Measuring Hydrogen in Indoor Air with a Selective Metal Oxide Semiconductor Sensor
Abstract
1. Introduction
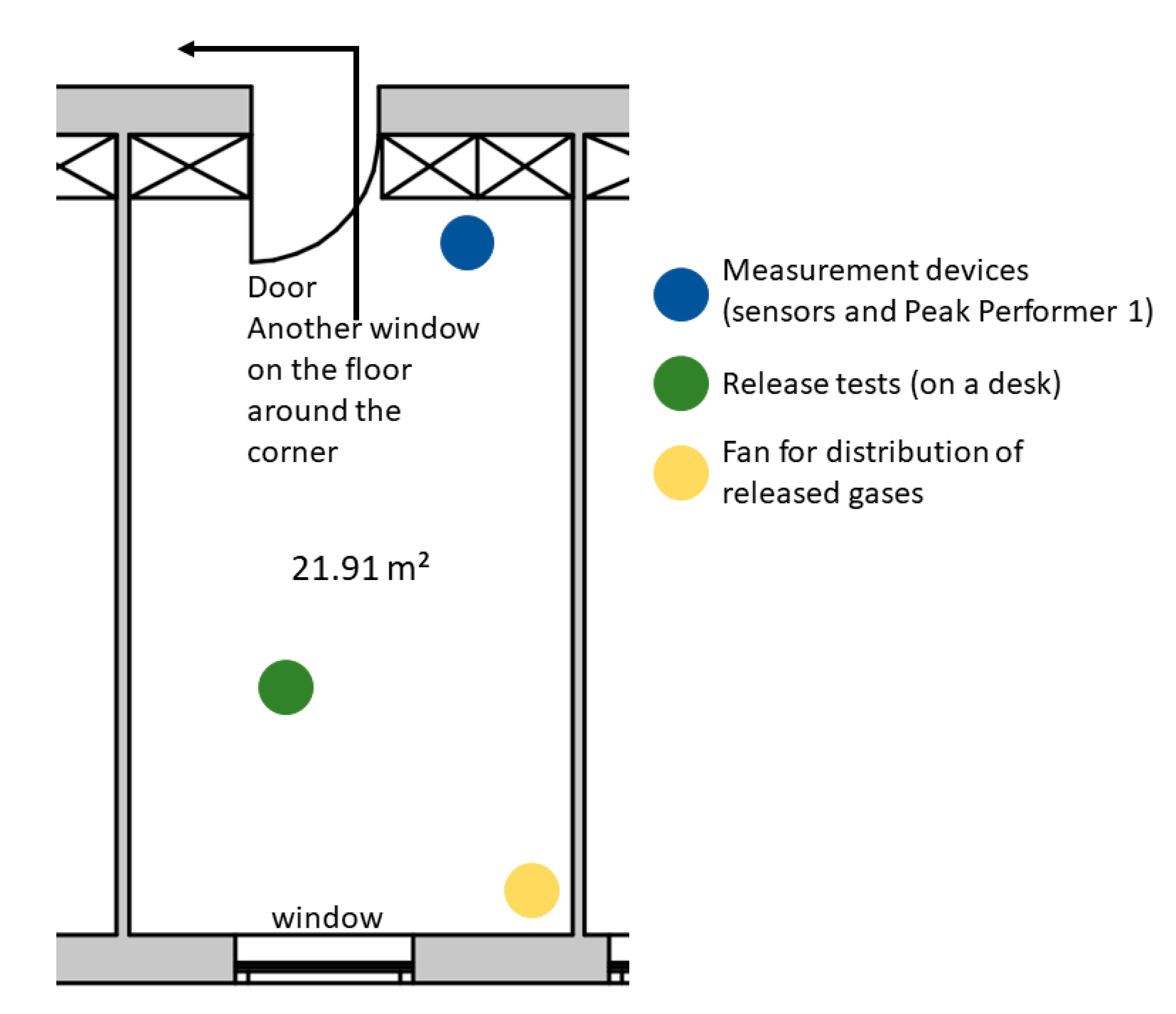
2. Experiments
3. Results
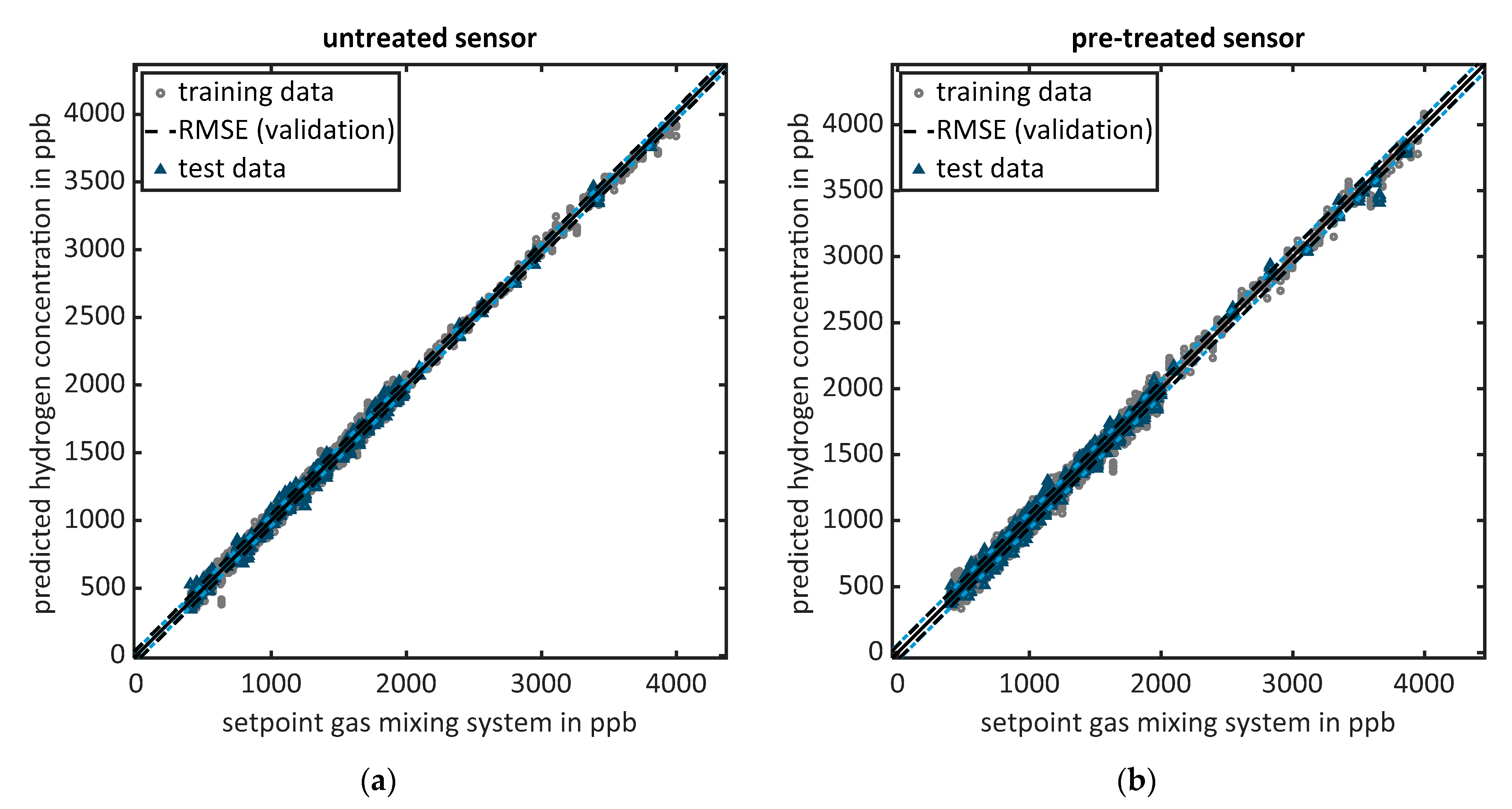
3.1. Lab Calibration
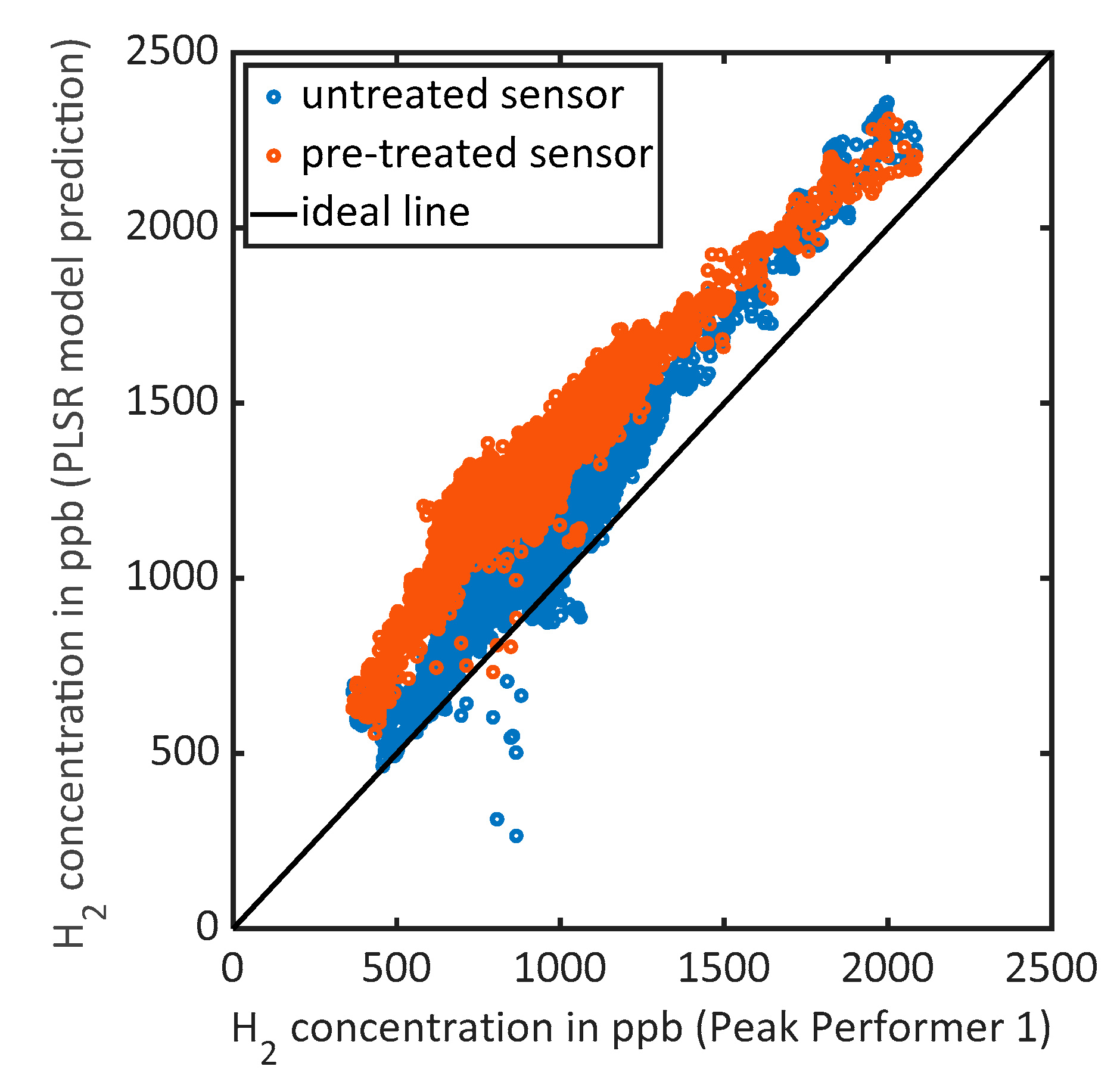
3.2. Validation in the Field
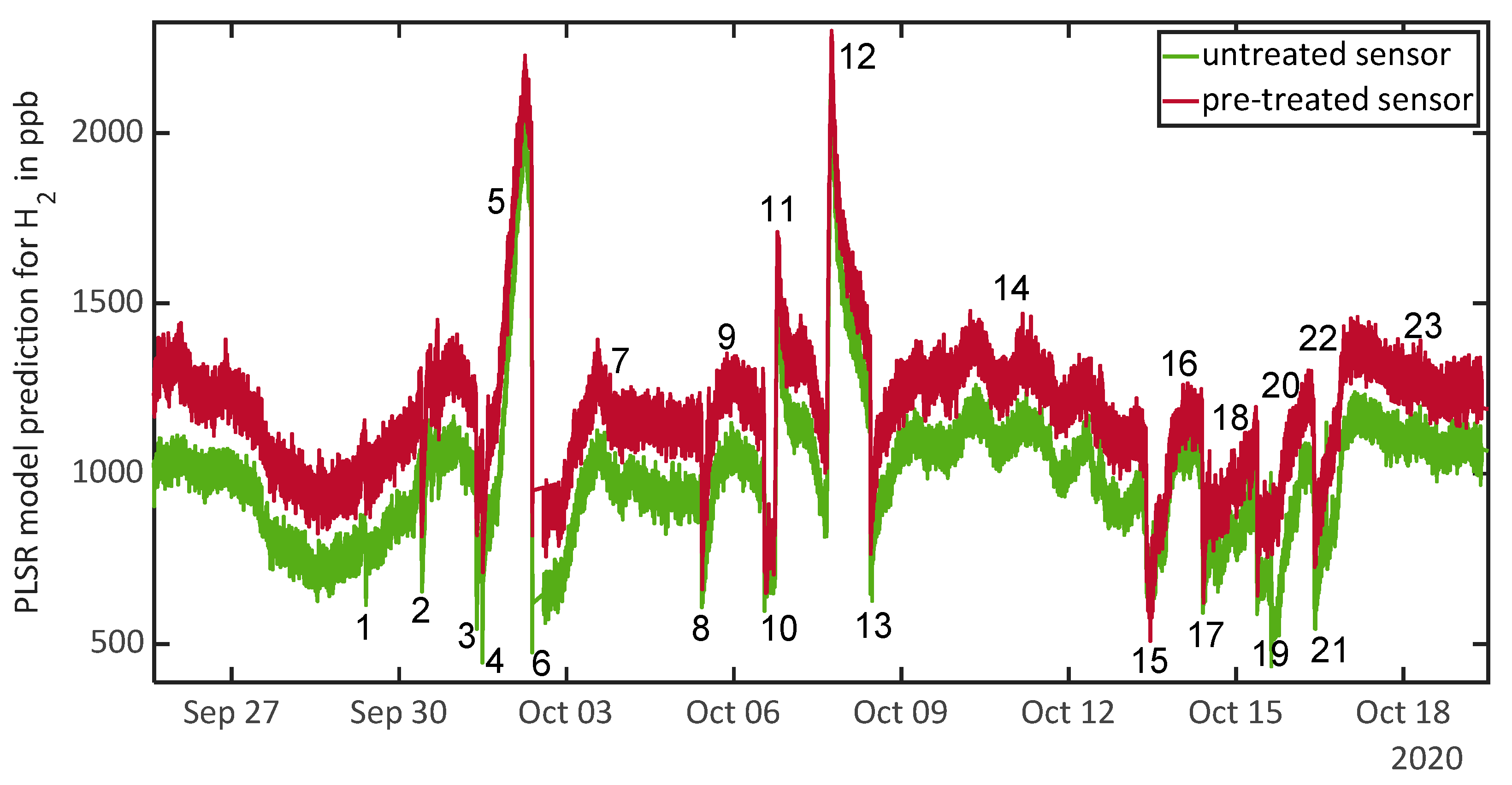
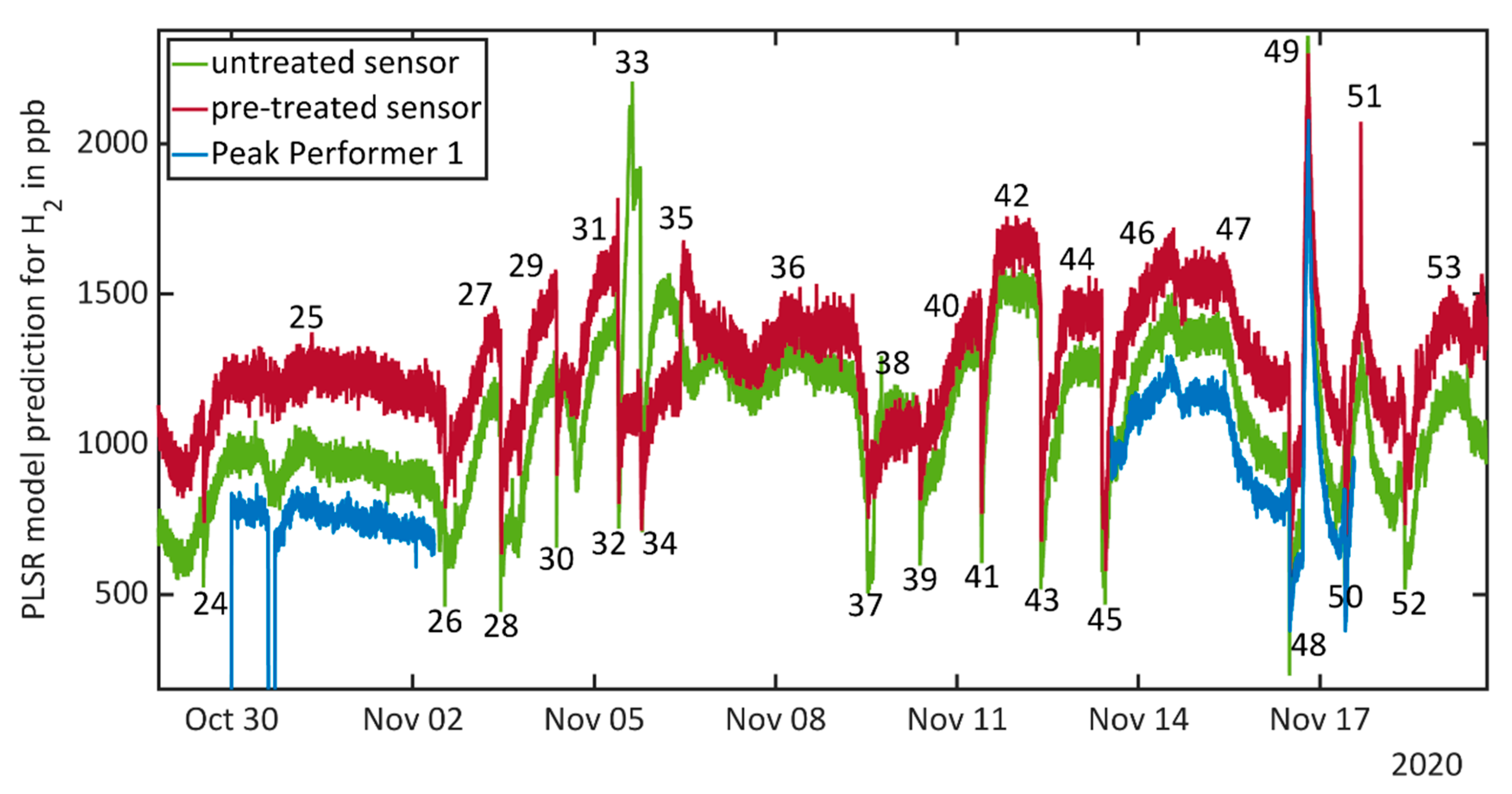
3.3. Results during Field Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number | Time | Type of event | Observation |
|---|---|---|---|
| 1 | 29 September, 09:35–09:48 | Door opened | Lower H2 concentration due to ventilation effect |
| 2 | 30 September, 09:24–09:58 | Window opened | Lower H2 concentration due to ventilation effect |
| 3 | 1 October, 09:10–09:30 | Window opened | Lower H2 concentration due to ventilation effect |
| 4 | 1 October, 11:47–12:05 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 5 | 1 October, 18:30–2 October, 06:30 | No specifiable event | Strong increase of H2 concentration over night |
| 6 | 2 October, 09:00–09:30 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 7 | 2 October, 14:00–5 October, 10:00 | Days without events and human presence | Maximum of H2 concentration at 3 October, 13:00 |
| 8 | 5 October, 10:10–10:30 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 9 | 5 October and 6 October | Several short periods of human presence | No increasing H2 concentration for short presence of one person |
| 10 | 6 October, 13:08–16:51 | Door and window opened | Lower H2 concentration due to ventilation effect |
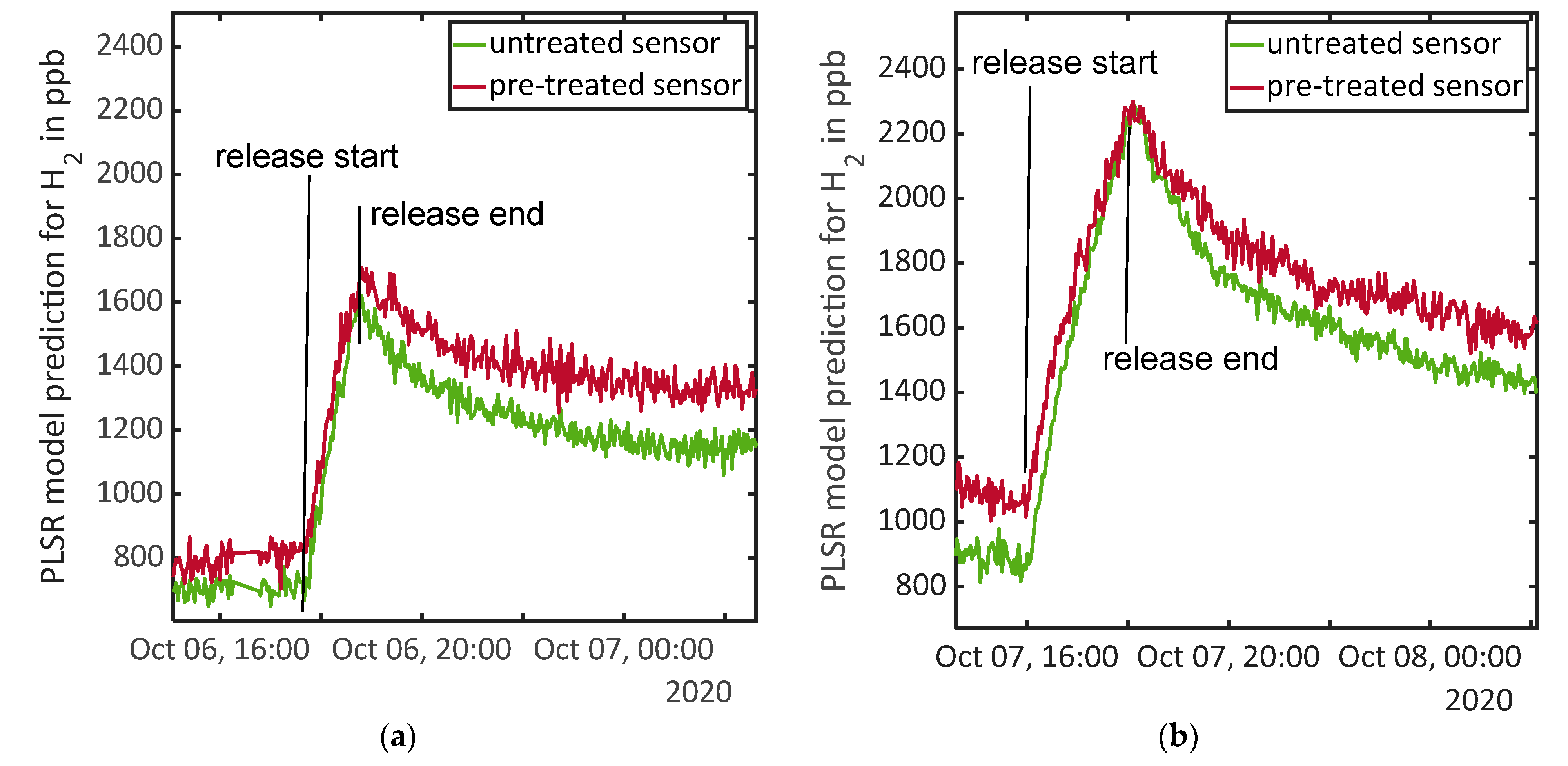
| 11 | 6 October, 17:42–18:44 | Release test: 1 ppm H2 | Compare Figure 3a |
| 12 | 7 October, 16:01–18:05 | Release test: 2 ppm H2 | Compare Figure 3b |
| 13 | 8 October, 10:46–11:00 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 14 | 8 October to 13 October | Days without events and human presence | Oscillating H2 concentration with maximums typically around 06:00–08:00 and minimums typically around 18:00–20:00 |
| 15 | 13 October, 09:25–14:00 | Door and window opened, human presence | Lower H2 concentration due to ventilation effect |
