A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management
Abstract
1. Introduction
2. Deterioration of Building Materials
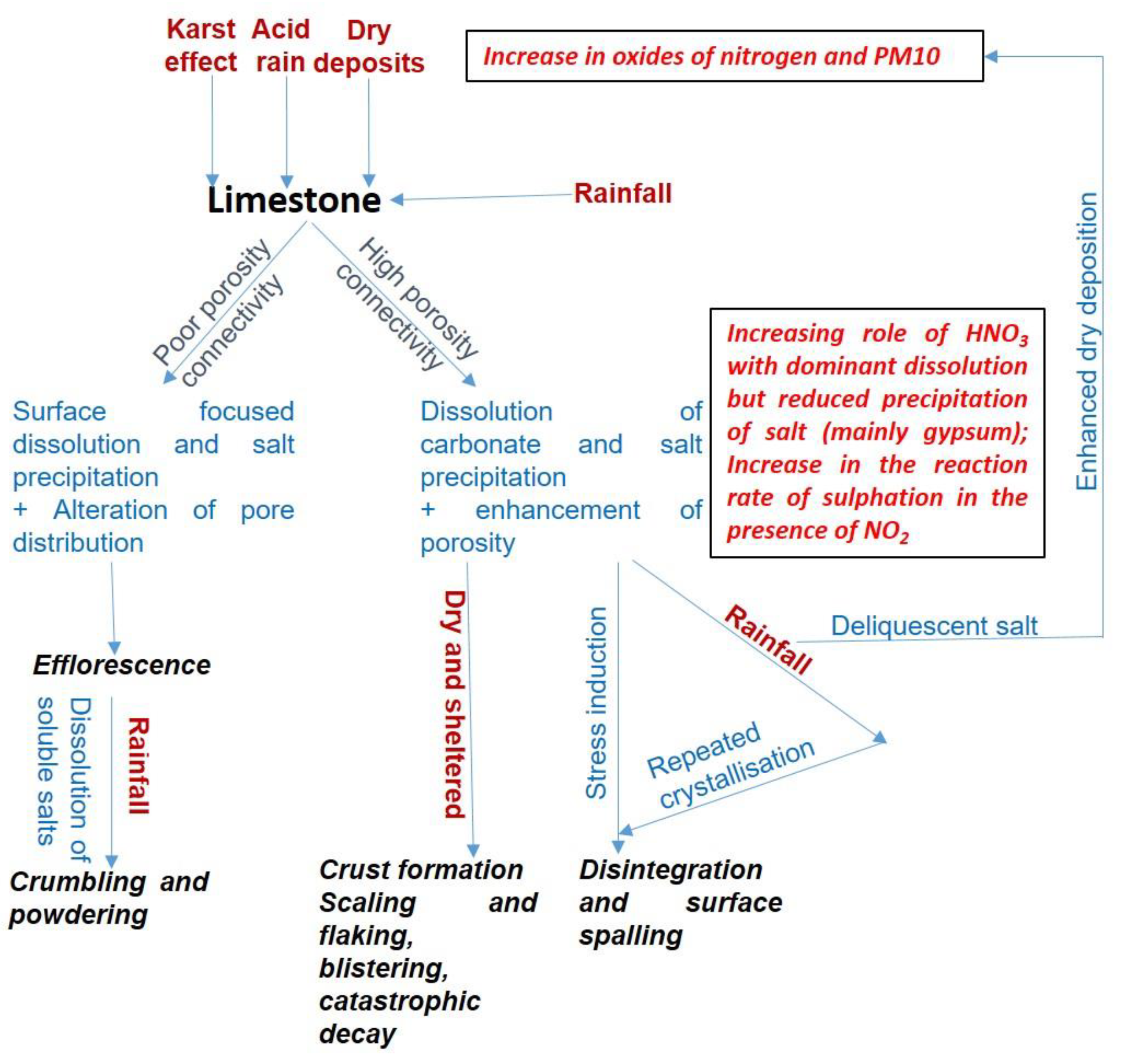
2.1. Carbonate Rocks (Limestone and Marble)
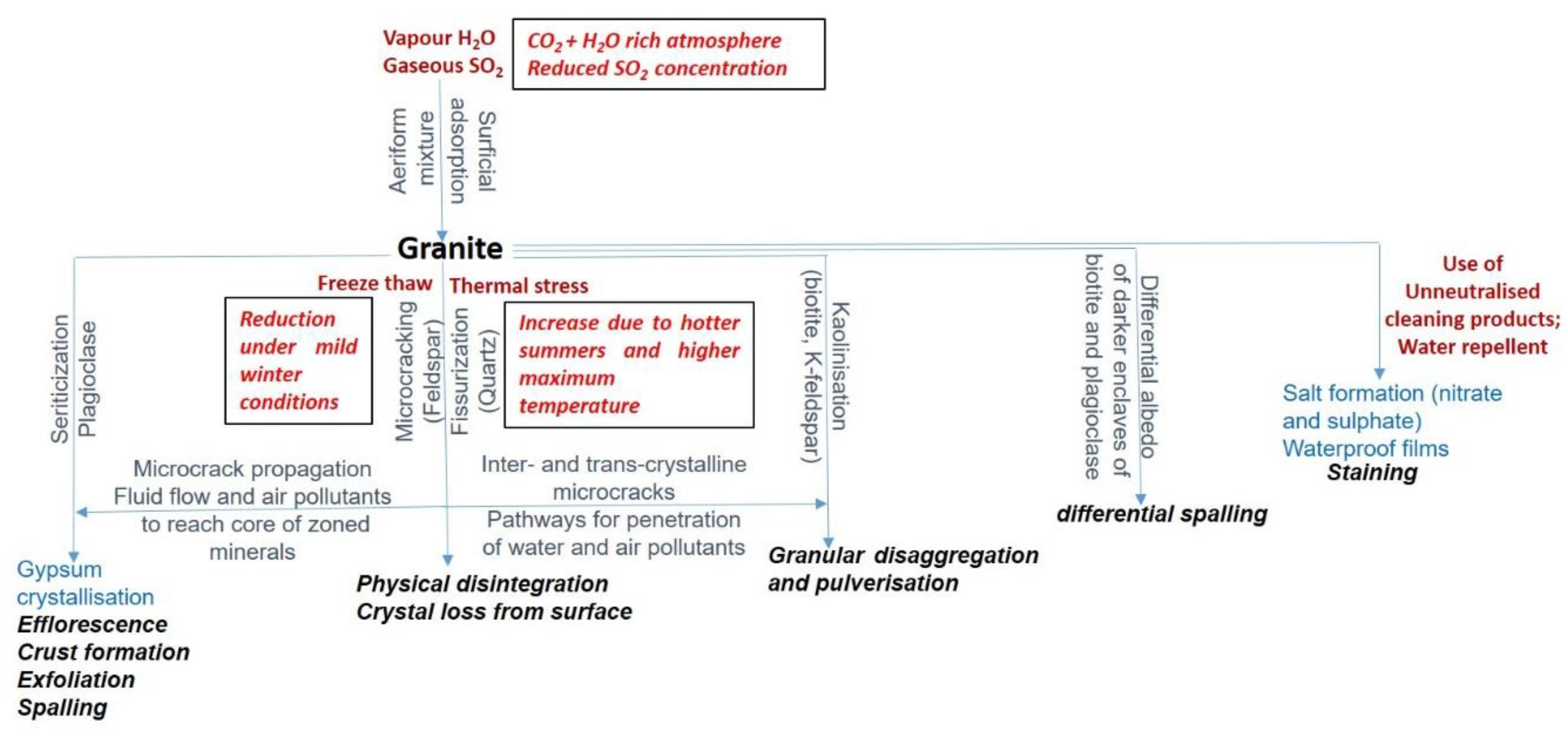
2.2. Granite
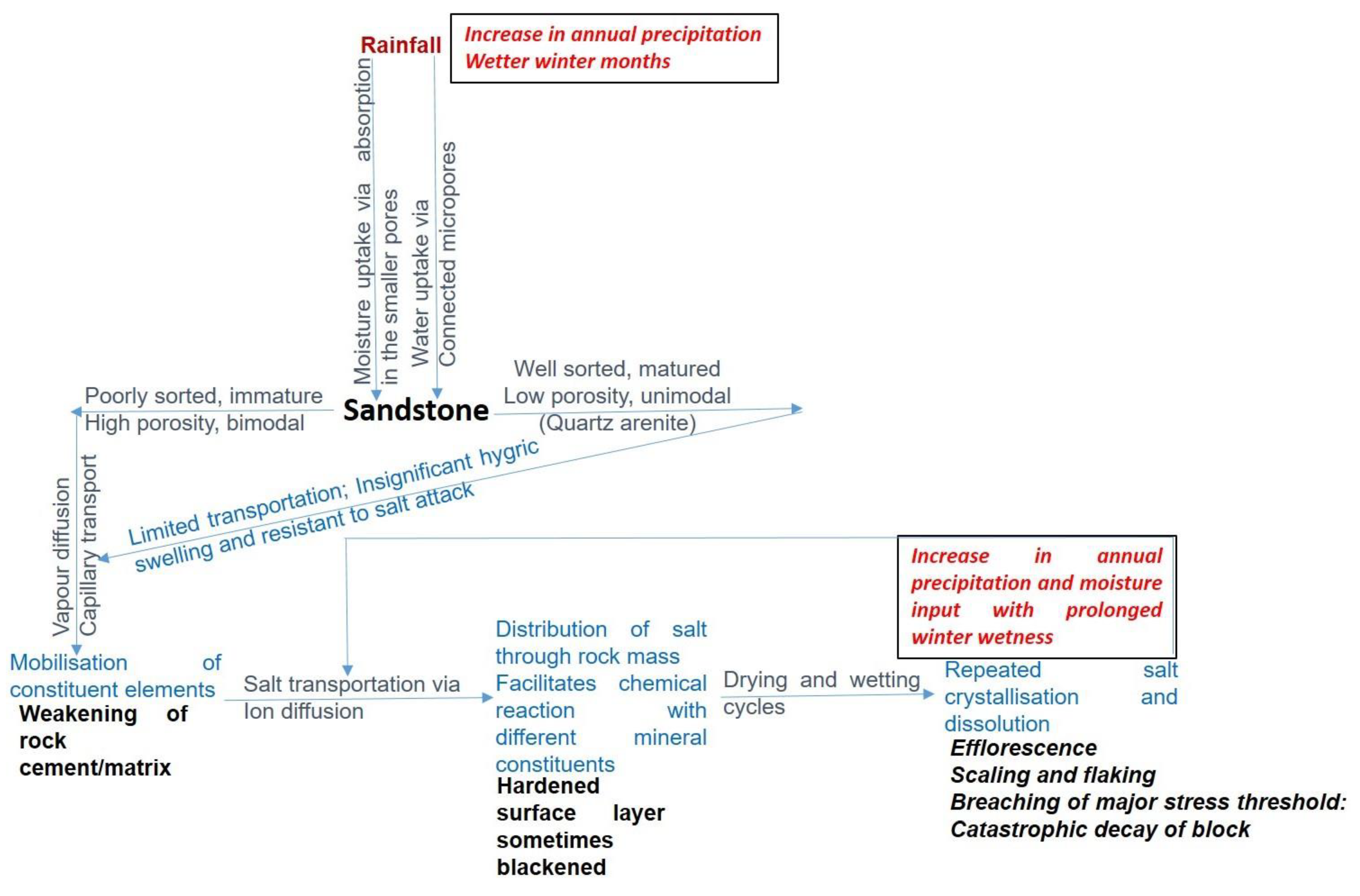
2.3. Sandstone
2.4. Flint
2.5. Slates
2.6. Lime Mortar
2.7. Bricks
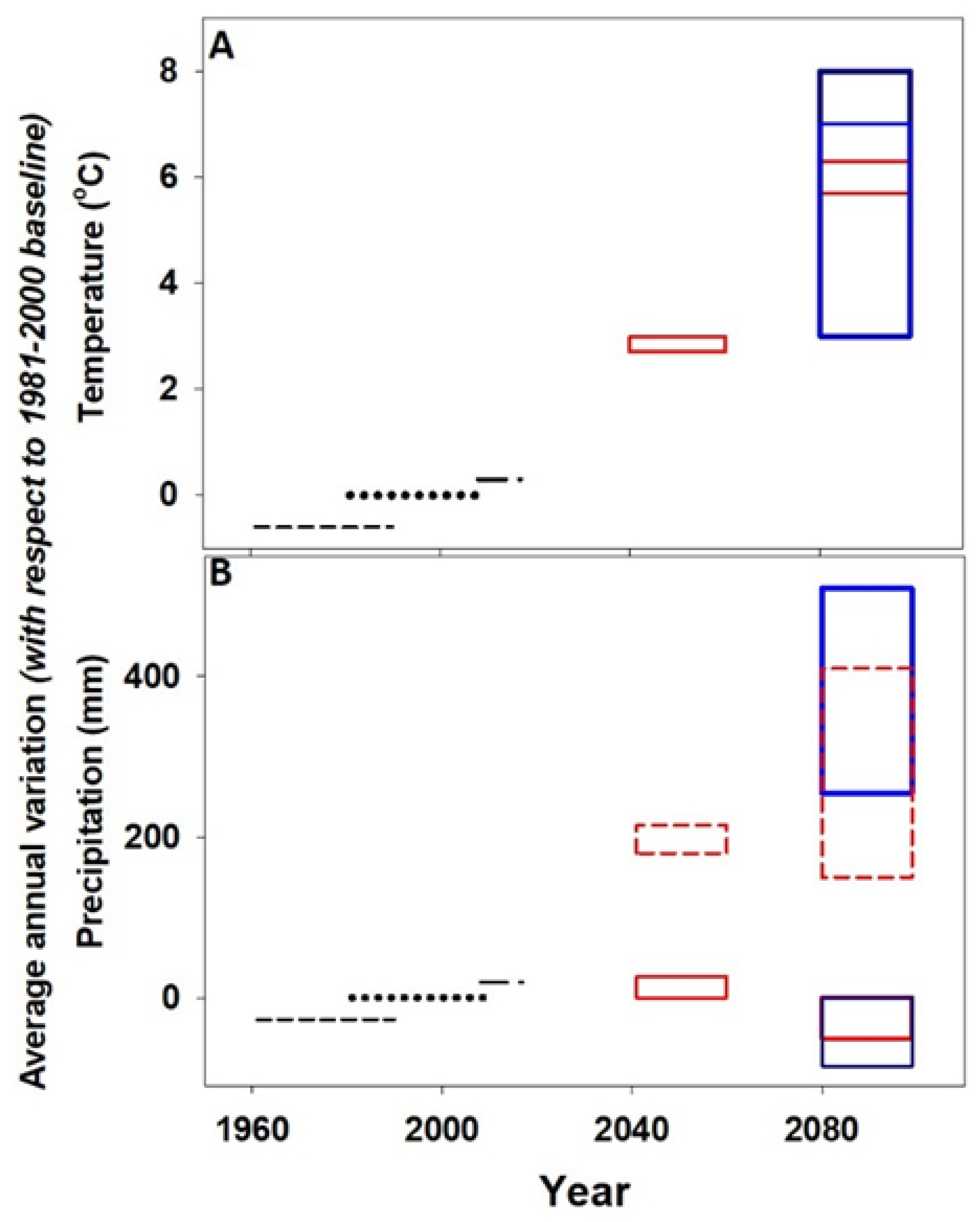
3. Deterioration of Building Stones in London: Future Directions of Study
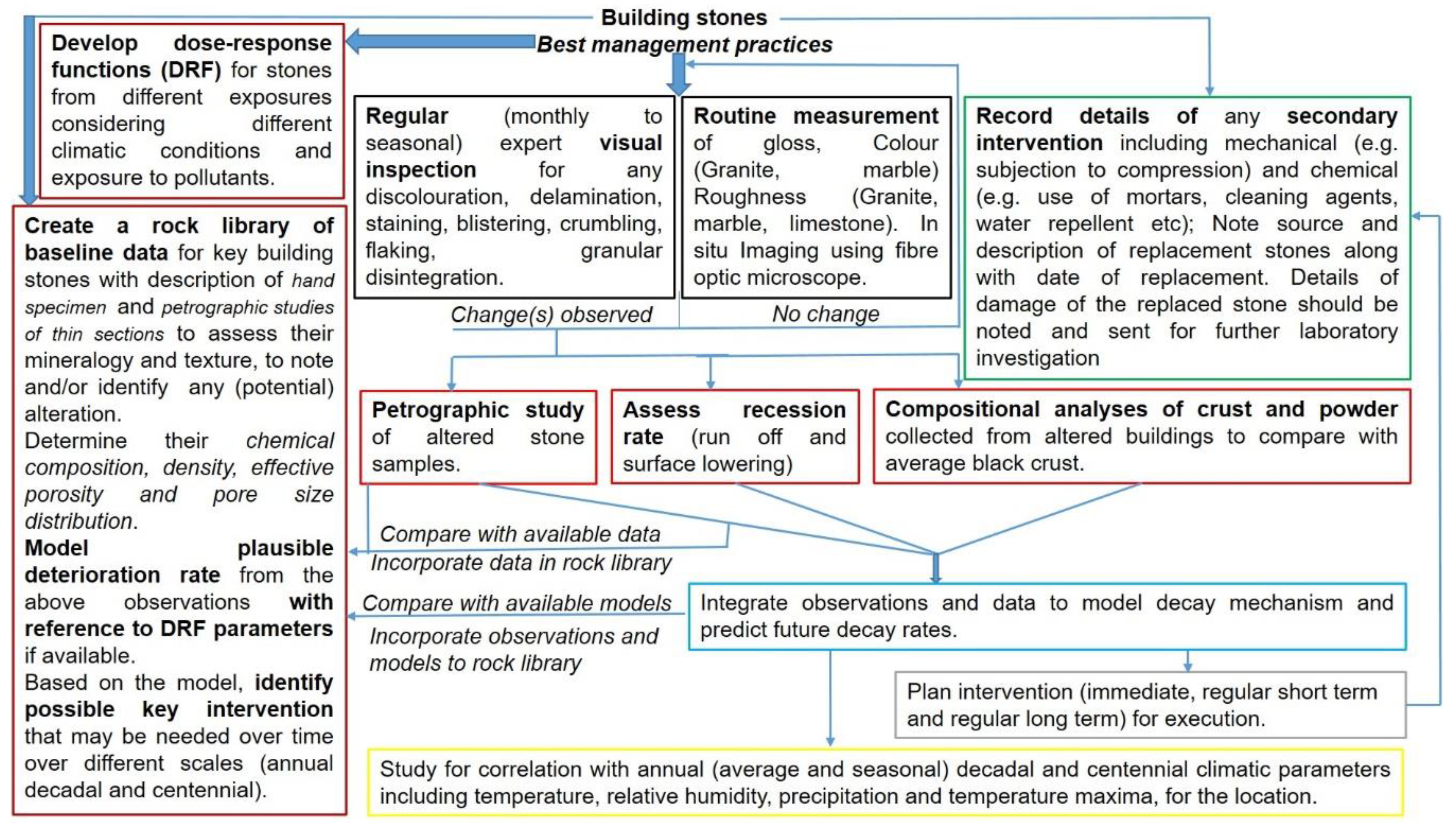
4. Monitoring Building Stones for Deterioration
4.1. Visual Inspection
4.2. Optical Microscopy and Other Imaging Techniques
