Organic Compounds and Suspended Particulate Matter in Snow of High Latitude Areas (Arctic and Antarctic)
Abstract
1. Introduction
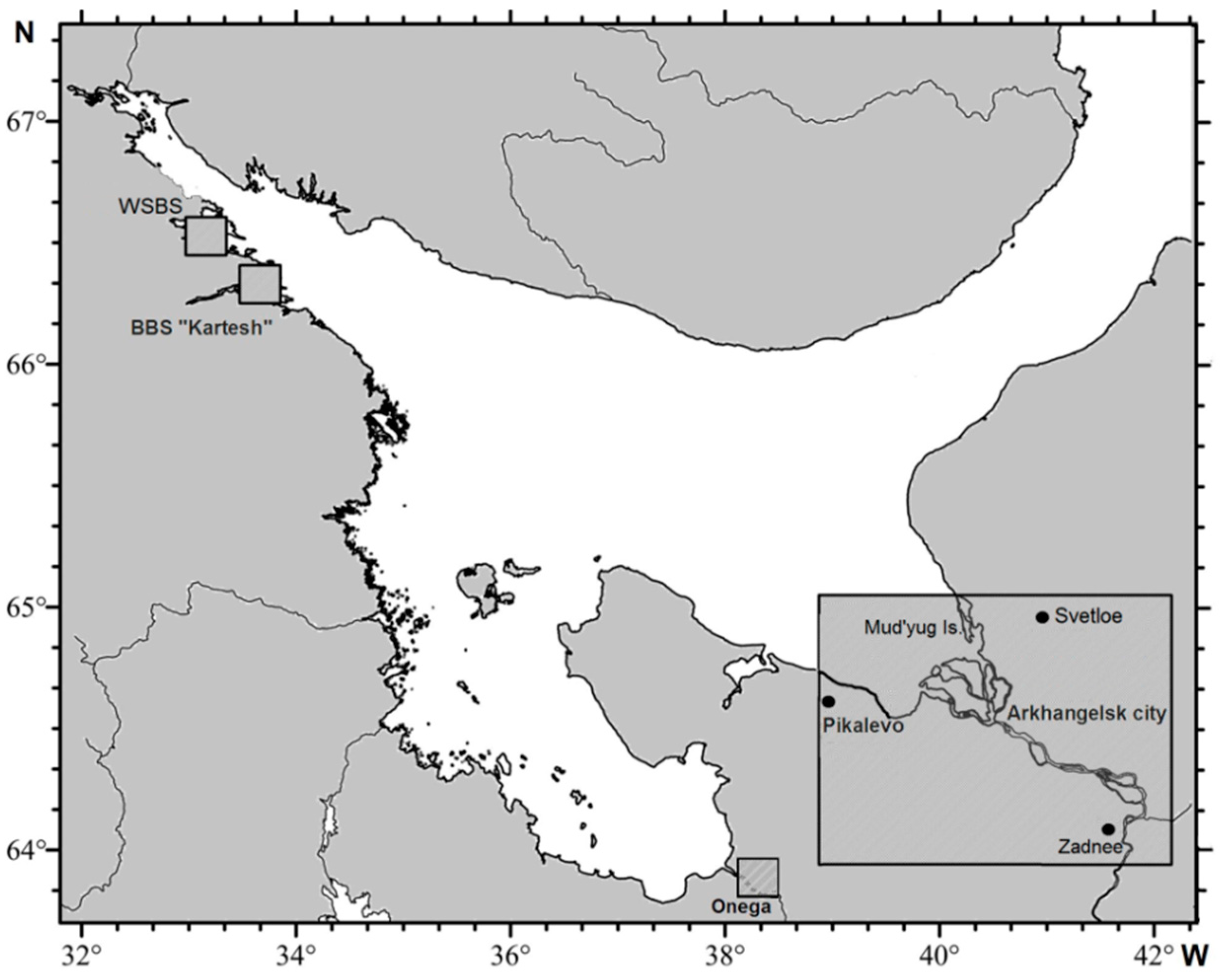
2. Methods of the Studies
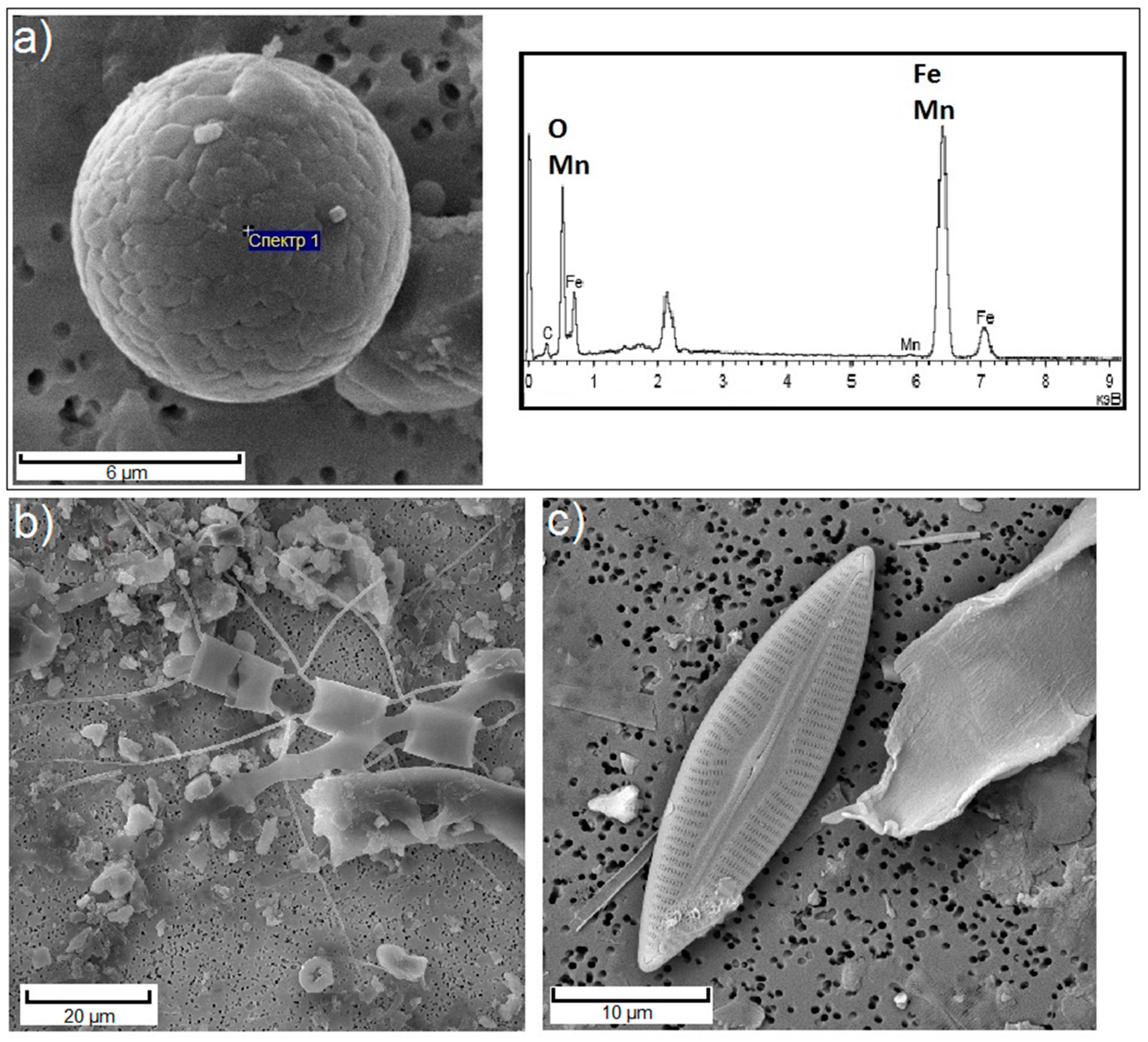
3. Results
3.1. Arctic
3.1.1. Background Areas
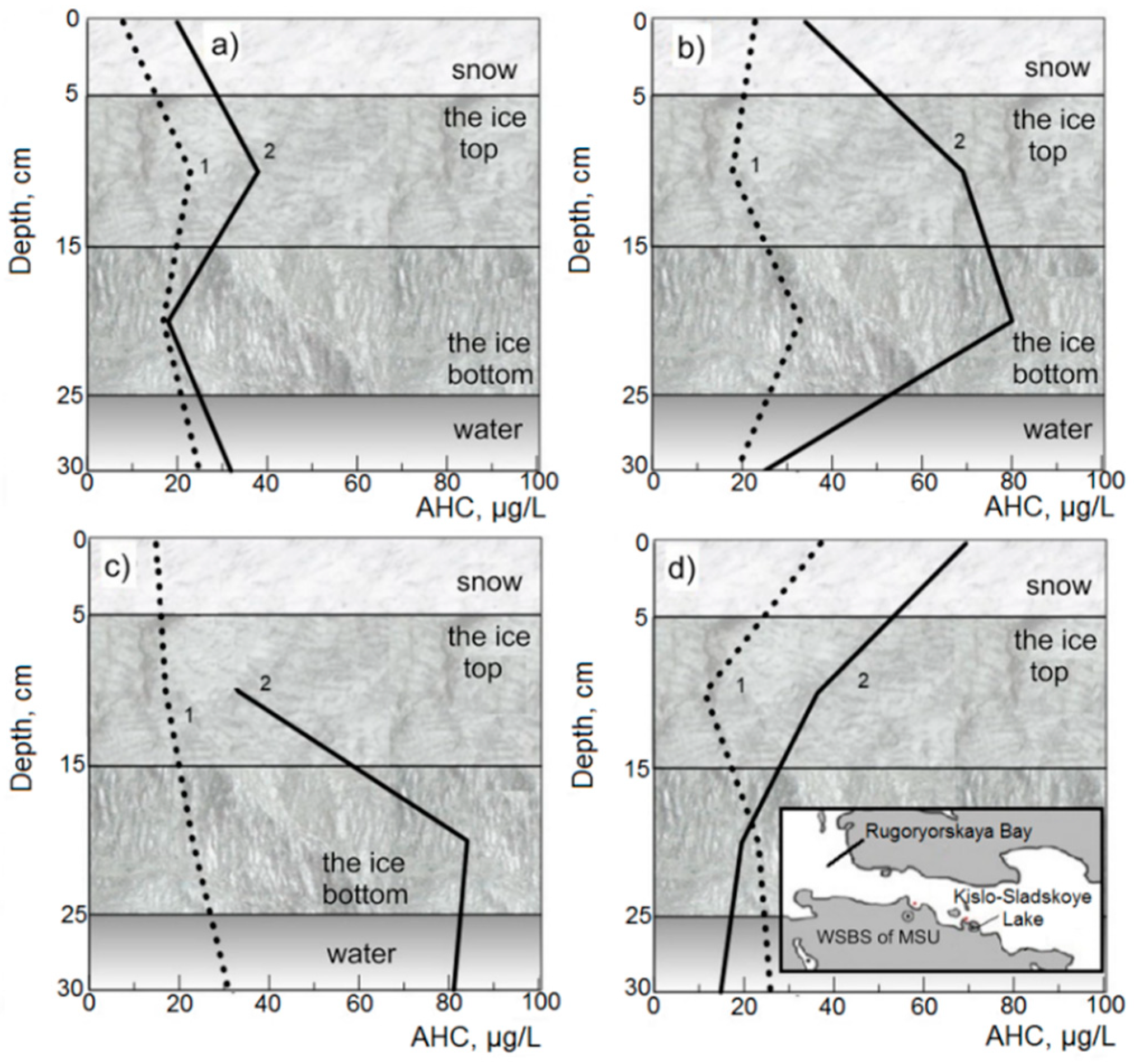
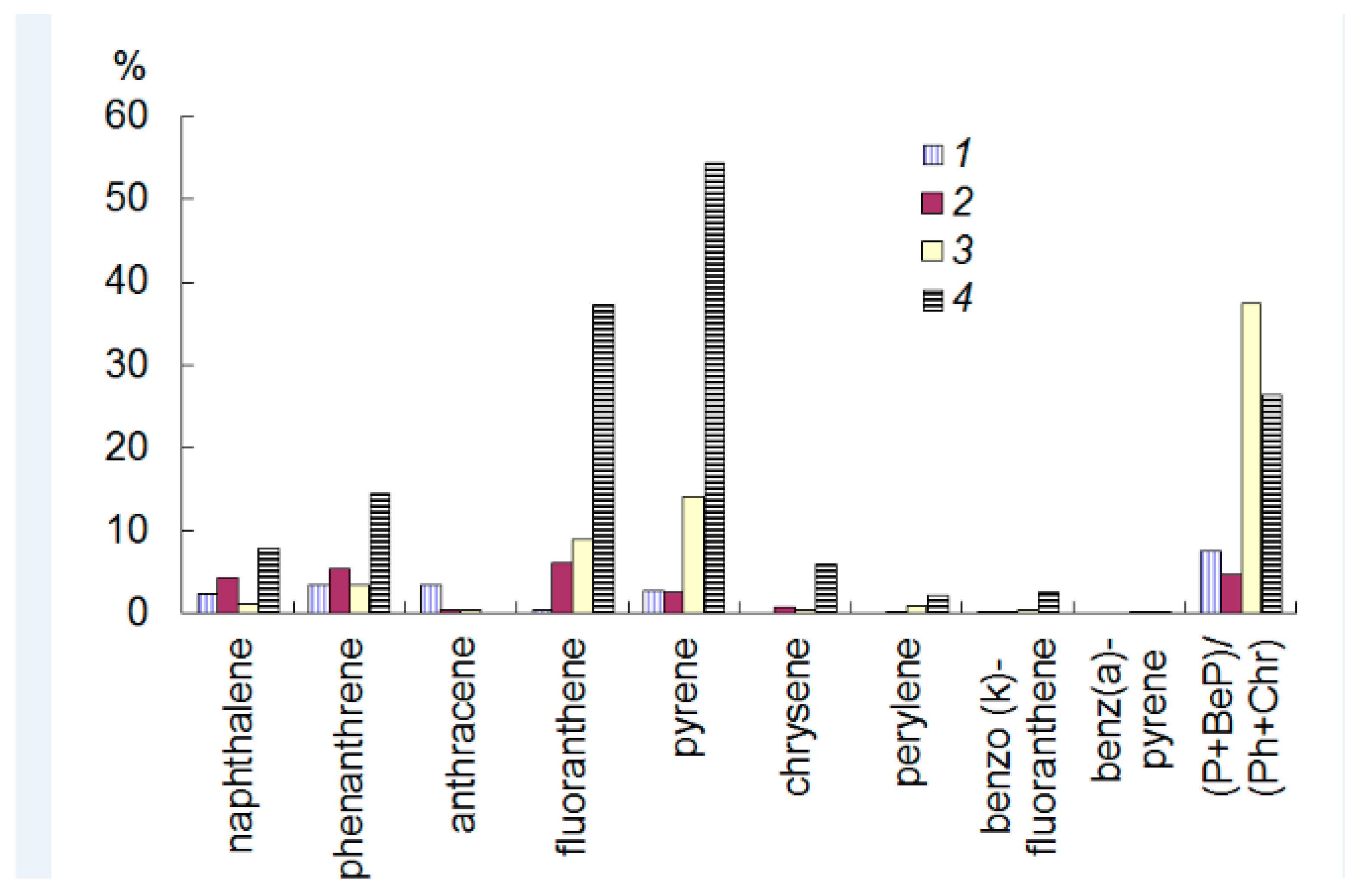
3.1.2. Impact Areas (White Sea)
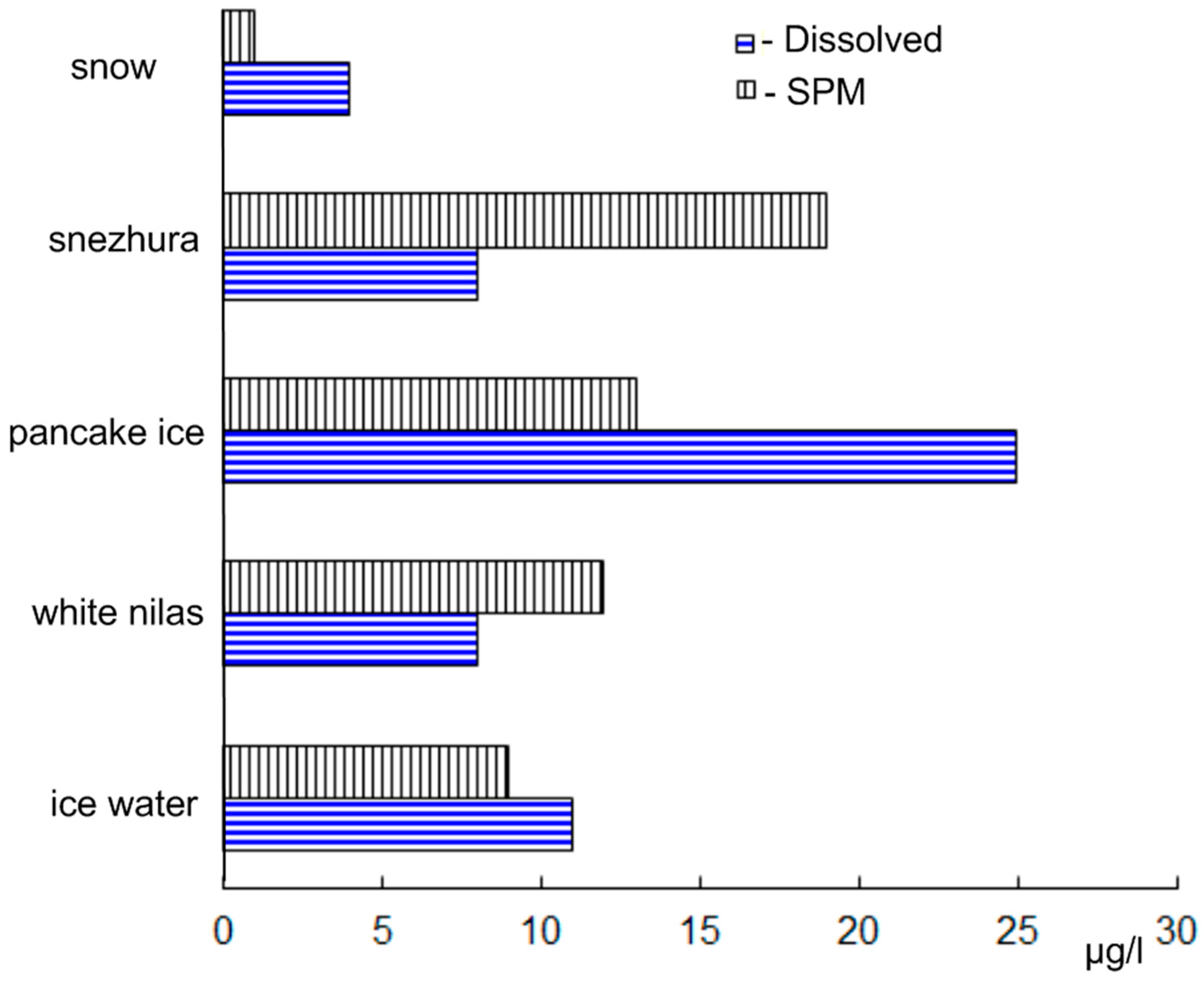
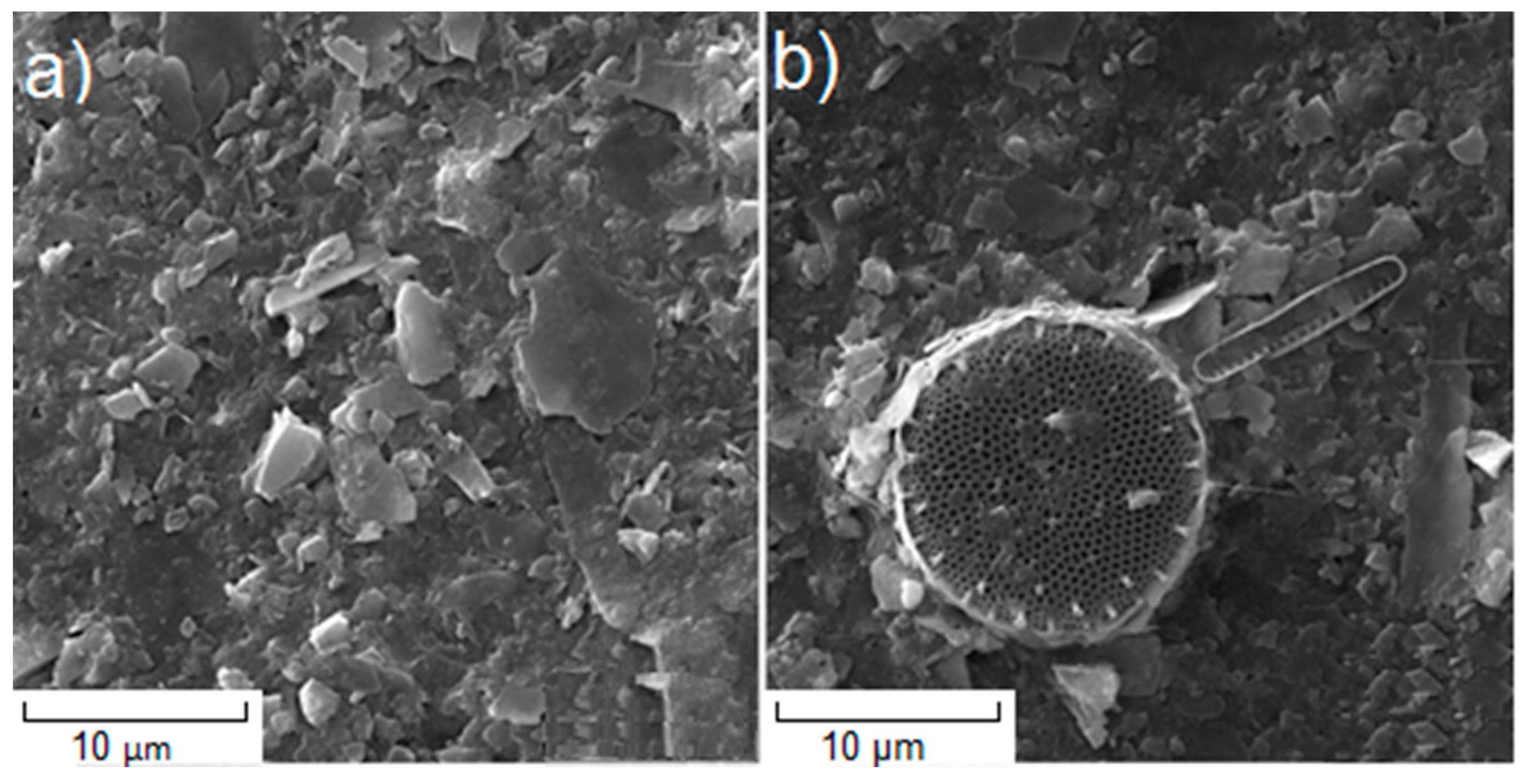
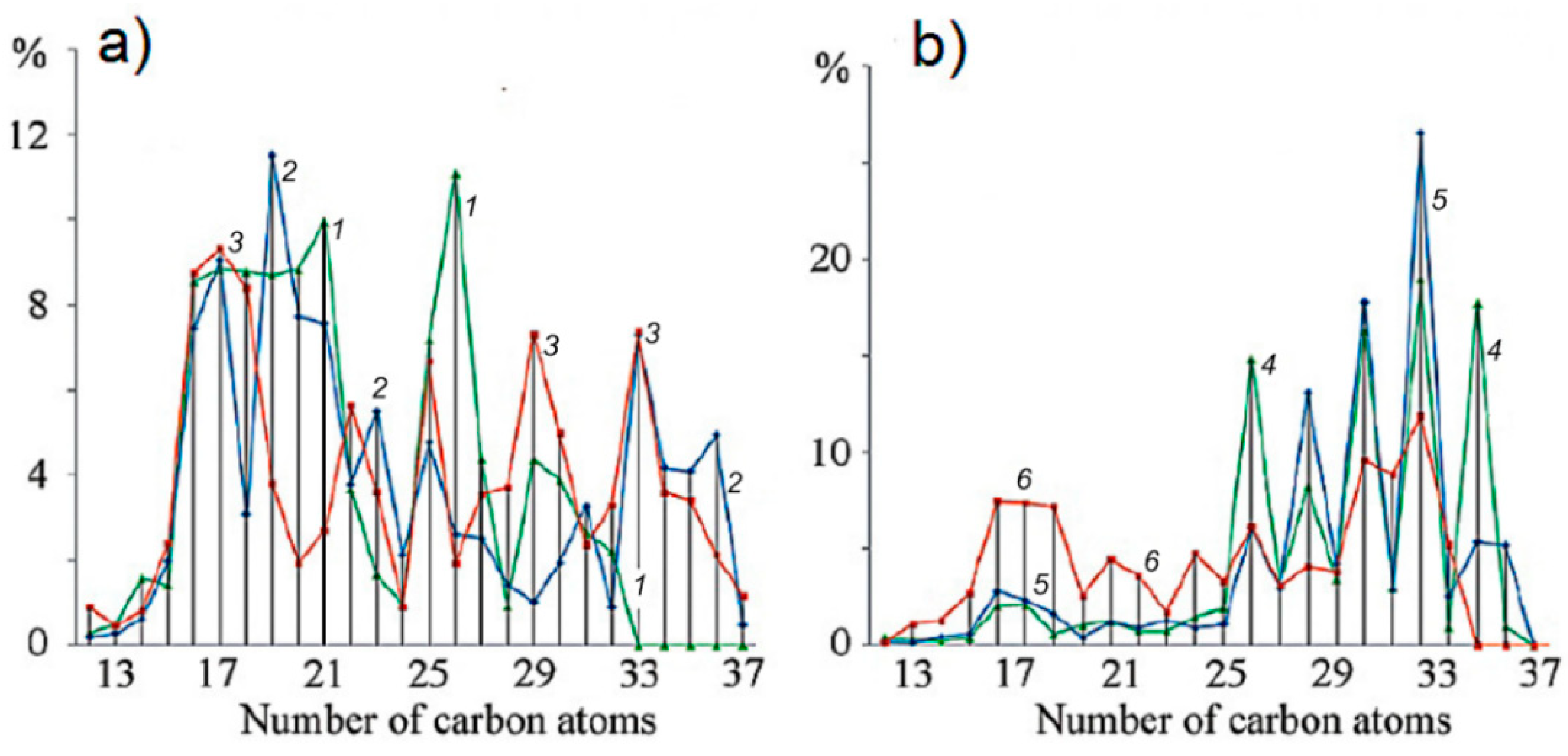
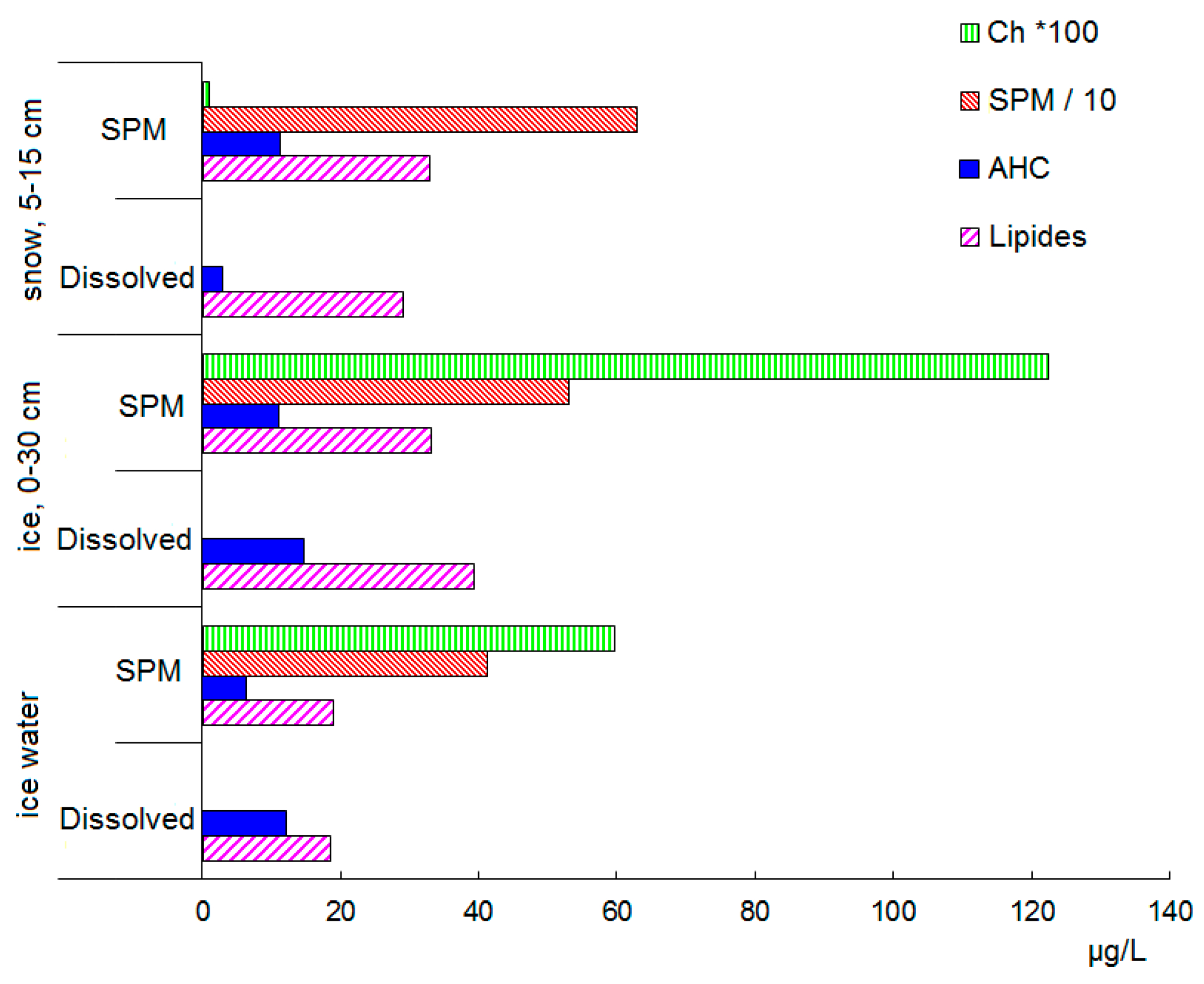
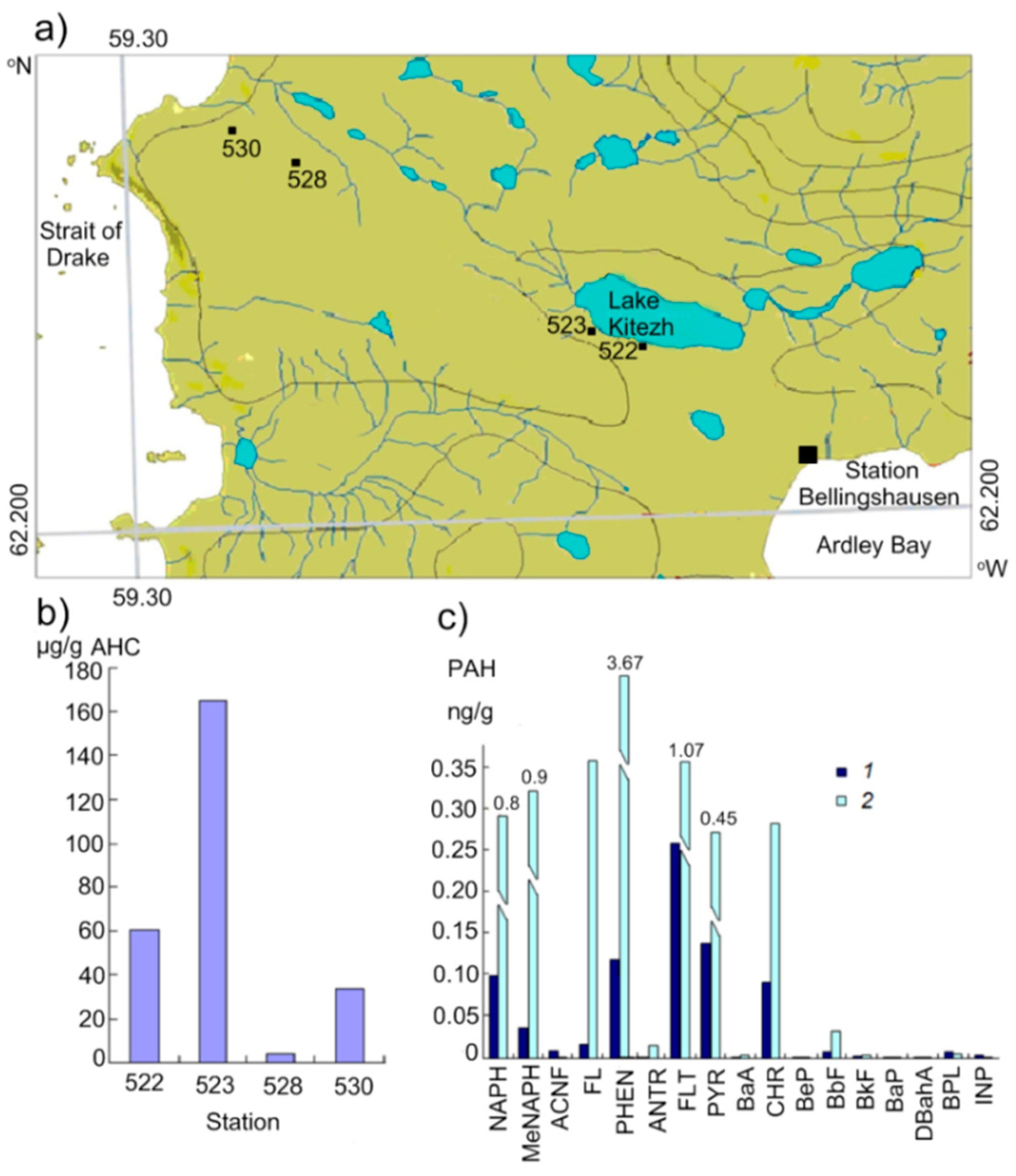
3.2. Antarctic
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lisitzin, A.P. Modern concepts of sedimentation in the oceans and seas. Ocean as a natural recorder of geospheres’ interaction. In The World Ocean; Lobkovsky, L.I., Lisitzin, A.P., Neiman, V.G., Romankevich, E.A., Flint, M.V., Yakovenko, O.I., Eds.; Nauchnyi Mir: Moscow, Russia, 2014; Volume 2, pp. 331–571. ISBN 978-5-91522-344-7. (In Russian) [Google Scholar]
- Mandrioli, P.; Negrini, M.G.; Cesari, G.; Morgan, G. Evidence for long range transport of biological and anthropogenic aerosol particles in the atmosphere. Grana 1984, 23, 43–53. [Google Scholar] [CrossRef]
- Currie, L.A.; Kessler, J.D.; Fletcher, R.A.; Dibb, J.A. Long range transport of biomass aerosol to Greenland: Multi-spectroscopic investigation of particles deposited in the snow. J. Radioanal Nucl. Chem. 2005, 263, 399–411. [Google Scholar] [CrossRef]
- Shevchenko, V.P. The Effect of Aerosols on the Environment and Marine Sedimentation in the Arctic; Nauka: Moscow, Russia, 2006; ISBN 5-02-032656-9. (In Russian) [Google Scholar] [CrossRef]
- Savvichev, A.S.; Rusanov, I.I.; Mitskevich, I.N.; Bairamov, I.T.; Lein, A.Y.; Lisitzin, A.P. Features of biogeochemical processes of the carbon cycle in the water column, bottom sediments, ice and snow cover of the Barents Sea. In Experience of System Oceanologic Studies in the Arctic; Lisitzin, A.P., Vinogradov, M.E., Romankevich, E.A., Eds.; Nauchnyi Mir: Moscow, Russia, 2001; pp. 394–408. ISBN 5-89176-160-2. (In Russian) [Google Scholar]
- Voskresensky, A.I.; Petrov, L.S. Climate features. In Arctic and Southern Oceans; Treshnikov, A.E., Salnikov, S.S., Eds.; Nauka: Leningrad, Russia, 1985; pp. 45–64. (In Russian) [Google Scholar]
- Kitaev, L.M. Spatio-temporal variability of snow depth in the Northern Hemisphere. Meteorol. Hydrol. 2002, 5, 20–25. (In Russian) [Google Scholar]
- Melnikov, I.A. Winter production of sea ice algae in the western Weddell Sea. J. Mar. Systems. 1998, 17, 195–205. [Google Scholar] [CrossRef]
