Online Measurement of PM2.5 at an Air Monitoring Supersite in Yangtze River Delta: Temporal Variation and Source Identification
Abstract
1. Introduction
2. Experiments
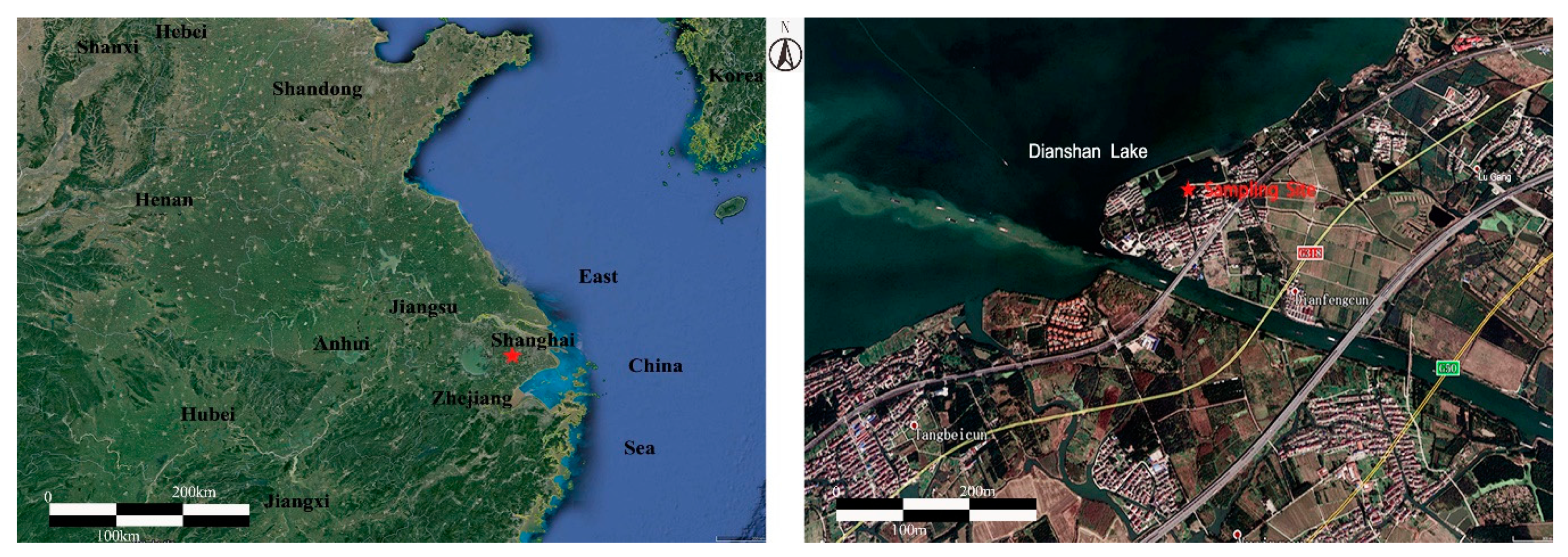
2.1. Field Observation
2.2. Measurements of Various Parameters
2.3. Sources Apportionment of PM2.5
2.3.1. Back Trajectory Cluster
2.3.2. PSCF Model
3. Results and Discussions
3.1. Dynamic Variations of PM2.5
3.1.1. Seasonal Variations of Chemical Composition in PM2.5
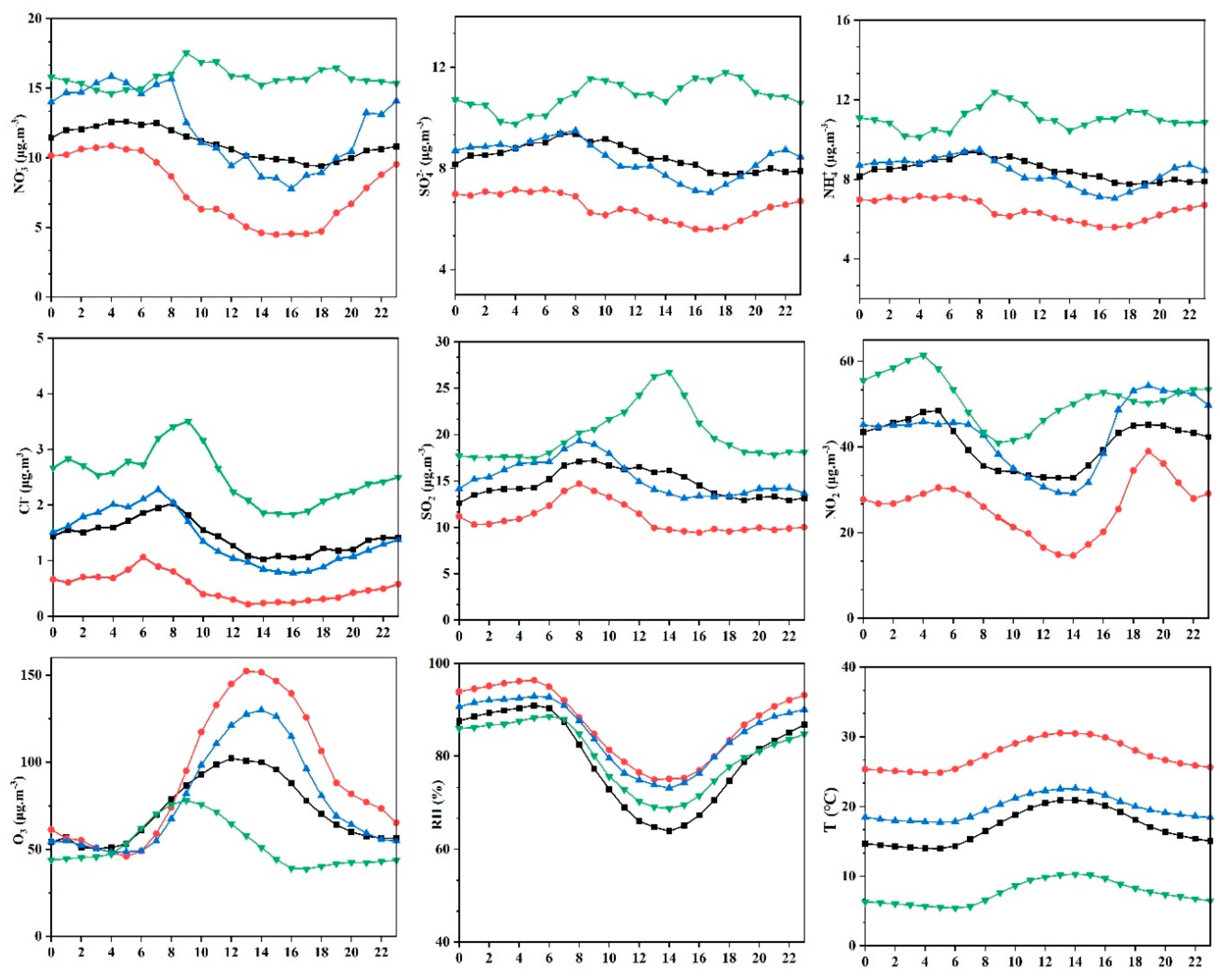
3.1.2. Diurnal Patterns of Water Soluble Ions in PM2.5
3.2. Source Identification
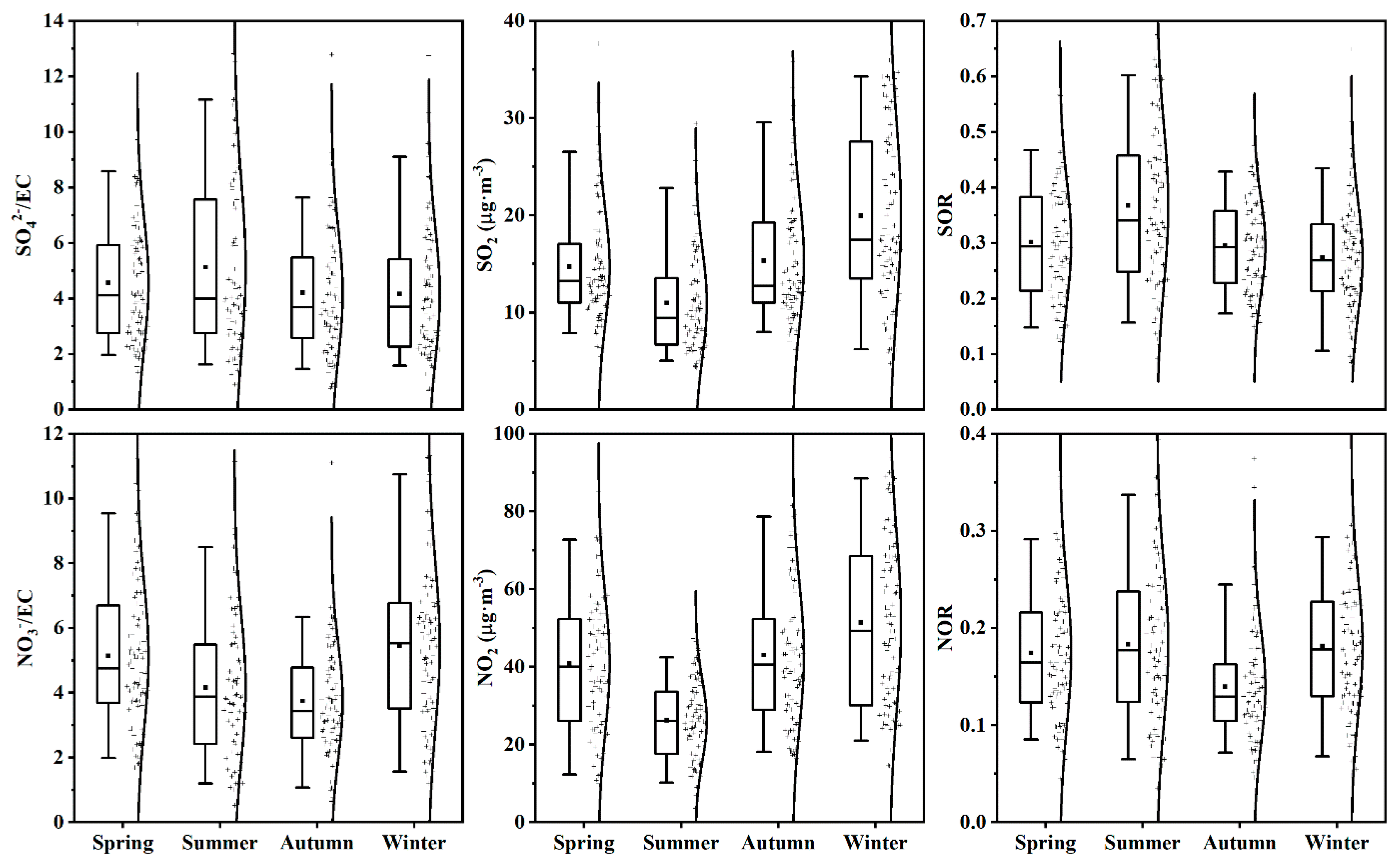
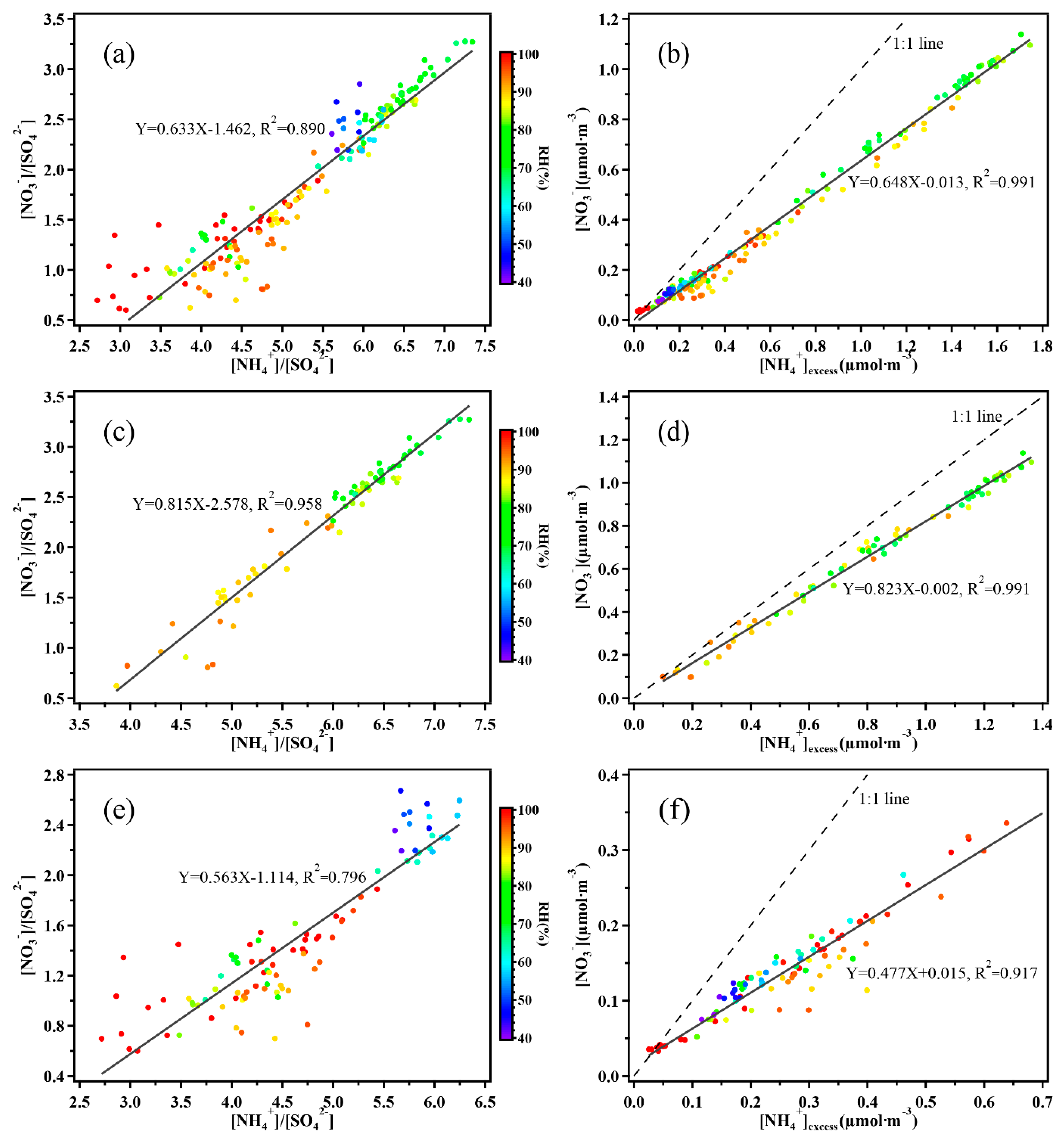
3.2.1. The Ratio of NO3−/EC and SO42−/EC, SOR and NOR
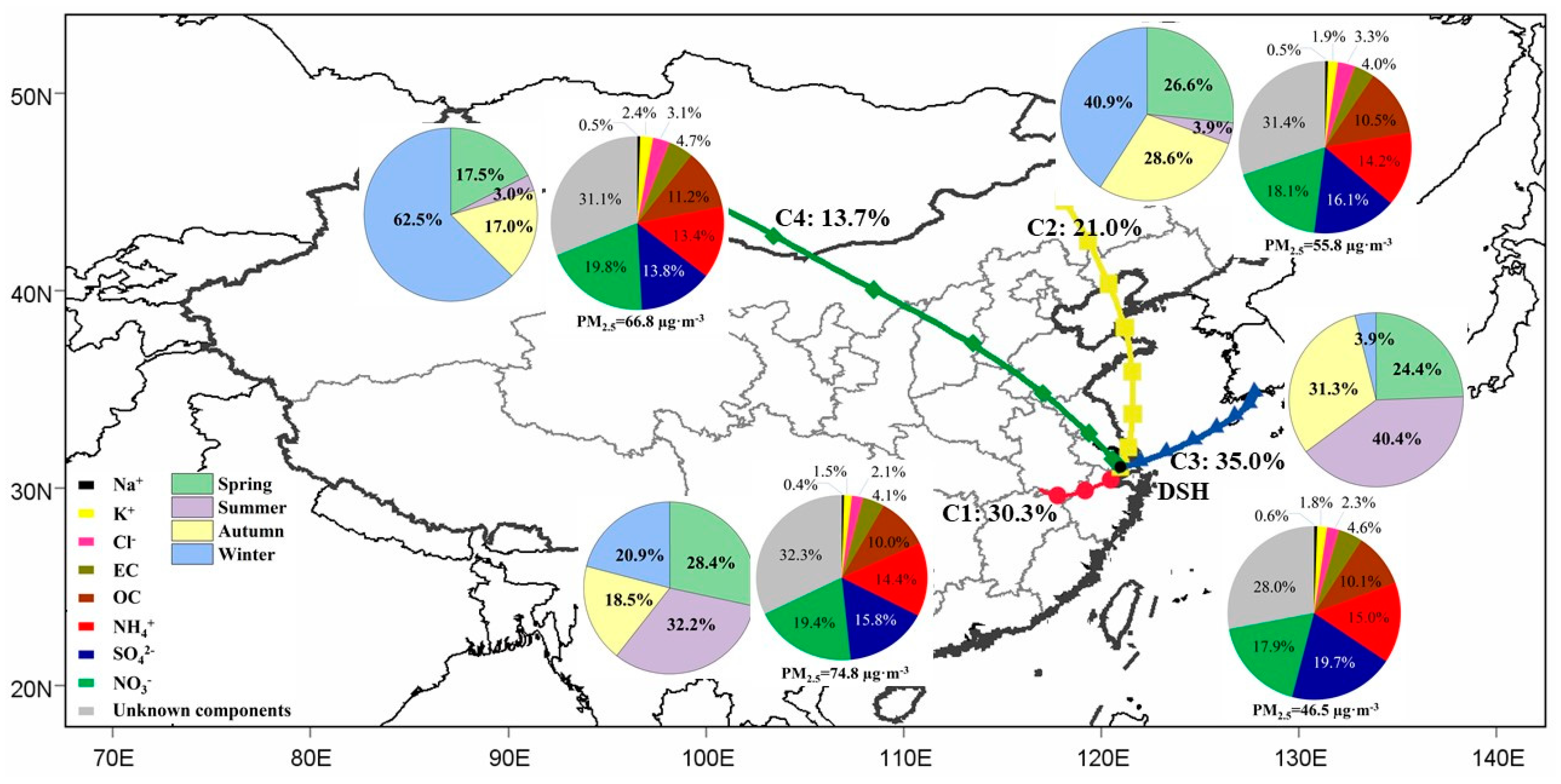
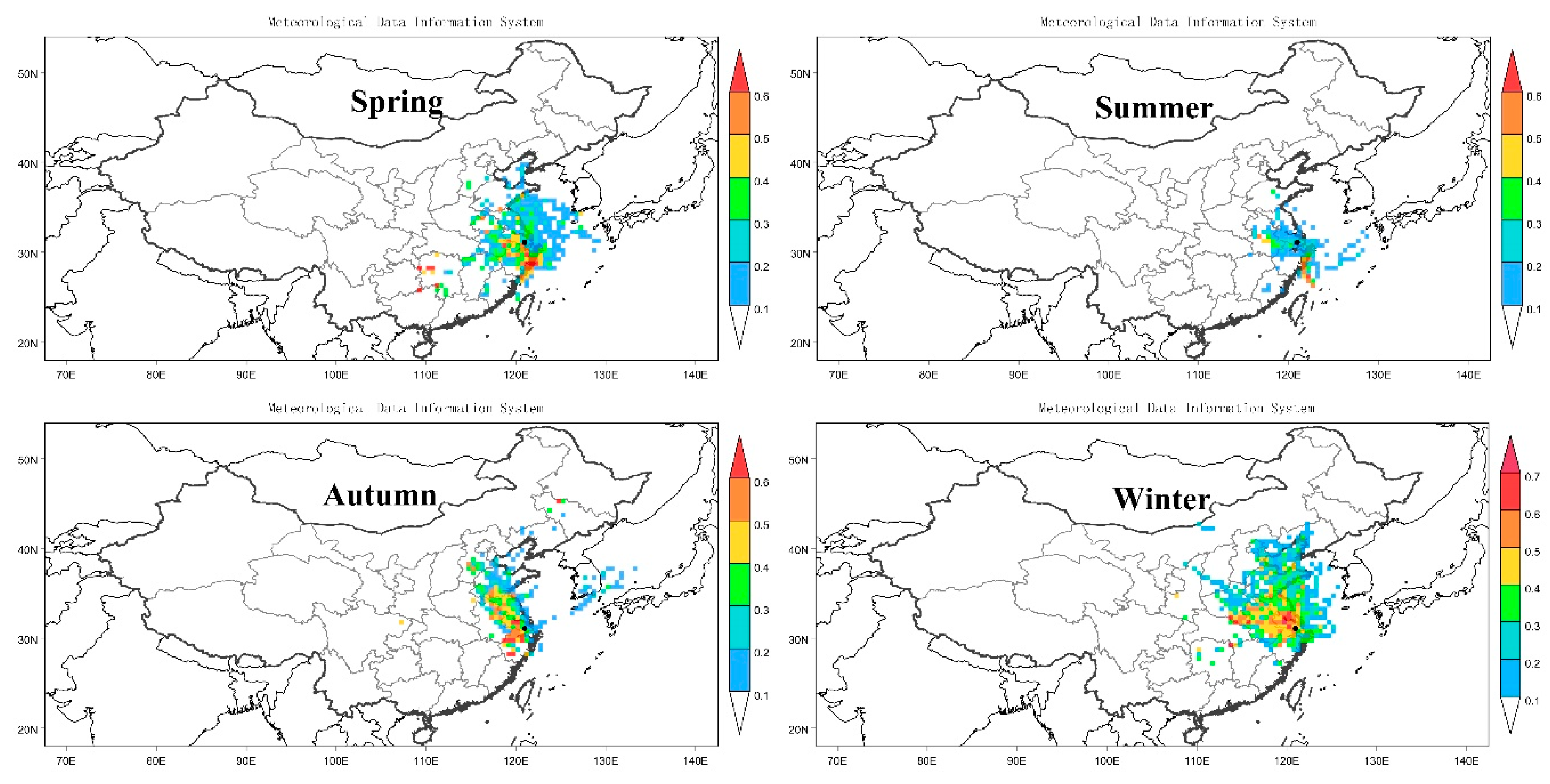
3.2.2. Cluster Analysis and PSCF
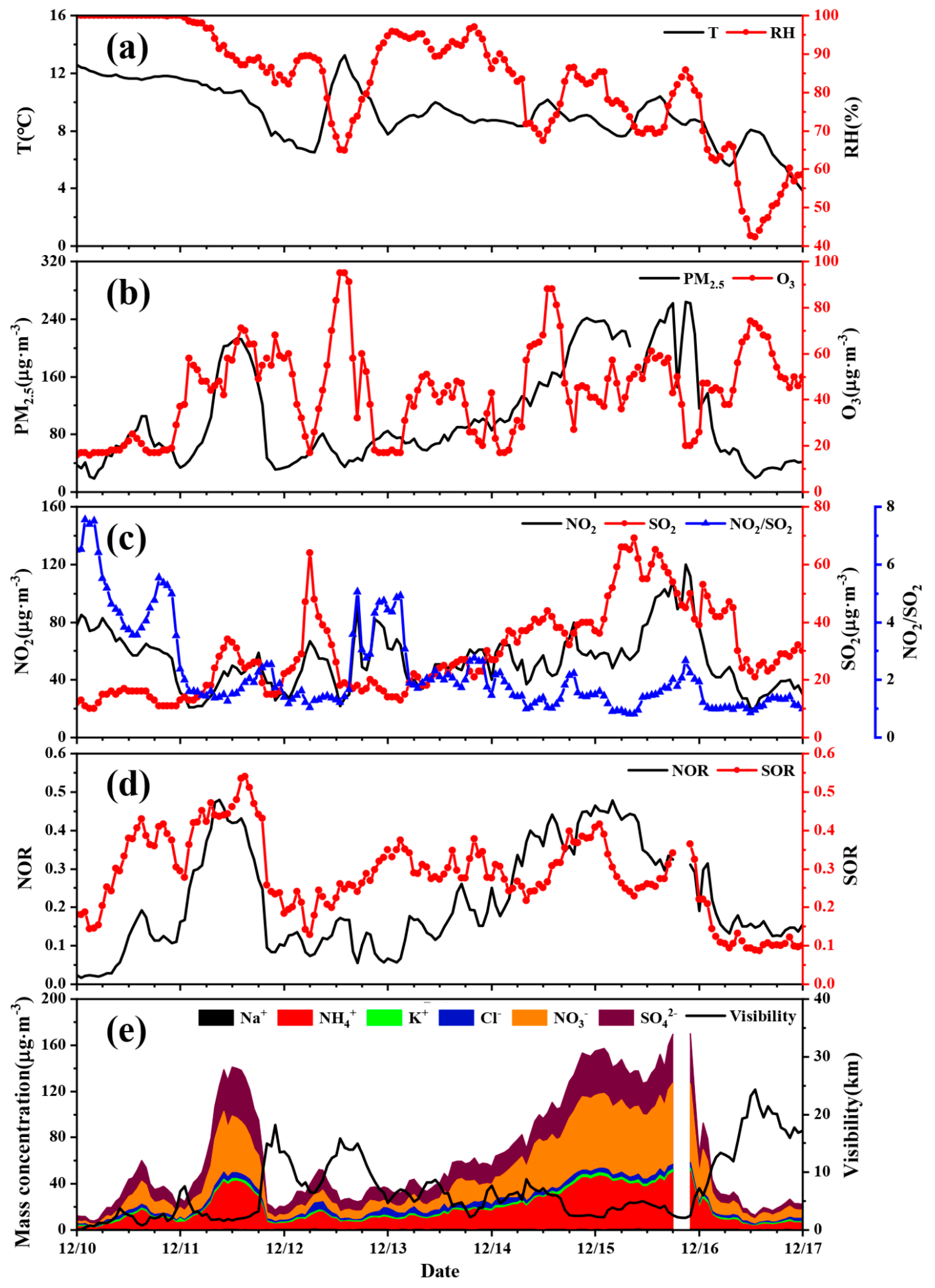
3.3. Pollution Episode
