Undiluted Measurement of the Particle Size Distribution of Different Oxygenated Biofuels in a Gasoline-Optimised DISI Engine
Abstract
:1. Introduction
2. Materials and Methods
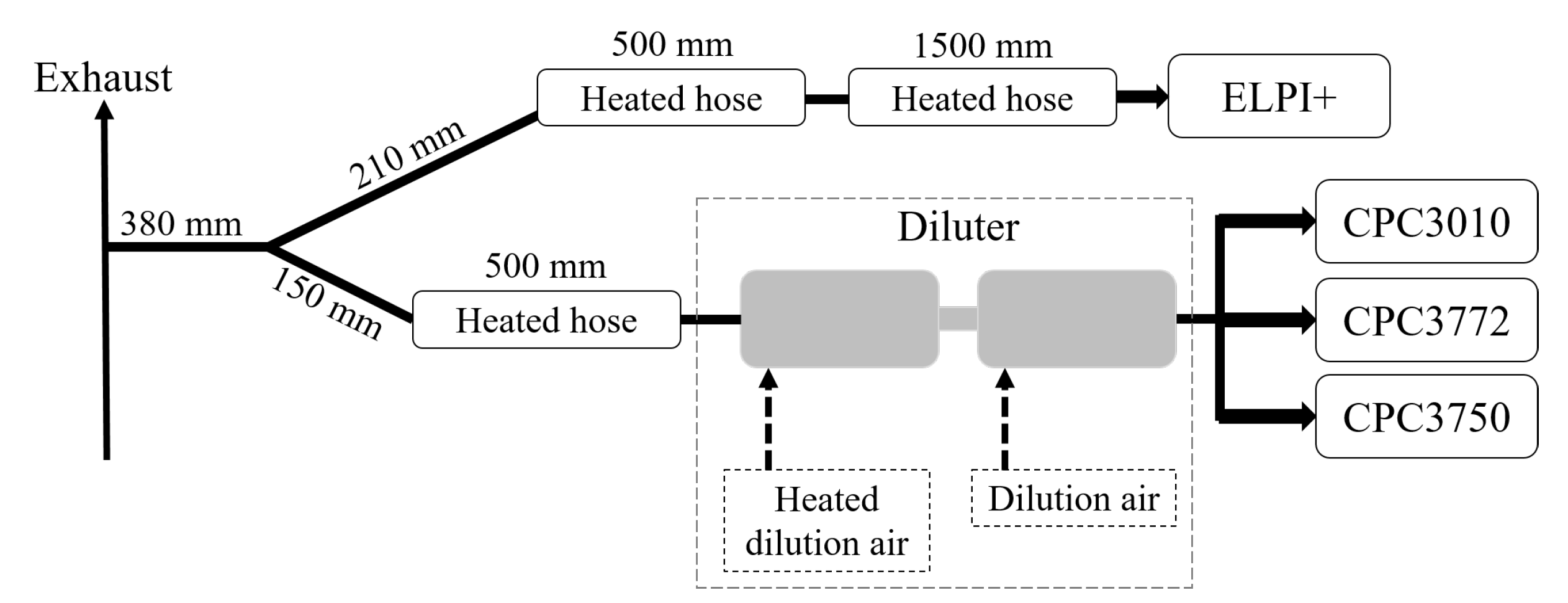
2.1. Experimental Setup
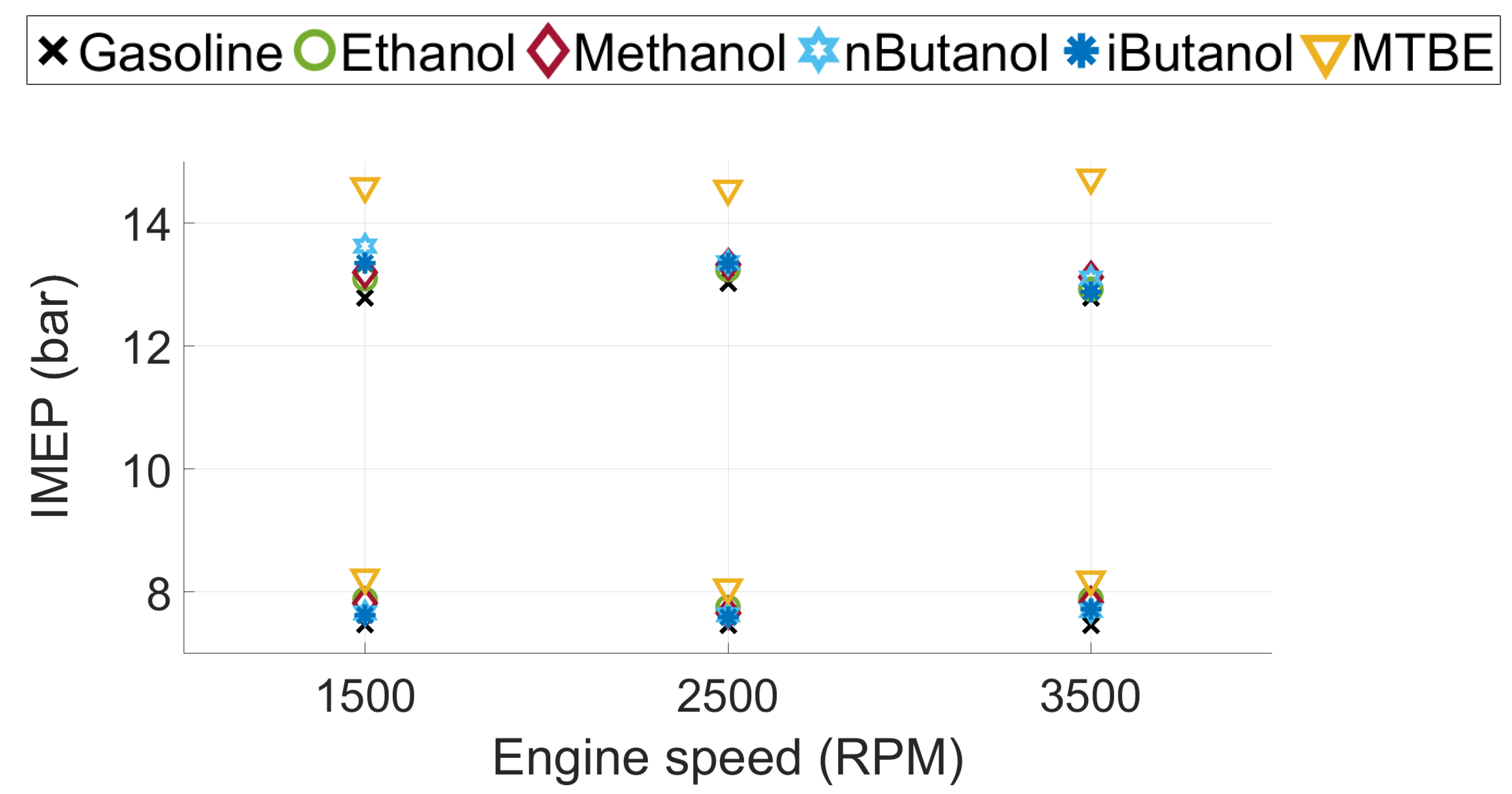
