Reactivity of a Carene-Derived Hydroxynitrate in Mixed Organic/Aqueous Matrices: Applying Synthetic Chemistry to Product Identification and Mechanistic Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. NMR Scale Reaction Procedures
2.2.1. Reaction in 0.6% D2O in DMSO-d6
2.2.2. Reactions in 4.2–50% D2O/DMSO-d6
2.2.3. Reactions in 100% H2O
2.2.4. Reactions with MeOD-d4
2.2.5. Reactions with EtOD-d6
2.2.6. Reactions with NaCl
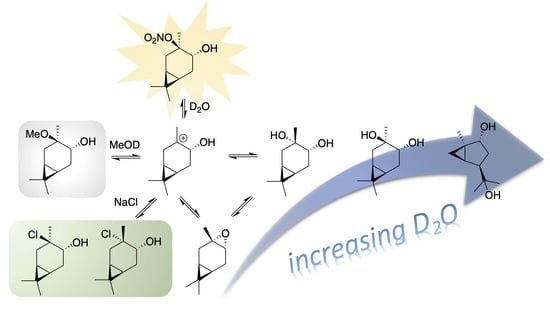
3. Results
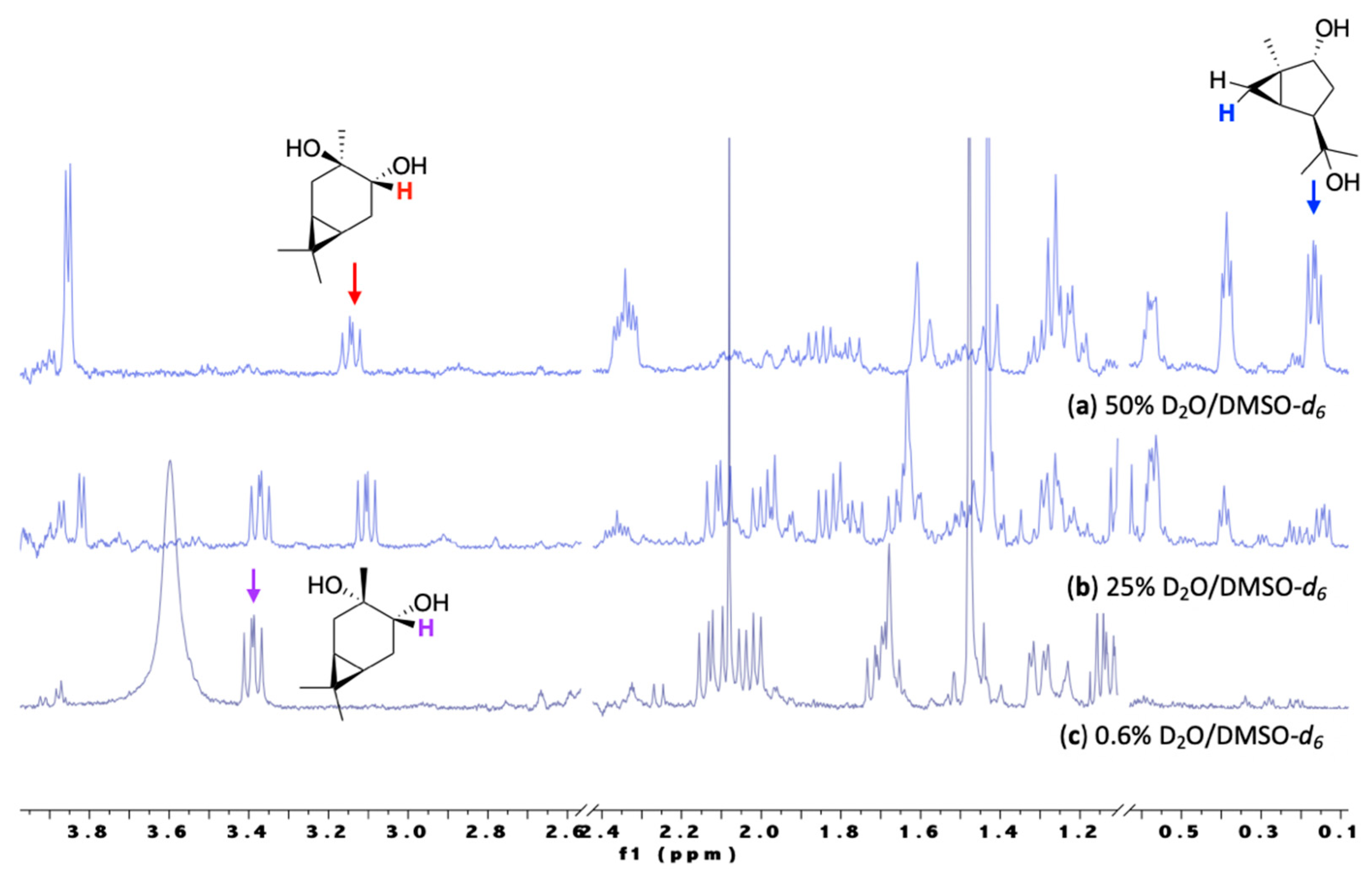
3.1. HN Reactivity in a Single-Phase Mixed D2O/DMSO-d6 Solution
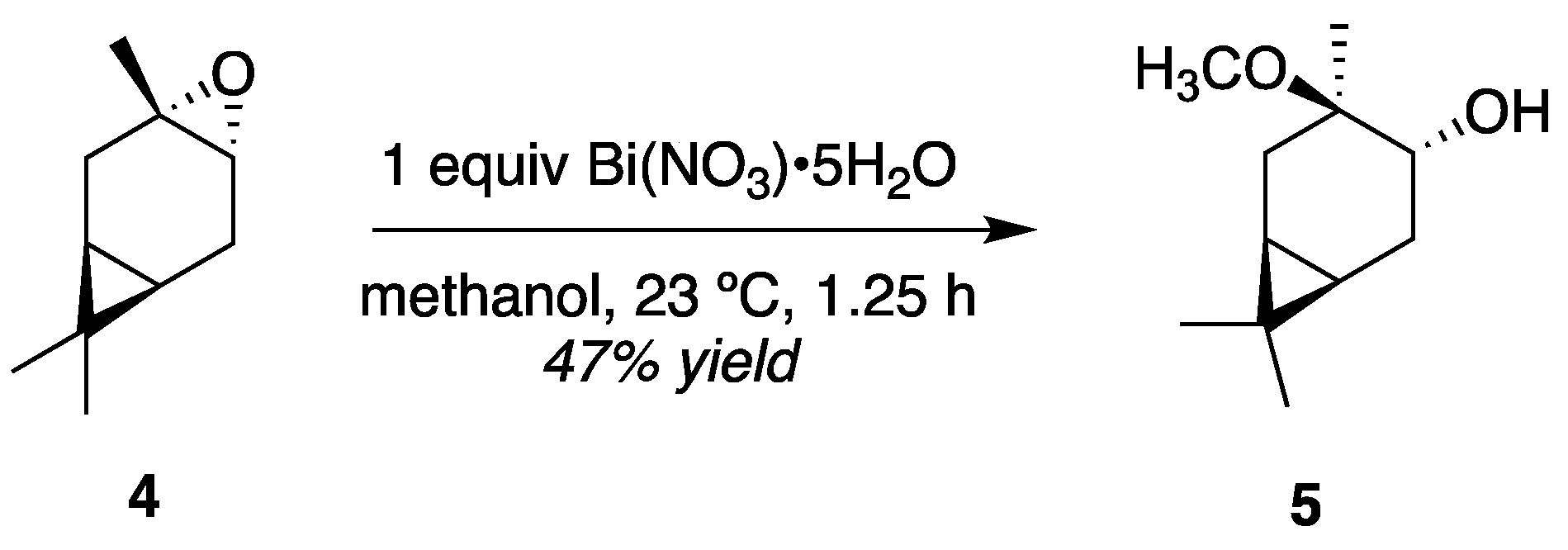
3.2. HN Reactivity in Single-Phase D2O/Alcohol Solutions
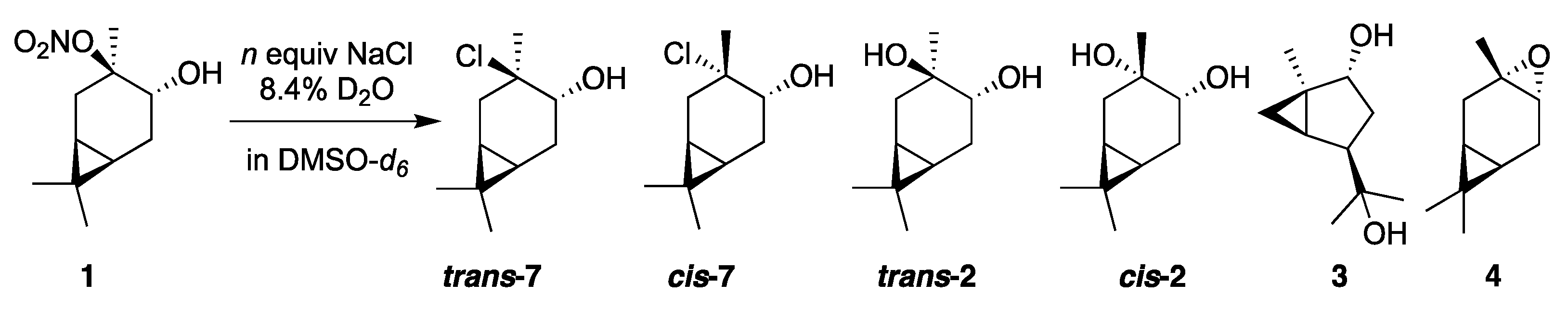
3.3. CHN Reactivity in D2O/DMSO-d6 Solutions Containing Cl-
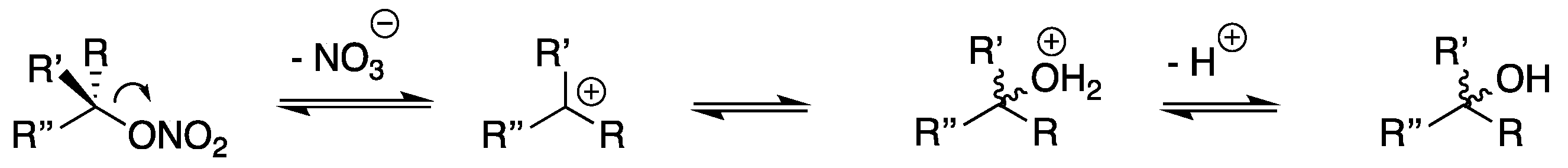
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wängberg, I.; Barnes, I.; Becker, K.H. Product and Mechanistic Study of the Reaction of NO3 Radicals with α-Pinene. Environ. Sci. Technol. 1997, 31, 2130–2135. [Google Scholar] [CrossRef]
- Hallquist, M.; Wängberg, I.; Ljungström, E.; Barnes, I.; Becker, K.H. Aerosol and Product Yields from NO3 Radical-Initiated Oxidation of Selected Monoterpenes. Environ. Sci. Technol. 1999, 33, 553–559. [Google Scholar] [CrossRef]
- Spittler, M.; Barnes, I.; Bejan, I.; Brockmann, K.J.; Benter, T.; Wirtz, K. Reactions of NO3 Radicals with Limonene and α-Pinene: Product and SOA Formation. Atmos. Environ. 2006, 40, 116–127. [Google Scholar] [CrossRef]
- Matsunaga, A.; Docherty, K.S.; Lim, Y.B.; Ziemann, P.J. Composition and Yields of Secondary Organic Aerosol Formed from OH Radical-Initiated Reactions of Linear Alkenes in the Presence of NOx: Modeling and Measurements. Atmos. Environ. 2009, 43, 1349–1357. [Google Scholar] [CrossRef]
- Kroll, J.H.; Seinfeld, J.H. Chemistry of Secondary Organic Aerosol: Formation and Evolution of Low-Volatility Organics in the Atmosphere. Atmos. Environ. 2008, 42, 3593–3624. [Google Scholar] [CrossRef]
