Dissolved and Suspended Forms of Metals and Metalloids in Snow Cover of Megacity: Partitioning and Deposition Rates in Western Moscow
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Snow Sampling
2.2. Laboratory Analysis and Data Processing
3. Results and Discussion
3.1. Physicochemical Properties and Macrocomposition of Snow Meltwater
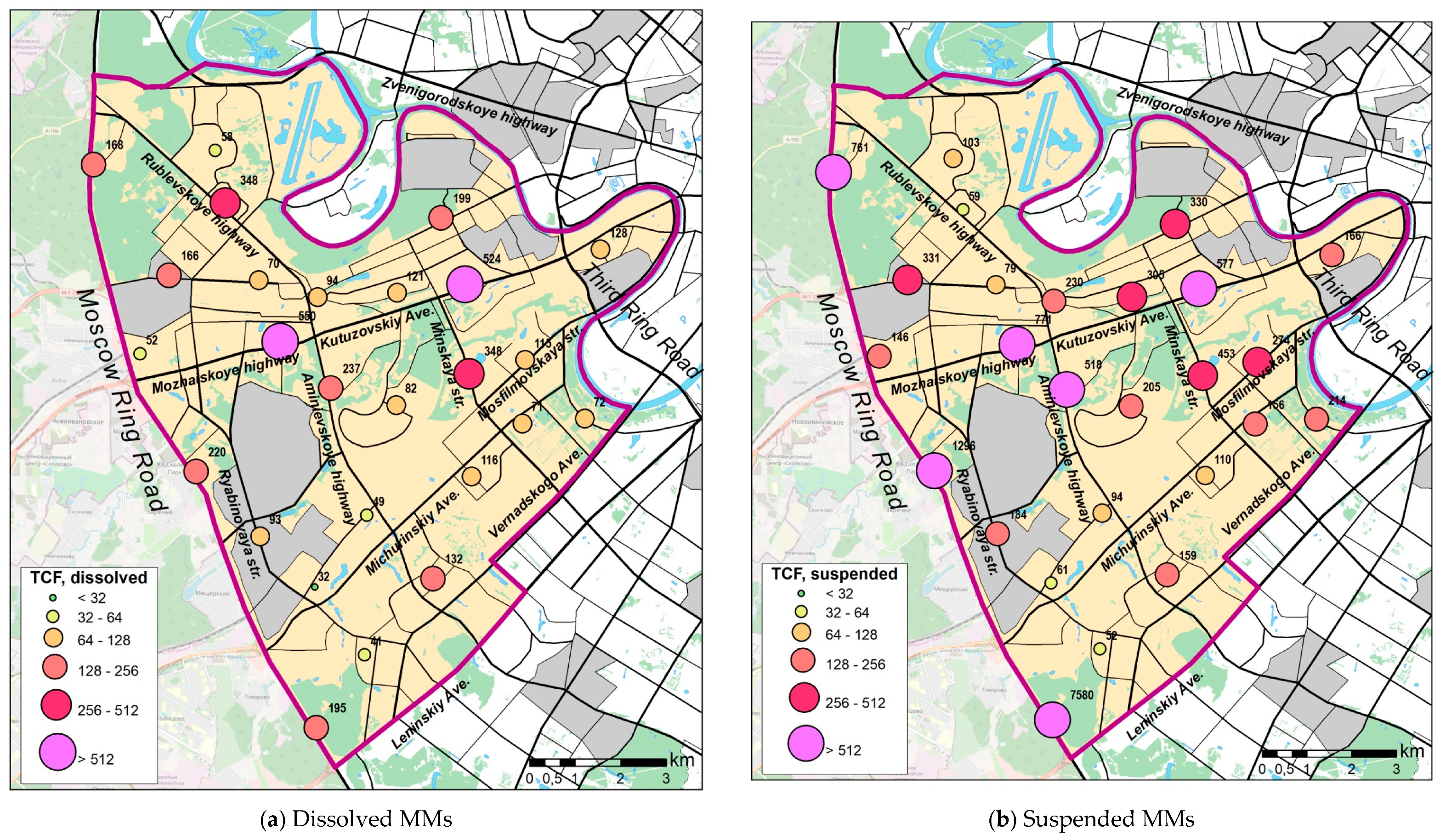
3.2. MMs Concentrations in Snow Cover
3.2.1. Dissolved Forms of MMs
3.2.2. Suspended Forms of MMs
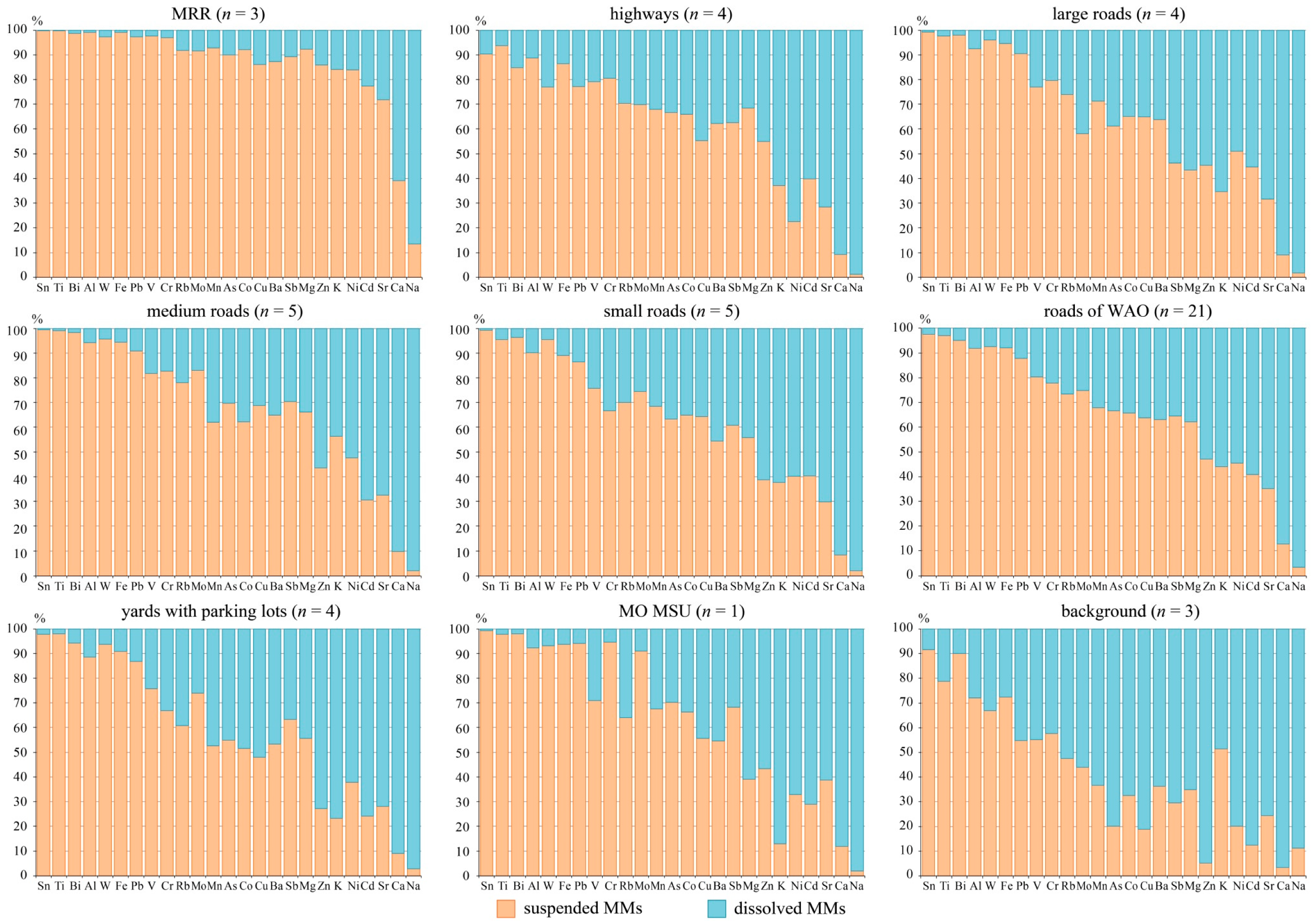
3.3. Partitioning of MMs
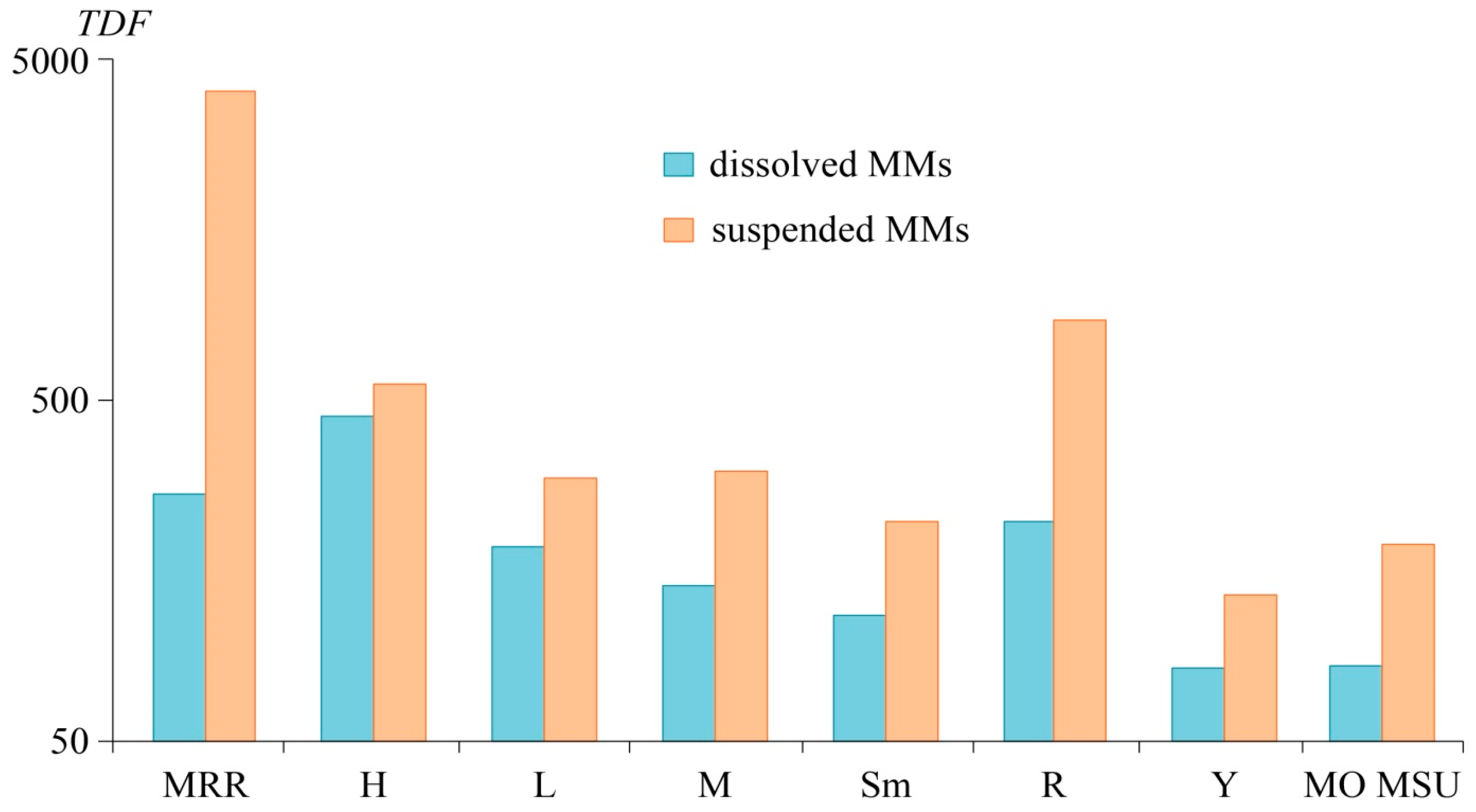
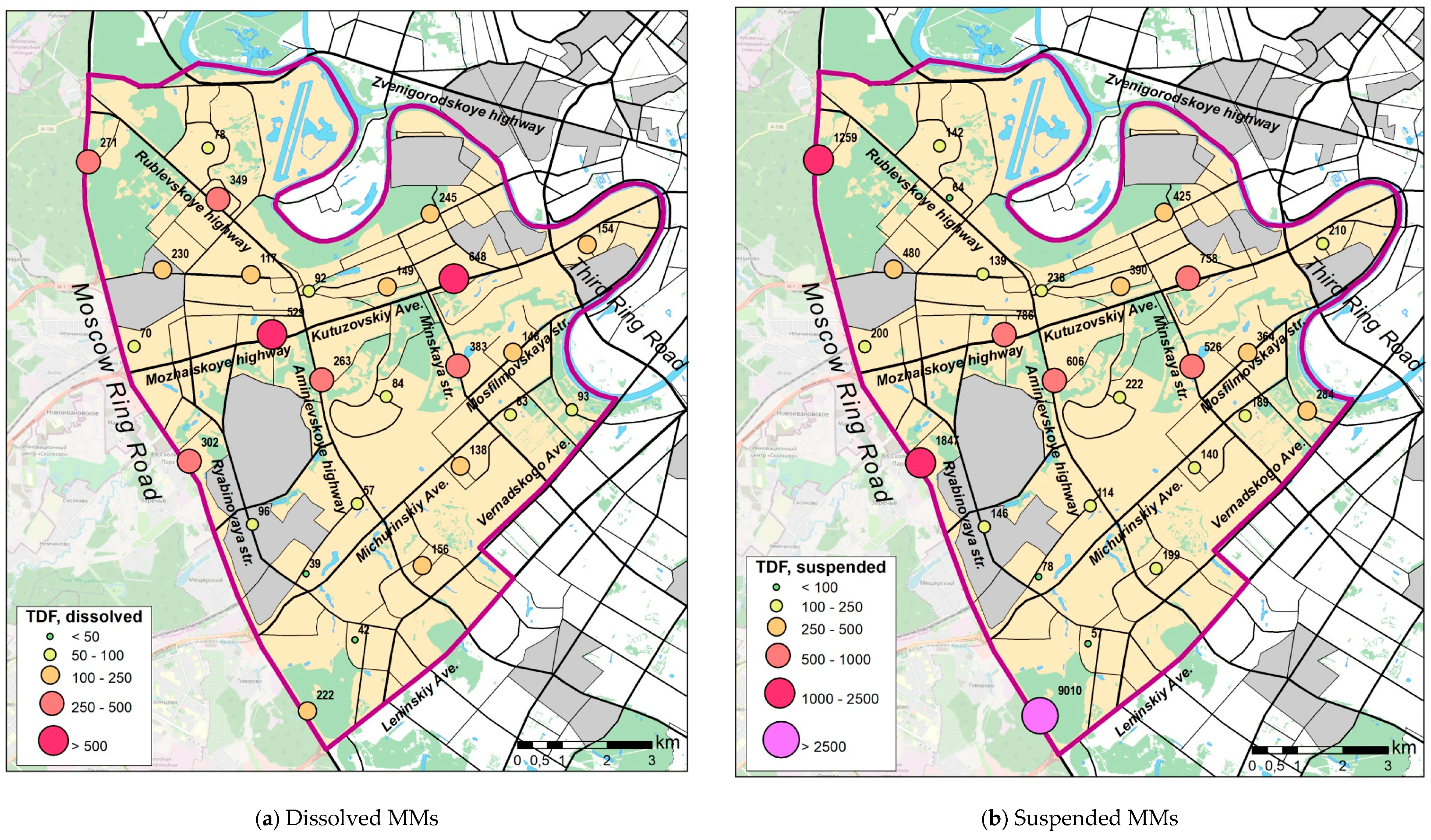
3.4. Deposition Rates of Dissolved and Suspended MMs
3.5. Indication of Sources (Cluster Analysis)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Burden of Disease from Ambient Air Pollution for 2012—Summary of Results; WHO: Geneva, Switzerland, 2014; 3p. [Google Scholar]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Veremchuk, L.V.; Tsarouhas, K.; Vitkina, T.I.; Mineeva, E.E.; Gvozdenko, T.A.; Antonyuk, M.V.; Rakitskii, V.N.; Sidletskaya, K.A.; Tsatsakis, A.M.; Golokhvast, K.S. Impact evaluation of environmental factors on respiratory function of asthma patients living in urban territory. Environ. Pollut. 2018, 235, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vasić, M.V.; Mihailović, A.; Kozmidis-Luburić, U.; Nemes, T.; Ninkov, J.; Zeremski-Škorić, T.; Antić, B. Metal contamination of short-term snow cover near urban crossroads: Correlation analysis of metal content and fine particles distribution. Chemosphere 2012, 86, 585–592. [Google Scholar] [CrossRef]
- Yanchenko, N.I.; Sukhodolov, A.P.; Slutskii, S.L. Improving the monitoring of the snow cover for emission components from primary aluminum production. Metallurgist 2015, 59, 187–192. [Google Scholar] [CrossRef]
- Moghadas, S.; Paus, K.H.; Muthanna, T.M.; Herrmann, I.; Marsalek, J.; Viklander, M. Accumulation of traffic-related trace metals in urban winter-long roadside snowbanks. Water Air Soil Pollut. 2015, 226, 404. [Google Scholar] [CrossRef]
- Talovskaya, A.V.; Yazikov, E.G.; Filimonenko, E.A.; Lata, J.-C.; Kim, J.; Shakhova, T.S. Characterization of solid airborne particles deposited in snow in the vicinity of urban fossil fuel thermal power plant (Western Siberia). Environ. Technol. 2018, 39, 2288–2303. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, C.; Ma, J.; Yin, M. Characteristics of the trace elements and arsenic, iodine and bromine species in snow in east-central China. Atmos. Environ. 2018, 174, 43–53. [Google Scholar] [CrossRef]
- Franz, T.P.; Eisenreich, S.J. Snow scavenging of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in Minnesota. Environ. Sci. Technol. 1998, 32, 1771–1778. [Google Scholar] [CrossRef]