- Rose, N.L.; Ruppel, M. Environmental archives of contaminant particles. In Environmental Contaminants, Developments in Paleoenvironmental Research 18; Blais, J.M., Rosen, M., Smol, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 187–221. [Google Scholar]
- Nemirovskaya, I.A. Oil in the Ocean (Pollution and Natural Flows); Nauchnyi Mir: Moscow, Russia, 2013; 432p, ISBN 978-5-91522-352-2. (In Russian) [Google Scholar]
- Korshenko, A. (Ed.) Marine Water Pollution. Annual Report 2018; Nauka: Moscow, Russia, 2019; 190p. (In Russian) [Google Scholar] [CrossRef]
- Savinov, V.; Larsen, L.H.; Green, N.; Korneev, O.; Rybalko, A.; Kochetkov, A. Monitoring of Hazardous Substances in the White Sea and Pechora Sea: Harmonisation with OSPAR’s Coordinated Environmental Monitoring Programme (CEMP); Akvaplan-niva: Tromsø, Norway, 2011; 71p, APN 414.5124. [Google Scholar]
- Lyutsarev, S.V. Determination of organic carbon in marine bottom sediments by dry burning method. Oceanology 1986, 26, 704–708. (In Russian) [Google Scholar]
- Melnikov, I.A. Ecosystems of Arctic Sea Ice; IORAN: Moscow, Russia, 1989. (In Russian) [Google Scholar] [CrossRef]
- Melnikov, I.A. Antarctic Sea Ice Ecosystems: A Comparative Analysis. In Arctic and Antarctic; Nauka: Mocow, Russia, 2003; Volume 36, pp. 149–164. (In Russian) [Google Scholar]
- Zubov, N.N. Ice of the Arctic; Glavsevmorput: Moscow, Russia, 1944; Volume 360. (In Russian) [Google Scholar]
- Pantyulin, A.N. Glaciation and ice of the White Sea according to observation data. In The White Sea System; Lisitzin, A.P., Ed.; Nauchnyi Mir: Moscow, Russia, 2012; Volume 2, pp. 120–131. ISBN 978-5-91522-319-5. (In Russian) [Google Scholar]
- Sazhin, A.F.; Ratkova, T.N. Population of seasonal ice of the White Sea. In The White Sea System; Lisitzin, A.P., Ed.; Nauchnyi Mir: Moscow, Russia, 2012; Volume 2, pp. 201–224. ISBN 978-5-91522-319-5. (In Russian) [Google Scholar]
- Sazhin, A.F.; Ratkova, T.N.; Kosobokova, K.N. Population of the coastal ice of the White Sea in the early spring period. Oceanology 2004, 44, 92–100. [Google Scholar]
- Cherepanov, N.V.; Kozlovsky, A.M. Typing of Antarctic sea ice according to the conditions of their formation. Probl. Arctic Antarct. 1973, 42, 55–58. (In Russian) [Google Scholar]
- Cherepanov, N.V.; Fedotov, V.I.; Tyshko, K.P. The crystal structure of sea ice. In Sea Ice; Gidrometeoizdat: St. Petersburg, Russia, 1997; pp. 36–37. (In Russian) [Google Scholar]