- Fiore, A.M.; Horowitz, L.W.; Purves, D.W.; Levy, H.; Evans, M.J.; Wang, Y.; Li, Q.; Yantosca, R.M. Evaluating the Contribution of Changes in Isoprene Emissions to Surface Ozone Trends over the Eastern United States. J. Geophys. Res. Atmos. 2005, 110, 1–13. [Google Scholar] [CrossRef]
- Von Kuhlmann, R.; Lawrence, M.G.; Pöschl, U.; Crutzen, P.J. Sensitivities in Global Scale Modeling of Isoprene. Atmos. Chem. Phys. 2004, 4, 1–17. [Google Scholar] [CrossRef]
- Horowitz, L.W.; Fiore, A.M.; Milly, G.P.; Cohen, R.C.; Perring, A.; Wooldridge, P.J.; Hess, P.G.; Emmons, L.K.; Lamarque, J.F. Observational Constraints on the Chemistry of Isoprene Nitrates over the Eastern United States. J. Geophys. Res. Atmos. 2007, 112, 1–13. [Google Scholar] [CrossRef]
- Ayres, B.R.; Allen, H.M.; Draper, D.C.; Brown, S.S.; Wild, R.J.; Jimenez, J.L.; Day, D.A.; Campuzano-Jost, P.; Hu, W.; De Gouw, J.; et al. Organic Nitrate Aerosol Formation via NO3 + Biogenic Volatile Organic Compounds in the Southeastern United States. Atmos. Chem. Phys 2015, 15, 13377–13392. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Luecken, D.J.; Xu, L.; Boyd, C.M.; Ng, N.L.; Baker, K.R.; Ayres, B.R.; Bash, J.O.; Baumann, K.; Carter, W.P.L.; et al. Modeling the Current and Future Roles of Particulate Organic Nitrates in the Southeastern United States. Environ. Sci. Technol. 2015, 49, 14195–14203. [Google Scholar] [CrossRef]
- Fisher, J.A.; Jacob, D.J.; Travis, K.R.; Kim, P.S.; Marais, E.A.; Miller, C.C.; Yu, K.; Zhu, L.; Yantosca, R.M.; Sulprizio, M.P.; et al. Organic Nitrate Chemistry and Its Implications for Nitrogen Budgets in an Isoprene- and Monoterpene-Rich Atmosphere: Constraints from Aircraft (SEAC4RS) and Ground-Based (SOAS) Observations in the Southeast US. Atmos. Chem. Phys. 2016, 16, 5969–5991. [Google Scholar] [CrossRef] [PubMed]
