Ground Level Isoprenoid Exchanges Associated with Pinus pinea Trees in A Mediterranean Turf
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site and Experimental Design
2.2. Isoprenoid Sampling
2.3. GC-MS Analyses of BVOCs
2.4. Statistical Analyses
3. Results
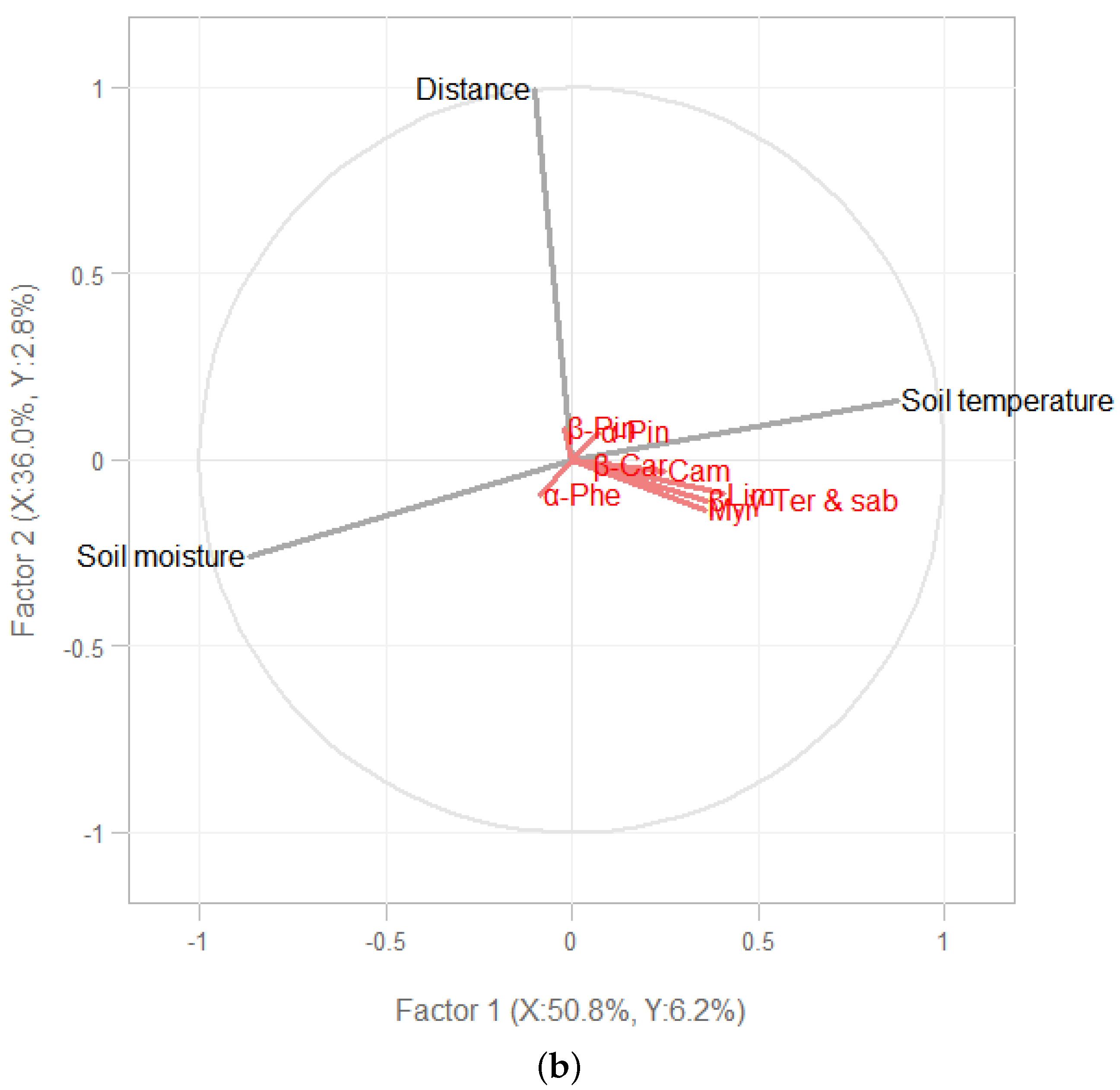
3.1. Soil Environmental Conditions
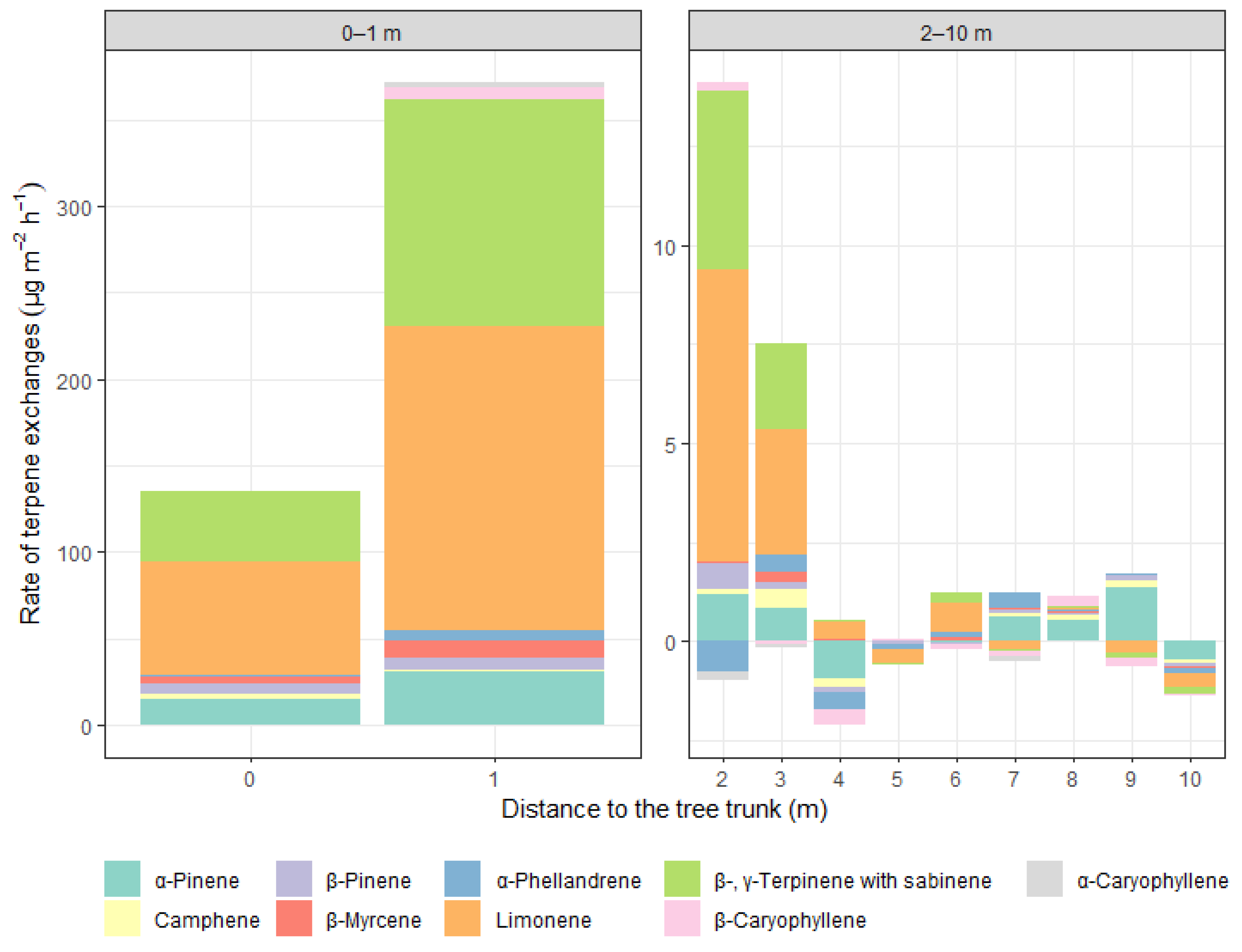
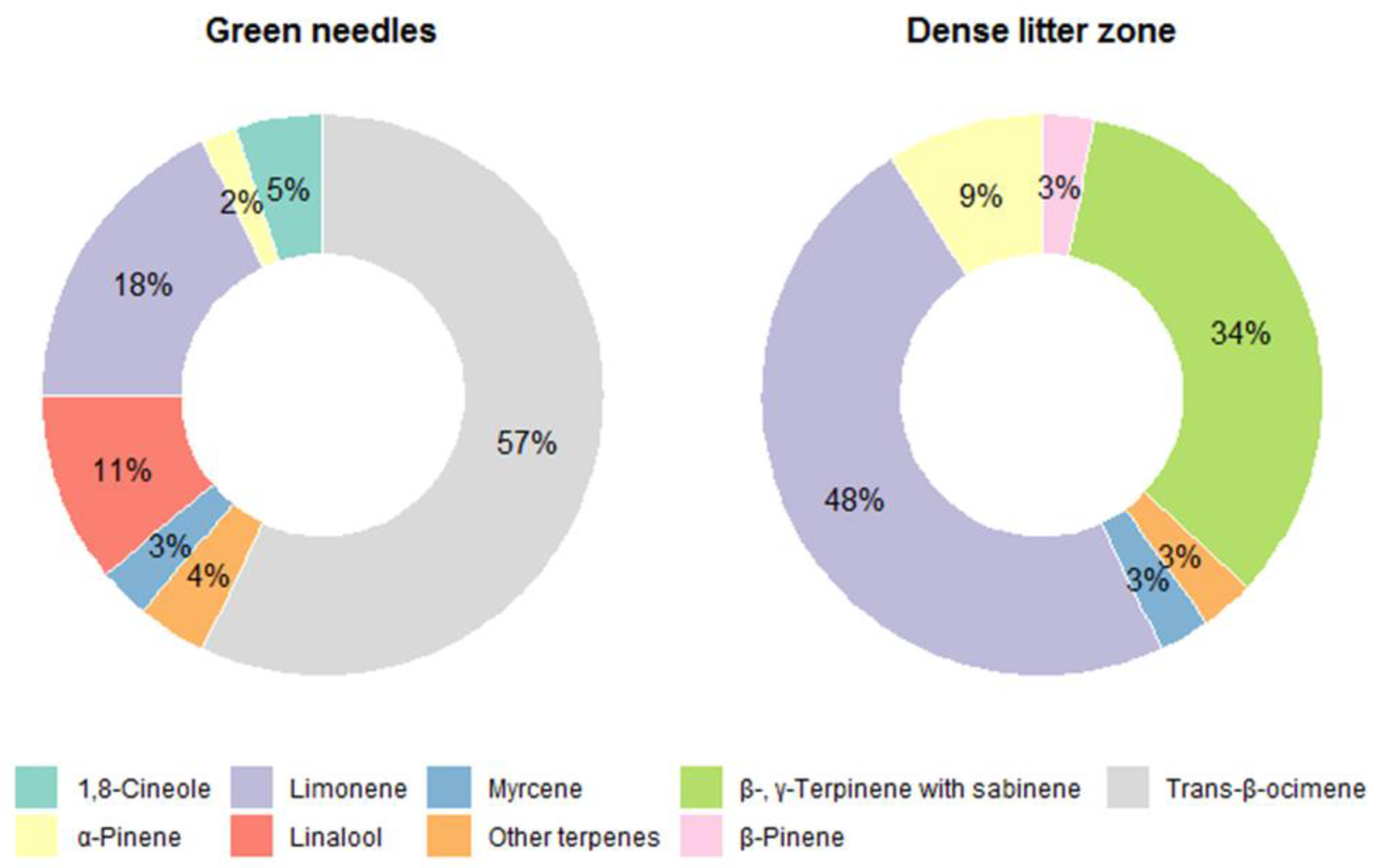
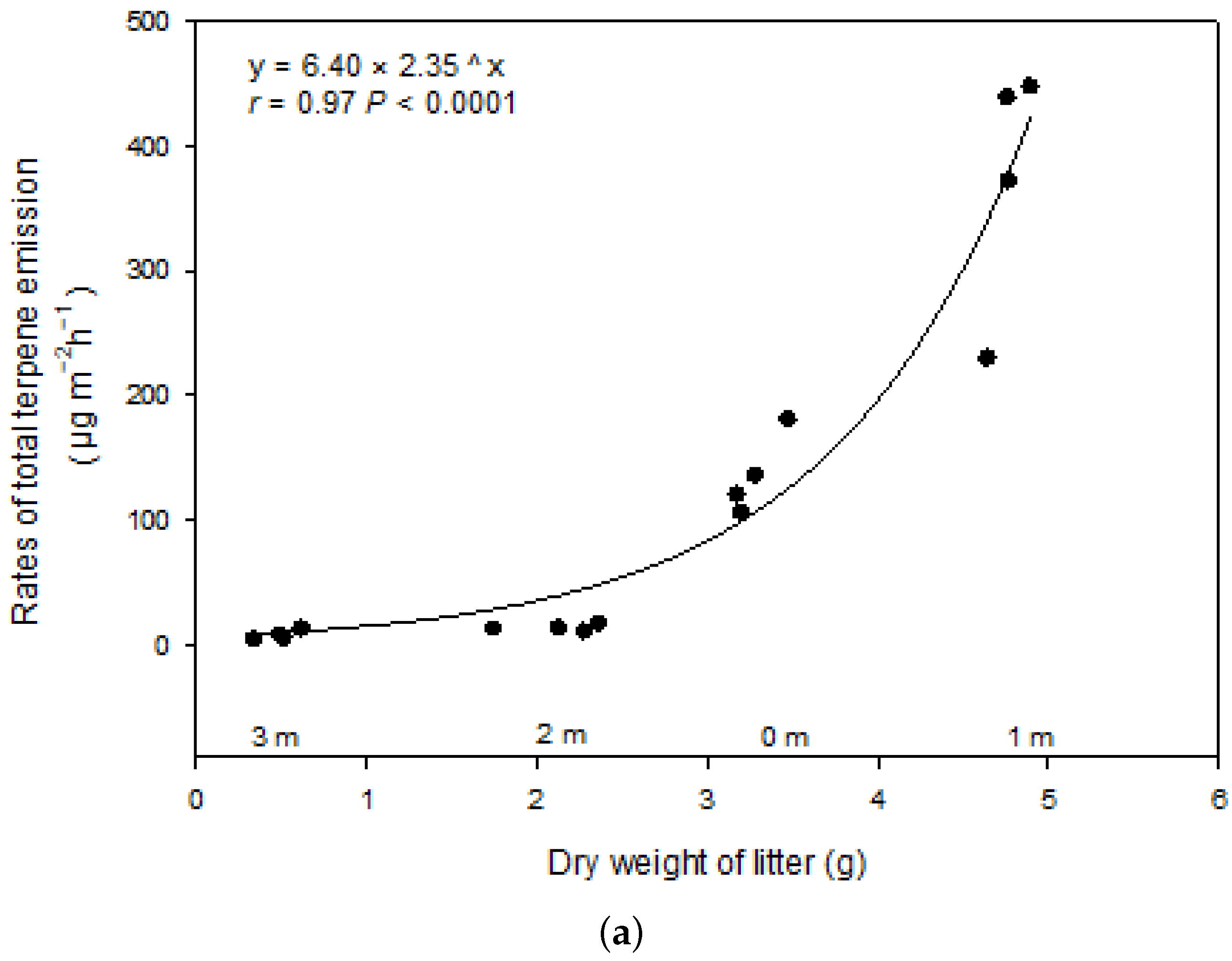
3.2. Terpene Exchange
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atkinson, R. Atmospheric chemistry of VOCs and NO(x). Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Peñuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef]
- Shindell, D.T.; Faluvegi, G.; Koch, D.M.; Schmidt, G.A.; Unger, N.; Bauer, S.E. Improved attribution of climate forcing to emissions. Science 2009, 326, 716–718. [Google Scholar] [CrossRef]
- Svendsen, S.H.; Priemé, A.; Voriskova, J.; Kramshøj, M.; Schostag, M.; Jacobsen, C.S.; Rinnan, R. Emissions of biogenic volatile organic compounds from arctic shrub litter are coupled with changes in the bacterial community composition. Soil Biol. Biochem. 2018, 120, 80–90. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compunds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Svendsen, S.H.; Lindwall, F.; Michelsen, A.; Rinnan, R. Biogenic volatile organic compound emissions along a high arctic soil moisture gradient. Sci. Total. Environ. 2016, 573, 131–138. [Google Scholar] [CrossRef]