- Sillanpää, N.; Koivusalo, H. Catchment-scale evaluation of pollution potential of urban snow at two residential catchments in southern Finland. Water Sci. Technol. 2013, 68, 2164–2170. [Google Scholar] [CrossRef]
- Gustaytis, M.A.; Myagkaya, I.N.; Chumbaev, A.S. Hg in snow cover and snowmelt waters in high-sulfide tailing regions (Ursk tailing dump site, Kemerovo region, Russia). Chemosphere 2018, 202, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhi, X.; Shen, Z.; Dai, Y.; Aini, G. Comparison between snowmelt-runoff and rainfall-runoff nonpoint source pollution in a typical urban catchment in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Telloli, C. Metal concentrations in snow samples in an urban area in the Po Valley. Int. J. Geosci. 2014, 5, 1116–1136. [Google Scholar] [CrossRef]
- Huber, M.; Welker, A.; Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: Occurrence, influencing factors, and partitioning. Sci. Total Environ. 2016, 541, 895–919. [Google Scholar] [CrossRef] [PubMed]
- Galfi, H.; Österlund, H.; Marsalek, J.; Viklander, M. Mineral and anthropogenic indicator inorganics in urban stormwater and snowmelt runoff: Sources and mobility patterns. Water Air Soil Pollut. 2017, 228, 263. [Google Scholar] [CrossRef]
- Vijayan, A.; Österlund, H.; Marsalek, J.; Viklander, M. Laboratory melting of late-winter urban snow samples: The magnitude and dynamics of releases of heavy metals and PAHs. Water Air Soil Pollut. 2019, 230, 182. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, X.; Mao, Y.; Yan, H.; Li, M.; Zhao, L. Chemical and mineral characteristics of melted snow-water in the Jilin Province, North-East China. J. Civ. Environ. Eng. 2016, 6, 1000248. [Google Scholar] [CrossRef]
- Golokhvast, K.S.; Manakov, Y.A.; Bykov, A.A.; Chayka, V.V.; Nikiforov, P.A.; Rogulin, R.S.; Romanova, T.Y.; Karabtsov, A.A.; Semenikhin, V.A. Some characteristics of dust particles in atmosphere of Kemerovo city according to pollution data of snow cover. IOP Conf. Ser. Earth Environ. Sci. 2017, 87, 042005. [Google Scholar] [CrossRef]
- Nazarenko, Y.; Fournier, S.; Kurien, U.; Rangel-Alvarado, R.B.; Nepotchatykh, O.; Seers, P.; Ariya, P.A. Role of snow in the fate of gaseous and particulate exhaust pollutants from gasoline-powered vehicles. Environ. Pollut. 2017, 223, 665–675. [Google Scholar] [CrossRef]
- Talovskaya, A.V.; Yazikov, E.G.; Osipova, N.A.; Lyapina, E.E.; Litay, V.V.; Metreveli, G.; Kim, J. Mercury pollution In snow cover around thermal power plants in cities (Omsk, Kemerovo, Tomsk Regions, Russia). Geogr. Environ. Sustain. 2019, 12, 132–147. [Google Scholar] [CrossRef][Green Version]
- Seleznev, A.; Yarmoshenko, I.; Malinovsky, G.; Ilgasheva, E.; Baglaeva, E.; Ryanskaya, A.; Kiseleva, D.; Gulyaeva, T. Snow-dirt sludge as an indicator of environmental and sedimentation processes in the urban environment. Sci. Rep. 2019, 9, 17241. [Google Scholar] [CrossRef]