- Perring, A.E.; Pusede, S.E.; Cohen, R.C. An Observational Perspective on the Atmospheric Impacts of Alkyl and Multifunctional Nitrates on Ozone and Secondary Organic Aerosol. Chem. Rev. 2013, 113, 5848–5870. [Google Scholar] [CrossRef] [PubMed]
- Rollins, A.W.; Smith, J.D.; Wilson, K.R.; Cohen, R.C. Real Time in Situ Detection of Organic Nitrates in Atmospheric Aerosols. Environ. Sci. Technol. 2010, 44, 5540–5545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, L.; Suresh, S.; Guo, H.; Weber, R.J.; Ng, N.L. Aerosol Characterization over the Southeastern United States Using High-Resolution Aerosol Mass Spectrometry: Spatial and Seasonal Variation of Aerosol Composition and Sources with a Focus on Organic Nitrates. Atmos. Chem. Phys. 2015, 15, 7307–7336. [Google Scholar] [CrossRef]
- Pankow, J.F.; Asher, W.E. SIMPOL.1: A Simple Group Contribution Method for Predicting Vapor Pressures and Enthalpies of Vaporization of Multifunctional Organic Compounds. Atmos. Chem. Phys. 2008, 8, 2773–2796. [Google Scholar] [CrossRef]
- Rollins, A.W.; Pusede, S.; Wooldridge, P.; Min, K.E.; Gentner, D.R.; Goldstein, A.H.; Liu, S.; Day, D.A.; Russell, L.M.; Rubitschun, C.L.; et al. Gas/Particle Partitioning of Total Alkyl Nitrates Observed with TD-LIF in Bakersfield. J. Geophys. Res. Atmos. 2013, 118, 6651–6662. [Google Scholar] [CrossRef]
- Compernolle, S.; Ceulemans, K.; Müller, J.F. Evaporation: A New Vapour Pressure Estimation Methodfor Organic Molecules Including Non-Additivity and Intramolecular Interactions. Atmos. Chem. Phys. 2011, 11, 9431–9450. [Google Scholar] [CrossRef]
- Ditto, J.C.; Joo, T.; Slade, J.H.; Shepson, P.B.; Ng, N.L.; Gentner, D.R. Nontargeted Tandem Mass Spectrometry Analysis Reveals Diversity and Variability in Aerosol Functional Groups across Multiple Sites, Seasons, and Times of Day. Environ. Sci. Technol. Lett. 2020, 7, 60–69. [Google Scholar] [CrossRef]