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Huang, Z.; Qiao, T.; Zhang, Y.; Xiu, G.; Yu, J. Chemical characterization, the transport pathways and potential sources of PM2.5 in Shanghai: Seasonal variations. Atmos. Res. 2015, 158, 66–78. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Chen, Y.; Chen, Z.; Xu, S. Contamination characteristics and possible sources of PM10 and PM2.5 in different functional areas of Shanghai, China. Atmos. Environ. 2013, 68, 221–229. [Google Scholar] [CrossRef]
- Liu, C.; Henderson, B.H.; Wang, D.; Yang, X.; Peng, Z.R. A land use regression application into assessing spatial variation of intra-urban fine particulate matter (PM2.5) and nitrogen dioxide (NO2) concentrations in City of Shanghai, China. Sci. Total Environ. 2016, 565, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, U.; Hellén, H.; Anttila, P.; Ferm, M. Size distribution and chemical composition of airborne particles in south-eastern Finland during different seasons and wildfire episodes in 2006. Sci. Total Environ. 2010, 408, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Cai, J.; Wang, H.; Wang, W.; Zhou, M.; Lou, S.; Chen, R.; Dai, H.; Chen, C.; Kan, H. PM2.5 constituents and hospital emergency-room visits in Shanghai, China. Environ. Sci. Technol. 2014, 48, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Salameh, D.; Detournay, A.; Pey, J.; Pérez, N.; Liguori, F.; Saraga, D.; Bove, M.C.; Brotto, P.; Cassola, F.; Massabò, D. PM2.5 chemical composition in five European Mediterranean cities: A 1-year study. Atmos. Res. 2015, 155, 102–117. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, H.R.; Wang, Z.W.; Lv, X.P.; Zhu, Z.M.; Zhang, G.; Wang, X.M. Fine particles (PM2.5) at a CAWNET background site in Central China: Chemical compositions, seasonal variations and regional pollution events. Atmos. Environ. 2014, 86, 193–202. [Google Scholar] [CrossRef]
- Tian, M.; Wang, H.B.; Chen, Y.; Yang, F.M.; Zhang, X.H.; Zou, Q.; Zhang, R.Q.; Ma, Y.L.; He, K.B. Characteristics of aerosol pollution during heavy haze events in Suzhou, China. Atmos. Chem. Phys. 2015, 16, 7357–7371. [Google Scholar] [CrossRef]