- Rangel-Alvarado, R.B.; Willis, C.E.; Kirk, J.L.; St Louis, V.L.; Amyot, M.; Bélanger, D.; Ariya, P.A. Athabasca oil sands region snow contains efficient micron and nano-sized ice nucleating particles. Environ. Pollut. 2019, 252, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, C.; De Toffol, S.; Lek, I.; Rauch, W.; Dallinger, R. Environmental impacts of urban snow management—The alpine case study of Innsbruck. Sci. Total Environ. 2007, 382, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.M.; Bergvall, C.; Westerholm, R. Emissions of particulate associated oxygenated and native polycyclic aromatic hydrocarbons from vehicles powered by ethanol/gasoline fuel blends. Fuel 2018, 214, 381–385. [Google Scholar] [CrossRef]
- Charola, A.E.; Rousset, B.; Bläuer, C. Deicing salts: An overview. In Proceedings of the SWBSS 2017; Laue, S., Ed.; Verlag der Fachhochschule: Potsdam, Germany, 2017; pp. 16–23. [Google Scholar]
- Ke, G.; Zhang, J.; Tian, B. Evaluation and selection of de-icing salt based on multi-factor. Materials 2019, 12, 912. [Google Scholar] [CrossRef]
- Novotny, E.V.; Murphy, D.; Stefan, H.G. Increase of urban lake salinity by road deicing salt. Sci. Total Environ. 2008, 406, 131–144. [Google Scholar] [CrossRef]
- Snieškienė, V.; Baležentienė, L.; Stankevičienė, A. Urban salt contamination impact on tree health and the prevalence of fungi agent in cities of the central Lithuania. Urban For. Urban Green. 2016, 19, 13–19. [Google Scholar] [CrossRef]
- Nikiforova, E.M.; Kasimov, N.S.; Kosheleva, N.E. Long-term dynamics of the anthropogenic salinization of soils in Moscow (by the example of the Eastern district). Eurasian Soil Sci. 2014, 47, 203–215. [Google Scholar] [CrossRef]
- Nikiforova, E.M.; Kasimov, N.S.; Kosheleva, N.E. Long-term dynamics of anthropogenic solonetzicity in soils of the Eastern okrug of Moscow under the impact of deicing salts. Eurasian Soil Sci. 2017, 50, 84–94. [Google Scholar] [CrossRef]
- Ordóñez-Barona, C.; Sabetski, V.; Millward, A.A.; Steenberg, J. De-icing salt contamination reduces urban tree performance in structural soil cells. Environ. Pollut. 2018, 234, 562–571. [Google Scholar] [CrossRef]
- Nelson, S.S.; Yonge, D.R.; Barber, M.E. Effects of road salts on heavy metal mobility in two Eastern Washington soils. J. Environ. Eng. 2009, 135, 505–510. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Y.; Fan, Z.; Oh, K. Accumulation of de-icing salts and its short-term effect on metal mobility in urban roadside soils. Bull. Environ. Contam. Toxicol. 2015, 94, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kul’bachevskii, A.O. Report on the State of the Environment in Moscow in 2018; Department of Nature Management and Environmental Protection of Moscow Government: Moscow, Russia, 2019; 247p.