4.3. Mineralogical and Geochemical Methods
4.4. Other Attributes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leissner, J.; Fuhrmann, C. Cultural Heritage and Climate Change: Are We at the Tipping Point? Instituto Italiano di Cultura: Brussels, Belgium, 2017; p. 221. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC 2013; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Field, C.B.; Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q. (Eds.) Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; IPCC 2012; Cambridge University Press: Cambridge, UK, 2012; p. 582. [Google Scholar]
- Fookes, P.G.; Lee, E.M. Climate variation: A simple geological perspective. Geol. Today 2007, 23, 66–73. [Google Scholar] [CrossRef]
- Lowe, J.A.; Bernie, D.; Bett, P.; Bricheno, L.; Brown, S.; Calvert, D.; Clark, R.; Eagle, K.; Edwards, T.; Fosser, G.; et al. UKCP18 Science Overview Report Version 2.0; Met Office, Hadley Centre: Exeter, UK, 2018; Updated March 2019. [Google Scholar]
- Nathanail, J.; Banks, V.J. Climate change: Implications for engineering geology practice. In Geological Society London Engineering Geology Special Publications; Geological Society: London, UK, 2009; Volume 22, pp. 65–82. [Google Scholar]
- Viles, H.A. Implications of future climate change for stone deterioration. In Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Geological Society: London, UK, 2002; Volume 205, pp. 407–418. [Google Scholar]
- Schneider, T.; O’Gorman, P.A.; Levine, X.J. Water vapor and the dynamics of climate changes. Rev. Geophys. 2010, 48, RG3001. [Google Scholar] [CrossRef]
- Satterthwaite, D. Cities’ contribution to global warming: Notes on the allocation of greenhouse gas emissions. Environ. Urban. 2008, 20, 539–549. [Google Scholar] [CrossRef]
- UN Climate Neutral Strategy. Greening The Blue. 5 June 2007. Available online: https://www.un.org/en/sections/general/un-and-sustainability/ (accessed on 26 July 2020).