- Albers, C.N.; Kramshøj, M.; Rinnan, R. Rapid mineralization of biogenic volatile organic compounds in temperate and Arctic soils. Biogeosciences 2018, 15, 3591–3601. [Google Scholar] [CrossRef]
- Leff, J.W.; Fierer, N. Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biol. Biochem. 2008, 40, 1629–1636. [Google Scholar] [CrossRef]
- Mu, Z.; Llusià, J.; Liu, D.; Ogaya, R.; Asensio, D.; Zhang, C.; Peñuelas, J. Seasonal and diurnal variations of plant isoprenoid emissions from two dominant species in Mediterranean shrubland and forest submitted to experimental drought. Atmos. Environ. 2018, 191, 105–115. [Google Scholar] [CrossRef]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef]
- Potard, K.; Monard, C.; Le Garrec, J.L.; Caudal, J.P.; Le Bris, N.; Binet, F. Organic amendment practices as possible drivers of biogenic Volatile Organic Compounds emitted by soils in agrosystems. Agric. Ecosyst. Environ. 2017, 250, 25–36. [Google Scholar] [CrossRef]
- Asensio, D.; Peñuelas, J.; Ogaya, R.; Llusià, J. Seasonal soil VOC exchange rates in a Mediterranean holm oak forest and their responses to drought conditions. Atmos. Environ. 2007, 41, 2456–2466. [Google Scholar] [CrossRef]
- He, N.P.; Han, X.G.; Pan, Q.M. Variations in the volatile organic compound emission potential of plant functional groups in the temperate grassland vegetation of inner Mongolia, China. J. Integr. Plant Biol. 2005, 47, 13–19. [Google Scholar] [CrossRef]