- Bityukova, V.R.; Saulskaya, T.D. Changes of the anthropogenic impact of Moscow industrial zones during the recent decades. Vestn. Mosk. Unviersiteta Seriya Geogr. 2017, 3, 24–33. [Google Scholar]
- Elansky, N.F.; Shilkin, A.V.; Ponomarev, N.A.; Semutnikova, E.G.; Zakharova, P.V. Weekly patterns and weekend effects of air pollution in the Moscow megacity. Atmos. Environ. 2020, 224, 117303. [Google Scholar] [CrossRef]
- Vinokurov, S.F.; Tarasova, N.P.; Trunova, A.N.; Sychkova, V.A. A comprehensive analysis of the content of heavy rare-earth elements and platinum in snow samples to assess the ecological hazard of air pollution in urban areas. Dokl. Earth Sci. 2017, 475, 771–774. [Google Scholar] [CrossRef]
- Dryabzhinskiy, O.; Zubkova, V.; Lebedeva, M. The chemical composition of snow as a component of the environment in the South-Western administrative district of Moscow. In Proceedings of the 25th International Scientific Conference on Economic and Social Development—XVII International Social Congress (ISC-2017), Moscow, Russia, 30–31 October 2017; pp. 58–68. [Google Scholar]
- Bogatyrev, L.G.; Zhilin, N.I.; Samsonova, V.P.; Yakushev, N.L.; Kirillova, N.P.; Benediktova, A.I.; Zemskov, P.I.; Karpuhin, M.M.; Ladonin, D.V.; Vartanov, A.N.; et al. Long-term monitoring of snow cover within natural and urbanized landscapes of the Moscow region. Vestn. Mosk. Unviersiteta Seriya Geogr. 2018, 2, 85–96. [Google Scholar]
- Voronina, L.P.; Morachevskaya, E.V.; Akishina, M.M.; Kozlova, O.N. Evaluation of environmental health of the Kolomenskoye Park under anthropogenic pressure from Moscow City. J. Soils Sediments 2019, 19, 3226–3234. [Google Scholar] [CrossRef]
- Khaustov, A.; Redina, M. Polycyclic aromatic hydrocarbons in the snow cover of Moscow (case study of the RUDN University campus). Polycycl. Aromat. Compd. 2019, 1–12. [Google Scholar] [CrossRef]
- Sokolov, L.S.; Astrakhan, E.D. Contamination of Moscow area by metals. Priroda 1993, 7, 68–73. [Google Scholar]
- Kasimov, N.S.; Perel’man, A.I.; Evseev, A.V.; Golubeva, N.I.; Nikiforova, E.M.; Lazukova, G.G. Ecogeochemistry of Urban Landscapes; Kasimov, N.S., Ed.; Moscow University Publishing House: Moscow, Russia, 1995; 336p. [Google Scholar]
- Klimenko, I.A.; Poliakov, V.A.; Sokolovskiĭ, L.G.; Aksenova, O.I.; Skvortsova, O.I.; Okhrimenko, S.E. The sanitary status of natural waters on the territory of Moscow (according to the results of the examination of chemical and radionuclide composition). Gig. Sanit. 2003, 5, 7–11. [Google Scholar]
- Kasimov, N.S.; Kosheleva, N.E.; Vlasov, D.V.; Terskaya, E.V. Geochemistry of snow cover within the Eastern district of Moscow. Vestn. Mosk. Unviersiteta Seriya Geogr. 2012, 4, 14–24. [Google Scholar]
- Galitskaya, I.V.; Rumyantseva, N.A. Snow-cover contamination in urban territories (Lefortovo district, Moscow). Ann. Glaciol. 2012, 53, 23–26. [Google Scholar] [CrossRef]
- Lebedev, A.T.; Polyakova, O.V.; Mazur, D.M.; Bol’shov, M.A.; Seregina, I.F. Estimation of contamination of atmosphere of Moscow in winter. J. Anal. Chem. 2012, 67, 1039–1049. [Google Scholar] [CrossRef]
- Ermakov, A.A.; Karpova, E.A.; Malysheva, A.G.; Mikhaylova, R.I.; Ryzhova, I.N. Monitoring of the chemical composition of snow cover pollution in the Moscow region. Gig. Sanit. 2014, 5, 88–94. [Google Scholar]
- Ermakov, A.A.; Karpova, E.A.; Malysheva, A.G.; Mikhaylova, R.I.; Ryzhova, I.N. Monitoring of the content of heavy metals and elements in the snow cover in agricultural soils at the territory of the Moscow region. Gig. Sanit. 2015, 94, 31–36. [Google Scholar]
- Bobrovskiy, Y.V.; Boguslavskiy, M.V.; Bykov, V.N.; Vedenin, V.A.; Gadenin, M.M.; Glushkova, V.G.; Gorinov, M.M. Great Atlas of Moscow; Shmidt, S.O., Ed.; Feoriya: Moscow, Russia, 2013; ISBN 978-5-91796-032-6. [Google Scholar]
- Yushkov, V.P.; Kurbatova, M.M.; Varentsov, M.I.; Lezina, E.A.; Kurbatov, G.A.; Miller, E.A.; Repina, I.A.; Artamonov, A.Y.; Kallistratova, M.A. Modeling an urban heat island during extreme frost in Moscow in January 2017. Izv. Atmos. Ocean. Phys. 2019, 55, 389–406. [Google Scholar] [CrossRef]
- Lokoshchenko, M.A. Urban heat island and urban dry island in Moscow and their centennial changes. J. Appl. Meteorol. Climatol. 2017, 56, 2729–2745. [Google Scholar] [CrossRef]
- Lokoshchenko, M.A. Snow cover and its modern changes in Moscow. Meteorol. Gidrol. 2005, 6, 71–82. [Google Scholar]
- Kuoppamäki, K.; Setälä, H.; Rantalainen, A.-L.; Kotze, D.J. Urban snow indicates pollution originating from road traffic. Environ. Pollut. 2014, 195, 56–63. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, Y.; Guo, X.; Liu, J. Characteristic contaminants in snowpack and snowmelt surface runoff from different functional areas in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 36256–36266. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, X.; Ma, Y.; Wang, L.; Wu, R.; Chen, B.; Wang, W. The concentrations, formations, relationships and modeling of sulfate, nitrate and ammonium (SNA) aerosols over China. Aerosol Air Qual. Res. 2017, 17, 84–97. [Google Scholar] [CrossRef]
- Eremina, I.D.; Vasil’chuk, J.Y. Temporal variations in chemical composition of snow cover in Moscow. Geogr. Environ. Sustain. 2019, 12, 148–158. [Google Scholar] [CrossRef][Green Version]
- NSAM № 499 AES/MS. Determination of the Elemental Composition of Rocks, Soils and Bottom Sediments by Atomic Emission and Mass Spectral Methods with Inductively Coupled Plasma; VIMS: Moscow, Russia, 2015. (In Russian) [Google Scholar]
- NSAM № 520 AES/MS. Determination of the Elemental Composition of Natural, Drinking, Sewage and Sea Waters by Atomic Emission and Mass Spectral Methods with Inductively Coupled Plasma; VIMS: Moscow, Russia, 2017. (In Russian) [Google Scholar]
- Saet, Y.E.; Revich, B.A.; Yanin, E.P.; Smirnova, R.S.; Basharkevich, E.L.; Onischenko, T.L.; Pavlova, L.N.; Trefilova, N.Y.; Achkasov, A.I.; Sarkisyan, S.S. Geochemistry of the Environment; Nedra: Moscow, Russia, 1990; ISBN 5-247-01127-9. [Google Scholar]
- Kasimov, N.S.; Kosheleva, N.E.; Nikiforova, E.M.; Vlasov, D.V. Benzo[a]pyrene in urban environments of eastern Moscow: Pollution levels and critical loads. Atmos. Chem. Phys. 2017, 17, 2217–2227. [Google Scholar] [CrossRef]
- Eremina, I.D.; Grigoriev, A.V. Acidity and chemical composition of snow cover in the city of Moscow and the Moscow region during 1999–2006. Vestn. Mosk. Unviersiteta Seriya Geogr. 2010, 3, 55–60. [Google Scholar]
- Johnson, C.C.; Demetriades, A.; Allen, M.A.; Cave, M.R.; Chenery, S.R.N.; Gowing, C.J.B.; Reeder, S.; Harrison, I. Mapping the Chemical Environment of Urban Areas; Johnson, C.C., Demetriades, A., Locutura, J., Ottesen, R.T., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; ISBN 978-0-470-67007-1. [Google Scholar]
- Prokof’eva, T.V.; Kiryushin, A.V.; Shishkov, V.A.; Ivannikov, F.A. The importance of dust material in urban soil formation: The experience on study of two young Technosols on dust depositions. J. Soils Sediments 2017, 17, 515–524. [Google Scholar] [CrossRef]
- Prokof’eva, T.; Shishkov, V.; Kiriushin, A. Calcium carbonate accumulations in Technosols of Moscow city. J. Soils Sediments 2020, 1–10. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Vlasov, D.V.; Kosheleva, N.E. Enrichment of road dust particles and adjacent environments with metals and metalloids in eastern Moscow. Urban Clim. 2020, 32, 100638. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Kosheleva, N.E.; Vlasov, D.V.; Nabelkina, K.S.; Ryzhov, A.V. Physicochemical properties of road dust in Moscow. Geogr. Environ. Sustain. 2019, 12, 96–113. [Google Scholar] [CrossRef]