- Aram, F.; García, E.H.; Solgi, E.; Mansournia, S. Urban green space cooling effect in cities. Heliyon 2019, 5, e01339. [Google Scholar] [CrossRef]
- Aktaş, Y.D.; Stocker, J.; Carruthers, D.; Hunt, J. A Sensitivity Study Relating to Local Urban Climate Modelling within the Built Environment. Procedia Eng. 2017, 198, 589–599. [Google Scholar] [CrossRef]
- Hunt, J.C.; Aktas, Y.D.; Mahalov, A.; Moustaoui, M.; Salamanca, F.; Georgescu, M. Climate change and growing megacities: Hazards and vulnerability. Proc. Inst. Civ. Eng. Eng. Sustain. 2017, 171, 314–326. [Google Scholar] [CrossRef]
- Paris Agreement under the United Nations Framework Convention on Climate Change. In Proceedings of the 21st Conference of the Parties, Paris, France, 12 December 2015.
- Siegfried, S.; Brimblecombe, P. Editorial to the Special Issue “urban use of rocks”. Environ. Earth Sci. 2013, 69, 1067–1069. [Google Scholar]
- Bell, F.G. Durability of carbonate rock as building stone with comments on its preservation. Environ. Geol. 1993, 21, 187–200. [Google Scholar] [CrossRef]
- Robinson, E. A Geology of the British Library. Available online: https://www.webarchive.org.uk/wayback/archive/20100427150116/http:/www.bl.uk/reshelp/experthelp/science/science@blevents/futureevents/geology_of_the_bl.pdf (accessed on 14 October 2016).