2.2. Test Fuels
2.3. Methodology
2.4. Data Acquisition and Analysis
3. Results
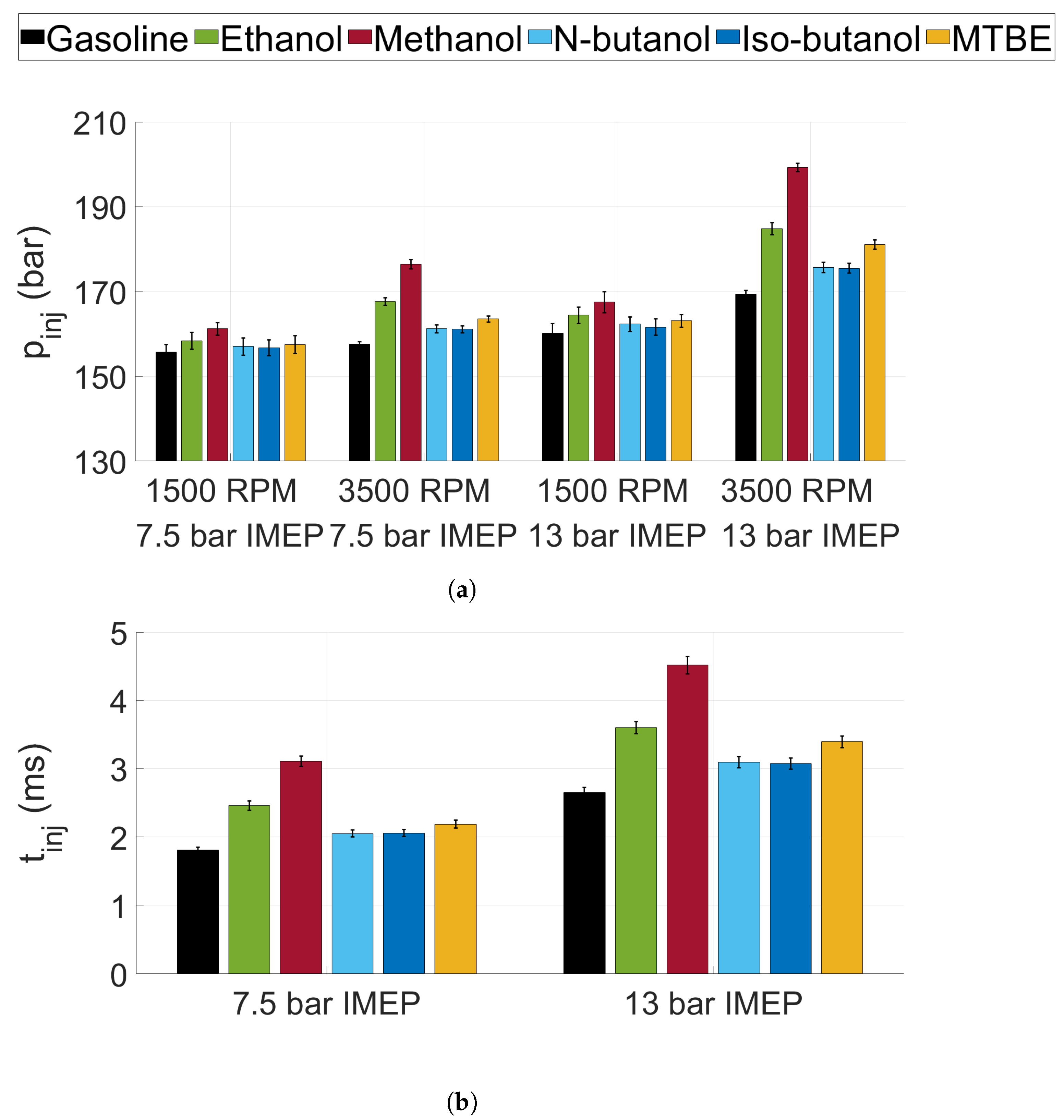
3.1. Injection Pressure and Duration
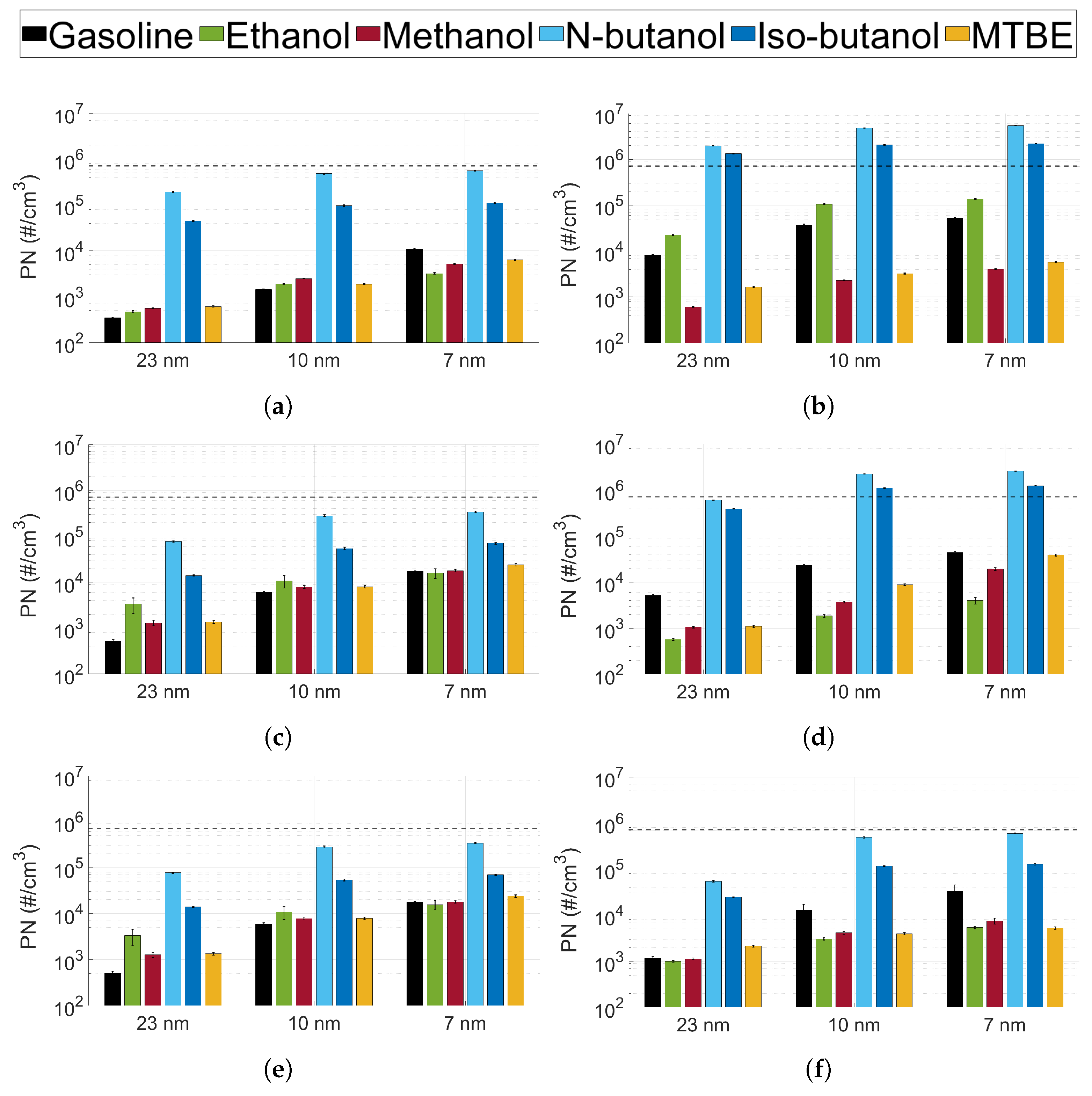
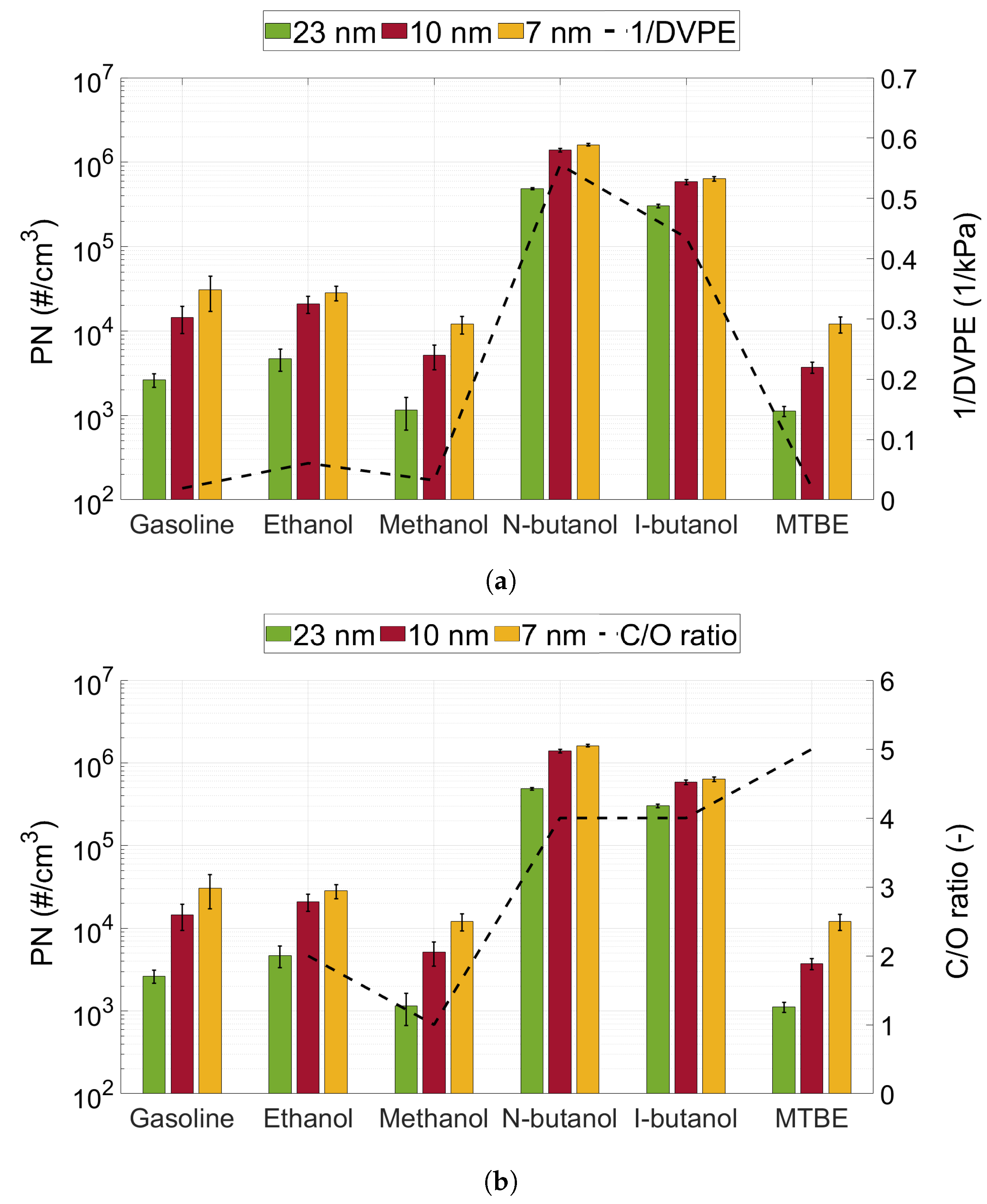