- Pathak, R.K.; Wu, W.S.; Wang, T. Summertime PM2.5 ionic species in four major cities of China: Nitrate formation in an ammonia-deficient atmosphere. Atmos. Chem. Phys. 2008, 9, 1711–1722. [Google Scholar] [CrossRef]
- Liu, X. Secondary formation of sulfate and nitrate during a haze episode in megacity Beijing, China. Aerosol Air Qual. Res. 2015, 15, 2246–2257. [Google Scholar] [CrossRef]
- Tian, M.; Wang, H.; Chen, Y.; Zhang, L.; Shi, G.; Liu, Y.; Yu, J.; Zhai, C.; Wang, J.; Yang, F. Highly time-resolved characterization of water-soluble inorganic ions in PM2.5 in a humid and acidic mega city in Sichuan Basin, China. Sci. Total Environ. 2016, 580, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Khoder, M.I. Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere 2002, 49, 675–684. [Google Scholar] [CrossRef]
- Russell, A.G.; Cass, G.R.; Seinfeld, J.H. On some aspects of nighttime atmospheric chemistry. Environ. Sci. Technol. 1986, 20, 1167–1172. [Google Scholar] [CrossRef]
- Pathak, R.K.; Wang, T.; Wu, W.S. Nighttime enhancement of PM2.5 nitrate in ammonia-poor atmospheric conditions in Beijing and Shanghai: Plausible contributions of heterogeneous hydrolysis of N2O5 and HNO3 partitioning. Atmos. Environ. 2011, 45, 1183–1191. [Google Scholar] [CrossRef]
- Chen, D.; Cui, H.; Zhao, Y.; Yin, L.; Lu, Y.; Wang, Q. A two-year study of carbonaceous aerosols in ambient PM2.5 at a regional background site for western Yangtze River Delta, China. Atmos. Res. 2016, 183, 351–361. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Lin, T.; Rose, N.L. Seasonal variation of carbonaceous pollutants in PM2.5 at an urban ‘supersite’ in Shanghai, China. Chemosphere 2015, 146, 238. [Google Scholar] [CrossRef]
- Ding, X.; Kong, L.; Du, C.; Zhanzakova, A.; Lin, W.; Fu, H.; Chen, J.; Xin, Y.; Cheng, T. Long-range and regional transported size-resolved atmospheric aerosols during summertime in urban Shanghai. Sci. Total Environ. 2017, 583, 334–343. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Li, J.; Wang, X.; Chen, H.; Chen, J.; Yang, X.; Gross, D.S.; Wang, H.; Qiao, L.; et al. A case study of the highly time-resolved evolution of aerosol chemical and optical properties in urban Shanghai, China. Atmos. Chem. Phys. 2013, 13, 3931–3944. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, X.Y.; Draxler, R.R. TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environ. Model. Softw. 2009, 24, 938–939. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.C. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. Discuss. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Lian, D.; Wang, X.; Wang, D.; Duan, Y.; Na, C.; Xiu, G. Atmospheric mercury speciation in Shanghai, China. Sci. Total Environ. 2016, 578, 460–468. [Google Scholar]
- NOAA. Available online: ftp://arlftp.arlhq.noaa.gov/pub/archives/reanalysis (accessed on 24 July 2020).