- Garcia-del-Cura, M.A.; Benavente, D.; Martinez-Martinez, J.; Cueto, N. Sedimentary structures and physical properties of travertine and carbonate tufa building stone. Constr. Build. Mater. 2012, 28, 456–467. [Google Scholar] [CrossRef]
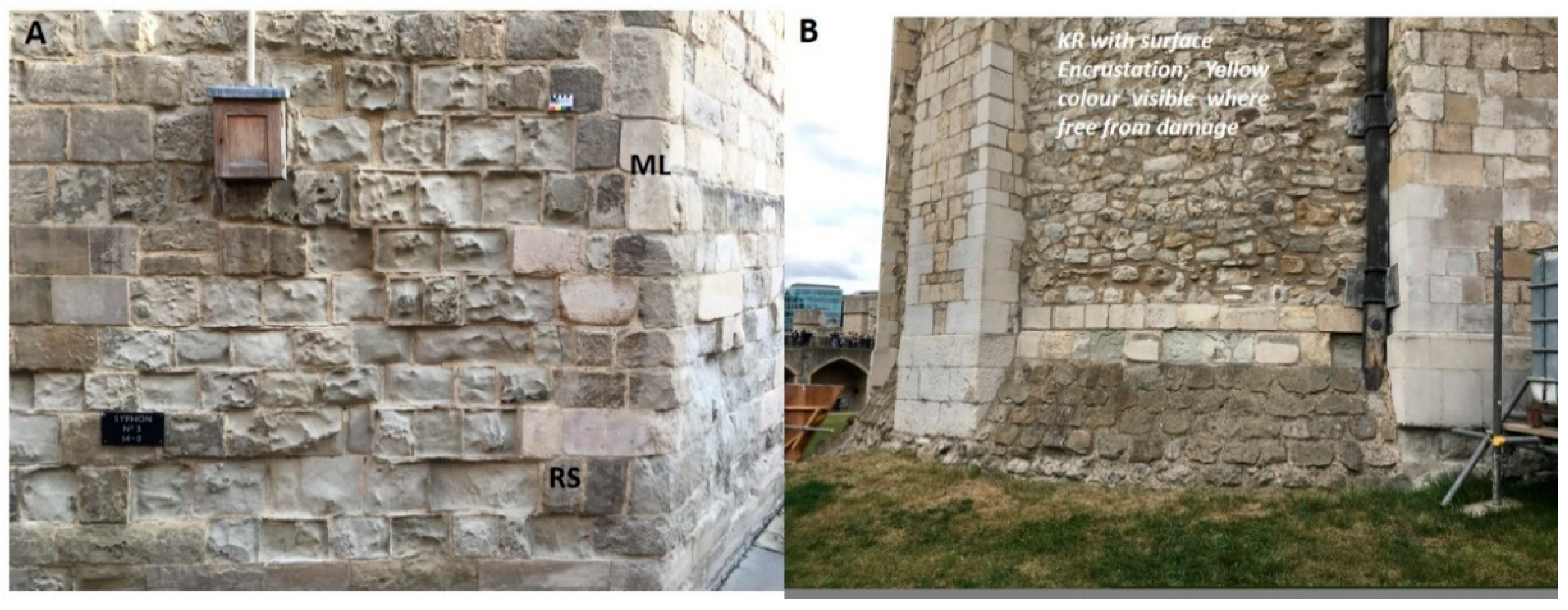
- Sabbioni, C.; Bonazza, A.; Zamagni, J.; Ghedini, N.; Grossi, C.M.; Brimblecombe, P. The Tower of London: A case study of stone decay in an urban area. In Air Pollution and Cultural; Saiz, C., Ed.; AA Balkema: London, UK, 2004; pp. 57–62. [Google Scholar]
- Building Stones and the Landscape British Geological Survey. Available online: https://www.bgs.ac.uk/discoveringGeology/geologyOfBritain/limestoneLandscapes/resourcesConflictsSustainability/buildingStones.html (accessed on 7 March 2020).