- Mäki, M.; Heinonsalo, J.; Hellén, H.; Bäck, J. Contribution of understorey vegetation and soil processes to boreal forest isoprenoid exchange. Biogeosciences 2017, 14, 1055–1073. [Google Scholar] [CrossRef]
- Aaltonen, H.; Aalto, J.; Kolari, P.; Pihlatie, M.; Pumpanen, J.; Kulmala, M.; Nikinmaa, E.; Vesala, T.; Bäck, J. Continuous VOC flux measurements on boreal forest floor. Plant Soil 2013, 369, 241–256. [Google Scholar] [CrossRef]
- Bäck, J.; Aaltonen, H.; Hellén, H.; Kajos, M.K.; Patokoski, J.; Taipale, R.; Pumpanen, J.; Heinonsalo, J. Variable emissions of microbial volatile organic compounds (MVOCs) from root-associated fungi isolated from Scots pine. Atmos. Environ. 2010, 44, 3651–3659. [Google Scholar] [CrossRef]
- Asensio, D.; Owen, S.M.; Llusià, J.; Peñuelas, J. The distribution of volatile isoprenoids in the soil horizons around Pinus halepensis trees. Soil Biol. Biochem. 2008, 40, 2937–2947. [Google Scholar] [CrossRef]
- Hellén, H.; Hakola, H.; Pystynen, K.H.; Rinne, J.; Haapanala, S. C2-C10 hydrocarbon emissions from a boreal wetland and forest floor. Biogeosciences 2006, 3, 167–174. [Google Scholar] [CrossRef]
- Tang, J.; Schurgers, G.; Rinnan, R. Process Understanding of Soil BVOC Fluxes in Natural Ecosystems: A Review. Rev. Geophys. 2019, 57, 966–986. [Google Scholar] [CrossRef]
- Hayward, S.; Muncey, R.J.; James, A.E.; Halsall, C.J.; Hewitt, C.N. Monoterpene emissions from soil in a Sitka spruce forest. Atmos. Environ. 2001, 35, 4081–4087. [Google Scholar] [CrossRef]