- Polissar, A.V.; Hopke, P.K.; Paatero, P.; Kaufmann, Y.J.; Hall, D.K.; Bodhaine, B.A.; Dutton, E.G.; Harris, J.M. The aerosol at Barrow, Alaska: Long-term trends and source locations. Atmos. Environ. 1999, 33, 2441–2458. [Google Scholar] [CrossRef]
- Wang, P.; Cao, J.J.; Shen, Z.X.; Han, Y.M.; Lee, S.C.; Huang, Y.; Zhu, C.S.; Wang, Q.Y.; Xu, H.M.; Huang, R.J. Spatial and seasonal variations of PM2.5 mass and species during 2010 in Xi’an, China. Sci. Total Environ. 2015, 508, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xing, Z.; Deng, J.; Du, K. Characterizing and sourcing ambient PM2.5 over key emission regions in China I: Water-soluble ions and carbonaceous fractions. Atmos. Environ. 2016, 135, 20–30. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, C.; Tao, J.; Zhang, L.; Liang, X.; Ma, J.; Gao, H.; Huang, T.; Zhang, K. Chemical characterization and source apportionment of PM2.5 in a semi-arid and petrochemical-industrialized city, Northwest China. Sci. Total Environ. 2016, 573, 1031–1040. [Google Scholar] [CrossRef]
- Lai, S.; Zhao, Y.; Ding, A.; Zhang, Y.; Song, T.; Zheng, J.; Ho, K.F.; Lee, S.C.; Zhong, L. Characterization of PM2.5 and the major chemical components during a 1-year campaign in rural Guangzhou, Southern China. Atmos. Res. 2016, 167, 208–215. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Guo, L.; Zhang, Y.; Zhang, G.; Wang, X. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, A.; Sen, A.; Saxena, P.; Saraswati; Mandal, T.K.; Sharma, S.K.; Sharma, C. Water soluble inorganic species of PM10 and PM2.5 at an urban site of Delhi, India: Seasonal variability and sources. Atmos. Res. 2016, 184, 112–125. [Google Scholar] [CrossRef]
- Zhao, P.S.; Dong, F.; He, D.; Zhao, X.J.; Zhang, X.L.; Zhang, W.Z.; Yao, Q.; Liu, H.Y. Characteristics of concentrations and chemical compositions for PM2.5 in the region of Beijing, Tianjin, and Hebei, China. Atmos. Chem. Phys. 2013, 13, 4631–4644. [Google Scholar] [CrossRef]
- Tao, J.; Shen, Z.; Zhu, C.; Yue, J.; Cao, J.; Liu, S.; Zhu, L.; Zhang, R. Seasonal variations and chemical characteristics of sub-micrometer particles (PM1) in Guangzhou, China. Atmos. Res. 2012, 118, 222–231. [Google Scholar] [CrossRef]
- Liu, X.G.; Li, J.; Qu, Y.; Han, T.; Hou, L.; Gu, J.; Chen, C.; Yang, Y.; Liu, X.; Yang, T. Formation and evolution mechanism of regional haze: A case study in the megacity Beijing, China. Atmos. Chem. Phys. 2013, 13, 4501–4514. [Google Scholar] [CrossRef]
- Liu, B.; Song, N.; Dai, Q.; Mei, R.; Sui, B.; Bi, X.; Feng, Y. Chemical composition and source apportionment of ambient PM2.5 during the non-heating period in Taian, China. Atmos. Res. 2016, 170, 23–33. [Google Scholar] [CrossRef]
- Arimoto, R.; Duce, R.A.; Savoie, D.L.; Prospero, J.M.; Talbot, R.; Cullen, J.D.; Tomza, U.; Lewis, N.F.; Ray, B.J. Relationships among aerosol constituents from Asia and the North Pacific during PEM-West A. J. Geophys. Res. Atmos. 1996, 101, 2011–2023. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Lu, Z.; Lowenthal, D.H.; Frazier, C.A.; Solomon, P.A.; Thuillier, R.H.; Magliano, K. Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos. Environ. 1996, 30, 2079–2112. [Google Scholar] [CrossRef]