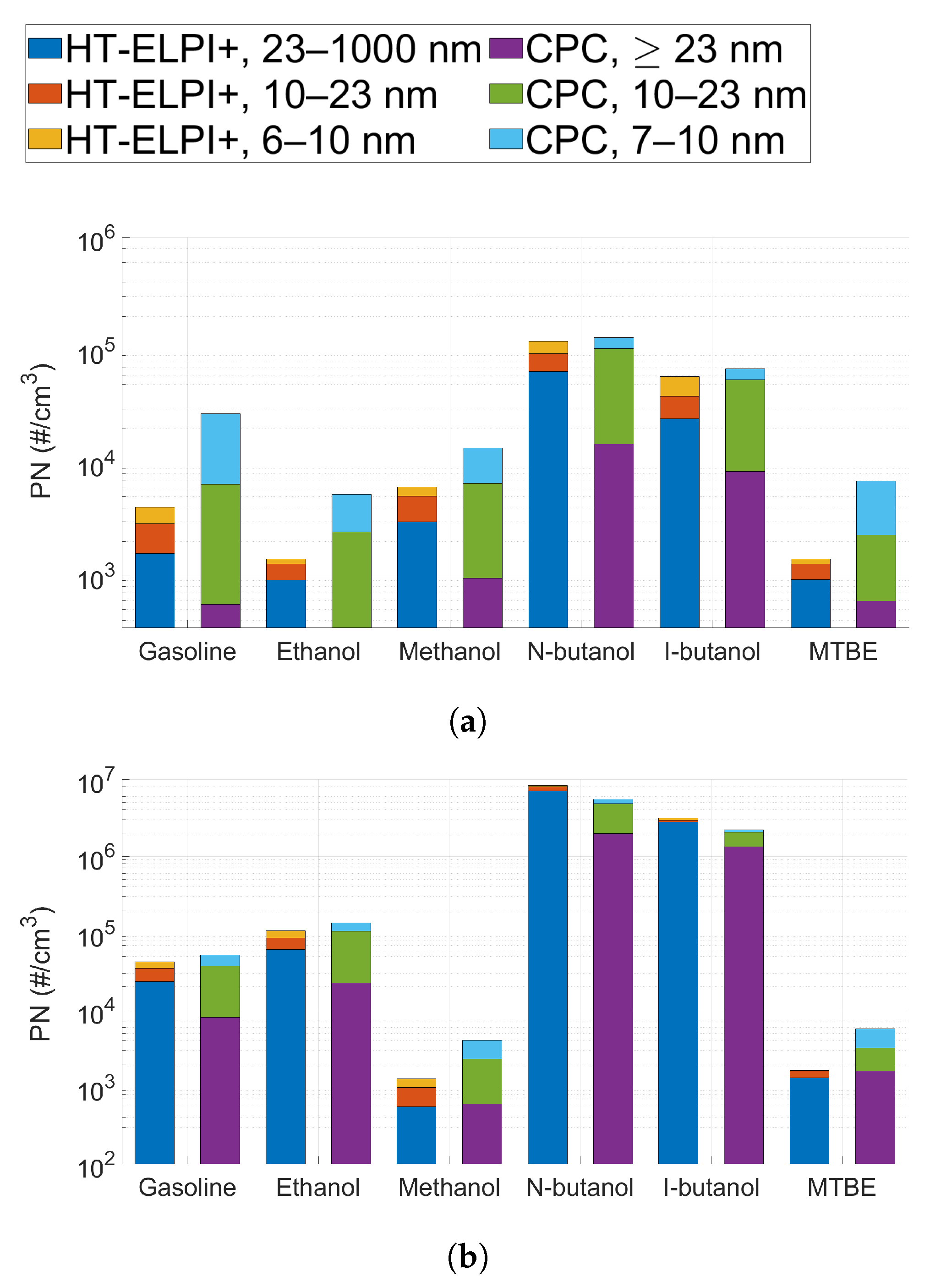
3.2. Particle Number Emissions
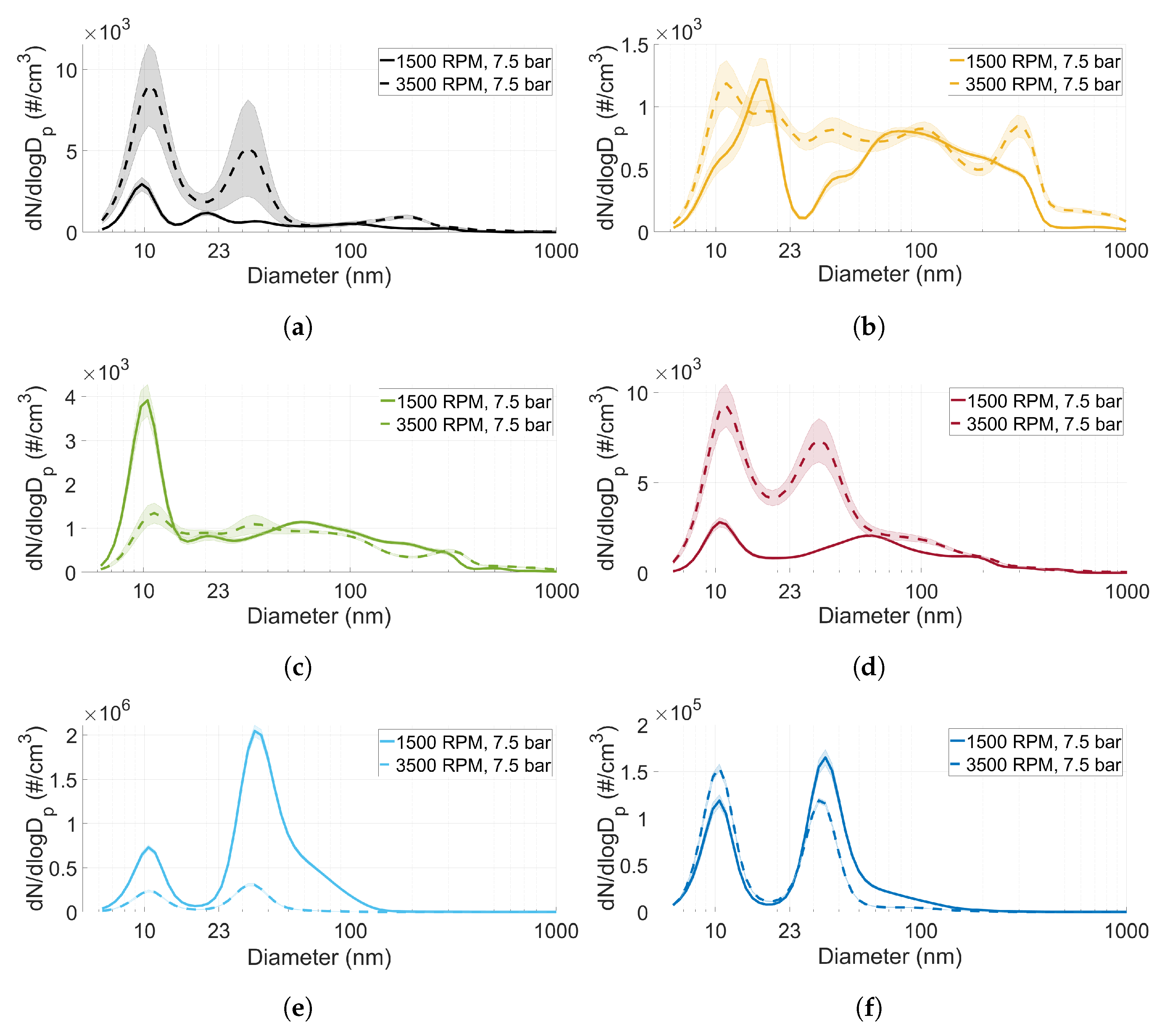
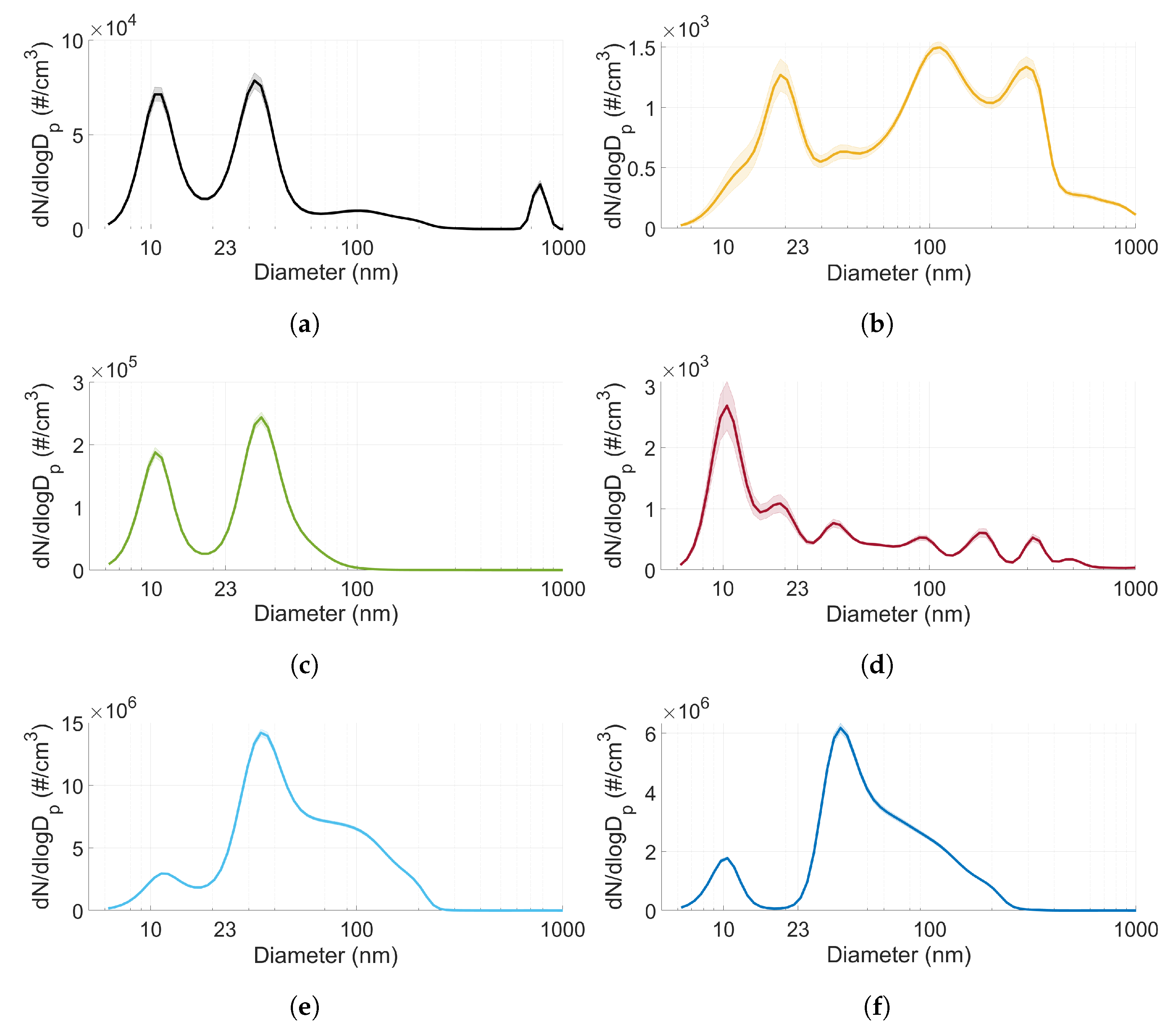
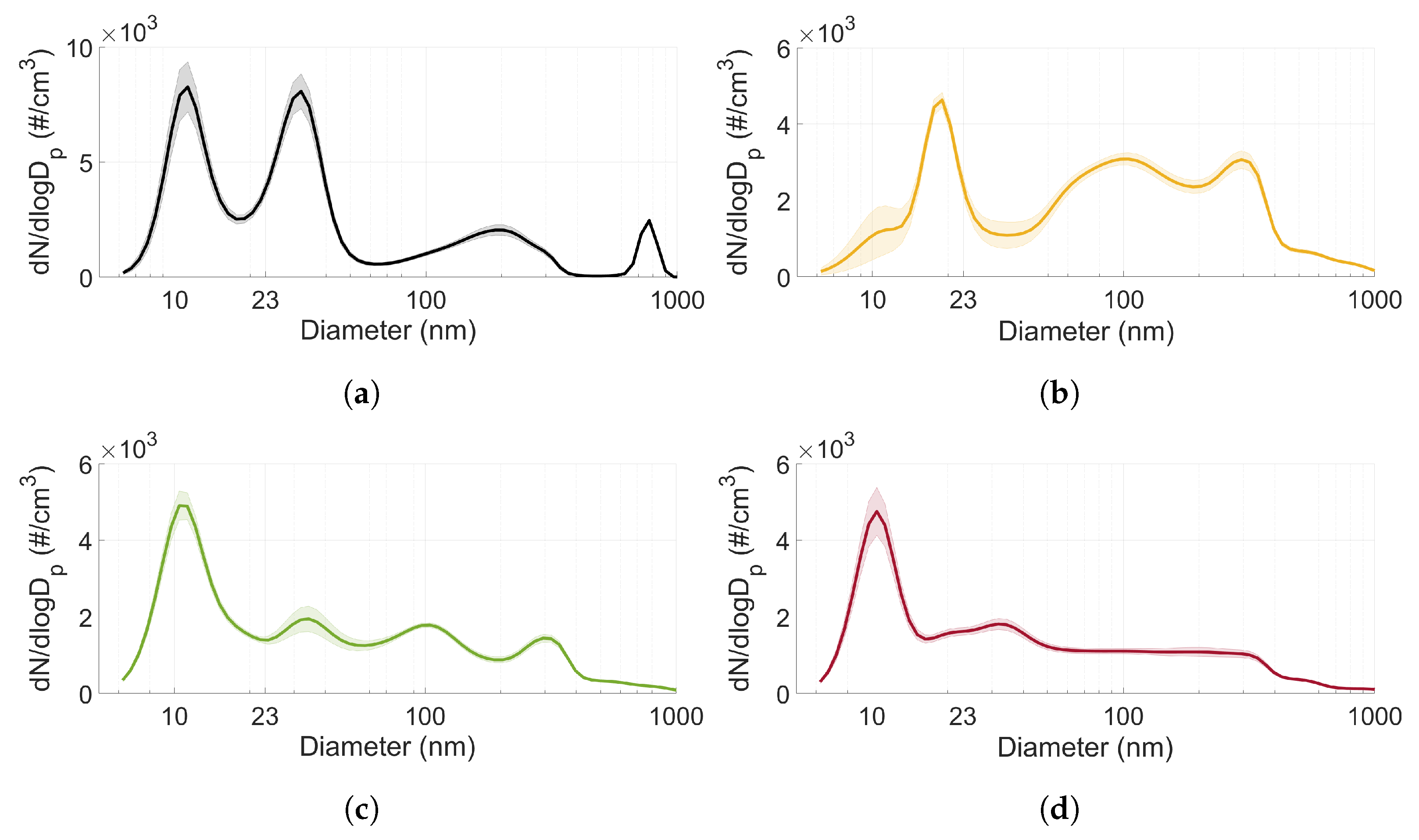
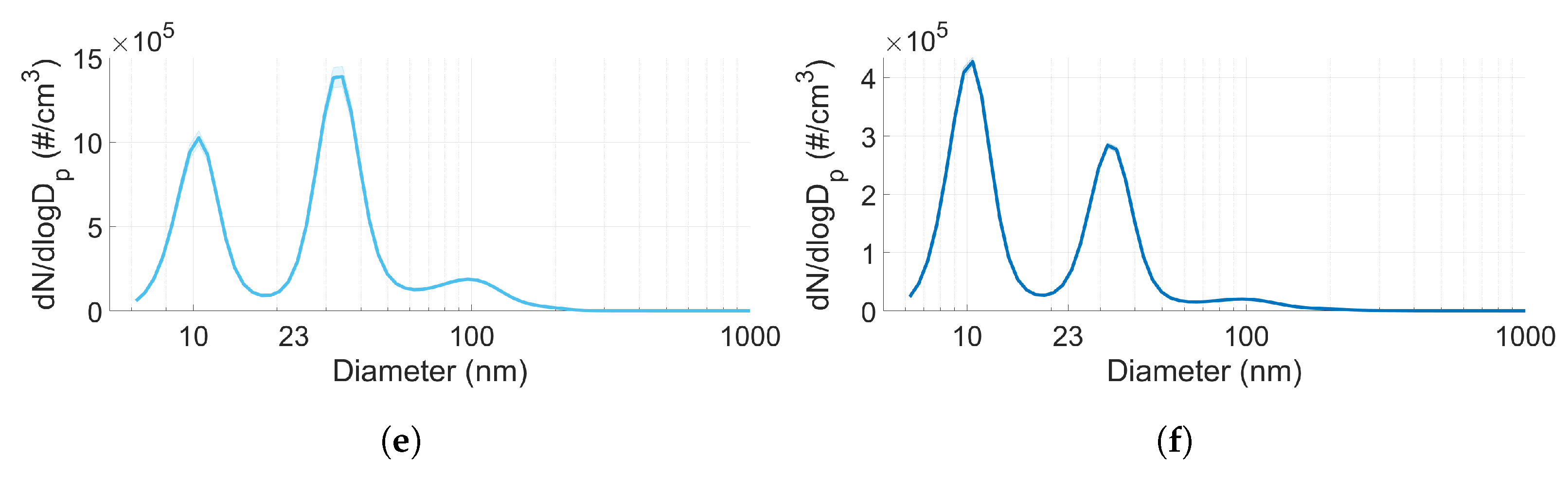
3.3. Particle Size Distribution
3.4. Comparison of Undiluted and Diluted Particle Measurements
3.5. Elemental Analysis
4. Discussion
5. Conclusions
- The particle number emissions increased with the utilisation of oxygenated biofuels, compared to gasoline, in a gasoline-optimised DISI engine.