- Shevchenko, V.P.; Filippov, A.S.; Novigatsky, A.N.; Gordeev, V.V.; Goryunova, N.V.; Demina, L.L. Dispersed sedimentary matter of freshwater and sea ice. In The White Sea System; Lisitzin, A.P., Ed.; Nauchnyi Mir: Moscow, Russia, 2012; Volume 2, pp. 169–201. ISBN 978-5-91522-319-5. (In Russian) [Google Scholar]
- Shevchenko, V.P.; Lisitsyn, A.P.; Vinogradova, A.A.; Starodymova, D.P.; Korobov, V.B.; Novigatsky, A.N.; Kokryatskaya, N.M.; Pokrovsky, O.S. Dispersed sedimentary matter of the atmosphere. Biogeochemistry of the Atmosphere, Ice and Water of the White Sea: The White Sea Environment Part I. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; Volume 81, pp. 9–46. [Google Scholar] [CrossRef]
- Nemirovskaya, I.A. Organic Compounds in the snow-ice cover of the White Sea. Biogeochemistry of the Atmosphere, Ice and Water of the White Sea: The White Sea Environment Part I. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; Volume 81, pp. 291–311. [Google Scholar] [CrossRef]
- Venkatesan, M.J. Occurrence and possible sources of perylene in marine sediments—A review. Mar. Chem. 1988, 25, 1–27. [Google Scholar] [CrossRef]
- Tolosa, I.; Mora, S.; Sheikholeslami, M.R.; Villeneuve, J.-P.; Bartocci, J.; Cattini, C. Aliphatic and aromatic hydrocarbons in coastal Caspian Sea sediments. Mar. Pollut. Bul. 2004, 48, 44–60. [Google Scholar] [CrossRef]
- Buinitsky, V.K. Sea Ice and Icebergs of Antarctica; LSU: Leningrad, Russia, 1973. (In Russian) [Google Scholar]
- Nemirovskaya, I.A.; Kravchishina, M.D.; Rejepova, Z.Y. Organic compounds and suspension in snow-ice covers and soils in the vicinities of the Russian Antarctic stations. Ice Snow 2015, 55, 114–126. [Google Scholar] [CrossRef]
- AMAP (Arctic Monitoring and Assessment Programme). Sources, Inputs and Concentrations of Petroleum Hydrocarbons, Polycyclic Aromatic Hydrocarbons, and other Contaminants Related to Oil and Gas Activities in the Arctic; AMAP: Oslo, Norway, 2007; Chapter 4; ISBN 978-82-7971-048-6. [Google Scholar]
- Bardin, V.I.; Korokevich, E.S.; Lebedev, V.L. Atlas of the Antarctic; Tolstikov, E.I., Avsyuk, G.A., Korotkevich, E.S., Eds.; Gidrometeoizdat: Leningrad, Russia, 1969; Volume 2. (In Russian) [Google Scholar]
- Lisitzin, A.P. Sea-Ice and Iceberg Sedimentation in the World Ocean. Recent and Past; Springer: Berlin/Heidelberg, Germany, 2002; 563р. [Google Scholar] [CrossRef]
- Vasilenko, V.N.; Nazarov, I.M.; Fridman, S.D. Monitoring of Snow Cover Pollution; Gidrometeoizdat: Leningrad, Russia, 1985. (In Russian) [Google Scholar]
- Boyarkina, A.P.; Baikovsky, V.V.; Vasiliev, N.V.; Glukhov, G.G.; Medvedev, M.A.; Pisareva, L.F.; Rezchikov, V.I.; Shelud’ko, S.I. Aerosols in Natural Archives of Siberia; Tomsk State University Publishers: Tomsk, Russia, 1985. (In Russian) [Google Scholar]