- Vlasov, D.V.; Shinkareva, G.L.; Kasimov, N.S. Metals and metalloids in bottom sediments of lakes and ponds of the eastern part of Moscow. Vestn. Mosk. Unviersiteta Seriya Geogr. 2019, 4, 43–52. [Google Scholar]
- Greinert, A.; Fruzińska, R.; Kostecki, J. Urban soils in Zielona Góra. In Technogenic Soils of Poland; Charzyński, P., Hulisz, P., Bednarek, R., Eds.; Polish Society of Soil Science: Toruń, Poland, 2013; pp. 31–54. [Google Scholar]
- Karanasiou, A.; Amato, F.; Moreno, T.; Lumbreras, J.; Borge, R.; Linares, C.; Boldo, E.; Alastuey, A.; Querol, X. Road dust emission sources and assessment of street washing effect. Aerosol Air Qual. Res. 2014, 14, 734–743. [Google Scholar] [CrossRef]
- Amato, F.; Alastuey, A.; de la Rosa, J.; Gonzalez Castanedo, Y.; Sánchez de la Campa, A.M.; Pandolfi, M.; Lozano, A.; Contreras González, J.; Querol, X. Trends of road dust emissions contributions on ambient air particulate levels at rural, urban and industrial sites in southern Spain. Atmos. Chem. Phys. 2014, 14, 3533–3544. [Google Scholar] [CrossRef]
- Ramírez, O.; Sánchez de la Campa, A.M.; Amato, F.; Moreno, T.; Silva, L.F.; de la Rosa, J.D. Physicochemical characterization and sources of the thoracic fraction of road dust in a Latin American megacity. Sci. Total Environ. 2019, 652, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Elumalai, S.P.; Pal, A.K. Rain pH estimation based on the particulate matter pollutants and wet deposition study. Sci. Total Environ. 2016, 563–564, 293–301. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Vlasov, D.V.; Kosheleva, N.E.; Nikiforova, E.M. Geochemistry of Landscapes of Eastern Moscow; APR: Moscow, Russia, 2016; ISBN 978-5-904761-62-2. [Google Scholar]
- Eremina, I.D.; Aloyan, A.E.; Arutyunyan, V.O.; Larin, I.K.; Chubarova, N.E.; Yermakov, A.N. Acidity and mineral composition of precipitation in Moscow: Influence of deicing salts. Izv. Atmos. Ocean. Phys. 2015, 51, 624–632. [Google Scholar] [CrossRef]
- Elansky, N.F.; Ponomarev, N.A.; Verevkin, Y.M. Air quality and pollutant emissions in the Moscow megacity in 2005–2014. Atmos. Environ. 2018, 175, 54–64. [Google Scholar] [CrossRef]
- Eremina, I.D. Chemical composition of atmospheric precipitation in Moscow and the trends of its long-term changes. Vestn. Mosk. Unviersiteta Seriya Geogr. 2019, 3, 3–10. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.G.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Alves, C.A.; Barbosa, C.; Rocha, S.; Calvo, A.; Nunes, T.; Cerqueira, M.; Pio, C.; Karanasiou, A.; Querol, X. Elements and polycyclic aromatic hydrocarbons in exhaust particles emitted by light-duty vehicles. Environ. Sci. Pollut. Res. 2015, 22, 11526–11542. [Google Scholar] [CrossRef]
- Hung-Lung, C.; Yao-Sheng, H. Particulate matter emissions from on-road vehicles in a freeway tunnel study. Atmos. Environ. 2009, 43, 4014–4022. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Lai, Y.-M.; Chang, S.-Y. Pollutant constituents of exhaust emitted from light-duty diesel vehicles. Atmos. Environ. 2012, 47, 399–406. [Google Scholar] [CrossRef]
- Almeida, S.; Pio, C.; Freitas, M.; Reis, M.; Trancoso, M. Source apportionment of fine and coarse particulate matter in a sub-urban area at the Western European Coast. Atmos. Environ. 2005, 39, 3127–3138. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Kaul, D.S.; Yang, F.; Sun, L.; Ning, Z. Source apportionment and water solubility of metals in size segregated particles in urban environments. Sci. Total Environ. 2015, 533, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.T.; Shields, L.G.; Sodeman, D.A.; Toner, S.M.; Prather, K.A. Comparison of oil and fuel particle chemical signatures with particle emissions from heavy and light duty vehicles. Atmos. Environ. 2006, 40, 5224–5235. [Google Scholar] [CrossRef]
- Cross, E.S.; Sappok, A.; Fortner, E.C.; Hunter, J.F.; Jayne, J.T.; Brooks, W.A.; Onasch, T.B.; Wong, V.W.; Trimborn, A.; Worsnop, D.R.; et al. Real-time measurements of engine-out trace elements: Application of a novel soot particle aerosol mass spectrometer for emissions characterization. J. Eng. Gas Turbines Power 2012, 134, 072801. [Google Scholar] [CrossRef]
- Pio, C.; Mirante, F.; Oliveira, C.; Matos, M.; Caseiro, A.; Oliveira, C.; Querol, X.; Alves, C.; Martins, N.; Cerqueira, M.; et al. Size-segregated chemical composition of aerosol emissions in an urban road tunnel in Portugal. Atmos. Environ. 2013, 71, 15–25. [Google Scholar] [CrossRef]
- Al-Momani, I. Trace elements in atmospheric precipitation at Northern Jordan measured by ICP-MS: Acidity and possible sources. Atmos. Environ. 2003, 37, 4507–4515. [Google Scholar] [CrossRef]
- Song, F.; Gao, Y. Size distributions of trace elements associated with ambient particular matter in the affinity of a major highway in the New Jersey–New York metropolitan area. Atmos. Environ. 2011, 45, 6714–6723. [Google Scholar] [CrossRef]
- Hu, S.; Herner, J.D.; Shafer, M.; Robertson, W.; Schauer, J.J.; Dwyer, H.; Collins, J.; Huai, T.; Ayala, A. Metals emitted from heavy-duty diesel vehicles equipped with advanced PM and NOX emission controls. Atmos. Environ. 2009, 43, 2950–2959. [Google Scholar] [CrossRef]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 2491–2504. [Google Scholar] [CrossRef]
- Penkała, M.; Ogrodnik, P.; Rogula-Kozłowska, W. Particulate matter from the road surface abrasion as a problem of non-exhaust emission control. Environments 2018, 5, 9. [Google Scholar] [CrossRef]
- Bencharif-Madani, F.; Ali-Khodja, H.; Kemmouche, A.; Terrouche, A.; Lokorai, K.; Naidja, L.; Bouziane, M. Mass concentrations, seasonal variations, chemical compositions and element sources of PM10 at an urban site in Constantine, northeast Algeria. J. Geochem. Explor. 2019, 206, 106356. [Google Scholar] [CrossRef]
- Vlasov, D.V.; Kasimov, N.S.; Kosheleva, N.E. Geochemistry of the road dust in the Eastern district of Moscow. Vestn. Mosk. Unviersiteta Seriya Geogr. 2015, 1, 23–33. [Google Scholar]
- Vlasov, D.V. Metals and metalloids in PM10 fraction of the road dust of Eastern Moscow. RUDN J. Ecol. Life Saf. 2017, 25, 529–539. [Google Scholar] [CrossRef][Green Version]
- Ermolin, M.S.; Fedotov, P.S.; Ivaneev, A.I.; Karandashev, V.K.; Fedyunina, N.N.; Burmistrov, A.A. A contribution of nanoscale particles of road-deposited sediments to the pollution of urban runoff by heavy metals. Chemosphere 2018, 210, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Krivcov, V.A.; Tobratov, S.A.; Vozorezov, A.V.; Komarov, M.M.; Zheleznova, O.S.; Solov’eva, E.A. The Natural Potential of the Landscapes of the Ryazan Oblast; Ryazan State University Named for S. Yesenin: Ryazan, Russia, 2011. [Google Scholar]
- Grotti, M.; Soggia, F.; Ardini, F.; Magi, E. Major and trace element partitioning between dissolved and particulate phases in Antarctic surface snow. J. Environ. Monit. 2011, 13, 2511. [Google Scholar] [CrossRef]
- Kosheleva, N.E.; Vlasov, D.V.; Korlyakov, I.D.; Kasimov, N.S. Contamination of urban soils with heavy metals in Moscow as affected by building development. Sci. Total Environ. 2018, 636, 854–863. [Google Scholar] [CrossRef]
- Ramírez, O.; Sánchez de la Campa, A.M.; Amato, F.; Catacolí, R.A.; Rojas, N.Y.; de la Rosa, J. Chemical composition and source apportionment of PM10 at an urban background site in a high–altitude Latin American megacity (Bogota, Colombia). Environ. Pollut. 2018, 233, 142–155. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency National Emissions Inventory 2017. Available online: https://www.epa.gov/air-emissions-inventories/2017-national-emissions-inventory-nei-data (accessed on 21 March 2020).