- Zheng, G.J.; Duan, F.K.; Su, H.; Ma, Y.L.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Tang, A. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Yue, D. Pollution properties of water-soluble secondary inorganic ions in atmospheric PM2.5 in the Pearl River Delta Region. Aerosol Air Qual. Res. 2015, 15, 1737–1747. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; An, S.Z. The variation of characteristics and formation mechanisms of aerosols in dust, haze, and clear days in Beijing. Atmos. Environ. 2006, 40, 6579–6591. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhao, P.S.; Xu, J.; Meng, W. Analysis of a winter regional haze event and its formation mechanism in the North China Plain. Atmos. Chem. Phys. 2013, 13, 5685–5696. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, G.; Tang, A.; Wang, Y.; An, Z. Chemical characteristics of PM2.5 and PM10 in Haze—Fog Episodes in Beijing. Environ. Sci. Technol. 2006, 40, 3148–3155. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Fu, P.; Jiang, Q.; Yang, T.; Li, J.; Ge, X. The impact of relative humidity on aerosol composition and evolution processes during wintertime in Beijing, China. Atmos. Environ. 2013, 77, 927–934. [Google Scholar] [CrossRef]


| Annual | Spring | Summer | Autumn | Winter | |
|---|---|---|---|---|---|
| Na+ | 0.31 ± 0.15 | 0.32 ± 0.16 | 0.26 ± 0.14 | 0.31 ± 0.09 | 0.34 ± 0.12 |
| K+ | 1.07 ± 1.02 | 0.454 ± 0.287 | 0.740 ± 0.402 | 1.51 ± 0.39 | 1.59 ± 1.68 |
| NH4+ | 8.59 ± 5.31 | 8.58 ± 4.43 | 6.87 ± 4.20 | 7.94 ± 4.22 | 10.9 ± 7.03 |
| Cl− | 1.52 ± 1.16 | 1.58 ± 1.02 | 0.64 ± 0.37 | 1.34 ± 0.81 | 2.49 ± 1.34 |
| NO3− | 11.2 ± 8.01 | 11.3 ± 6.66 | 8.47 ± 6.45 | 9.53 ± 6.09 | 15.53 ± 10.29 |
| SO42− | 9.92 ± 5.47 | 9.49 ± 4.47 | 9.74 ± 6.29 | 9.67 ± 4.83 | 10.76 ± 6.06 |
| WSIIs | 32.62 ± 18.68 | 31.73 ± 15.09 | 26.68 ± 16.54 | 30.33 ± 14.82 | 41.62 ± 23.85 |
| OC | 6.22 ± 3.12 | 5.58 ± 2.49 | 5.34 ± 2.56 | 5.91 ± 2.70 | 7.87 ± 4.01 |
| EC | 2.59 ± 1.44 | 2.37 ± 1.19 | 2.00 ± 0.89 | 2.73 ± 1.42 | 3.14 ± 1.81 |
| PM2.5 | 59.82 ± 31.68 | 58.56 ± 24.78 | 48.54 ± 23.56 | 55.89 ± 25.84 | 75.73 ± 42.53 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| Percentage (%) | 30.33 | 21.18 | 35.90 | 12.59 |
| Na+ | 0.32 | 0.30 | 0.27 | 0.35 |
| K+ | 1.10 | 1.05 | 0.85 | 1.60 |
| NH4+ | 10.84 | 7.91 | 6.98 | 8.97 |
| Cl− | 1.58 | 1.84 | 1.05 | 2.10 |
| NO3− | 14.53 | 10.12 | 8.34 | 13.22 |
| SO42− | 11.8 | 8.98 | 9.17 | 9.21 |
| OC | 7.46 | 5.87 | 4.71 | 7.50 |
| EC | 3.10 | 2.25 | 2.12 | 3.12 |
| PM2.5 | 74.82 | 55.84 | 46.51 | 66.81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Yan, L.; Xiu, G. Online Measurement of PM2.5 at an Air Monitoring Supersite in Yangtze River Delta: Temporal Variation and Source Identification. Atmosphere 2020, 11, 789. https://doi.org/10.3390/atmos11080789
Duan L, Yan L, Xiu G. Online Measurement of PM2.5 at an Air Monitoring Supersite in Yangtze River Delta: Temporal Variation and Source Identification. Atmosphere. 2020; 11(8):789. https://doi.org/10.3390/atmos11080789
Chicago/Turabian StyleDuan, Lian, Lei Yan, and Guangli Xiu. 2020. "Online Measurement of PM2.5 at an Air Monitoring Supersite in Yangtze River Delta: Temporal Variation and Source Identification" Atmosphere 11, no. 8: 789. https://doi.org/10.3390/atmos11080789
APA StyleDuan, L., Yan, L., & Xiu, G. (2020). Online Measurement of PM2.5 at an Air Monitoring Supersite in Yangtze River Delta: Temporal Variation and Source Identification. Atmosphere, 11(8), 789. https://doi.org/10.3390/atmos11080789