- Lisitzin, A.P. Oceanic Sedimentation: Lithology and Geochemistry; American Geophysical Union: Washington, DC, USA, 1996; 400p, ISBN 087590243X. [Google Scholar]
- Lisitzin, A.P. Marine ice-rafting as a new type of sedimentogenesis in the Arctic and novel approaches to studying sedimentary processes. Russ. Geol. Geophys. 2010, 51, 12–47. [Google Scholar] [CrossRef]
- Eicken, H.; Reimnitz, E.; Alexandrov, V.; Martin, T.; Kassens, H.; Viehoff, T. Sea-ice processes in the Laptev Sea and their importance for sediment export. Cont. Shelf Res. 1997, 17, 205–233. [Google Scholar] [CrossRef]
- Pfirman, S.; Wollenburg, I.; Thiede, J.; Lange, M. Lithogenic sediment on Arctic pack ice: Potential aeolian flux and contribution to deep sea sediments. In Paleoclimatology and Paleometeorology: Modern and Past Patterns of Global Atmospheric Transport; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 463–493. [Google Scholar] [CrossRef]
- Novigatsky, A.N.; Lisitzin, A.P. Concentration, composition, and fluxes of dispersed sedimentary material in the snow and Iie cover of the polar Arctic. Oceanology 2019, 59, 406–410. [Google Scholar] [CrossRef]
- Nemirovskaya, I.A.; Novigatskii, A.N. Hydrocarbons in the snow and ice cover and waters of the Arctic Ocean. Geochem. Int. 2003, 41, 585–594. [Google Scholar]
- Vinogradova, A.A. Anthropogenic pollutants in the Russian Arctic atmosphere: Sources and sinks in spring and summer. Atmos. Environ. 2000, 34, 5151–5160. [Google Scholar] [CrossRef]
- Vinogradova, A.A.; Ponomareva, T.Y. Atmospheric transport of anthropogenic impurities to the Russian Arctic (1986–2010). Atmos. Ocean Opt. 2012, 25, 414–422. [Google Scholar] [CrossRef]
- Vinogradova, A.A. Distant evaluation of the influence of air pollution on remote areas. Izvestiya Atmos. Ocean Phys. 2015, 51, 712–722. [Google Scholar] [CrossRef]
- Hirdman, D.; Sodemann, H.; Eckhardt, S.; Burkhart, J.F.; Jefferson, A.; Mefford, T.; Quinn, P.K.; Sharma, S.; Ström, J.; Stohl, A. Source identification of short-lived air pollutants in the Arctic using statistical analysis of measurement data and particle dispersion model output. Atmos. Chem. Phys. 2010, 10, 669–693. [Google Scholar] [CrossRef]
- Vasil’ev, L.Y.; Vodovozova, T.E. Climate. In The White Sea System. Natural Environment of the Catchment Area of the White Sea; Lisitzin, A.P.I., Nemirovskaya, I.A., Shevchenko, V.P., Eds.; Nauchnyi Mir: Moscow, Russia, 2010; pp. 50–69. (In Russian) [Google Scholar]