- For the oxygenated fuels, the highest number of emissions was seen for n-butanol, and the lowest number was seen for MTBE.
- The particle size distribution seems to depend on the type of fuel used. MTBE exhibited a significantly higher ratio of accumulation mode particles compared to the other fuels (including gasoline).
- Gasoline exhibited a higher mass ratio of zinc and phosphorus in the particles than the biofuels.
- For spray-guided DISI engines, the volatility of the fuel is the main fuel property to consider for the optimisation of the fuel injection in regard to decreasing particle emissions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Normalised random error | |
| Excess air ratio | |
| Mean value | |
| Standard deviation | |
| bTDC | before top dead center |
| C | constant for the confidence level (1.96) |
| CA | Crank angle |
| CO | Carbon monoxide |
| C/O | Carbon oxygen ratio |
| CPC | Condensation particle counter |
| DISI | Direct-injected spark-ignited |
| DVPE | Dry vapour pressure equivalent |
| ECU | Engine control unit |
| EDXRF | Energy-Dispersive X-ray Fluorescence |
| EOI | End of injection |
| HC | Unburned hydrocarbons |
| HOV | Heat of vaporisation |
| HT-ELPI+ | High-temperature Electrical Low-Pressure Impactor |
| i-butanol | Iso-butanol |
| ICE | Internal combustion engine |
| IMEP | Indicated mean effective pressure |
| KLSA | Knock limited spark advance |
| LHV | Lower heating value |
| mass flow of air | |
| mass flow of fuel | |
| MBT | Maximum brake torque |
| MON | Motor octane number |
| MTBE | Methyl tert-butyl ether |
| n | number of samples |
| NI | National Instruments |
| NOx | Nitrogen oxide |
| In-cylinder pressure | |
| PFI | Port-fuel injected |
| Injection pressure | |
| PM | Particle mass |
| PN | Particle number |