- Zare, A.; Romer, P.S.; Nguyen, T.; Keutsch, F.N.; Skog, K.; Cohen, R.C. A Comprehensive Organic Nitrate Chemistry: Insights into the Lifetime of Atmospheric Organic Nitrates. Atmos. Chem. Phys. 2018, 18, 15419–15436. [Google Scholar] [CrossRef]
- Romonosky, D.E.; Nguyen, L.Q.; Shemesh, D.; Nguyen, T.B.; Epstein, S.A.; Martin, D.B.C.; Vanderwal, C.D.; Gerber, R.B.; Nizkorodov, S.A. Absorption Spectra and Aqueous Photochemistry of β-Hydroxyalkyl Nitrates of Atmospheric Interest. Mol. Phys. 2015, 113, 2179–2190. [Google Scholar] [CrossRef]
- Hu, K.S.; Darer, A.I.; Elrod, M.J. Thermodynamics and Kinetics of the Hydrolysis of Atmospherically Relevant Organonitrates and Organosulfates. Atmos. Chem. Phys. 2011, 11, 8307–8320. [Google Scholar] [CrossRef]
- Darer, A.I.; Cole-Filipiak, N.C.; O’Connor, A.E.; Elrod, M.J. Formation and Stability of Atmospherically Relevant Isoprene-Derived Organosulfates and Organonitrates. Environ. Sci. Technol. 2011, 45, 1895–1902. [Google Scholar] [CrossRef]
- Morales, A.C.; Jayarathne, T.; Slade, J.H.; Laskin, A.; Shepson, P.B. The Production and Hydrolysis of Organic Nitrates from OH Radical Oxidation of β-Ocimene. Atmos. Chem. Phys. 2021, 21, 129–145. [Google Scholar] [CrossRef]
- Rindelaub, J.D.; Borca, C.H.; Hostetler, M.A.; Slade, J.H.; Lipton, M.A.; Slipchenko, L.V.; Shepson, P.B. The Acid-Catalyzed Hydrolysis of an α-Pinene-Derived Organic Nitrate: Kinetics, Products, Reaction Mechanisms, and Atmospheric Impact. Atmos. Chem. Phys. 2016, 16, 15425–15432. [Google Scholar] [CrossRef]
- Jacobs, M.I.; Burke, W.J.; Elrod, M.J. Kinetics of the Reactions of Isoprene-Derived Hydroxynitrates: Gas Phase Epoxide Formation and Solution Phase Hydrolysis. Atmos. Chem. Phys. 2014, 14, 8933–8946. [Google Scholar] [CrossRef]