- Nazarenko, Y.; Rangel-Alvarado, R.B.; Kos, G.; Kurien, U.; Ariya, P.A. Novel aerosol analysis approach for characterization of nanoparticulate matter in snow. Environ. Sci. Pollut. Res. 2017, 24, 4480–4493. [Google Scholar] [CrossRef]
- AMAP (Arctic Monitoring and Assessment Program). Pollution of Arctic: A Report on Environmental Conditions in Arctic; AMAP: St. Petersburg, Russia, 1998; 188p. (In Russian) [Google Scholar]
- Gershuni, G.Z.; Zhukhvitskii, E.M. Convective Stability of Incompressible Fluid; Nauka: Moscow, Russia, 1972; 392p. (In Russian) [Google Scholar] [CrossRef]
- Izmailov, V.V. Transport and Transformation of Oil Pollution of the Arctic Ocean; Gidrometeoizdat: St. Petersburg, Russia, 1999; 139p, ISBN 5-286-01251-5. (In Russian) [Google Scholar]
- Nemirovskaya, I.A.; Kravchishina, M.D. Biogeochemical features of the distribution of organic compounds and particulate matter in the snow-ice cover in East Antarctica. Geochem. Int. 2015, 53, 430–440. [Google Scholar] [CrossRef]
- Radionov, V.F.; Sveshnikov, A.M. Microstructure parameters of aerosol particles on the East Antarctic Coast. In Newsletter of the Russian Antarctic Expedition; Gidrometeoizdat: Saint Petersburg, Russia, 1999; Volume 119, pp. 64–72. (In Russian) [Google Scholar]
- Nemirovskaya, I.A. Organic compounds in the Antarctic coastal ecosystem. Russ. Meteorol. Hydrol. 2020, 45, 105–117. [Google Scholar] [CrossRef]
- Nemirovskaya, I.A. Sedimentary Matter and Organic Compounds in the Aerosols and Surface Waters along the Transatlantic Section. Geochem. Int. 2017, 55, 367–379. [Google Scholar] [CrossRef]
- Balks, M.R.; Paetzold, R.F.; Kimble, J.H. Effects of hydrocarbons spills on the temperature and moisture regimes of Creosols in the Ross Sea region. Antarct. Sci. 2002, 14, 319–326. [Google Scholar] [CrossRef]
- Kim, M.; Kennicutt, M.C., II; Qian, Y. Molecular and stable carbon isotopic characterization of PAH contaminants at McMurdo Station, Antarctica. Mar. Pollut. Bull. 2006, 52, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Kukucka, P.; Lammel, G.; Dvorská, A. Contamination of Antarctic snow by polycyclic aromatic hydrocarbons dominated by combustion sources in the polar region. Environ. Chem. 2010, 7, 504–513. [Google Scholar] [CrossRef]
- Na, G.; Gao, Y.; Li, R.; Gao, H.; Ye, J.; Zhang, Z. Occurrence and sources of polycyclic aromatic hydrocarbons in atmosphere and soil from 2013 to 2019 in the Fildes Peninsula, Antarctica. Mar. Pollut. Bull. 2020, 156, 111–173. [Google Scholar] [CrossRef]