- Amato, F.; Favez, O.; Pandolfi, M.; Alastuey, A.; Querol, X.; Moukhtar, S.; Bruge, B.; Verlhac, S.; Orza, J.A.G.; Bonnaire, N.; et al. Traffic induced particle resuspension in Paris: Emission factors and source contributions. Atmos. Environ. 2016, 129, 114–124. [Google Scholar] [CrossRef]
- Emelyanov, E.M. Physicochemical barriers. In The Barrier Zones in the Ocean; Springer: Berlin/Heidelberg, Germany, 2005; pp. 15–24. ISBN 978-3-540-25391-4. [Google Scholar]
- Bauer, S.; Conrad, S.; Ingri, J. Geochemistry of tungsten and molybdenum during freshwater transport and estuarine mixing. Appl. Geochem. 2018, 93, 36–48. [Google Scholar] [CrossRef]
- Adamiec, E.; Wieszała, R.; Strzebońska, M.; Jarosz-Krzemińska, E. An attempt to identify traffic related elements in snow. Geol. Geophys. Environ. 2013, 39, 317. [Google Scholar] [CrossRef]
- Zhang, L.; Michelangeli, D.V.; Taylor, P.A. Numerical studies of aerosol scavenging by low-level, warm stratiform clouds and precipitation. Atmos. Environ. 2004, 38, 4653–4665. [Google Scholar] [CrossRef]
- Di Marco, V.; Tapparo, A.; Badocco, D.; D’Aronco, S.; Pastore, P.; Giorio, C. Metal ion release from fine particulate matter sampled in the Po Valley to an aqueous solution mimicking fog water: Kinetics and solubility. Aerosol Air Qual. Res. 2020, 20, 720–729. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Lychagin, M.Y.; Chalov, S.R.; Shinkareva, G.L. Paragenetic associations of chemical elements in landscapes. Vestn. Mosk. Unviersiteta Seriya Geogr. 2019, 6, 20–28. [Google Scholar]
- Pan, Y.P.; Wang, Y.S. Atmospheric wet and dry deposition of trace elements at 10 sites in Northern China. Atmos. Chem. Phys. 2015, 15, 951–972. [Google Scholar] [CrossRef]
- Carling, G.T.; Fernandez, D.P.; Johnson, W.P. Dust-mediated loading of trace and major elements to Wasatch Mountain snowpack. Sci. Total Environ. 2012, 432, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Moiseenko, T.; Baranov, D. Snowpack as indicators of atmospheric pollution: The Valday Upland. Atmosphere 2020, 11, 462. [Google Scholar] [CrossRef]
- Westerlund, C.; Viklander, M.; Bäckström, M. Seasonal variations in road runoff quality in Luleå, Sweden. Water Sci. Technol. 2003, 48, 93–101. [Google Scholar] [CrossRef]
- Rangel-Alvarado, R.B.; Nazarenko, Y.; Ariya, P.A. Snow-borne nanosized particles: Abundance, distribution, composition, and significance in ice nucleation processes. J. Geophys. Res. Atmos. 2015, 120, 11760–11774. [Google Scholar] [CrossRef]
- Göbel, P.; Dierkes, C.; Coldewey, W.G. Storm water runoff concentration matrix for urban areas. J. Contam. Hydrol. 2007, 91, 26–42. [Google Scholar] [CrossRef]
- Sister, V.; Koretsky, V. Engineering-Environmental Protection of Water System of the Northern Megapolis in Winter Period; Center MSUIE: Moscow, Russia, 2004. [Google Scholar]
- Borsato, A.; Johnston, V.E.; Frisia, S.; Miorandi, R.; Corradini, F. Temperature and altitudinal influence on karst dripwater chemistry: Implications for regional-scale palaeoclimate reconstructions from speleothems. Geochim. Cosmochim. Acta 2016, 177, 275–297. [Google Scholar] [CrossRef]
- Fabretti, J.-F.; Sauret, N.; Gal, J.-F.; Maria, P.-C.; Schärer, U. Elemental characterization and source identification of PM2.5 using Positive Matrix Factorization: The Malraux road tunnel, Nice, France. Atmos. Res. 2009, 94, 320–329. [Google Scholar] [CrossRef]
- Lawrence, S.; Sokhi, R.; Ravindra, K. Quantification of vehicle fleet PM10 particulate matter emission factors from exhaust and non-exhaust sources using tunnel measurement techniques. Environ. Pollut. 2016, 210, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Morera-Gómez, Y.; Alonso-Hernández, C.M.; Santamaría, J.M.; Elustondo, D.; Lasheras, E.; Widory, D. Levels, spatial distribution, risk assessment, and sources of environmental contamination vectored by road dust in Cienfuegos (Cuba) revealed by chemical and C and N stable isotope compositions. Environ. Sci. Pollut. Res. 2020, 27, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Tanner, P.A.; Ma, H.-L.; Yu, P.K.N. Fingerprinting metals in urban street dust of Beijing, Shanghai, and Hong Kong. Environ. Sci. Technol. 2008, 42, 7111–7117. [Google Scholar] [CrossRef]
- Apeagyei, E.; Bank, M.S.; Spengler, J.D. Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos. Environ. 2011, 45, 2310–2323. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Bezberdaya, L.A.; Vlasov, D.V.; Lychagin, M.Y. Metals, metalloids, and benzo[a]pyrene in PM10 particles of soils and road dust of Alushta city. Eurasian Soil Sci. 2019, 52, 1608–1621. [Google Scholar] [CrossRef]
- Konstantinova, E.; Minkina, T.; Konstantinov, A.; Sushkova, S.; Antonenko, E.; Kurasova, A.; Loiko, S. Pollution status and human health risk assessment of potentially toxic elements and polycyclic aromatic hydrocarbons in urban street dust of Tyumen city, Russia. Environ. Geochem. Health 2020, 1–24. [Google Scholar] [CrossRef]
- Wahlin, P.; Berkowicz, R.; Palmgren, F. Characterisation of traffic-generated particulate matter in Copenhagen. Atmos. Environ. 2006, 40, 2151–2159. [Google Scholar] [CrossRef]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef]
- Amato, F.; Pandolfi, M.; Moreno, T.; Furger, M.; Pey, J.; Alastuey, A.; Bukowiecki, N.; Prevot, A.S.H.; Baltensperger, U.; Querol, X. Sources and variability of inhalable road dust particles in three European cities. Atmos. Environ. 2011, 45, 6777–6787. [Google Scholar] [CrossRef]
- Hulskotte, J.H.J.; Roskam, G.D.; Denier van der Gon, H.A.C. Elemental composition of current automotive braking materials and derived air emission factors. Atmos. Environ. 2014, 99, 436–445. [Google Scholar] [CrossRef]
- Goddard, S.L.; Williams, K.R.; Robins, C.; Brown, R.J.C. Determination of antimony and barium in UK air quality samples as indicators of non-exhaust traffic emissions. Environ. Monit. Assess. 2019, 191, 641. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.G.; Xu, N.; Dalka, T.M.; Maricq, M.M. Airborne brake wear debris: Size distributions, composition, and a comparison of dynamometer and vehicle tests. Environ. Sci. Technol. 2003, 37, 4060–4069. [Google Scholar] [CrossRef]