- Cortés, D.A.; Elrod, M.J. Kinetics of the Aqueous Phase Reactions of Atmospherically Relevant Monoterpene Epoxides. J. Phys. Chem. A 2017, 121, 9297–9305. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, K.T.; Crounse, J.D.; Schulze, B.C.; Bates, K.H.; Teng, A.P.; Xu, L.; Allen, H.M.; Wennberg, P.O. Rapid Hydrolysis of Tertiary Isoprene Nitrate Efficiently Removes NOx from the Atmosphere. Proc. Natl. Acad. Sci. USA 2021, 117, 33011–33016. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ng, N.L. Chemical Composition and Hydrolysis of Organic Nitrate Aerosol Formed from Hydroxyl and Nitrate Radical Oxidation of α-Pinene and β-Pinene. Atmos. Chem. Phys. Discuss. 2019, 19, 12749–12766. [Google Scholar] [CrossRef]
- Boyd, C.M.; Sanchez, J.; Xu, L.; Eugene, A.J.; Nah, T.; Tuet, W.Y.; Guzman, M.I.; Ng, N.L. Secondary Organic Aerosol Formation from the β-Pinene+NO3 System: Effect of Humidity and Peroxy Radical Fate. Atmos. Chem. Phys. 2015, 15, 7497–7522. [Google Scholar] [CrossRef]
- Bean, J.K.; Hildebrandt Ruiz, L. Gas-Particle Partitioning and Hydrolysis of Organic Nitrates Formed from the Oxidation of α-Pinene in Environmental Chamber Experiments. Atmos. Chem. Phys. 2016, 16, 2175–2184. [Google Scholar] [CrossRef]
- Sato, K. Detection of Nitrooxypolyols in Secondary Organic Aerosol Formed from the Photooxidation of Conjugated Dienes under High-NOx Conditions. Atmos. Environ. 2008, 42, 6851–6861. [Google Scholar] [CrossRef]
- Rindelaub, J.D.; McAvey, K.M.; Shepson, P.B. The Photochemical Production of Organic Nitrates from α-Pinene and Loss via Acid-Dependent Particle Phase Hydrolysis. Atmos. Environ. 2015, 100, 193–201. [Google Scholar] [CrossRef]
- McKnight, E.A.; Kretekos, N.P.; Owusu, D.; LaLonde, R.L. Technical Note: Preparation and Purification of Atmospherically Relevant Alpha-Hydroxynitrate Esters of Monoterpenes. Atmos. Chem. Phys. Discuss. 2019, 1–23. [Google Scholar] [CrossRef]
- US EPA. Estimation Programs Interface SuiteTM for Microsoft® Windows; v. 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2021.