- Préndez, M.; Barra, C.; Toledo-Neira, C.; Richter, P. Alkanes and polycyclic aromatic hydrocarbons in marine surficial sediment near Antarctic stations at Fildes Peninsula, King George Island. Antarct. Sci. 2011, 578–588. [Google Scholar] [CrossRef]
- Kotlyakov, V.M. 137 years after the discovery of Antarctica. Russ. Meteorol. Hydrol. 2020, 45, 5–13, ISSN 0130-2906. [Google Scholar]
- Richter, W.; Bormann, P. Weather and climate. In The Schirmacher Oasis, Queen Maud Land, East Antarctica and its Surrounding; Bormann, P., Fritzsche, D., Eds.; Justus Perthes Verlag: Gotha, Germany, 1995; pp. 207–220. [Google Scholar]
- Abakumov, U.V. The Sources and Composition of Humus in Some Soils of West Antarctica. Eurasian Soil Sci. 2010, 43, 499–508. [Google Scholar] [CrossRef]

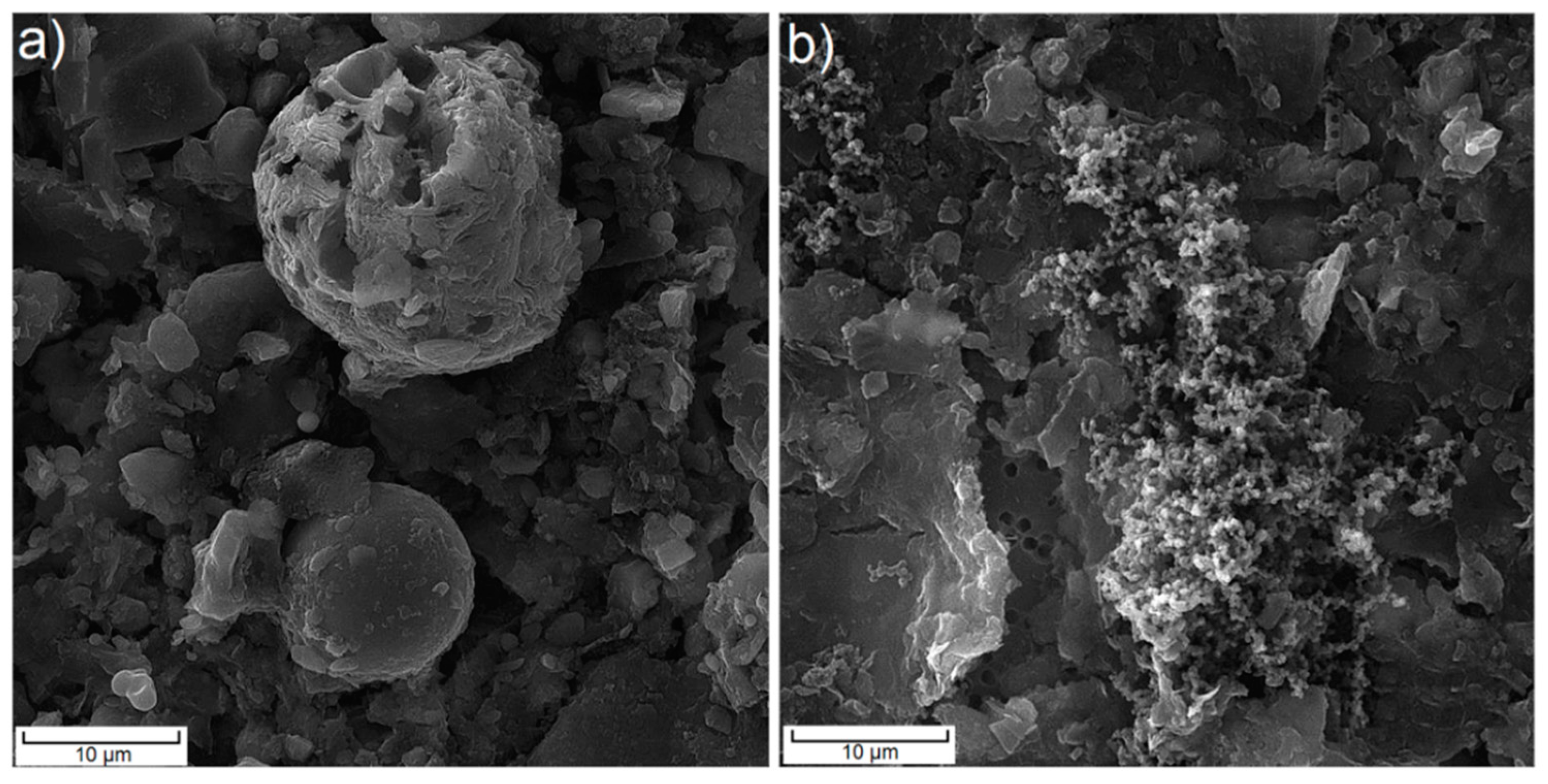
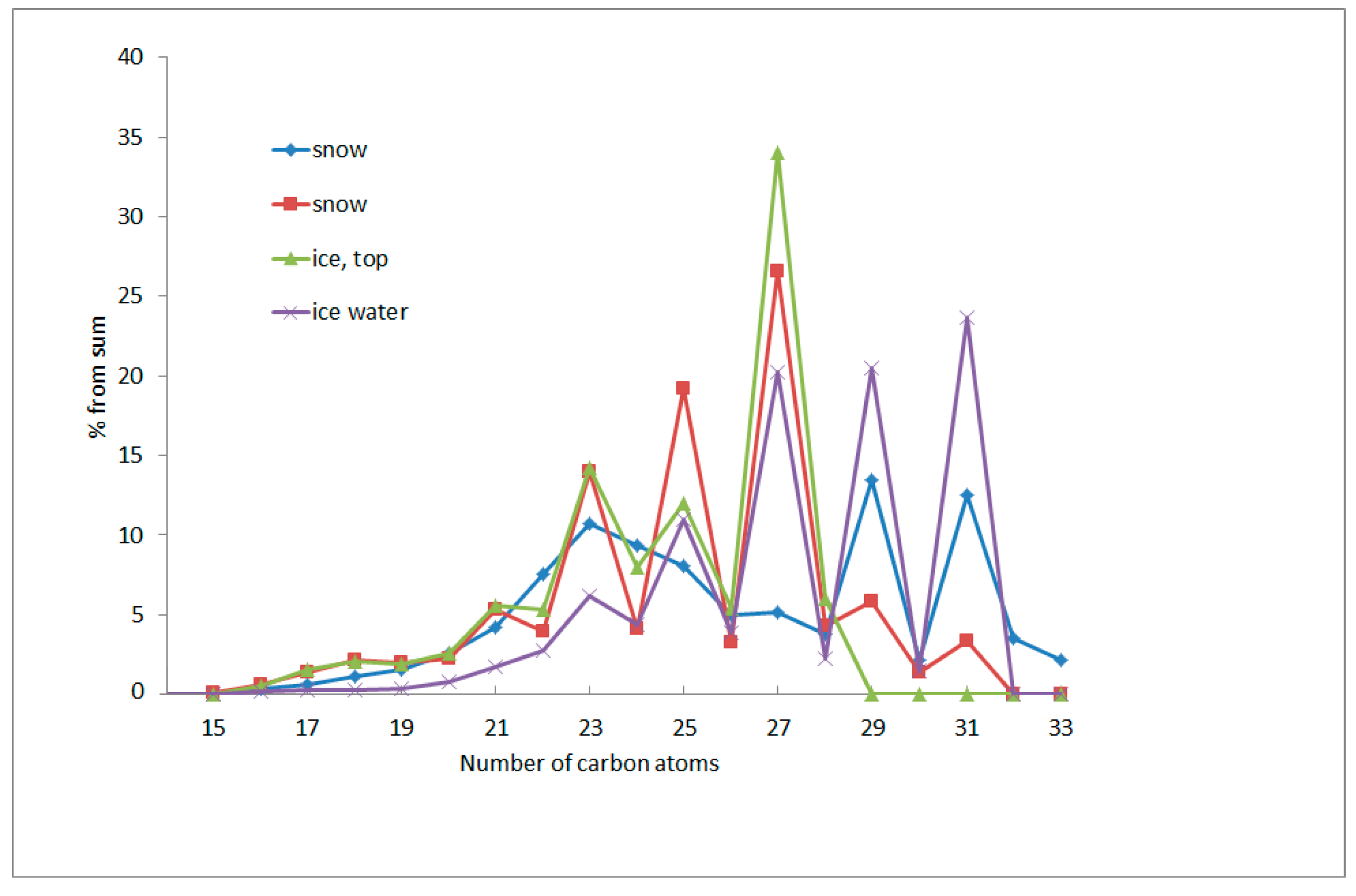

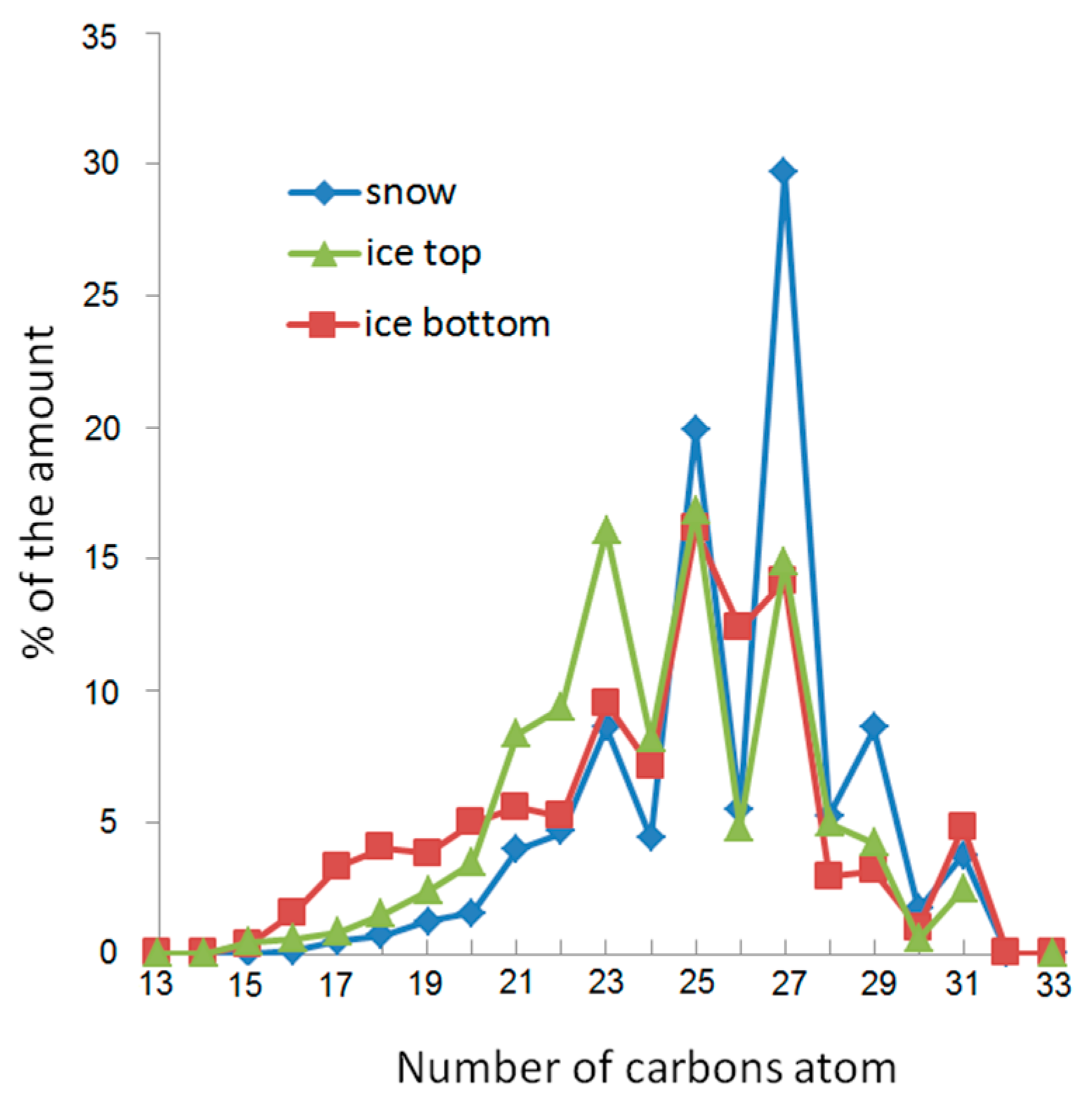
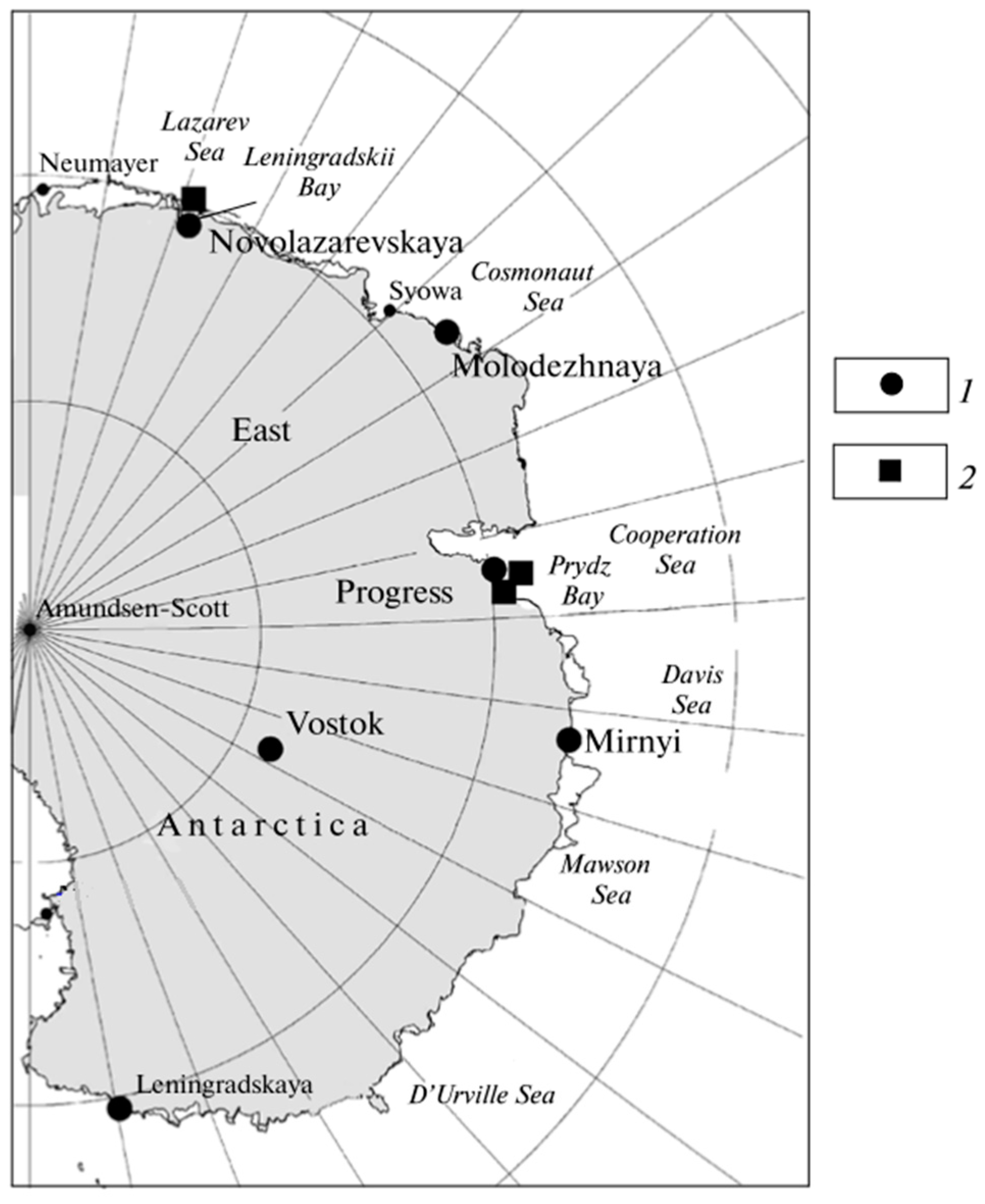

| Object | Form | AHCs | PAHs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N * | Σ, μg/L | L/H * | CPI23-35 | N * | Σ, ng/L | FL ** | P + BP | ||
| FL + P | Ph + Ch | ||||||||
| Arctic Ocean, Mendeleev Rise | |||||||||
| Snow | Dissolv. | 7 | 5 | 1.1 | 1.2 | 2 | 2 | 0.64 | 0.54 |
| SPM | 5 | 5 | 0.7 | 1.2 | 2 | 3 | 0.65 | 0.28 | |
| Melt pond | Dissolv. | 4 | 4 | 0.8 | 1.1 | 2 | 8 | 0.82 | 0.63 |
| SPM | 4 | 8 | 1.2 | 1.2 | 2 | 17 | 0.70 | 0.88 | |
| Ice 0–25 | Dissolv. | 4 | 6 | 1.2 | 1.1 | 4 | 6 | 0.85 | 0.73 |
| cm | SPM | 4 | 6 | 1.3 | 1.2 | 4 | 21 | 0.93 | 0.45 |
| Northern Barents Sea, Franz Victoria Trough | |||||||||
| Snow | Dissolv. | 8 | 14 | 1.3 | 1.7 | 8 | 30 | 0.64 | 0.66 |
| SPM | 2 | 18 | 0.3 | 2.2 | 2 | 50 | 0.70 | 1.35 | |
| Ice | Dissolv. | 1 | 25 | 1.8 | 1.2 | 1 | 110 | 0.92 | 0.10 |
| 0–50 cm | SPM | 1 | 148 | 0.3 | 1.9 | 1 | 47 | 0.62 | 0.39 |
| Description Samples | SPM, mg/L | Corg | Chl a | * Pheo a | Lipids Dissol./SPM | AHCs Dissol./SPM |
|---|---|---|---|---|---|---|
| µg/L | ||||||
| Lazareva Sea, Alasheeva Aiord (67°34′ S, 46°05′ E), 2008 | ||||||
| Snezhura | – | – | – | – | 9/105 | 2/6 |
| Ice water | – | – | – | – | 14/18 | 11/9 |
| Lazareva Sea, Atka Fiord (70°32′ S, 08°07′89″ E), 2010 | ||||||
| Sludge | 4.37 | 61 | 2.895 | 4.414 | 26/28 | 13/19 |
| Sludge | 1.51 | 74 | 2.282 | 3.828 | 31/61 | 17/33 |
| Ice water | 0.39 | 24 | 0.347 | 0.228 | 14/19 | 7/14 |
| Cooperation Sea (67°40′042″ S, 51°194′ E), 2010 | ||||||
| Pancake ice | 0.52 | 43 | 0.236 | 0.204 | 11/44 | 6/31 |
| Ice water | 0.35 | 18 | 0.044 | 0.033 | 9/18 | 6/10 |
| Prudes Bay, Talla Fiord (64°04′32″ S, 70°39′48″ E), 2014 | ||||||
| Snow, 0-5 cm | 0.80 | 25 | 0.030 | 0.020 | /11 | /4 |