| 16 | 13 October, 15:00 | Release test: toluene | Increase of H2 concentration 3 h after release (18:00) |
| 17 | 14 October, 09:30–10:05 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 18 | 8 October to 13 October | Sporadic human presence, no specifiable events | Slow increase of H2 concentration over day and night |
| 19 | 15 October, 09:00–09:30 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 20 | 15 October, 15:00 | Release test: acetone | Untreated sensor reacts to acetone release with a short peak and displays lower concentration afterwards. Three hours after release both sensors indicate increasing H2 concentration |
| 21 | 16 October, 09:40–10:10 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 22 | 16 October, 14:50 and 18:00 | Release test: acetone and toluene | Untreated sensor reacts to release with a short peak and displays lower concentration afterwards. Five hours after first release both sensors indicate increasing H2 concentration |
| 23 | 17 October to 19 October | Days without events and human presence | Almost constant H2 concentration |
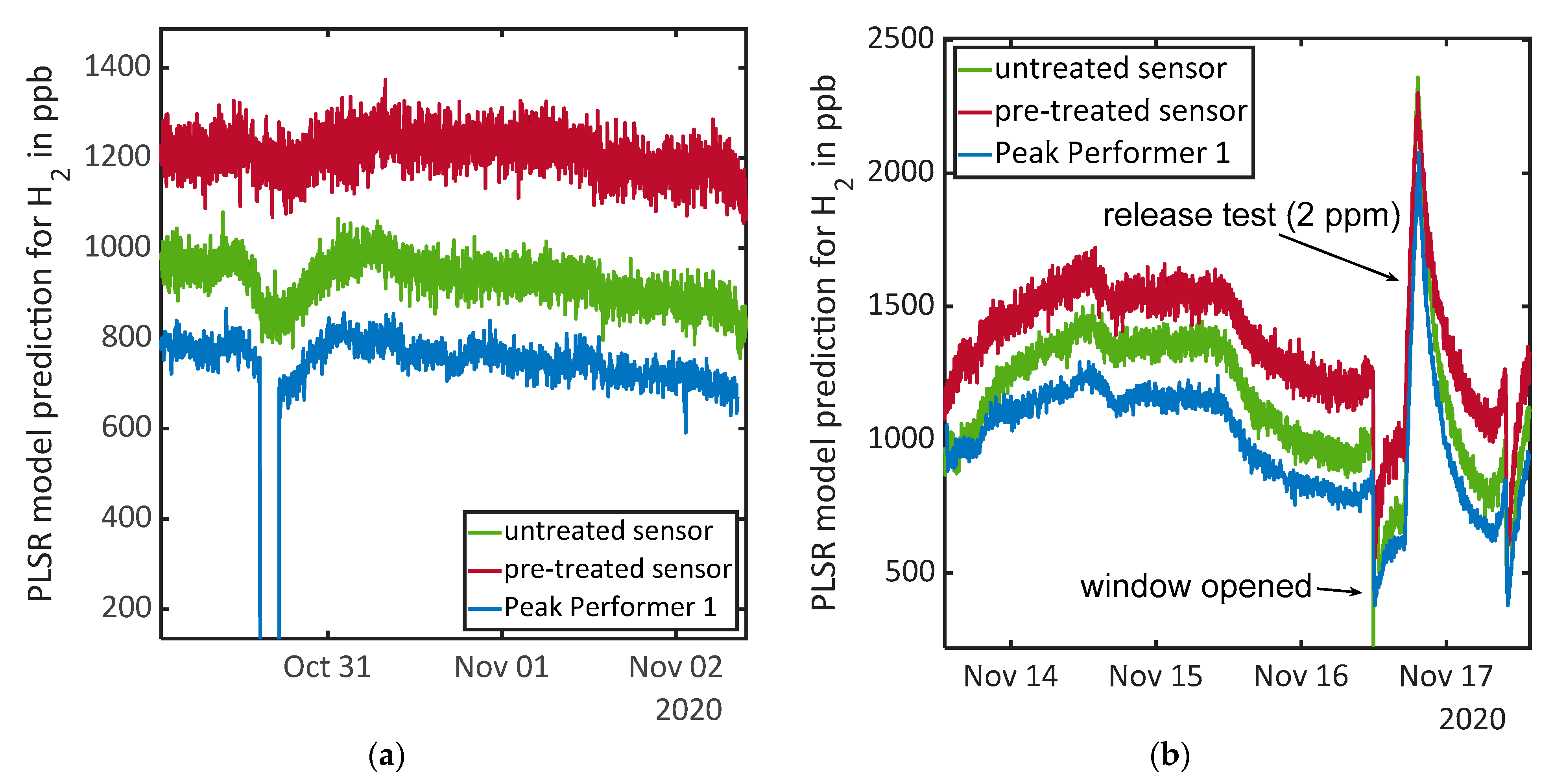
| 24 | 29 October, 12:55–13:10 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 25 | 29 October to 2 November | Days without events and human presence | Almost constant H2 concentration |
| 26 | 2 November, 12:40–12:55 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 27 | 2 November, 16:50 | Release test: toluene | Short reaction of untreated sensor upon release, constant increase of H2 concentration over day and night |
| 28 | 3 November, 10:55–11:10 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 29 | 3 November, 15:30 | Release test: acetone followed by defect of the pump, human presence during fixing | Three hours after release test increase of H2 concentration (coincides with present person for pump fixing) |
| 30 | 4 November, 09:00–09:15 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 31 | 4 November, 16:22 | Release test: acetone | Untreated sensor signal drops again upon release, increase of H2 concentration 2 h after release |
