | NA | S | S | S | R | Rural PHCC, canteen |
| Staphylococcus simulans | 1 (0.2) | NA | S | S | S | R | Rural PHCC, outdoor |
| Staphylococcus urealyticus | 55 (10.2) | NA | S | S | S | R | Rural PHCC, treatment room |
| Staphylococcus warneri | 9 (1.8) | NA | S | S | S | R | Urban PHCC, vaccination room |
| Gram-negative cocci | |||||||
| Kingella kingae | 50 (10.2) | NA | S | NA | NA | NA | Urban PHCC, waiting room |
| Neisseria gonorrhoeae | 5 (1.0) | R | NA | NA | NA | NA | Urban PHCC, outdoor |
| Neisseria meningitidis | 11 (2.0) | NA | S | NA | NA | NA | Rural PHCC, waiting room |
| Gram-negative rods | |||||||
| Yersinia pseudotuberculosis | 18 (3.3) | R | S | S | S | S | Rural PHCC, waiting room |
| ATM Aztreonam, CTX Cefotaxime, GEN Gentamicin, IPM Imipenem, TIC Ticarcillin | |||||||
| PHCC | Air | Surface | EDC | Indoor Environmental Conditions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Coliforms | Total Aerobic Mesophilic Bacteria | Total Coliforms | Total Aerobic Mesophilic Bacteria | Total Coliforms | Temperature (°C) | Relative Humidity (%) | Carbon Dioxide (ppm—Mean) | |||
| Air | Total Aerobic Mesophilic Bacteria | Rural | 0.082 | 0.393 | 0.408 | 0.937 ** | - | 0.214 | −0.738 * | 0.857 ** |
| Urban | 0.412 | −0.250 | −0.612 | 0.056 | 0.757 * | 0.357 | 0.072 | 0.536 | ||
| Total Coliforms | Rural | 0.612 | −0.167 | 0.000 | - | 0.204 | −0.247 | −0.204 | ||
| Urban | 0.408 | −0.167 | −0.214 | 0.764 * | 0.204 | 0.309 | 0.612 | |||
| Surface | Total Aerobic Mesophilic Bacteria | Rural | 0.000 | 0.216 | - | −0.071 | −0.821 * | 0.321 | ||
| Urban | 0.204 | 0.019 | 0.356 | 0.179 | 0.234 | −0.286 | ||||
| Total Coliforms | Rural | 0.412 | - | 0.612 | −0.408 | 0.204 | ||||
| Urban | 0.214 | −0.255 | 0.000 | 0.000 | −0.612 | |||||
| EDC | Total Aerobic Mesophilic Bacteria | Rural | - | 0.306 | −0.487 | 0.559 | ||||
| Urban | −0.047 | −0.430 | 0.774 * | −0.412 | ||||||
| Total Coliforms | Rural | - | - | - | ||||||
| Urban | 0.579 | 0.135 | 0.401 | |||||||
| Indoor Environmental Conditions | Temperature (°C) | Rural | 0.000 | 0.071 | ||||||
| Urban | −0.523 | 0.179 | ||||||||
| Relative Humidity (%) | Rural | −0.393 | ||||||||
| Urban | 0.054 | |||||||||
| Location | N | Ranks | Test Statistics | ||||
|---|---|---|---|---|---|---|---|
| Mean Rank | Sum of Ranks | Mann-Whitney U | p | ||||
| Air | Total Aerobic Mesophilic Bacteria | Rural | 7 | 7.79 | 54.50 | 22.500 | 0.798 |
| Urban | 7 | 7.21 | 50.50 | ||||
| Total | 14 | ||||||
| Total Coliforms | Rural | 7 | 7.50 | 52.50 | 24.500 | 1.000 | |
| Urban | 7 | 7.50 | 52.50 | ||||
| Total | 14 | ||||||
| Surface | Total Aerobic Mesophilic Bacteria | Rural | 7 | 4.57 | 32.00 | 4.000 | 0.009 * |
| Urban | 7 | 10.43 | 73.00 | ||||
| Total | 14 | ||||||
| Total Coliforms | Rural | 7 | 7.50 | 52.50 | 24.500 | 1.000 | |
| Urban | 7 | 7.50 | 52.50 | ||||
| Total | 14 | ||||||
| EDC | Total Aerobic Mesophilic Bacteria | Rural | 7 | 5.57 | 39.00 | 11.000 | 0.081 |
| Urban | 7 | 9.43 | 66.00 | ||||
| Total | 14 | ||||||
| Total Coliforms | Rural | 7 | 6.50 | 45.50 | 17.500 | 0.142 | |
| Urban | 7 | 8.50 | 59.50 | ||||
| Total | 14 | ||||||
| Indoor Environmental Conditions | Temperature (°C) | Rural | 7 | 4.00 | 28.00 | 0.000 | 0.002 * |
| Urban | 7 | 11.00 | 77.00 | ||||
| Total | 14 | ||||||
| Relative Humidity (%) | Rural | 7 | 7.38 | 59.00 | 23.000 | 0.343 | |
| Urban | 7 | 9.63 | 77.00 | ||||
| Total | 14 | ||||||
| Carbon Dioxide (ppm—Mean) | Rural | 7 | 10.29 | 72.00 | 5.000 | 0.013 * | |
| Urban | 7 | 4.71 | 33.00 | ||||
| 14 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, A.; Almeida, B.; Paciência, I.; Cavaleiro Rufo, J.; Ribeiro, E.; Carolino, E.; Viegas, C.; Uva, A.S.; Verde, S.C. Bacterial Contamination in Health Care Centers: Differences between Urban and Rural Settings. Atmosphere 2021, 12, 450. https://doi.org/10.3390/atmos12040450
Monteiro A, Almeida B, Paciência I, Cavaleiro Rufo J, Ribeiro E, Carolino E, Viegas C, Uva AS, Verde SC. Bacterial Contamination in Health Care Centers: Differences between Urban and Rural Settings. Atmosphere. 2021; 12(4):450. https://doi.org/10.3390/atmos12040450
Chicago/Turabian StyleMonteiro, Ana, Beatriz Almeida, Inês Paciência, João Cavaleiro Rufo, Edna Ribeiro, Elisabete Carolino, Carla Viegas, António Sousa Uva, and Sandra Cabo Verde. 2021. "Bacterial Contamination in Health Care Centers: Differences between Urban and Rural Settings" Atmosphere 12, no. 4: 450. https://doi.org/10.3390/atmos12040450
APA StyleMonteiro, A., Almeida, B., Paciência, I., Cavaleiro Rufo, J., Ribeiro, E., Carolino, E., Viegas, C., Uva, A. S., & Verde, S. C. (2021). Bacterial Contamination in Health Care Centers: Differences between Urban and Rural Settings. Atmosphere, 12(4), 450. https://doi.org/10.3390/atmos12040450