- Mäki, M.; Aaltonen, H.; Heinonsalo, J.; Hellén, H.; Pumpanen, J.; Bäck, J. Boreal forest soil is a significant and diverse source of volatile organic compounds. Plant Soil 2019, 441, 89–110. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Lauber, C.L.; Fierer, N. Microbial consumption and production of volatile organic compounds at the soil-litter interface. Biogeochemistry 2010, 99, 97–107. [Google Scholar] [CrossRef]
- Smolander, A.; Ketola, R.A.; Kotiaho, T.; Kanerva, S.; Suominen, K.; Kitunen, V. Volatile monoterpenes in soil atmosphere under birch and conifers: Effects on soil N transformations. Soil Biol. Biochem. 2006, 38, 3436–3442. [Google Scholar] [CrossRef]
- Stahl, P.D.; Parkin, T.B. Microbial production of volatile organic compounds in soil microcosms. Soil Sci. Soc. Am. J. 1996, 60, 821–828. [Google Scholar] [CrossRef]
- Paavolainen, L.; Kitunen, V.; Smolander, A. Inhibition of nitrification in forest soil by monoterpenes. Plant Soil 1998, 205, 147–154. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef]
- Rossabi, S.; Choudoir, M.; Helmig, D.; Hueber, J.; Fierer, N. Volatile Organic Compound Emissions From Soil Following Wetting Events. J. Geophys. Res. Biogeosciences 2018, 123, 1988–2001. [Google Scholar] [CrossRef]
- Greenberg, J.P.; Asensio, D.; Turnipseed, A.; Guenther, A.B.; Karl, T.; Gochis, D. Contribution of leaf and needle litter to whole ecosystem BVOC fluxes. Atmos. Environ. 2012, 59, 302–311. [Google Scholar] [CrossRef]