- Kolehmainen, E.; Laihia, K.; Heinaenen, M.; Rissanen, K.; Froehlich, R.; Korvola, J.; Maenttaeri, P.; Kauppinen, R. Oxygen-Containing Bicyclic Monoterpenes. 1H, 13C and 17O NMR Spectroscopic and X-ray Diffraction Studies of Seven Oxidation Products of (+)-3-Carene. J. Chem. Soc. Perkin Trans. 1993, 2, 641–648. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Yang, X.; Li, W.; Xue, L.; Wang, T.; Chen, J.; Wang, W. Mixed Chloride Aerosols and Their Atmospheric Implications: A Review. Aerosol Air Qual. Res. 2017, 17, 878–887. [Google Scholar] [CrossRef]
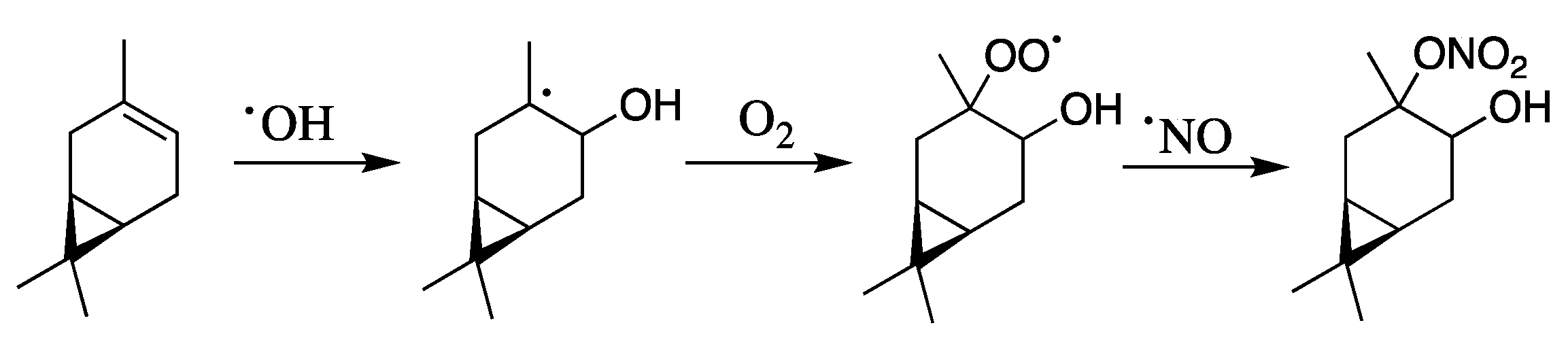
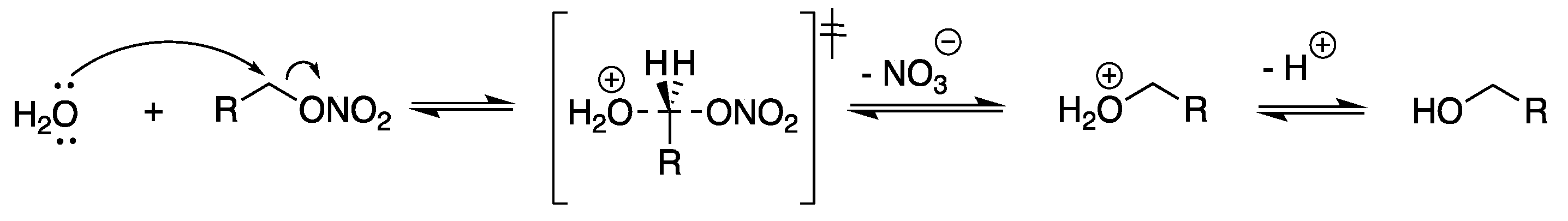


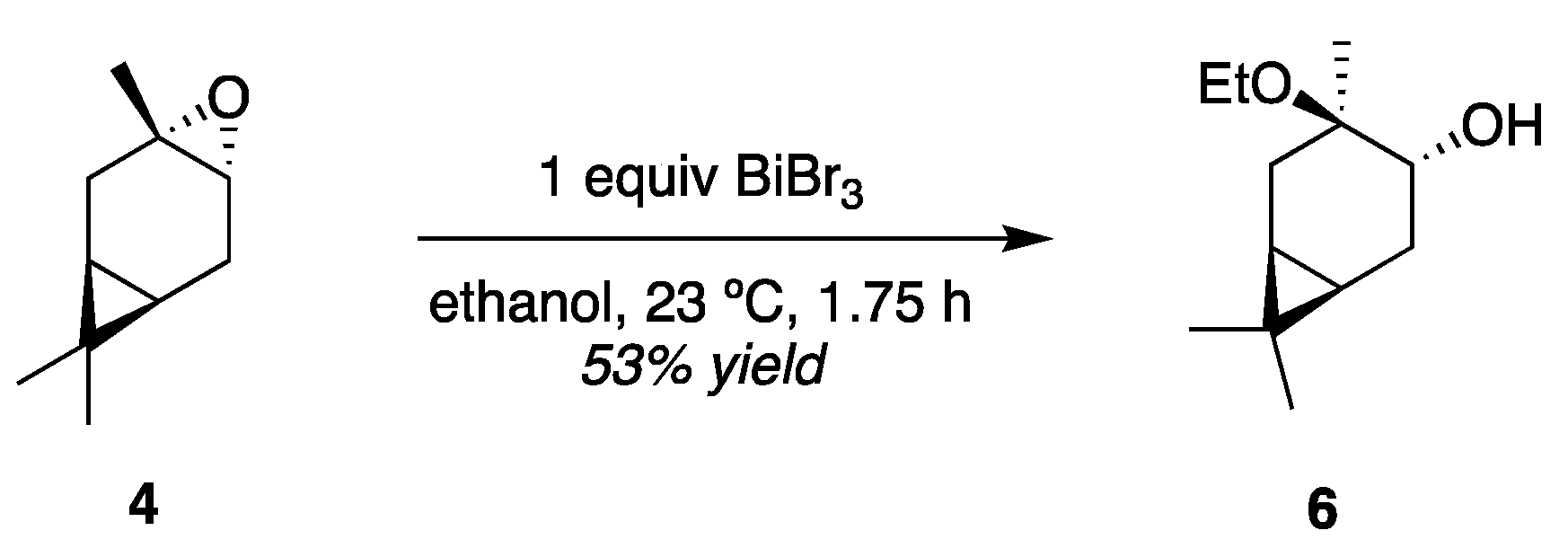
| ID | Structure | 1H NMR (ppm) |
|---|---|---|
| trans-2 | 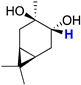 | 3.11 (dd) |
| cis-2 | 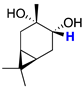 | 3.41 (dd) |
| 3 | 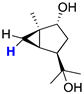 | 0.14 (dt) |
| 4 |  | 2.79 (brs) |
| 5 | 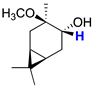 | 3.43 (dd) 2 |
| 6 | 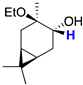 | 3.42 (dd) 3 |
| trans-7 |  | 3.33 (dd) |
| cis-7 | 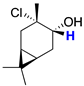 | 3.94 (t) |
| Entry | %D2O | cis-2 | trans-2 | 3 | 4 |
|---|---|---|---|---|---|
| 1 | 100 1 | n.d. | n.d. | 0.68 2 | n.d. |
| 2 | 50 | n.d. | 0.33 3 | 0.67 3 | n.d. |