| 32 | 5 November, 09:26–09:41 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 33 | 5 November, 15:10 | Release test: acetone and toluene. Unidentified event due to construction inside the building | Very strong increase in signal of the untreated sensor long before the release tests. Release then again causes a drop in the untreated sensor’s signal, and 2.5 h after release also the pre-treated sensor signal increases slightly. |
| 34 | 5 November, 18:30–18:50 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 35 | 6 November, 10:03 | Release test: limonene | Pre-treated sensor detects limonene release immediately, untreated sensor shows increasing H2 concentration after 5–6 h, after 10 h signals go parallel again |
| 36 | 6 November to 9 November | Days without events and human presence | Almost constant H2 concentration, slight oscillation with maximum at 06:30 and minimums at 15:00 and 16:30 |
| 37 | 9 November, 12:21–13:01 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 38 | 9 November, 18:00 | Release test: ethanol | One outlier towards higher concentration for untreated sensor upon release, almost constant H2 concentration |
| 39 | 10 November, 09:10–09:25 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 40 | 10 November, 14:30 | Release test: isopopanol | Four hours after first release both sensors indicate increasing H2 concentration |
| 41 | 11 November, 09:28–09:48 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 42 | 11 November, 15:49 | Release test: xylene | Increase of H2 concentration until release test, constant concentration afterwards |
| 43 | 12 November, 09:15–09:30 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 44 | 12 November, 15:08 | Release test: toluene and xylene | Increase of the H2 concentration over the day (independent from release) and constant value after 19:00 |
| 45 | 13 November, 09:28–11:06 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 46 | 13 November, 14:30 | Release test: acetone and ethanol | Small drop in sensor signal for untreated sensor, ascending H2 concentration over day and night |
