Effects of Rock Powder Additions to Cattle Slurry on Ammonia and Greenhouse Gas Emissions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Slurry Collection, Treatments, and Site
2.2. Emission Measurement Design
2.3. Data Analysis
3. Results
3.1. Gaseous Emissions
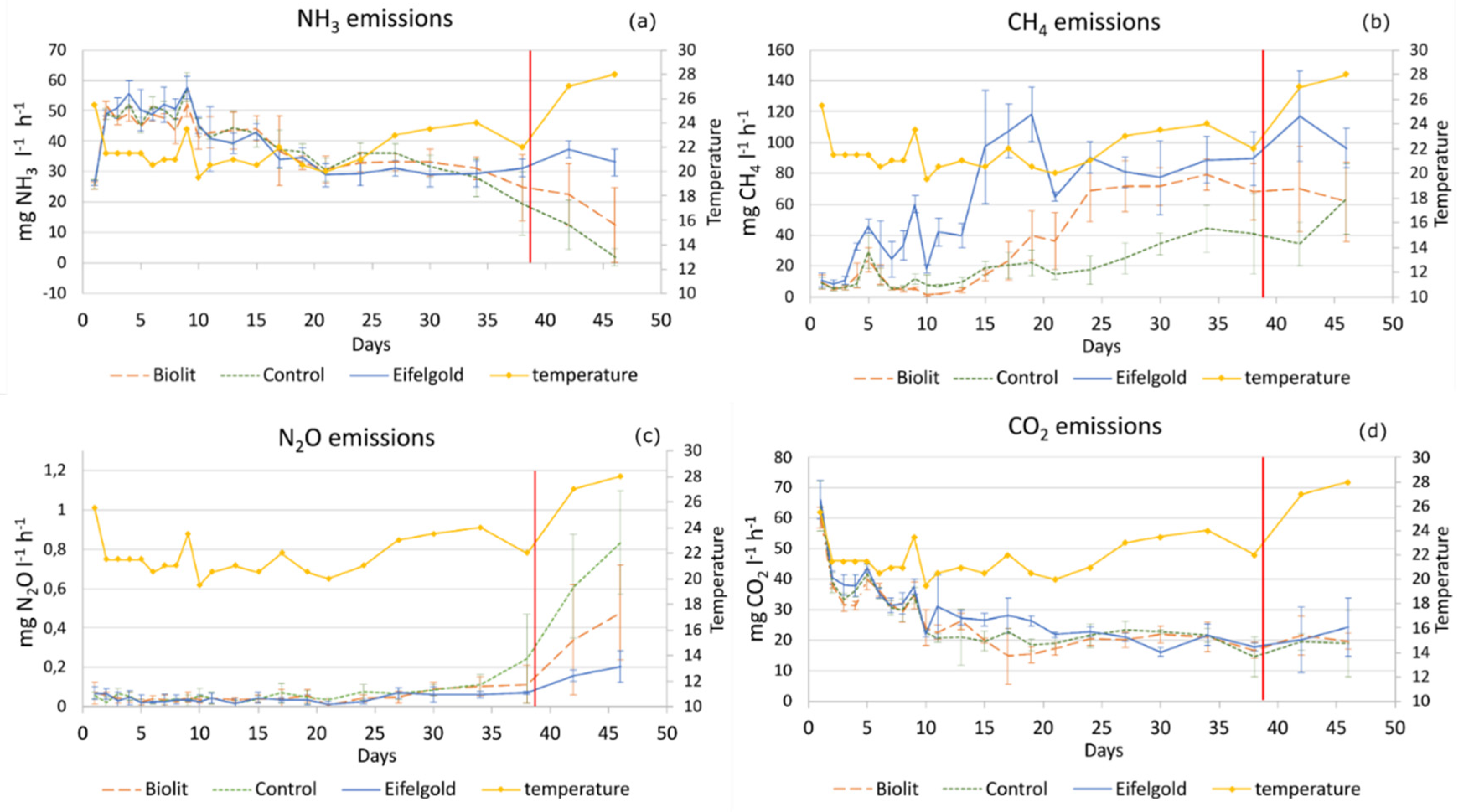
3.1.1. NH3 Emissions
3.1.2. CH4 Emissions
3.1.3. N2O Emissions
3.1.4. CO2 Emissions
3.2. Total Greenhouse Gas Emissions
3.3. Slurry Physicochemical Properties
4. Discussion
4.1. NH3 Emissions
4.2. CH4, CO2, and N2O Emissions
4.3. Physicochemical Properties
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- European Commission, DG Climate Action; European Environment Agency. Annual European Union Greenhouse Gas Inventory 1990–2017 and Inventory Report 2019: Submission under the United Nations Framework Convention on Climate Change and the Kyoto Protocol; European Environment Agency: København, Copenhagen, 2019. Available online: https://www.eea.europa.eu/publications/european-union-greenhouse-gas-inventory-2019/at_download/file (accessed on 18 August 2021).