- Asensio, D.; Peñuelas, J.; Llusià, J.; Ogaya, R.; Filella, I. Interannual and interseasonal soil CO2 efflux and VOC exchange rates in a Mediterranean holm oak forest in response to experimental drought. Soil Biol. Biochem. 2007, 39, 2471–2484. [Google Scholar] [CrossRef]
- Asensio, D.; Peñuelas, J.; Filella, I.; Llusià, J. On-line screening of soil VOCs exchange responses to moisture, temperature and root presence. Plant Soil 2007, 291, 249–261. [Google Scholar] [CrossRef]
- Van Roon, A.; Parsons, J.R.; Te Kloeze, A.M.; Govers, H.A.J. Fate and transport of monoterpenes through soils. Part I. Prediction of temperature dependent soil fate model input-parameters. Chemosphere 2005, 61, 599–609. [Google Scholar]
- Insam, H.; Seewald, M.S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Veres, P.R.; Behrendt, T.; Klapthor, A.; Meixner, F.X.; Williams, J. Volatile Organic Compound emissions from soil: Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry (PTR-TOF-MS) for the real time observation of microbial processes. Biogeosci. Discuss. 2014, 11, 12009–12038. [Google Scholar] [CrossRef]
- Gray, C.M.; Monson, R.K.; Fierer, N. Emissions of volatile organic compounds during the decomposition of plant litter. J. Geophys. Res. Biogeosci. 2010, 115, 115. [Google Scholar] [CrossRef]
- Loreto, F.; Nascetti, P.; Graverini, A.; Mannozzi, M. Emission and content of monoterpenes in intact and wounded needles of the Mediterranean Pine, Pinus pinea. Funct. Ecol. 2000, 14, 589–595. [Google Scholar] [CrossRef]