| 47 | 13 November–16 November | Days without events and human presence | Decreasing H2 concentration between 13:30 and 17:00 on first day and after 11:30 over the whole night on second day |
| 48 | 16 November, 11:55–12:20 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 49 | 16 November, 17:06–19:20 | Release test: 2 ppm H2 | Compare Figure 4b |
| 50 | 17 November, 09:54–10:24 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 51 | 17 November, 18:24 | Release test: ethanol | Maximum in H2 concentration at 16:30, outlier in pre-treated signal due to pump switching, no signal change upon release, increasing H2 concentration after 06:30 |
| 52 | 18 November, 09:36–09:56 | Door and window opened | Lower H2 concentration due to ventilation effect |
| 53 | 19 November, 12:02–16:02 | Release test: carbon monoxide (tea candle) | Pre-treated sensor signal shows slight increase of H2 concentration during burning candle |
References
- Spaul, W.A. Building-related factors to consider in indoor air quality evaluations. J. Allergy Clin. Immunol. 1994, 94, 385–389. [Google Scholar] [CrossRef]
- Settimo, G.; Manigrasso, M.; Avino, P. Indoor Air Quality: A Focus on the European Legislation and State-of-the-Art Research in Italy. Atmosphere 2020, 11, 370. [Google Scholar] [CrossRef]
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Krzyzanowski, M.; Cohen, A. Update of WHO air quality guidelines. Air Qual. Atmos. Health 2008, 1, 7–13. [Google Scholar] [CrossRef]
- Pettenkofer, M. Besprechung Allgemeiner auf die Ventilation bezüglicher Fragen; J.G. Cottaísche Buchhandlung: München, Germany, 1858. [Google Scholar]
- Szczurek, A.; Maciejewska, M.; Pietrucha, T. CO2 and Volatile Organic Compounds as indicators of IAQ. In Proceedings of the 36th AIVC Conference—Effective Ventilation in High Performance Buildings, Madrid, Spain, 23–24 September 2015; pp. 118–127. [Google Scholar]
- Rovelli, S.; Cattaneo, A.; Fazio, A.; Spinazzè, A.; Borghi, F.; Campagnolo, D.; Dossi, C.; Cavallo, D. VOCs Measurements in Residential Buildings: Quantification via Thermal Desorption and Assessment of Indoor Concentrations in a Case-Study. Atmosphere 2019, 10, 57. [Google Scholar] [CrossRef]