- Reid, J.S.; Koppmann, R.; Eck, T.F.; Eleuterio, D.P. A review of biomass burning emissions part II: Intensive physical properties of biomass burning particles. Atmos. Chem. Phys. 2005, 5, 799–825. [Google Scholar] [CrossRef]
- Grivas, G.; Cheristanidis, S.; Chaloulakou, A.; Koutrakis, P.; Mihalopoulos, N. Elemental composition and source apportionment of fine and coarse particles at traffic and urban background locations in Athens, Greece. Aerosol Air Qual. Res. 2018, 18, 1642–1659. [Google Scholar] [CrossRef]
- Achad, M.; Caumo, S.; de Castro Vasconcellos, P.; Bajano, H.; Gómez, D.; Smichowski, P. Chemical markers of biomass burning: Determination of levoglucosan, and potassium in size-classified atmospheric aerosols collected in Buenos Aires, Argentina by different analytical techniques. Microchem. J. 2018, 139, 181–187. [Google Scholar] [CrossRef]


| Parameter | Roads * | R (n = 21) | Y (n = 4) | MO MSU (n = 1) | B (n = 3) | ||||
|---|---|---|---|---|---|---|---|---|---|
| MRR (n = 3) | H (n = 4) | L (n = 4) | M (n = 5) | Sm (n = 5) | |||||
| рН | 8.0 | 8.4 | 8.6 | 8.2 | 8.0 | 8.2 | 7.5 | 7.3 | 5.6 |
| Mineralization, mg/L | 144 | 233 | 87 | 73 | 58 | 113 | 35 | 32 | 6.4 |
| Solid particles, mg/L | 93 | 83 | 28 | 31 | 24 | 48 | 15 | 17 | 9.0 |
| Dust load, mg/m2 per day | 133 | 102 | 35 | 41 | 30 | 62 | 20 | 21 | 9.0 |
| Snow cover thickness, cm | 50 | 42 | 46 | 45 | 42 | 45 | 45 | 45 | 61 |
| Water supply, L/m2 | 91 | 73 | 83 | 80 | 77 | 80 | 79 | 77 | 67 |
| Na+, μeq/L | 1126 | 2357 | 505 | 594 | 436 | 951 | 262 | 236 | 8 |
| K+, μeq/L | 9 | 25 | 14 | 9 | 11 | 14 | 16 | 28 | 1 |
| Mg2+, μeq/L | 17 | 20 | 38 | 12 | 12 | 19 | 9 | 19 | 4 |
| Ca2+, μeq/L | 1039 | 1459 | 707 | 498 | 394 | 773 | 226 | 178 | 69 |
| Cl–, μeq/L | 1400 | 3353 | 607 | 719 | 434 | 1229 | 278 | 240 | 7 |
| NO3–, μeq/L | 22 | 28 | 29 | 25 | 26 | 26 | 24 | 29 | 24 |
| SO42–, μeq/L | 92 | 104 | 79 | 63 | 82 | 83 | 70 | 59 | 15 |
| HCO3–, μeq/L | 678 | 374 | 550 | 305 | 310 | 420 | 141 | 134 | 38 |
| MMs | Dissolved Forms | Suspended Forms | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roads (n = 21) | Yards (n = 4) | MO MSU (n = 1) | Background (n = 3) | Roads (n = 21) | Yards (n = 4) | MO MSU (n = 1) | Background (n = 3) | |||||||||||||
| Mean | Min. | Max. | Mean | Min. | Max. | Mean | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Mean | Min. | Max. | |
| Na | 21870 | 2830 | 85900 | 6022 | 1960 | 15620 | 5430 | 193 | 170 | 210 | 932 | 69 | 10907 | 103 | 54 | 172 | 109 | 24 | 17 | 38 |
| Mg | 233 | 100 | 840 | 107 | 100 | 120 | 230 | 50 * | 50 * | 50 * | 1110 | 102 | 12610 | 145 | 83 | 228 | 148 | 28 | 22 | 39 |
| Al | 75 | 31 | 227 | 65 | 39 | 111 | 53 | 58 | 47 | 69 | 4216 | 351 | 48798 | 526 | 299 | 822 | 638 | 165 | 103 | 270 |
| K | 531 | 100 | 1210 | 617 | 260 | 950 | 1110 | 50 * | 50 * | 50 * | 836 | 106 | 8660 | 157 | 85 | 242 | 164 | 61 | 36 | 108 |
| Ca | 15498 | 2820 | 46160 | 4535 | 2800 | 5800 | 3570 | 1387 | 1230 | 1690 | 4010 | 251 | 44960 | 462 | 227 | 778 | 478 | 50 | 30 | 81 |
| Ti | 2.0 | 0.3 * | 13 | 0.85 | 0.55 | 1.1 | 1.0 | 1.1 | 1.0 | 1.2 | 345 | 30 | 3900 | 48 | 29 | 76 | 45 | 8.3 | 1.9 | 18 |
| V | 0.92 | 0.2 * | 2.9 | 0.76 | 0.2 * | 1.7 | 1.1 | 0.2 * | 0.2 * | 0.2 * | 12 | 1.1 | 135 | 2.1 | 1.4 | 2.7 | 2.8 | 0.27 | 0.16 | 0.45 |
| Cr | 0.66 | 0.5 * | 3.9 | 0.5 * | 0.5 * | 0.5 * | 0.5 * | 0.5 * | 0.5 * | 0.5 * | 11 | 0.44 | 140 | 1.1 | 0.59 | 1.6 | 8.8 | 0.71 | 0.53 | 1.0 |
| Mn | 11 | 3.8 | 84 | 12 | 3.2 | 34 | 3.9 | 4.5 | 3.2 | 6.2 | 55 | 5.5 | 603 | 8.4 | 4.5 | 14 | 8.1 | 3.0 | 1.2 | 5.8 |
| Fe | 93 | 25 | 440 | 68 | 37 | 93 | 46 | 49 | 43 | 56 | 3848 | 452 | 39098 | 680 | 383 | 1036 | 678 | 139 | 94 | 218 |
| Co | 0.28 | 0.12 | 1.0 | 0.18 | 0.1 | 0.3 | 0.11 | 0.071 | 0.054 | 0.091 | 1.6 | 0.13 | 19 | 0.19 | 0.11 | 0.31 | 0.22 | 0.037 | 0.02 | 0.058 |
| Ni | 4.6 | 1.7 * | 28 | 1.7 * | 1.7 * | 1.7 * | 3.6 | 1.7 * | 1.7 * | 1.7 * | 6.1 | 0.51 | 76 | 1.1 | 0.67 | 1.4 | 1.7 | 0.45 | 0.3 | 0.67 |
| Cu | 5.2 | 2.5 | 13 | 4.1 | 2.8 | 4.9 | 4.9 | 2.8 | 2.5 | 3.1 | 17 | 2.1 | 121 | 3.8 | 2.5 | 4.7 | 6.1 | 0.65 | 0.58 | 0.7 |
| Zn | 16 | 8.7 | 29 | 18 | 12 | 23 | 24 | 22 | 15 | 31 | 33 | 3.9 | 311 | 6.9 | 3.7 | 9.2 | 18 | 1.1 | 0.63 | 1.7 |
| As | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.05 * | 0.27 | 0.013 | 2.6 | 0.077 | 0.014 | 0.11 | 0.12 | 0.013 | 0.01 | 0.016 |
| Rb | 0.43 | 0.22 | 0.88 | 0.35 | 0.25 | 0.52 | 0.34 | 0.19 | 0.17 | 0.23 | 3.1 | 0.42 | 32 | 0.56 | 0.33 | 0.81 | 0.60 | 0.2 | 0.12 | 0.38 |
| Sr | 14 | 4.9 | 40 | 7.2 | 5.2 | 11 | 7.0 | 1.2 | 1.0 | 1.3 | 17 | 1.6 | 191 | 2.7 | 1.8 | 4.1 | 4.4 | 0.41 | 0.23 | 0.74 |
| Mo | 0.26 | 0.025 * | 3.4 | 0.071 | 0.025 * | 0.16 | 0.025 * | 0.025 * | 0.025 * | 0.025 * | 0.71 | 0.083 | 7.0 | 0.17 | 0.1 | 0.21 | 0.25 | 0.02 | 0.015 | 0.025 |
| Cd | 0.068 | 0.019 | 0.61 | 0.064 | 0.028 | 0.12 | 0.061 | 0.033 | 0.017 | 0.047 | 0.053 | 0.008 | 0.42 | 0.018 | 0.009 | 0.025 | 0.025 | 0.006 | 0.001 * | 0.012 |
| Sn | 0.015 | 0.005 * | 0.21 | 0.012 | 0.005 * | 0.033 | 0.005 * | 0.005 * | 0.005 * | 0.005 * | 1.3 | 0.35 | 9.6 | 0.59 | 0.38 | 0.75 | 0.56 | 0.063 | 0.04 * | 0.098 |
| Sb | 0.38 | 0.1 | 4.0 | 0.12 | 0.095 | 0.16 | 0.12 | 0.068 | 0.063 | 0.074 | 0.79 | 0.11 | 8.0 | 0.21 | 0.16 | 0.25 | 0.26 | 0.029 | 0.022 | 0.044 |
| Ba | 9.2 | 4.2 | 31 | 6.7 | 4.1 | 8.6 | 7.7 | 2.7 | 2.1 | 3.3 | 34 | 4.9 | 341 | 7.5 | 5.8 | 9.9 | 9.3 | 1.7 | 0.95 | 3.2 |
| W | 0.11 | 0.02 * | 0.88 | 0.02 * | 0.02 * | 0.02 * | 0.02 * | 0.02 * | 0.02 * | 0.02 * | 2.6 | 0.25 | 28 | 0.33 | 0.19 | 0.47 | 0.27 | 0.045 | 0.025 * | 0.068 |
| Pb | 0.35 | 0.13 | 1.8 | 0.28 | 0.18 | 0.44 | 0.54 | 0.70 | 0.50 | 0.83 | 5.0 | 0.46 | 38 | 1.8 | 1.5 | 2.0 | 8.6 | 0.84 | 0.67 | 1.0 |
| Bi | 0.003 | 0.001 * | 0.029 | 0.002 | 0.001 * | 0.006 | 0.001 * | 0.001 * | 0.001 * | 0.001 * | 0.094 | 0.023 | 0.66 | 0.035 | 0.031 | 0.036 | 0.047 | 0.011 | 0.007 | 0.02 |
| Object (Number of Mixed Samples) | Values of Contamination Factor CF | TCF | |||||
|---|---|---|---|---|---|---|---|
| >50 | 25–50 | 10–25 | 5–10 | 3–5 | |||
| MRR (n = 3) | DS | Na134 | – | Ca15Sr14 | W7K7Co5 | Mo4Mg4Ba4V3 | 194 |
| SP | Ca385W258Co219Ti203Mg199Sr198Na194 Mo141Al128Fe122Zn120Sb113Mn88As86 Ba85Cu78Cr78Ni66Rb66Sn63K60 | Cd31Bi25 | Pb19 | – | – | 3212 | |
| Highways (n = 4) | DS | Na280 | – | Sr23Ca21K20W19Sn11 | Bi8Co6Mo6Mg5Ni5V5 | Ba4Sb3Fe3Ti3 | 414 |