| 3 | 25 | 0.25 ± 0.02 | 0.21 ± 0.01 | 0.13 ± 0.03 | 0.03 ± 0.00 |
| 4 | 16.7 | 0.42 ± 0.04 | 0.22 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 |
| 5 | 8.4 | 0.46 ± 0.04 | 0.10 ± 0.01 | 0.02 ± 0.03 | 0.05 ± 0.01 |
| 6 | 4.2 | 0.53 ± 0.01 | 0.10 ± 0.01 | n.d. | 0.04 ± 0.00 |
| 7 | 0.6 | 0.91 ± 0.02 | n.d. | n.d. | 0.08 ± 0.05 |
| Entry | % D2O | Solvent | 5 or 6 | Cis-2 | Trans-2 | 3 | 4 |
|---|---|---|---|---|---|---|---|
| 1 | 16.7 | MeOD-d4 | 0.27 ± 0.02 | n.d. | 0.19 ± 0.02 | 0.39 ± 0.02 | n.d. |
| 2 | 0 | 0.51 ± 0.06 | n.d. | n.d. | 0.20 ± 0.03 | n.d. | |
| 3 | 16.7 | EtOD-d6 | n.d. | 0.05 ± 0.03 | 0.30 ± 0.01 | 0.24 ± 0.02 | 0.04 ± 0.04 |
| 4 | 0 | n.d. | 0.36 ± 0.01 | 0.13 ± 0.03 | 0.09 ± 0.01 | n.d. |
| Entry | n Molar Equiv. NaCl | Trans-7 | Cis-7 | Cis-2 | Trans-2 | 3 | 4 |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.06 ± 0.01 | 0.19 ± 0.09 | 0.49 ± 0.25 | 0.08 ± 0.02 | 0.04 ± 0.03 | 0.03 ± 0.02 |
| 2 | 5 | 0.16 ± 0.08 | 0.18 ± 0.09 | 0.27 ± 0.15 | 0.08 ± 0.03 | 0.06 ± 0.03 | 0.02 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAlister, A.B.; Vesto, J.I.; Huang, A.; Wright, K.A.; McLaughlin Sta. Maria, E.J.; Bailey, G.M.; Kretekos, N.P.; Baldwin, P.R.; Carrasquillo, A.J.; LaLonde, R.L. Reactivity of a Carene-Derived Hydroxynitrate in Mixed Organic/Aqueous Matrices: Applying Synthetic Chemistry to Product Identification and Mechanistic Implications. Atmosphere 2021, 12, 1617. https://doi.org/10.3390/atmos12121617