- Tista, M.; Gager, M.; Gaisbauer, S.; Ullrich, B. European Union Emission Inventory Report 1990–2017 under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP); Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-9480-078-7. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, D.M.; Taylor, M.; Bindi, S.; Brown, I.; Camilloni, A.; Diedhiou, R.; Djalante, K.L.; Ebi, F. Impacts of 1.5 °C Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Thomas, F., Ed.; Stocker, Working Group I Co-Chair, University of Bern [and nine others]; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Schneidemesser Ev Kutzner, R.; Münster, A.; Staudt, E.; Saar, D. Landwirtschaft, Ammoniak und Luftverschmutzung; IASS Fact Sheet: Berlin, Germany, 2016. [Google Scholar]
- Sonneveld, M.P.W.; Schröder, J.J.; Vos JA de Monteny, G.J.; Mosquera, J.; Hol, J.M.G.; Lantinga, E.A.; Verhoeven, F.P.M.; Bouma, J. A whole-farm strategy to reduce environmental impacts of nitrogen. J. Environ. Q. 2008, 37, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Paulot, F.; Jacob, D.J.; Pinder, R.W.; Bash, J.O.; Travis, K.; Henze, D.K. Ammonia emissions in the United States, European Union, and China derived by high-resolution inversion of ammonium wet deposition data: Interpretation with a new agricultural emissions inventory (MASAGE_NH3). J. Geophys. Res. Atmos. 2014, 119, 4343–4364. [Google Scholar] [CrossRef]
- Clemens, J.; Bergmann, S.; Vandré, R. Reduced ammonia emissions from slurry after self-acidification with organic supplements. Environ. Technol. 2002, 23, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R.E. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Kistner-Othmer, M. Einstreu von Steinmehl in Hühnerställe zur Verbesserung des Hofdüngers und der Stallluft. Ph.D. Thesis, Gesamthochschule Kassel, Kassel, Germany, 1989. [Google Scholar]
- Snoek, H.; Wülfrath, H. Das Buch vom Steinmehl: Entstehung, Verwendung und Bedeutung im Land- und Gartenbau; Orac: Wien, Austria, 1983; p. 144. [Google Scholar]
- Shah, G.A.; Shah, G.M.; Rashid, M.I.; Groot, J.C.J.; Traore, B.; Lantinga, E.A. Bedding additives reduce ammonia emission and improve crop N uptake after soil application of solid cattle manure. J. Environ. Manag. 2018, 209, 195–204. [Google Scholar] [CrossRef]
- Shah, G.M.; Shah, G.A.; Groot, J.C.J.; Oenema, O.; Lantinga, E.A. Irrigation and lava meal use reduce ammonia emission and improve N utilization when solid cattle manure is applied to grassland. Agric. Ecosyst. Environ. 2012, 160, 59–65. [Google Scholar] [CrossRef]
- Witter, E.; Kirchmann, H. Peat, zeolite and basalt as adsorbents of ammoniacal nitrogen during manure decomposition. Plant Soil 1989, 115, 43–52. [Google Scholar] [CrossRef]
- Zaied, H. Untersuchungen zum Einfluß verschiedener Zusätze auf den Rotteverlauf, die Stickstoffdynamik und die Kompostqualität bei der Kompostierung von Reststoffen aus der Legehennenhaltung; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1999. [Google Scholar]