| RDE | Real driving emissions |
| RON | Research octane number |
| RPM | Revolutions per minute |
| SI | Spark-ignited |
| SOI | Start of injection |
| Temperature after three-way catalyst | |
| Exhaust temperature | |
| Injection duration | |
| TWC | Three-way catalyst |
Appendix A. Particle Loss Calculations
| Type of Loss | CPCs | HT-ELPI+ | ||||
|---|---|---|---|---|---|---|
| 10 nm | 100 nm | 1000 nm | 10 nm | 100 nm | 1000 nm | |
| Thermophoresis | 27.8 | 24.4 | ||||
| Sedimentation | 7.0 × 10 | 8.7 × 10 | 0.34 | 2.7 × 10 | 0.03 | 1.36 |
| Diffusion | 3.0 | 0.14 | 0.02 | 5.1 | 0.24 | 0.04 |
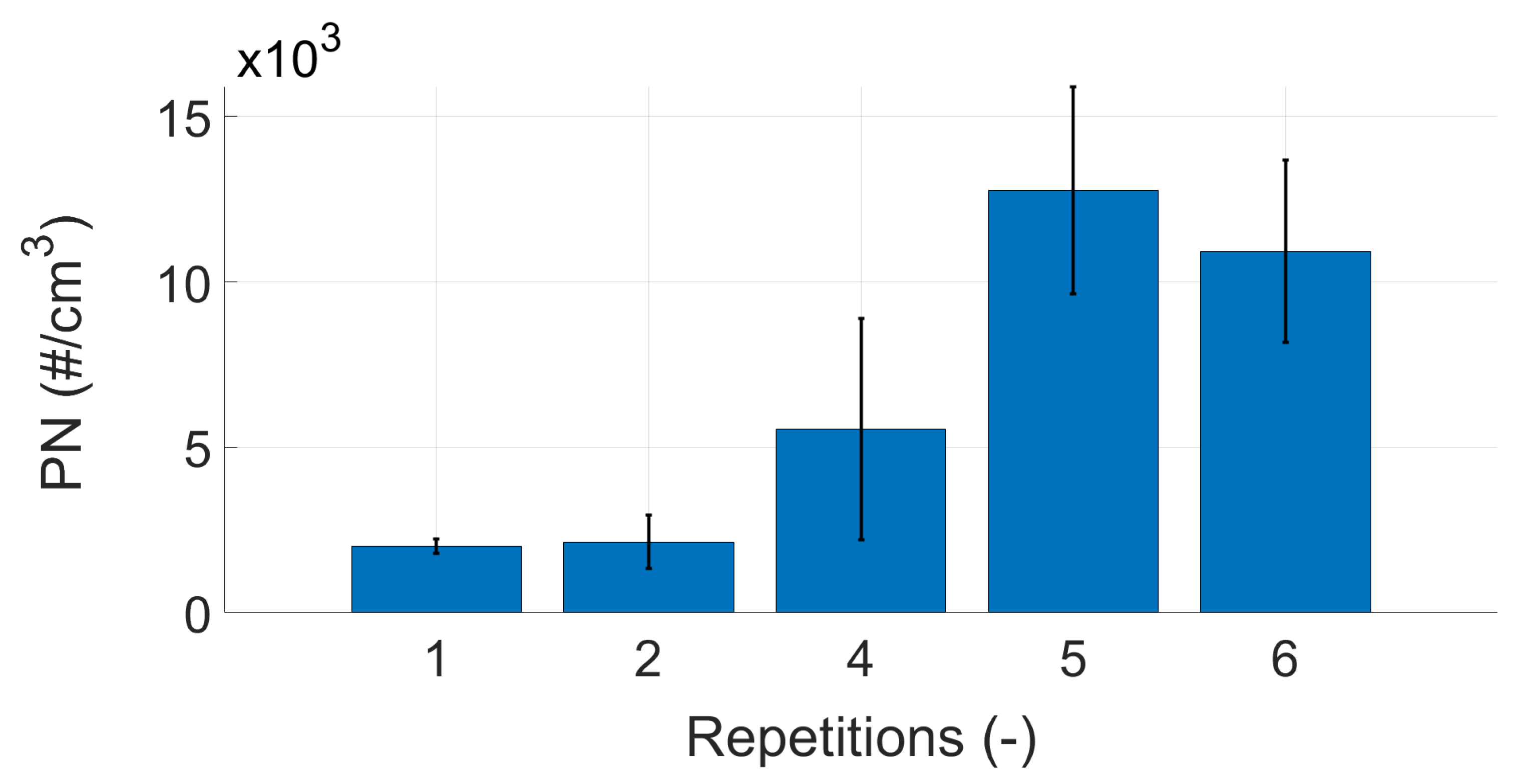
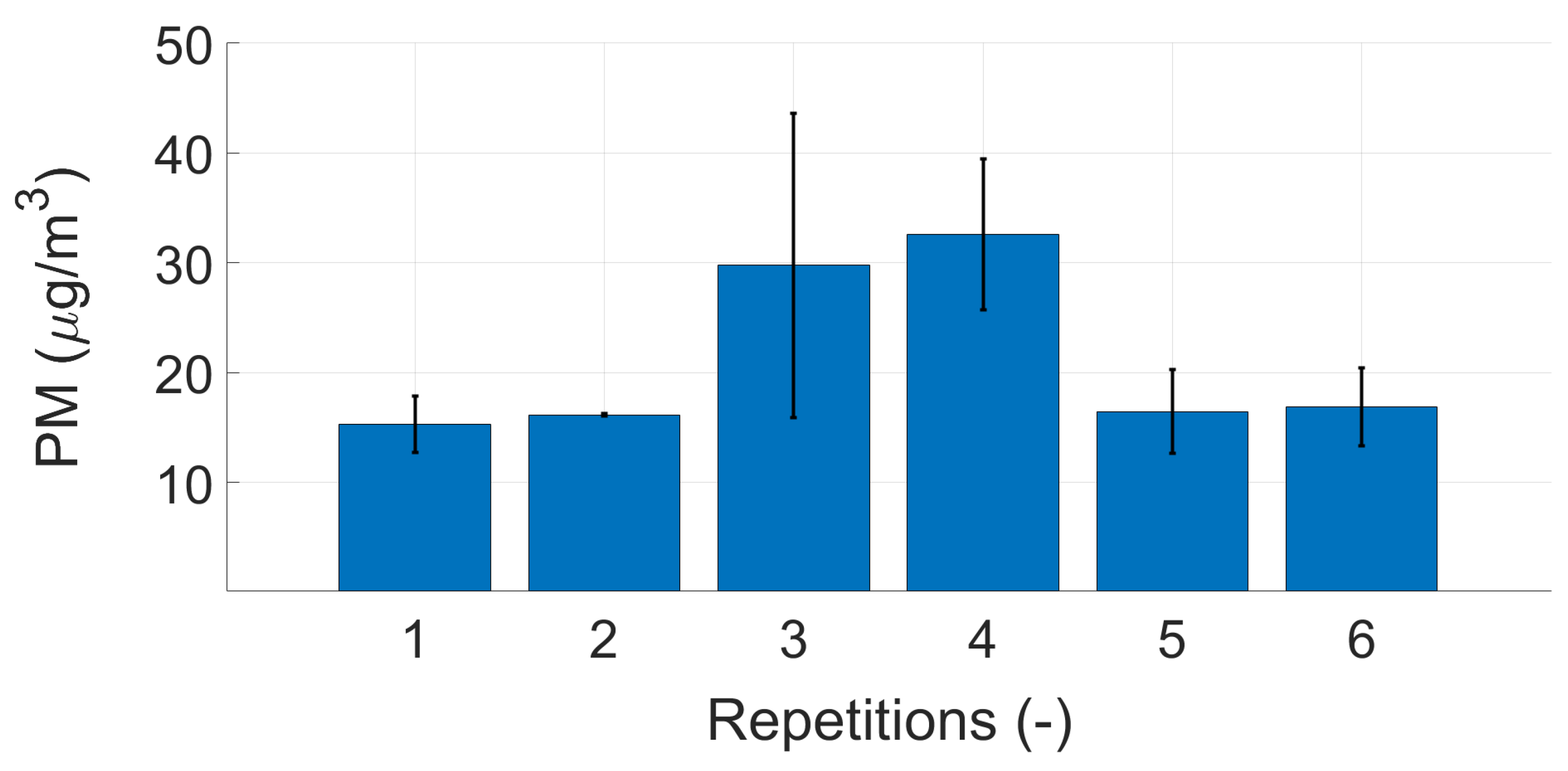
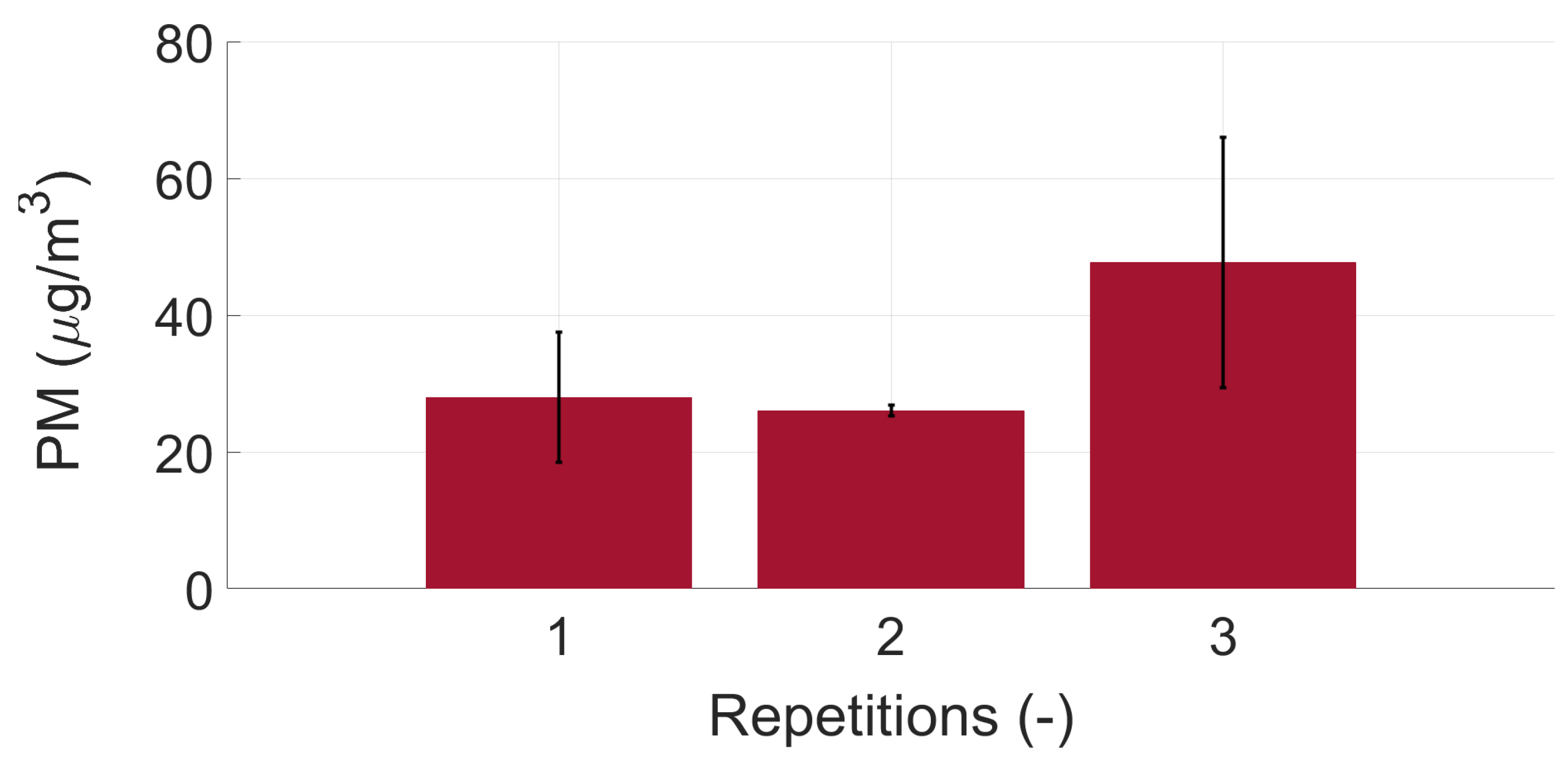
Appendix B. Repeatability Measurements

References
- Rönkkö, T.; Kuuluvainen, H.; Karjalainen, P.; Keskinen, J.; Hillamo, R.; Niemi, J.V.; Pirjola, L.; Timonen, H.J.; Saarikoski, S.; Saukko, E.; et al. Traffic is a major source of atmospheric nanocluster aerosol. Proc. Natl. Acad. Sci. USA 2017, 114, 7549–7554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, F.; Knorsch, T.; Laidig, C.; Wiese, W. A Review of the Requirements for Injection Systems and the Effects of Fuel Quality on Particulate Emissions from GDI Engines. In International Powertrains, Fuels & Lubricants Meeting; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Becker, S.; Soukup, J.M.; Sioutas, C.; Cassee, F.R. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp. Lung Res. 2003, 29, 29–44. [Google Scholar] [CrossRef] [PubMed]
- European Vehicle Emissions Standards—Euro 7 for Cars, Vans, Lorries and Buses. Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12313-European-vehicle-emissions-standards-Euro-7-for-cars-vans-lorries-and-buses_en/ (accessed on 18 May 2021).