| Ice, 0-20 cm | 1.05 | 20 | 0.052 | 0.018 | /10 | /5 |
| Lazarev Sea, Leningradskii Fiord (70°03′486″ S, 12°22′59″ E), 2012 | ||||||
| Snow, 5–15 cm | 0.12 | 9 | 0 | 0 | 11/18 | 5/11 |
| Ice, 0–30, cm | 0.32 | 5 | 0.190 | 5.901 | 12/27 | 9/13 |
| Study Area | Object | Horizon, cm | Lipides | AHC | Chl a | Corg | SPM, mg/L |
|---|---|---|---|---|---|---|---|
| µg/L | |||||||
| Station Molodezhnaya, 67°40′ S, 45°51′ E | |||||||
| Lagernoe Lake, 2010 | Snow Ice | 0–5 0−30 | 10/17 * 11/33 | 7/12 8/20 | 0.008 0.041 | 16 – | 0.32 0.33 |
| 2019 | Snow | 0–10 | –/47 | –/28 | 0.025 | 0 | |
| Snow | 0–10 | –/82 | –/56 | 0.105 | – | 0.87 | |
| Observatory Mirny, 66°34′ S, 93°00′ E | |||||||
| 2010 | Firn | 0−10 | 17/18 | 6/8 | 0.009 | 0 | 0.09 |
| Ice | 0−30 | 15/120 | 7/73 | 0.232 | 132 | 0.23 | |
| Ice barrier station Novolazarevskaya, 70°03′ S, 11°35′ E | |||||||
| 2010 | Snow | 0−10 | 18/33 | 20/10 | 0.004 | 78 | 0.18 |
| Ice | 0−30 | 16/12 | 9/7 | 0.052. | 1 | 0.23 | |
| Station Novolazarevskaya, 70°46′ S, 11°50′ E | |||||||
| Stantsionnoe Lake, 2012 | Snow | 0−15 | 31/42 | 9/22 | 0.011 | 66 | 4.15 |
| Ice | 0−25 | 24/63 | 8/25 | 0.052 | 78 | 1.84 | |
| Verkhnee Lake, 2012 | Snow | 5−15 | 22/410 | 9/360 | 0.073 | 235.2 | 0.78 |
| Ice | 0−80 | 18/64 | 10/35 | 0.042 | 24.4 | 0.68 | |
| Verkhnee Lake, 2019 | Snow | 0–10 | N.d.**/53 | N.d./35 | 0.102 | 218 | 0.982 |
| Glubokoe Lake, 2019 | Snow | 0−10 | N.d./88 | N.d./72 | 0.045 | 125 | 1.06 |
| Geodezistov Lake, 2019 Area around the station | Firn | 0−10 | N.d./180 | N.d./124 | 0.147 | 713. | 0.998 |
| Snow Moss | 0–10 – | N.d./75 – | N.d./40 18–89 | 0.072 – | 297 3.33–9.21 | 1.519 – | |
| Station Progress, Stepped Lake, 69°22′97″S, 76°22′65″E | |||||||
| 2008 | Snow *** | 0−10 | 49/33 | 37/22 | N.d. | N.d. | 1.55 |
| Snow *** | 10−20 | 31/134 | 26/82 | « | « | 3.12 | |
| Ice | 0−20 | 50/119 | 42/50 | « | « | 4.30 | |
| 2010 | Snow *** | 5−10 | 33/74 | 22/56 | 0.005 | « | 1.00 |
| Ice | 0−25 | 50/119 | 42/50 | 0.025 | « | 0.37 | |
| 2012 | Snow | 5−15 | 29/33 | 11/15 | 0.010 | 17.6 | 0.67 |
| Ice | 0−30 | 39/33 | 12/15 | 1.22 | 0.9 | 0.50 | |
| 2014 | Snow | 5−15 | /31 | /10 | 0.002 | N.d. | 0.21 |
| Ice | 0−60 | /20 | /6 | 0.008 | « | 0.50 | |
| Station Bellingshausen 62°10′59′′ S, 58°57′00′′ E | |||||||
| Kitezh Lake, 2012 Area around the station, 2012 Area around the station, 2019 | Snow *** | 0−20 | 14/35 | 7/19 | 0.203 | 73 | 4.21 |
| Suga | 0−3 | 10/14 | 5/7 | 0.230 | 61 | 7.02 | |
| Ice | 0−20 | 15/24 | 7/12 | 0.182 | 0 | 1.81 | |
| Moss – Moss – | 304–1640 – | 24–601 18–142 | – – | 15.50–26.87 8.62–17.28 | – – | ||