- Staudt, M.; Bertin, N.; Frenzel, B.; Seufert, G. Seasonal variation in amount and composition of monoterpenes emitted by young Pinus pinea trees—Implications for emission modeling. J. Atmos. Chem. 2000, 35, 77–99. [Google Scholar] [CrossRef]
- Lin, C.; Owen, S.M.; Peñuelas, J. Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol. Biochem. 2007, 39, 951–960. [Google Scholar] [CrossRef]
- Rinnan, R.; Gierth, D.; Bilde, M.; Rosenørn, T.; Michelsen, A. Off-season biogenic volatile organic compound emissions from heath mesocosms: Responses to vegetation cutting. Front. Microbiol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Steeghs, M.; Bais, H.P.; De Gouw, J.; Goldan, P.; Kuster, W.; Northway, M.; Fall, R.; Vivanco, J.M. Proton-transfer-reaction mass spectrometry as a new tool for real time analysis of root-secreted volatile organic compounds in Arabidopsis. Plant Physiol. 2004, 135, 47–58. [Google Scholar] [CrossRef]
- Gray, C.M.; Fierer, N. Impacts of nitrogen fertilization on volatile organic compound emissions from decomposing plant litter. Glob. Chang. Biol. 2012, 18, 739–748. [Google Scholar] [CrossRef]
- Karl, T.G.; Spirig, C.; Rinne, J.; Stroud, C.; Prevost, P.; Greenberg, J.; Fall, R.; Guenther, A. Virtual disjunct eddy covariance measurements of organic compound fluxes from a subalpine forest using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2002, 2, 279–291. [Google Scholar] [CrossRef]