- Scully, R.R.; Basner, M.; Nasrini, J.; Lam, C.; Hermosillo, E.; Gur, R.C.; Moore, T.; Alexander, D.J.; Satish, U.; Ryder, V.E. Effects of acute exposures to carbon dioxide on decision making and cognition in astronaut-like subjects. NPJ Microgravity 2019, 5, 17. [Google Scholar] [CrossRef]
- Liu, Y.; Misztal, P.K.; Xiong, J.; Tian, Y.; Arata, C.; Weber, R.J.; Nazaroff, W.W.; Goldstein, A.H. Characterizing sources and emissions of volatile organic compounds in a northern California residence using space- and time-resolved measurements. Indoor Air 2019, 29, 630–644. [Google Scholar] [CrossRef]
- Leidinger, M.; Sauerwald, T.; Reimringer, W.; Ventura, G.; Schütze, A. Selective detection of hazardous VOCs for indoor air quality applications using a virtual gas sensor array. J. Sens. Sens. Syst. 2014, 3, 253–263. [Google Scholar] [CrossRef]
- Schütze, A.; Baur, T.; Leidinger, M.; Reimringer, W.; Jung, R.; Conrad, T.; Sauerwald, T. Highly Sensitive and Selective VOC Sensor Systems Based on Semiconductor Gas Sensors: How to? Environments 2017, 4, 20. [Google Scholar] [CrossRef]
- Lee, A.P.; Reedy, B.J. Temperature modulation in semiconductor gas sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Schleyer, R.; Bieber, E.; Wallasch, M. Das Luftmessnetz des Umweltbundesamtes; Umweltbundesamt: Dessau-Roßlau, Germany, 2013. [Google Scholar]
- Barnes, D.H.; Wofsy, S.C.; Fehlau, B.P.; Gottlieb, E.W.; Elkins, J.W.; Dutton, G.S.; Novelli, P.C. Hydrogen in the atmosphere: Observations above a forest canopy in a polluted environment. J. Geophys. Res. Space Phys. 2003, 108, 4197. [Google Scholar] [CrossRef]
- Ehhalt, D.H.; Rohrer, F. The tropospheric cycle of H 2: A critical review. Tellus B Chem. Phys. Meteorol. 2009, 61, 500–535. [Google Scholar] [CrossRef]
- Grant, A.; Archibald, A.T.; Cooke, M.C.; Nickless, G.; Shallcross, D.E. Modelling the oxidation of 15 VOCs to track yields of hydrogen. Atmos. Sci. Lett. 2010, 11, 265–269. [Google Scholar] [CrossRef]