| SP | Ca64 | W47Co31Ti31Sr29 V29Mg28Na27 | Zn24Mo22Fe20Sn17Al17Sb16 Cu15Ba14As13Mn13Rb11K10 | Cd7Bi6Ni6Cr6 | Pb4 | 481 | |
| Large roads (n = 4) | DS | Na60 | Mo36 | Sb17K11Ca10 | Sr9Mg9 | V4Co3 | 156 |
| SP | – | – | Ca24W21Cu17Mo16Zn14Sr13Co12 Sn12Sb11Mg11V11Ti10As10Na10 | Fe9Ba8Al7Rb6Mn6 Bi6Cd5K5Ni5Pb5 | Cr4 | 235 | |
| Medium roads (n = 5) | DS | Na71 | – | – | Sr9Ca7K7Cd6Mn5Co5 | V4Mg3Mo3 | 116 |
| SP | Cu22Ca21W19Mo18Sn14Sb13V13 Sr13Zn12Ti11Mg11Co11As11Na10Fe10 | Ba8Al7Rb6K6Mn6Bi5 | Cd4Ni4Cr4Pb4 | 240 | |||
| Small roads (n = 5) | DS | Na52 | – | – | K9Sr7Ca6V6Mo5 | Sb4Ba4Ni4Mg3 | 98 |
| SP | – | – | Mo17V15Ca13Sn12W12 Cu12Sb11As10Zn10Fe10 | Sr9Co9Ti8Mg7Na6 Ba6Al5Mn5Bi5 | Cd4Ni4Rb4K4Pb3Cr3 | 179 | |
| Average for roads (n = 21) | DS | Na113 | – | Sr12Ca11K11Mo10 | Sb6W6Mg5V5 | Co4Sn3Ba3 | 187 |
| SP | Ca80W57 | Co44V44Sr42Ti41Mg40Na39 Mo36Zn30Fe28Sb27Cu25 | As22Sn21Ba20Mn19 Al25Rb15Cr15K14Ni14 | Cd9Bi8Pb6 | – | 695 | |
| Yards with car parking lots (n = 4) | DS | Na31 | – | K12 | Sr6 | V4Ca3 | 66 |
| SP | – | – | – | Sn9Ca9Mo8V8Sb7W7Sr6 Zn6As6Ti6Cu6Co5Mg5Fe5 | Ba4Na4Al3Cd3Bi3 | 103 | |
| MO MSU (n = 1) | DS | Na28 | – | K22 | V6Sr6Mg5 | – | 71 |
| SP | – | – | Zn16Mo13Cr12 Sr11V10Pb10Ca10 | Cu9As9Sn9Sb9W6 Co6Ba5Ti5Mg5Fe5Na5 | Cd4Bi4Al4Ni4 | 156 | |
| MMs | Dissolved Forms | Suspended Forms | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roads (n = 21) | Yards (n = 4) | MO MSU (n = 1) | Background (n = 3) | Roads (n = 21) | Yards (n = 4) | MO MSU (n = 1) | Background (n = 3) | |||||||||||||
| Mean | Min. | Max. | Mean | Min. | Max. | Mean | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Mean | Min. | Max. | |
| Na | 27511 | 3489 | 98917 | 7695 | 2160 | 19791 | 6666 | 211 | 154 | 275 | 1189 | 95 | 13321 | 134 | 60 | 233 | 134 | 25 | 16 | 35 |
| Mg | 299 | 107 | 932 | 137 | 110 | 152 | 282 | 54 | 45 | 69 | 1415 | 115 | 15400 | 188 | 91 | 310 | 181 | 29 | 21 | 35 |
| Al | 95 | 41 | 247 | 85 | 48 | 150 | 66 | 61 | 55 | 65 | 5382 | 383 | 59597 | 683 | 330 | 1115 | 783 | 170 | 99 | 244 |
| K | 665 | 122 | 1588 | 783 | 353 | 1288 | 1363 | 54 | 45 | 69 | 1060 | 122 | 10577 | 204 | 94 | 328 | 201 | 62 | 35 | 97 |
| Ca | 19997 | 3476 | 61603 | 5851 | 3086 | 7865 | 4383 | 1475 | 1191 | 1705 | 5104 | 309 | 54910 | 603 | 250 | 1054 | 587 | 51 | 38 | 74 |
| Ti | 2.4 | 0.31 | 14 | 1.1 | 0.75 | 1.5 | 1.3 | 1.2 | 0.9 | 1.6 | 440 | 42 | 4764 | 63 | 32 | 103 | 55 | 8.4 | 1.8 | 16 |
| V | 1.2 | 0.21 | 3.7 | 0.93 | 0.27 | 2.2 | 1.4 | 0.22 | 0.18 | 0.27 | 15 | 1.2 | 165 | 2.6 | 1.5 | 3.4 | 3.4 | 0.28 | 0.16 | 0.41 |
| Cr | 0.84 | 0.52 | 4.9 | 0.63 | 0.55 | 0.68 | 0.61 | 0.54 | 0.45 | 0.69 | 13 | 0.48 | 171 | 1.4 | 0.65 | 2.2 | 11 | 0.76 | 0.48 | 0.99 |
| Mn | 14 | 4.3 | 110 | 16 | 3.5 | 46 | 4.8 | 5.1 | 3.1 | 8.5 | 71 | 6.7 | 737 | 11 | 5.0 | 19 | 10 | 3.0 | 1.1 | 5.3 |
| Fe | 116 | 34 | 479 | 89 | 41 | 126 | 57 | 52 | 42 | 65 | 4898 | 492 | 47751 | 884 | 422 | 1405 | 833 | 144 | 91 | 197 |
| Co | 0.36 | 0.14 | 1.4 | 0.24 | 0.13 | 0.41 | 0.13 | 0.074 | 0.067 | 0.082 | 2.1 | 0.16 | 23 | 0.25 | 0.12 | 0.42 | 0.27 | 0.037 | 0.027 | 0.053 |
| Ni | 5.6 | 1.9 | 36 | 2.2 | 1.9 | 2.4 | 4.4 | 1.9 | 1.6 | 2.4 | 7.7 | 0.56 | 93 | 1.4 | 0.74 | 1.8 | 2.1 | 0.47 | 0.35 | 0.64 |
| Cu | 6.6 | 3.0 | 17 | 5.2 | 3.6 | 6.6 | 6 | 3.1 | 2.5 | 3.9 | 21 | 2.3 | 148 | 4.9 | 2.8 | 6.0 | 7.5 | 0.7 | 0.62 | 0.79 |
| Zn | 21 | 13 | 37 | 24 | 15 | 31 | 29 | 25 | 15 | 43 | 42 | 4.3 | 380 | 9.0 | 4.0 | 12 | 22 | 1.2 | 0.61 | 1.6 |
| As | 0.064 | 0.052 | 0.084 | 0.064 | 0.055 | 0.068 | 0.061 | 0.054 | 0.045 | 0.069 | 0.35 | 0.016 | 3.2 | 0.1 | 0.015 | 0.14 | 0.14 | 0.013 | 0.011 | 0.015 |
| Rb | 0.55 | 0.23 | 1.2 | 0.45 | 0.32 | 0.7 | 0.42 | 0.21 | 0.16 | 0.25 | 3.9 | 0.46 | 39 | 0.73 | 0.37 | 1.1 | 0.74 | 0.21 | 0.11 | 0.34 |
| Sr | 18 | 6.09 | 53 | 9.3 | 5.7 | 16 | 8.7 | 1.3 | 0.99 | 1.8 | 22 | 2.0 | 233 | 3.4 | 2.0 | 5.6 | 5.5 | 0.42 | 0.27 | 0.67 |
| Mo | 0.32 | 0.03 | 4.0 | 0.091 | 0.028 | 0.2 | 0.031 | 0.027 | 0.023 | 0.034 | 0.9 | 0.09 | 8.5 | 0.21 | 0.11 | 0.27 | 0.31 | 0.021 | 0.019 | 0.023 |
| Cd | 0.087 | 0.025 | 0.8 | 0.082 | 0.035 | 0.16 | 0.075 | 0.036 | 0.016 | 0.049 | 0.067 | 0.009 | 0.52 | 0.024 | 0.01 | 0.035 | 0.031 | 0.006 | 0.001 | 0.011 |
| Sn | 0.017 | 0.005 | 0.23 | 0.016 | 0.006 | 0.045 | 0.006 | 0.005 | 0.005 | 0.007 | 1.7 | 0.38 | 12 | 0.75 | 0.42 | 0.95 | 0.69 | 0.066 | 0.039 | 0.089 |
| Sb | 0.47 | 0.12 | 4.8 | 0.16 | 0.1 | 0.22 | 0.15 | 0.074 | 0.06 | 0.1 | 1.0 | 0.12 | 9.8 | 0.27 | 0.17 | 0.34 | 0.32 | 0.03 | 0.021 | 0.04 |
| Ba | 12 | 5.2 | 39 | 8.4 | 5.6 | 12 | 9.5 | 3.0 | 2.0 | 4.6 | 43 | 5.4 | 417 | 9.6 | 6.4 | 13 | 11 | 1.7 | 0.92 | 2.9 |
| W | 0.14 | 0.021 | 0.96 | 0.025 | 0.022 | 0.027 | 0.025 | 0.022 | 0.018 | 0.027 | 3.3 | 0.3 | 34 | 0.42 | 0.21 | 0.64 | 0.33 | 0.046 | 0.034 | 0.061 |
| Pb | 0.44 | 0.15 | 1.9 | 0.36 | 0.23 | 0.6 | 0.66 | 0.76 | 0.48 | 1.1 | 6.3 | 0.5 | 46 | 2.3 | 1.7 | 2.7 | 11 | 0.91 | 0.65 | 1.1 |
| Bi | 0.003 | 0.001 | 0.032 | 0.003 | 0.0013 | 0.007 | 0.0012 | 0.0011 | 0.0009 | 0.0014 | 0.12 | 0.025 | 0.81 | 0.044 | 0.04 | 0.048 | 0.058 | 0.012 | 0.007 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasov, D.; Vasil’chuk, J.; Kosheleva, N.; Kasimov, N. Dissolved and Suspended Forms of Metals and Metalloids in Snow Cover of Megacity: Partitioning and Deposition Rates in Western Moscow. Atmosphere 2020, 11, 907. https://doi.org/10.3390/atmos11090907
Vlasov D, Vasil’chuk J, Kosheleva N, Kasimov N. Dissolved and Suspended Forms of Metals and Metalloids in Snow Cover of Megacity: Partitioning and Deposition Rates in Western Moscow. Atmosphere. 2020; 11(9):907. https://doi.org/10.3390/atmos11090907
Chicago/Turabian StyleVlasov, Dmitry, Jessica Vasil’chuk, Natalia Kosheleva, and Nikolay Kasimov. 2020. "Dissolved and Suspended Forms of Metals and Metalloids in Snow Cover of Megacity: Partitioning and Deposition Rates in Western Moscow" Atmosphere 11, no. 9: 907. https://doi.org/10.3390/atmos11090907
APA StyleVlasov, D., Vasil’chuk, J., Kosheleva, N., & Kasimov, N. (2020). Dissolved and Suspended Forms of Metals and Metalloids in Snow Cover of Megacity: Partitioning and Deposition Rates in Western Moscow. Atmosphere, 11(9), 907. https://doi.org/10.3390/atmos11090907