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions; SAE WCX Digital Summit; SAE International: Warrendale, PA, USA, 2021. [Google Scholar]
- Raza, M.; Chen, L.; Leach, F.; Ding, S. A Review of Particulate Number (PN) Emissions from Gasoline Direct Injection (GDI) Engines and Their Control Techniques. Energies 2018, 6, 1417. [Google Scholar] [CrossRef] [Green Version]
- Seong, H.; Choi, S.; Zaluzec, N.J.; Lee, S.; Wu, T.; Shao, H.; Remias, J.E. Identification of engine oil-derived ash nanoparticles and ash formation process for a gasoline direct-injection engine. Environ. Pollut. 2021, 272, 116390. [Google Scholar] [CrossRef]
- Abdul-Khalek, I.S.; Kittelson, D.B.; Graskow, B.R.; Wei, Q. Diesel Exhaust Particle Size: Measurement Issues and Trends. In International Congress & Exposition; SAE International: Warrendale, PA, USA, 1998. [Google Scholar] [CrossRef]
- Miller, A.L.; Stipe, C.B.; Habjan, M.C.; Ahlstrand, G.G. Role of Lubrication Oil in Particulate Emissions from a Hydrogen-Powered Internal Combustion Engine. Environ. Sci. Technol. 2007, 41, 6828–6835. [Google Scholar] [CrossRef]
- Khuong, L.S.; Masjuki, H.H.; Zulkifli, N.W.M.; Mohamad, E.N.; Kalam, M.A.; Alabdulkarem, A.; Arslan, A.; Mosarof, M.H.; Syahir, A.Z.; Jamshaid, M. Effect of gasoline–bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Adv. 2017, 7, 15005–15019. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, S.; Lazzaro, M.; Sementa, P.; Vaglieco, B.M.; Catapano, F. Use of Renewable Oxygenated Fuels in Order to Reduce Particle Emissions from a GDI High Performance Engine. In SAE 2011 World Congress & Exhibition; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Price, P.; Twiney, B.; Stone, R.; Kar, K.; Walmsley, H. Particulate and Hydrocarbon Emissions from a Spray Guided Direct Injection Spark Ignition Engine with Oxygenate Fuel Blends. In SAE World Congress & Exhibition; SAE International: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.L.; Asa-Awuku, A.; Jung, H.; Johnson, K.C.; Durbin, T.D. The impact of ethanol and iso-butanol blends on gaseous and particulate emissions from two passenger cars equipped with spray-guided and wall-guided direct injection SI (spark ignition) engines. Energy 2015, 82, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Anselmi, P.; Matrat, M.; Starck, L.; Duffour, F. Combustion characteristics of oxygenated fuels Ethanol-and Butanol-gasoline fuel blends, and their impact on performance, emissions and Soot Index. In 2019 JSAE/SAE Powertrains, Fuels and Lubricants; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Herreros, J.M.; Wang, J.; Cracknell, R. Impact of fuel and injection system on particle emissions from a GDI engine. Appl. Energy 2014, 132, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, S.; Lazzaro, M.; Sementa, P.; Vaglieco, B.M.; Catapano, F. Particle Size Distributions from a DI High Performance SI Engine Fuelled with Gasoline-Ethanol Blended Fuels. In Proceedings of the 10th International Conference on Engines & Vehicles, Warrendale, PA, USA, 11 September 2011; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Qin, J.; Li, X.; Pei, Y. Effects of Combustion Parameters and Lubricating Oil on Particulate Matter Emissions from a Turbo-Charged GDI Engine Fueled with Methanol/Gasoline Blends. In Proceedings of the SAE 2014 International Powertrain, Fuels & Lubricants Meeting, Warrendale, PA, USA, 13 October 2014; SAE International: Warrendale, PA, USA, 2014. [Google Scholar] [CrossRef]
- Wang, X.; Ge, Y.; Liu, L.; Peng, Z.; Hao, L.; Yin, H.; Ding, Y.; Wang, J. Evaluation on toxic reduction and fuel economy of a gasoline direct injection- (GDI-) powered passenger car fueled with methanol–gasoline blends with various substitution ratios. Appl. Energy 2015, 157, 134–143. [Google Scholar] [CrossRef]
- Yu, X.; Guo, Z.; He, L.; Dong, W.; Sun, P.; Shi, W.; Du, Y.; He, F. Effect of gasoline/n-butanol blends on gaseous and particle emissions from an SI direct injection engine. Fuel 2018, 229, 1–10. [Google Scholar] [CrossRef]
- Vojtisek-Lom, M.; Beranek, V.; Stolcpartova, J.; Pechout, M.; Klir, V. Effects of n-Butanol and Isobutanol on Particulate Matter Emissions from a Euro 6 Direct-injection Spark Ignition Engine During Laboratory and on-Road Tests. SAE Int. J. Engines 2015, 8, 2338–2350. [Google Scholar] [CrossRef]
- Larsson, T.; Prasath, A.; Olofsson, U.; Erlandsson, A. Undiluted Measurement of sub 10 nm Non-Volatile and Volatile Particle Emissions from a DISI Engine Fueled with Gasoline and Ethanol. In SAE WCX Digital Summit; SAE International: Warrendale, PA, USA, 2021. [Google Scholar]
- Salamanca, M.; Sirignano, M.; D’Anna, A. Particulate Formation in Premixed and Counter-flow Diffusion Ethylene/Ethanol Flames. Energy Fuels 2012, 26, 6144–6152. [Google Scholar] [CrossRef]
- Lee, Z.; Park, S. Particulate and gaseous emissions from a direct-injection spark ignition engine fueled with bioethanol and gasoline blends at ultra-high injection pressure. Renew. Energy 2020, 149, 80–90. [Google Scholar] [CrossRef]
- Bonatesta, F.; Chiappetta, E.; La Rocca, A. Part-load particulate matter from a GDI engine and the connection with combustion characteristics. Appl. Energy 2014, 124, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Lyyränen, J.; Jokiniemi, J.; Kauppinen, E.I.; Backman, U.; Vesala, H. Comparison of Different Dilution Methods for Measuring Diesel Particle Emissions. Aerosol Sci. Technol. 2004, 38, 12–23. [Google Scholar] [CrossRef]
- Suresh, A.; Johnson, J.H. A Study of the Dilution Effects on Particle Size Measurement from a Heavy-Duty Diesel Engine with EGR. In SAE 2001 World Congress; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Kayes, D.; Hochgreb, S. Investigation of the Dilution Process for Measurement of Particulate Matter from Spark-Ignition Engines. In International Fall Fuels and Lubricants Meeting and Exposition; SAE International: Warrendale, PA, USA, 1998. [Google Scholar] [CrossRef]
- Alozie, N.S.; Peirce, D.; Lindner, A.; Winklmayr, W.; Ganippa, L. Influence of Dilution Conditions on Diesel Exhaust Particle Measurement Using a Mixing Tube Diluter. In SAE 2014 World Congress & Exhibition; SAE International: Warrendale, PA, USA, 2014. [Google Scholar] [CrossRef]
- Dekati. Dekati Diluter. 2020. Available online: https://www.dekati.com/wp-content/uploads/dekatidiluterservices.pdf (accessed on 1 February 2021).
- Bernemyr, H.; Erlandsson, A. Comparison of Two Dilution and Conditioning Systems for Particle Number Measurements along the Exhaust After-Treatment System of an HD Diesel Engine. In SAE WCX Digital Summit; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Dekati. Dekati High Temperature ELPI+. 2018. Available online: https://www.dekati.com/products/high-temperature-elpi/ (accessed on 1 February 2021).
- Babazadeh Shayan, S.; Seyedpour, S.M.; Ommi, F. Effect of oxygenates blending with gasoline to improve fuel properties. Chin. J. Mech. Eng. 2012, 25, 792–797. [Google Scholar] [CrossRef]
- Perricone, G.; Matĕjka, V.; Alemani, M.; Wahlström, J.; Olofsson, U. A Test Stand Study on the Volatile Emissions of a Passenger Car Brake Assembly. Atmosphere 2019, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 1999. [Google Scholar]
- Vanhanen, J.; Svedberg, M.; Miettinen, E.; Salo, J.P.; Väkevä, M. Dekati Diluter Characterization in the 1-20 nm Particle Size Range. 2017. Available online: https://www.researchgate.net/publication/319416891_Dekati_Diluter_characterization_in_the_1-20_nm_particle_size_range (accessed on 1 February 2021).
- Giechaskiel, B.; Schindler, W.; Jörgl, H.; Vescoli, V.; Bergmann, A.; Silvis, W. Accuracy of Particle Number Measurements from Partial Flow Dilution Systems. In Proceedings of the 10th International Conference on Engines Vehicles, Warrendale, PA, USA, 11 September 2011; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Leach, F.; Stone, R.; Fennell, D.; Hayden, D.; Richardson, D.; Wicks, N. Predicting the particulate matter emissions from spray-guided gasoline direct-injection spark ignition engines. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2017, 231, 717–730. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Arndt, M.; Schindler, W.; Bergmann, A.; Drossinos, Y.; Silvis, W. Sampling of Non-Volatile Vehicle Exhaust Particles: A Simplified Guide. SAE Int. J. Engines 2012, 5, 379–399. [Google Scholar] [CrossRef]
- Ferraro, F.; Russo, C.; Schmitz, R.; Hasse, C.; Sirignano, M. Experimental and numerical study on the effect of oxymethylene ether-3 (OME3) on soot particle formation. Fuel 2021, 286, 119353. [Google Scholar] [CrossRef]
- Hua, Y.; Liu, F.; Wu, H.; Lee, C.F.; Li, Y. Effects of alcohol addition to traditional fuels on soot formation: A review. Int. J. Engine Res. 2021, 22, 1395–1420. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, Y.; Pan, M.; Zhuang, Y.; Qiu, L.; Zhou, T.; Liu, Y. Morphology analysis of soot particles from a modern diesel engine fueled with different types of oxygenated fuels. Fuel 2020, 267, 117248. [Google Scholar] [CrossRef]
- Verma, P.; Jafari, M.; Rahman, S.A.; Pickering, E.; Stevanovic, S.; Dowell, A.; Brown, R.; Ristovski, Z. The impact of chemical composition of oxygenated fuels on morphology and nanostructure of soot particles. Fuel 2020, 259, 116167. [Google Scholar] [CrossRef]
- Stevens, E.; Steeper, R. Piston Wetting in an Optical DISI Engine: Fuel Films, Pool Fires, and Soot Generation. In SAE 2001 World Congress; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Hua, Y.; Wu, H.; fon Lee, C.; Shi, Z. Experimental and kinetic studies of soot formation in methanol-gasoline coflow diffusion flames. J. Energy Inst. 2019, 92, 38–50. [Google Scholar] [CrossRef]
- Liu, F.; Hua, Y.; Wu, H.; Lee, C.F.; He, X. Effect of Alcohol Addition to Gasoline on Soot Distribution Characteristics in Laminar Diffusion Flames. Chem. Eng. Technol. 2018, 41, 897–906. [Google Scholar] [CrossRef]
- David, K.; Simone, H. Mechanisms of Particulate Matter Formation in Spark-Ignition Engines. 1. Effect of Engine Operating Conditions. Environ. Sci. Technol. 1999, 33, 3957–3967. [Google Scholar] [CrossRef]
- Camacho, J.; Lieb, S.; Wang, H. Evolution of size distribution of nascent soot in n- and i-butanol flames. Proc. Combust. Inst. 2013, 34, 1853–1860. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Rönkkö, T.; Pirjola, L.; Ntziachristos, L.; Heikkilä, J.; Karjalainen, P.; Hillamo, R.; Keskinen, J. Vehicle Engines Produce Exhaust Nanoparticles Even When Not Fueled. Environ. Sci. Technol. 2014, 48, 2043–2050. [Google Scholar] [CrossRef]
- Phosphorus—US Environmental Protection Agency. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/phosphorus.pdf (accessed on 27 October 2021).