- Forster, G.L.; Sturges, W.T.; Fleming, Z.L.; Bandy, B.J.; Emeis, S. A year of H 2 measurements at Weybourne Atmospheric Observatory, UK. Tellus B Chem. Phys. Meteorol. 2012, 64, 17771. [Google Scholar] [CrossRef]
- Marthinsen, D.; Fleming, S.E. Excretion of Breath and Flatus Gases by Humans Consuming High-Fiber Diets. J. Nutr. 1982, 112, 1133–1143. [Google Scholar] [CrossRef]
- Tomlin, J.; Lowis, C.; Read, N.W. Investigation of normal flatus production in healthy volunteers. Gut 1991, 32, 665–669. [Google Scholar] [CrossRef]
- Tadesse, K.; Smith, D.; Eastwood, M.A. Breath Hydrogen and Methane Excretion Patterns in Normal man and in Clinical Practices. J. Expimental Physiol. 1980, 65, 85–97. [Google Scholar] [CrossRef]
- Levitt, M.D. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969, 281, 122–127. [Google Scholar] [CrossRef]
- Sone, Y.; Tanida, S.; Matsubara, K.; Kojima, Y.; Kato, N.; Takasu, N.; Tokura, H. Everyday Breath Hydrogen Excretion Profile in Japanese Young Female Students. J. Physiol. Anthr. Appl. Hum. Sci. 2000, 19, 229–237. [Google Scholar] [CrossRef][Green Version]
- Appel, J.; Schulz, R. Hydrogen metabolism in organisms with oxygenic photosynthesis: Hydrogenases as important regulatory devices for a proper redox poising? J. Photochem. Photobiol. B Biol. 1998, 47, 1–11. [Google Scholar] [CrossRef]
- Nandi, R.; Sengupta, S. Microbial Production of Hydrogen: An Overview. Crit. Rev. Microbiol. 1998, 24, 61–84. [Google Scholar] [CrossRef]
- Rüffer, D.; Hoehne, F.; Bühler, J. New digital metal-oxide (MOx) sensor platform. Sensors 2018, 18, 1052. [Google Scholar] [CrossRef]
- Schultealbert, C.; Baur, T.; Schütze, A.; Sauerwald, T. Investigating the role of hydrogen in the calibration of MOS gas sensors for indoor air quality monitoring. In Proceedings of the Indoor Air Conference, Philadelphia, PA, USA, 22–27 July 2018. [Google Scholar]
- Meng, X.; Zhang, Q.; Zhang, S.; He, Z. The Enhanced H2 Selectivity of SnO2 Gas Sensors with the Deposited SiO2 Filters on Surface of the Sensors. Sensors 2019, 19, 2478. [Google Scholar] [CrossRef] [PubMed]