McAlister AB, Vesto JI, Huang A, Wright KA, McLaughlin Sta. Maria EJ, Bailey GM, Kretekos NP, Baldwin PR, Carrasquillo AJ, LaLonde RL. Reactivity of a Carene-Derived Hydroxynitrate in Mixed Organic/Aqueous Matrices: Applying Synthetic Chemistry to Product Identification and Mechanistic Implications. Atmosphere. 2021; 12(12):1617. https://doi.org/10.3390/atmos12121617
Chicago/Turabian StyleMcAlister, Addison B., James I. Vesto, Aaron Huang, Kathryn A. Wright, Emily J. McLaughlin Sta. Maria, Gabriela M. Bailey, Nicole P. Kretekos, Petra R. Baldwin, Anthony J. Carrasquillo, and Rebecca Lyn LaLonde. 2021. "Reactivity of a Carene-Derived Hydroxynitrate in Mixed Organic/Aqueous Matrices: Applying Synthetic Chemistry to Product Identification and Mechanistic Implications" Atmosphere 12, no. 12: 1617. https://doi.org/10.3390/atmos12121617
APA StyleMcAlister, A. B., Vesto, J. I., Huang, A., Wright, K. A., McLaughlin Sta. Maria, E. J., Bailey, G. M., Kretekos, N. P., Baldwin, P. R., Carrasquillo, A. J., & LaLonde, R. L. (2021). Reactivity of a Carene-Derived Hydroxynitrate in Mixed Organic/Aqueous Matrices: Applying Synthetic Chemistry to Product Identification and Mechanistic Implications. Atmosphere, 12(12), 1617. https://doi.org/10.3390/atmos12121617