- Mu, Z.; Llusià, J.; Liu, D.; Ogaya, R.; Asensio, D.; Zhang, C.; Peñuelas, J. Profile of foliar isoprenoid emissions from Mediterranean dominant shrub and tree species under experimental nitrogen deposition. Atmos. Environ. 2019, 216, 116951. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Llusià, J. Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol. Plant. 2007, 131, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Dumergues, L.; Solignac, G.; Torres, L. Biogenic emissions from Pinus halepensis: A typical species of the Mediterranean area. Atmo. Res. 2005, 74, 37–48. [Google Scholar] [CrossRef]
- Tava, A.; Berardo, N.; Odoardi, M. Composition of essential oil of tall fescue. Phytochemistry 1991, 30, 1455–1458. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Z.; Llusià, J.; Peñuelas, J. Ground Level Isoprenoid Exchanges Associated with Pinus pinea Trees in A Mediterranean Turf. Atmosphere 2020, 11, 809. https://doi.org/10.3390/atmos11080809
Mu Z, Llusià J, Peñuelas J. Ground Level Isoprenoid Exchanges Associated with Pinus pinea Trees in A Mediterranean Turf. Atmosphere. 2020; 11(8):809. https://doi.org/10.3390/atmos11080809
Chicago/Turabian StyleMu, Zhaobin, Joan Llusià, and Josep Peñuelas. 2020. "Ground Level Isoprenoid Exchanges Associated with Pinus pinea Trees in A Mediterranean Turf" Atmosphere 11, no. 8: 809. https://doi.org/10.3390/atmos11080809
APA StyleMu, Z., Llusià, J., & Peñuelas, J. (2020). Ground Level Isoprenoid Exchanges Associated with Pinus pinea Trees in A Mediterranean Turf. Atmosphere, 11(8), 809. https://doi.org/10.3390/atmos11080809