- Gordon, T.; Chen, L.; Fine, J.; Schlesinger, R.; Su, W.; Kimmel, T.; MO, A. Pulmonary effects of inhaled zinc oxide in human subjects, guinea pigs, rats, and rabbits. Am. Ind. Hyg. Assoc. J. 1992, 53, 503–509. [Google Scholar] [CrossRef] [PubMed]
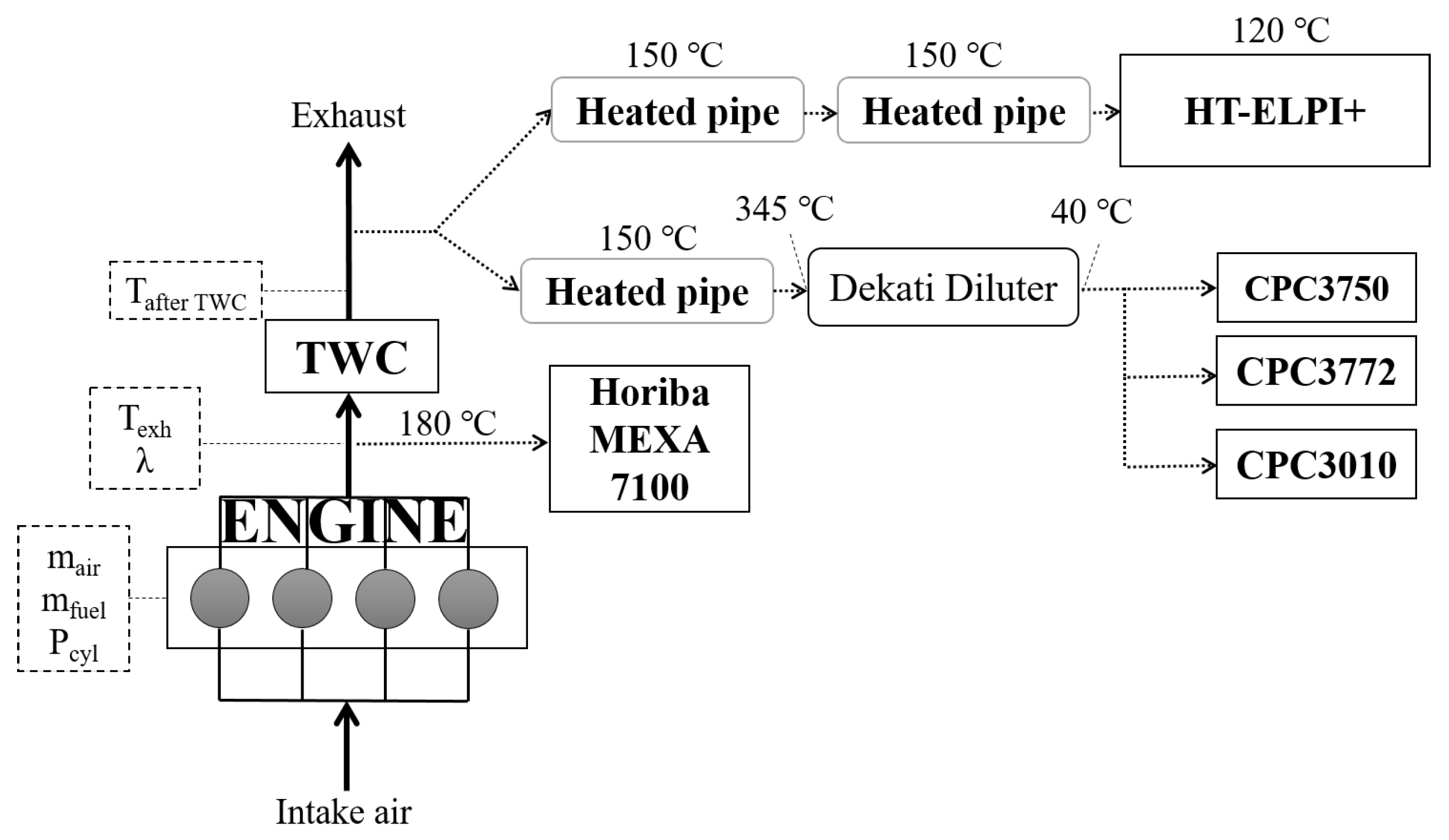

| Displacement volume | 1969 cc |
| Stroke | 93.2 mm |
| Bore | 82 mm |
| Connecting rod | 143.8 mm |
| Compression ratio | 10.3 |
| Number of cylinders | 4 (inline) |
| Peak power | 225 kW (5700 RPM) |
| Peak injection pressure | 200 bar |
| Fuel | RON (-) | MON (-) | DVPE (kPa) | LHV (MJ/kg) | Oxygen Content (wt-%) |
|---|---|---|---|---|---|
| Gasoline | 93.7 | 84.9 | 52.5 | 43 | 0 |
| Ethanol | 107.2 | 89.5 | 16.5 | 26.8 | 34.7 |
| Methanol | 108 | 88.7 | 30.9 | 19.7 | 49.9 |
| N-butanol | 98 | 84.7 | <9.0 (2.3) | 33.1 | 21.6 |
| I-butanol | 104.6 | 89.6 | <9.0 (1.8) | 33.2 | 21.6 |
| MTBE | 116 [32] | 101 [32] | 53.6 | 38.2 | 18.2 |
| Measurement | Sensor | Range | Uncertainty |
|---|---|---|---|
| ( % FS) | |||
| In-cylinder pressure | Kistler 6045A32420 | 0–250 Bar | 0.4 |
| Kistler Noridc AB, | |||
| Jonsered, Sweden | |||
| Lambda | Bosch O2-sensor LSU 4.9 | 0.65–∞ | 0.7 ( = 1) |
| Via National Instruments, | |||
| Budapest, Hungary | |||
| CO emissions | Horiba MEXA-7100 | 0–7000 ppm | |
| HC emissions | Horiba Europe, | 0–5000 ppm | 2 |
| NOx emissions | Guthenburg, Sweden | 0–5000 ppm | |
| Particle number ≥ 23 nm | TSI CPC3010 | ≤10,000 #/cm3 | 10 |
| TSI Europe, | |||
| Aachen, Germany | |||
| Particle number ≥ 10 nm | TSI CPC3772 | ≤10,000 #/cm3 | 10 |
| TSI Europe, | |||
| Aachen, Germany | |||
| Particle number ≥ 7 nm | TSI CPC3750 | ≤10 #/cm3 | 5 |
| TSI Europe, | |||
| Aachen, Germany | |||
| Particle size distribution | Dekati HT-ELPI+ | 6 nm–10 μm | 20 |
| Dekati, | |||
| Kangasala, Finland |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsson, T.; Olofsson, U.; Christiansen Erlandsson, A. Undiluted Measurement of the Particle Size Distribution of Different Oxygenated Biofuels in a Gasoline-Optimised DISI Engine. Atmosphere 2021, 12, 1493. https://doi.org/10.3390/atmos12111493
Larsson T, Olofsson U, Christiansen Erlandsson A. Undiluted Measurement of the Particle Size Distribution of Different Oxygenated Biofuels in a Gasoline-Optimised DISI Engine. Atmosphere. 2021; 12(11):1493. https://doi.org/10.3390/atmos12111493
Chicago/Turabian StyleLarsson, Tara, Ulf Olofsson, and Anders Christiansen Erlandsson. 2021. "Undiluted Measurement of the Particle Size Distribution of Different Oxygenated Biofuels in a Gasoline-Optimised DISI Engine" Atmosphere 12, no. 11: 1493. https://doi.org/10.3390/atmos12111493
APA StyleLarsson, T., Olofsson, U., & Christiansen Erlandsson, A. (2021). Undiluted Measurement of the Particle Size Distribution of Different Oxygenated Biofuels in a Gasoline-Optimised DISI Engine. Atmosphere, 12(11), 1493. https://doi.org/10.3390/atmos12111493