- Schultealbert, C.; Uzun, I.; Baur, T.; Sauerwald, T.; Schütze, A. Siloxane treatment of metal oxide semiconductor gas sensors in temperature-cycled operation—Sensitivity and selectivity. J. Sens. Sens. Syst. 2020, 9, 283–292. [Google Scholar] [CrossRef]
- Hyodo, T.; Baba, Y.; Wada, K.; Shimizu, Y.; Egashira, M. Hydrogen sensing properties of SnO2 varistors loaded with SiO2 by surface chemical modification with diethoxydimethylsilane. Sens. Actuators B Chem. 2000, 64, 175–181. [Google Scholar] [CrossRef]
- Tournier, G.; Pijolat, C. Selective filter for SnO-based gas sensor: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Katsuki, A.; Fukui, K. H2 selective gas sensor based on SnO2. Sens. Actuators B Chem. 1998, 52, 30–37. [Google Scholar] [CrossRef]
- Schultealbert, C.; Baur, T.; Schütze, A.; Böttcher, S.; Sauerwald, T. A novel approach towards calibrated measurement of trace gases using metal oxide semiconductor sensors. Sens. Actuators B Chem. 2017, 239, 390–396. [Google Scholar] [CrossRef]
- Schultealbert, C.; Baur, T.; Schütze, A.; Sauerwald, T. Facile Quantification and Identification Techniques for Reducing Gases over a Wide Concentration Range Using a MOS Sensor in Temperature-Cycled Operation. Sensors 2018, 18, 744. [Google Scholar] [CrossRef]
- Baur, T.; Schultealbert, C.; Schütze, A.; Sauerwald, T. Novel method for the detection of short trace gas pulses with metal oxide semiconductor gas sensors. J. Sens. Sens. Syst. 2018, 7, 411–419. [Google Scholar] [CrossRef]
- Bastuck, M.; Baur, T.; Schütze, A. DAV3E—A MATLAB toolbox for multivariate sensor data evaluation. J. Sens. Sens. Syst. 2018, 7, 489–506. [Google Scholar] [CrossRef]
- Amann, J. Möglichkeiten und Grenzen des Einsatzes von Halbleitergassensoren im temperaturzyklischen Betrieb für die Messung der Innenraumluftqualität—Kalibrierung, Feldtest, Validierung. Master’s Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2021. [Google Scholar]
- Bastuck, M. Improving the Performance of Gas Sensor Systems with Advanced Data Evaluation, Operation, and Calibration Methods; Shaker Verlag GmbH: Düren, Germany, 2019; ISBN 978-3-8440-7075-0. [Google Scholar]