- Sajeev, E.P.; Winiwarter, W.; Amon, B. Greenhouse gas and ammonia emissions from different stages of liquid manure management chains: Abatement options and emission interactions. J. Environ. Q. 2018, 47, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, D.A.C.; Theodoro, S.H. Enabling food security through use of local rocks and minerals. Extr. Ind. Soc. 2020, 7, 480–487. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2021, 150976. in press, corrected proof. [Google Scholar] [CrossRef]
- Fyfe, W.S.; Leonardos, O.H.; Theodoro, S.H. Sustainable farming with native rocks: The transition without revolution. Ann. Braz. Acad. Sci. 2006, 78, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoro, S.H.; Leonardos, O.H. The use of rocks to improve family agriculture in Brazil. An. Da Acad. Bras. De Ciências 2006, 78, 721–730. [Google Scholar] [CrossRef]
- Van Straaten, P. Agrogeology: The Use of Rocks for Crops; Enviroquest Ltd.: Cambridge, ON, Canada, 2007; p. 440. [Google Scholar]
- Jones, D.L.; Cross, P.; Withers, P.J.A.; DeLuca, T.H.; Robinson, D.A.; Quilliam, R.S.; Harris, I.M.; Chadwick, D.R.; Edwards-Jones, G. REVIEW: Nutrient stripping: The global disparity between food security and soil nutrient stocks. J. Appl. Ecol. 2013, 50, 851–862. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Silva Oliveira, M.L.; Silva Oliveira, L.F.; Homrich Schneider, I.A.; Kautzmann, R.M. Application of andesite rock as a clean source of fertilizer for eucalyptus crop: Evidence of sustainability. J. Cleaner Prod. 2020, 256, 120432. [Google Scholar] [CrossRef]
- Leonardos, O.H.; Theodoro, S.H.; Assad, M.L. Remineralization for sustainable agriculture: A tropical perspective from a Brazilian viewpoint. Nutr. Cycl. Agroecosyst. 2000, 56, 3–9. [Google Scholar] [CrossRef]
- Abbott, L.K.; Manning, D.A.C. Soil health and related ecosystem services in organic agriculture. SAR 2015, 4, 116. [Google Scholar] [CrossRef]
- Renforth, P.; Washbourne, C.-L.; Taylder, J.; Manning, D.A.C. Silicate production and availability for mineral carbonation. Environ. Sci. Technol. 2011, 45, 2035–2041. [Google Scholar] [CrossRef]
- Bian, Z.; Miao, X.; Lei, S.; Chen, S.-E.; Wang, W.; Struthers, S. The challenges of reusing mining and mineral-processing wastes. Science 2012, 337, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Franks, D.M. Reclaiming the neglected minerals of development. Extr. Ind. Soc. 2020, 7, 453–460. [Google Scholar] [CrossRef]
- Harley, A.D.; Gilkes, R.J. Factors influencing the release of plant nutrient elements from silicate rock powders: A geochemical overview. Nutr. Cycl. Agroecosyst. 2000, 56, 11–36. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Naidu, R. Environmentally safe release of plant available potassium and micronutrients from organically amended rock mineral powder. Environ. Geochem. Health 2020, 43, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.C.; Rogers, J.R.; Choi, W.J. Silicates, Silicate Weathering, and Microbial Ecology. Geomicrobiol. J. 2001, 18, 3–19. [Google Scholar] [CrossRef]
- Uroz, S.; Kelly, L.C.; Turpault, M.-P.; Lepleux, C.; Frey-Klett, P. The mineralosphere concept: Mineralogical control of the distribution and function of mineral-associated bacterial communities. Trends Microbiol. 2015, 23, 751–762. [Google Scholar] [CrossRef]