| Station | Study Area | AHCs, µg/L | Pr/Ph | CPI | Σ(C12–24) | Dominating Peaks | ||
|---|---|---|---|---|---|---|---|---|
| Σo/Σe | C12–24 | C25–37 | Σ(C25–37) | |||||
| Snow | ||||||||
| Molodezhnaya | Glubokoe Lake | 28.2 | 0.62 | 0.97 | 0.93 | 1.02 | 1.77 | C17, C19, C21 |
| Promernoe Lake | 56.48 | 0.7 | 1.45 | 1.45 | 1.46 | 1.6 | C19, C20, C21 | |
| Glubokoe Lake | 72.4 | 0.72 | 1.15 | 0.83 | 1.62 | 0.97 | C27, C29, C31 | |
| Geodexistov Lake | 124.2 | 0.33 | 4.35 | 0.82 | 6.63 | 0.15 | C27, C29, C31 | |
| Verkhnee Lake | 40.2 | 0.42 | 2.78 | 0.62 | 3.88 | 0.17 | C25, C27, C29, C31 | |
| Prilednikovoe Lake | 34.27 | 0.8 | 1.15 | 0.86 | 1.52 | 0.91 | C16, C17, C25 | |
| Moss | ||||||||
| Novolazarevsk-aya | 56 (soil under moss) | 31.55 | 0.48 | 2.82 | 1.64 | 3.36 | 0.28 | C25, C27, C29, C31 |
| 55 | 22.88 | 0.31 | 2.17 | 2.16 | 2.19 | 1.02 | C19, C21, C23 | |
| 56 | 27.88 | 0.77 | 2.59 | 1.56 | 4.60 | 0.89 | C21, C23, C25 | |
| 61 | 49.21 | 0.85 | 2.06 | 1.38 | 3.88 | 1.28 | C19, C21, C23 | |
| Glubokoe-1 Lake | 84.28 | 0.60 | 3.27 | 2.15 | 3.64 | 0.22 | C27, C29, C31 | |
| Belligshausen | Glubokoe-2 Lake | 40.14 | 0.38 | 2.47 | 2.01 | 3.11 | 1.04 | C21, C23, C25, C27 |
| Glubokoe-4 Lake | 49.94 | 0.42 | 1.78 | 1.31 | 2.28 | 0.74 | C23, C25, C27, C29 | |
| Norma-1 Lake | 29.78 | 0.50 | 1.79 | 1.23 | 2.83 | 1.08 | C25, C27, C29 | |
| Norma-2 Lake | 87.81 | 3.73 | 1.63 | 1.26 | 2.33 | 1.29 | C21, C23, C25, C27 | |
| Norma-3 Lake | 142.14 | 1.18 | 2.01 | 1.24 | 3.24 | 0.84 | C21, C23, C25, C27 | |
| Station 01-2 | 46.09 | 0.36 | 1.50 | 0.88 | 2.20 | 0.66 | C17, C19, C21 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemirovskaya, I.A.; Shevchenko, V.P. Organic Compounds and Suspended Particulate Matter in Snow of High Latitude Areas (Arctic and Antarctic). Atmosphere 2020, 11, 928. https://doi.org/10.3390/atmos11090928
Nemirovskaya IA, Shevchenko VP. Organic Compounds and Suspended Particulate Matter in Snow of High Latitude Areas (Arctic and Antarctic). Atmosphere. 2020; 11(9):928. https://doi.org/10.3390/atmos11090928
Chicago/Turabian StyleNemirovskaya, Inna A., and Vladimir P. Shevchenko. 2020. "Organic Compounds and Suspended Particulate Matter in Snow of High Latitude Areas (Arctic and Antarctic)" Atmosphere 11, no. 9: 928. https://doi.org/10.3390/atmos11090928
APA StyleNemirovskaya, I. A., & Shevchenko, V. P. (2020). Organic Compounds and Suspended Particulate Matter in Snow of High Latitude Areas (Arctic and Antarctic). Atmosphere, 11(9), 928. https://doi.org/10.3390/atmos11090928