- Leidinger, M.; Schultealbert, C.; Neu, J.; Schütze, A.; Sauerwald, T. Characterization and calibration of gas sensor systems at ppb level—a versatile test gas generation system. Meas. Sci. Technol. 2018, 29, 015901. [Google Scholar] [CrossRef]
- Helwig, N.; Schüler, M.; Bur, C.; Schütze, A.; Sauerwald, T. Gas mixing apparatus for automated gas sensor characterization. Meas. Sci. Technol. 2014, 25, 055903. [Google Scholar] [CrossRef]
- Baur, T.; Bastuck, M.; Schultealbert, C.; Sauerwald, T.; Schütze, A. Random gas mixtures for efficient gas sensor calibration. J. Sens. Sens. Syst. 2020, 9, 411–424. [Google Scholar] [CrossRef]
- Loh, W.-L. On Latin Hypercube Sampling. Ann. Stat. 1996, 24, 2058–2080. [Google Scholar] [CrossRef]
- Lilliefors, H.W. On the Kolmogorov-Smirnov Test for Normality with Mean and Variance Unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Martin-Luengo, M.A.; Yates, M.; Rojo, E.S.; Huerta Arribas, D.; Aguilar, D.; Ruiz Hitzky, E. Sustainable p-cymene and hydrogen from limonene. Appl. Catal. A Gen. 2010, 387, 141–146. [Google Scholar] [CrossRef]
- Fleischer, M.; Kornely, S.; Weh, T.; Frank, J.; Meixner, H. Selective gas detection with high-temperature operated metal oxides using catalytic filters. Sens. Actuators B Chem. 2000, 69, 205–210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultealbert, C.; Amann, J.; Baur, T.; Schütze, A. Measuring Hydrogen in Indoor Air with a Selective Metal Oxide Semiconductor Sensor. Atmosphere 2021, 12, 366. https://doi.org/10.3390/atmos12030366
Schultealbert C, Amann J, Baur T, Schütze A. Measuring Hydrogen in Indoor Air with a Selective Metal Oxide Semiconductor Sensor. Atmosphere. 2021; 12(3):366. https://doi.org/10.3390/atmos12030366
Chicago/Turabian StyleSchultealbert, Caroline, Johannes Amann, Tobias Baur, and Andreas Schütze. 2021. "Measuring Hydrogen in Indoor Air with a Selective Metal Oxide Semiconductor Sensor" Atmosphere 12, no. 3: 366. https://doi.org/10.3390/atmos12030366
APA StyleSchultealbert, C., Amann, J., Baur, T., & Schütze, A. (2021). Measuring Hydrogen in Indoor Air with a Selective Metal Oxide Semiconductor Sensor. Atmosphere, 12(3), 366. https://doi.org/10.3390/atmos12030366