- Christensen, M.L.; Sommer, S.G. Manure Characterisation and Inorganic Chemistry 2013. In Animal Manure: Recycling, Treatment, and Management; Jensen, L.S., Christensen, M.L., Sommer, S.G., Schmidt, T., Eds.; John Wiley & Sons Inc.: Chichester, UK, 2013; pp. 41–65. ISBN 9781118676677. [Google Scholar]
- Blume, H.-P. Handbuch der Bodenuntersuchung; John Wiley & Sons: Berlin, Germany, 2000; ISBN 978-3-527-19080-5. [Google Scholar]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Overmeyer, V.; Holtkamp, F.; Clemens, J.; Büscher, W.; Trimborn, M. Dynamics of different buffer systems in slurries based on time and temperature of storage and their visualization by a new mathematical tool. Anim. Open Access J. 2020, 10, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinuccio, E.; Berg, W.; Balsari, P. Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmos. Environ. 2008, 42, 2448–2459. [Google Scholar] [CrossRef] [Green Version]
- Parker, D.B.; Casey, K.D.; Todd, R.W.; Waldrip, H.M.; Marek, G.M.; Auvermann, B.W.; Marek, T.H.; Webb, K.; Willis, W.M.; Pemberton, B.; et al. Improved chamber systems for rapid, real-time nitrous oxide emissions from manure and soil. Trans. ASABE 2017, 60, 1235–1258. [Google Scholar] [CrossRef]
- Duncan, E.W.; Dell, C.J.; Kleinman, P.J.A.; Beegle, D.B. Nitrous oxide and ammonia emissions from injected and broadcast-applied dairy slurry. J. Environ. Q. 2017, 46, 36–44. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC); Organisation for Economic Co-operation and Development (OECD); International Energy Agency (IEA). Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 1997; Volume 3. [Google Scholar]
- Berg, W.; Brunsch, R.; Pazsiczki, I. Greenhouse gas emissions from covered slurry compared with uncovered during storage. Agric. Ecosyst. Environ. 2006, 112, 129–134. [Google Scholar] [CrossRef]
- Fangueiro, D.; Coutinho, J.; Chadwick, D.; Moreira, N.; Trindade, H. Effect of cattle slurry separation on greenhouse gas and ammonia emissions during storage. J. Environ. Q. 2008, 37, 2322–2331. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Brookman, S.K.E.; Smith, K.A.; Cumby, T.; Williams, A.G.; McCrory, D.F. Crusting of stored dairy slurry to abate ammonia emissions: Pilot-scale studies. J. Environ. Q. 2005, 34, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Cumby, T.; Lapworth, J.; Misselbrook, T.; Williams, A. Natural crusting of slurry storage as an abatement measure for ammonia emissions on dairy farms. Biosyst. Eng. 2007, 97, 464–471. [Google Scholar] [CrossRef]
- Wood, J.D.; Gordon, R.J.; Wagner-Riddle, C.; Dunfield, K.E.; Madani, A. Relationships between dairy slurry total solids, gas emissions, and surface crusts. J. Environ. Q. 2012, 41, 694–704. [Google Scholar] [CrossRef]
- Adams, R.S.; Stevenson, F.J. Ammonium Sorption and Release from Rocks and Minerals. Soil Sci. Soci. Am. J. 1964, 28, 345. [Google Scholar] [CrossRef]
- Mersi, W.V.; Kuhnert-Finkernagel, R.; Schinner, F. The influence of rock powders on microbial activity of three forest soils. Z. Für Pflanz. Und Bodenkd. 1992, 155, 29–33. [Google Scholar] [CrossRef]
- Sparling, G.P.; BG, O.; Vaugha, D. Microbial biomass and activity in soils amended with glucose. Soil Biol. Biochem. 1981, 13, 301–307. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J.; Vitousek, P.M. Ecological Stoichiometry; Princeton University Press: Princeton, NJ, USA, 2003; p. 464. [Google Scholar]
- Li, J.; Mavrodi, D.V.; Dong, Y. Effect of rock dust-amended compost on the soil properties, soil microbial activity, and fruit production in an apple orchard from the Jiangsu province of China. Arch. Agron. Soil Sci. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Carson, J.K.; Rooney, D.; Gleeson, D.B.; Clipson, N. Altering the mineral composition of soil causes a shift in microbial community structure. FEMS Microbiol. Ecol. 2007, 61, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, J.K.; Campbell, L.; Rooney, D.; Clipson, N.; Gleeson, D.B. Minerals in soil select distinct bacterial communities in their microhabitats. FEMS Microbiol. Ecol. 2009, 67, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nature reviews. Microbiology 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Perski, H.J.; Schönheit, P.; Thauer, R.K. Sodium dependence of methane formation in methanogenic bacteria. FEBS Lett. 1982, 143, 323–326. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S.G.; Jensen, L.S.; Christensen, M.L.; Schmidt, T. Animal Manure Recycling: Treatment and Management; Sommer, S.G., Christensen, M.L., Schmidt, T., Jensen, L.S., Eds.; Wiley: Chichester, UK, 2013. [Google Scholar]
- Sommer, S.G.; Husted, S. The chemical buffer system in raw and digested animal slurry. J. Agric. Sci. 1995, 124, 45–53. [Google Scholar] [CrossRef]
- Oenema, O.; Bannink, A.; Sommer, S.G.; Velthof, G.L. Gaseous Nitrogen Emissions from Livestock Farming Systems. In Nitrogen in the Environment: Sources, Problems, and Management; Follett, R.F., Hatfield, J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Sommer, S.G.; Petersen, S.O.; Søgaard, H.T. Greenhouse Gas Emission from Stored Livestock Slurry. J. Environ. Q. 2000, 29, 744–751. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; The Minerological Society: London, UK, 2013; p. 498. [Google Scholar]
- Zhang, G.; Kang, J.; Wang, T.; Zhu, C. Review and outlook for agromineral research in agriculture and climate mitigation. Soil Res. 2018, 56, 113. [Google Scholar] [CrossRef]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Bodeling; US Geological Survey: Menlo Park, CA, USA, 2004.
- Brennan, R.F.; Penrose, B.; Bell, R.W. Micronutrients limiting pasture production in Australia. Crop Pasture Sci. 2019, 70, 1053. [Google Scholar] [CrossRef]
- Dove, H.; Masters, D.G.; Thompson, A.N. New perspectives on the mineral nutrition of livestock grazing cereal and canola crops. Anim. Prod. Sci. 2016, 56, 1350. [Google Scholar] [CrossRef]
- Masters, D.G.; Norman, H.C.; Thomas, D.T. Minerals in pastures—Are we meeting the needs of livestock? Crop Pasture Sci. 2019, 70, 1184. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Vicca, S.; Goll, D.S.; Hagens, M.; Hartmann, J.; Janssens, I.A.; Neubeck, A.; Verbruggen, E. Is the climate change mitigation effect of enhanced silicate weathering governed by biological processes? Glob. Change Biol. 2021, 00, 1–16. [Google Scholar] [CrossRef] [PubMed]

| Major Elements in wt% | Trace Elements in ppm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Particle Size (μm) | pH | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | SO3 | Cu | Zn | Mn | |
| Biolit | 10% < 2.3 90% < 79.4 | 9.9 | 57.07 | 14.59 | 8.01 | 3.99 | 3.00 | 3.86 | 2.91 | 0.35 | 0.25 | 20 | 83 | 1059 |
| Eifelgold | 10% < 2.3 90% < 77.7 | 10 | 43.37 | 14.36 | 11.22 | 9.06 | 11.06 | 3.16 | 3.38 | 0.51 | 0.21 | 52 | 78 | 1433 |
| Minerals | Biolit | Eifelgold |
|---|---|---|
| Quartz | 18.97 | - |
| K feldspar | 13.37 | 9.17 |
| Plagioclase | 43.50 | 13.81 |
| Amphibole | 2.14 | - |
| Pyroxene | - | 44.59 |
| Olivine | - | 7.46 |
| Leucite | - | 10.63 |
| Illite/Muscovite | 8.74 | 3.45 |
| Chlorite | 10.55 | - |
| Iron oxides | - | 10.89 |
| Titanium oxides | 2.73 | - |
| Treatment | Gaseous Emissions | ||||
|---|---|---|---|---|---|
| NH3 | CO2 | CH4 | N2O | Total GHG | |
| mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | |
| Control | 217.47 (12.34) | 408.42b (12.97) | 106.37c (14.19) | 0.615a (0.091) | 2831.79c (113.06) |
| Biolit | 217.99 (4.045) | 397.12b (9.940) | 164.28b (15.44) | 0.465b (0.226) | 3390.35b (159.20) |
| Eifelgold | 231.83 (7.666) | 445.86a (8.820) | 326.46a (22.01) | 0.302b (0.080) | 7395.28a (243.49) |
| Before Trial | After Trial | |||
|---|---|---|---|---|
| Control | Eifelgold | Biolit | ||
| Dry residue (%) | 7.5 | 7.4 | 12.2 | 11.8 |
| Water content (%) | 92.5 | 92.7 | 87.8 | 88.2 |
| pH | 7 | 7.4 | 7.6 | 7.5 |
| Organic matter (kg/cbm) | 56.3 | 50.1 | 48.6 | 48.1 |
| Total N (kg/m³) | 3.32 | 2.15 | 2.56 | 2.25 |
| NH4-N (kg/m³) | 1.75 | 0.56 | 0.87 | 0.712 |
| P2O5 (kg/m³) | 1.28 | 1.45 | 1.68 | 1.59 |
| K2O (kg/m³) | 3.93 | 4.49 | 6.12 | 4.57 |
| MgO (kg/m³) | 0.77 | 0.89 | 3.16 | 2.67 |
| CaO (kg/m³) | 1.5 | 1.73 | 3.52 | 2.98 |
| S (kg/m³) | 0.35 | 0.39 | 0.422 | 0.411 |
| Cu (g m³) | 1.78 | 2.08 | 4.43 | 2.89 |
| Zn (g/m³) | 9.83 | 11.5 | 14.3 | 14.9 |
| Na (g/m³) | 116 | 146 | 925 | 181 |
| Mn (g/m³) | 16 | 19 | 67 | 66 |
| C/N (kg/m³) | 9.8 | 13.5 | 11.0 | 12.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swoboda, P.; Hamer, M.; Stotter, M.; Döring, T.F.; Trimborn, M. Effects of Rock Powder Additions to Cattle Slurry on Ammonia and Greenhouse Gas Emissions. Atmosphere 2021, 12, 1652. https://doi.org/10.3390/atmos12121652
Swoboda P, Hamer M, Stotter M, Döring TF, Trimborn M. Effects of Rock Powder Additions to Cattle Slurry on Ammonia and Greenhouse Gas Emissions. Atmosphere. 2021; 12(12):1652. https://doi.org/10.3390/atmos12121652
Chicago/Turabian StyleSwoboda, Philipp, Martin Hamer, Michael Stotter, Thomas F. Döring, and Manfred Trimborn. 2021. "Effects of Rock Powder Additions to Cattle Slurry on Ammonia and Greenhouse Gas Emissions" Atmosphere 12, no. 12: 1652. https://doi.org/10.3390/atmos12121652
APA StyleSwoboda, P., Hamer, M., Stotter, M., Döring, T. F., & Trimborn, M. (2021). Effects of Rock Powder Additions to Cattle Slurry on Ammonia and Greenhouse Gas Emissions. Atmosphere, 12(12), 1652. https://doi.org/10.3390/atmos12121652