- Palmer, T.J. Limestone petrography and durability in English Jurassic freestones. In Proceedings of the England’s Heritage Stone: Proceedings of a Conference, Tempest Anderson Hall, York, UK, 15–17 March 2005; pp. 65–78. [Google Scholar]
- Ingham, J.P. Predicting the frost resistance of building stone. Q. J. Eng. Geol. Hydrogeol. 2005, 38, 387–399. [Google Scholar] [CrossRef]
- Cherblanc, F.; Berthonneau, J.; Bromblet, P. Role of hydro-mechanical coupling in the damage process of limestones used in historical buildings. In Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Glasgow, UK, 6–10 September 2016. [Google Scholar]
- Morse, J.W.; Arvidson, R.S. The Dissolution Kinetics of Major Sedimentary Carbonate Minerals. Earth Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Reddy, M.M. Acid rain damage to carbonate stones: A quantitative assessment based on the aqueous geochemistry of rainfall runoff from stone. Earth Surf. Process. Landf. 1988, 13, 335–354. [Google Scholar] [CrossRef]
- Grossi, C.M.; Murray, M. Characteristics of carbonate building stones that influence the dry deposition of acidic gases. Constr. Build. Mater. 1999, 13, 101–108. [Google Scholar] [CrossRef]
- Cooper, T.P.; O’Brien, P.F.; Jeffrey, D.W. Rates of Deterioration of Portland Limestone in an Urban Environment. Stud. Conserv. 1992, 37, 228–238. [Google Scholar]
- Inkpen, R.; Viles, H.; Moses, C.; Baily, B. Modelling the impact of changing atmospheric pollution levels on limestone erosion rates in central London, 1980–2010. Atmos. Environ. 2012, 61, 476–481. [Google Scholar] [CrossRef]
- Auras, M.; Beer, S.; Bundschuh, P.; Eichhorn, J.; Mach, M.; Scheuvens, D.; Schorling, M.; Schumann, J.V.; Snethlage, R.; Weinbruch, S. Traffic-related emissions and their impact on historic buildings: Implications from a pilot study at two German cities. Environ. Earth Sci. 2013, 69, 1135–1147. [Google Scholar] [CrossRef]
- Lipfert, F.W. Atmospheric damage to calcareous stones: Comparison and reconciliation of recent experimental findings. Atmos. Environ. 1989, 23, 415–429. [Google Scholar] [CrossRef]
- Bonazza, A.; Palmira, M.; Sabbioni, C.; Grossi, C.; Brimblecombe, P. Mapping the impact of climate change on surface recession of carbonate buildings in Europe. Sci. Total Environ. 2009, 407, 2039–2050. [Google Scholar] [CrossRef]
- Doehne, E. Salt weathering: A selective review. In Geological Society London Special Publication Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Siegesmund, S., Weiss, T., Vollbrecht, A., Eds.; Geological Society of London: London, UK, 2002; Volume 205, pp. 51–64. [Google Scholar]
- Gulotta, D.; Bertoldi, M.; Bortolotto, S.; Fermo, P.; Piazzalunga, A.; Toniolo, L. The Angera stone: A challenging conservation issue in the polluted environment of Milan (Italy). Environ. Earth Sci. 2013, 69, 1085–1094. [Google Scholar] [CrossRef]
- Smith, B.J.; Viles, H.A. Rapid, catastrophic decay of building limestones: Thoughts on causes, effects and consequences. In Heritage Weathering and Conservation; Taylor and Francis: London, UK, 2003; pp. 191–197. [Google Scholar]
- Török, A. Morphology and detachment mechanism of weathering crusts of porous limestone in the urban environment of Budapest. Cent. Eur. Geol. 2007, 50, 225–240. [Google Scholar] [CrossRef]
- Haynie, F.H. Theoretical model of soiling of surfaces by airborne particles in aerosols: Research, risk assessment and Control Strategies. In Proceedings of the Second US-Dutch International Symposium, Williamsburg, VA, USA, 19–4 May 1986; Lee, S.D., Ed.; Lewis Publishers: Williamsburg, VA, USA, 1986. [Google Scholar]
- Grossi, C.M.; Esbert, R.M.; Alonso, F.J. Soiling of building stones in urban environments. Build. Environ. 2003, 38, 147–159. [Google Scholar] [CrossRef]
- Ascaso, C.; Wierzchos, J.; Souza-Egipsy, V.; de los Rı’os, A.; Delgado, J.; Rodrigues, J. In situ evaluation of the biodeteriorating action of microorganisms and the effects of biocides on carbonate rock of the Jeronimos Monastery (Lisbon). Biodeterior. Biodegrad. 2002, 49, 1–12. [Google Scholar] [CrossRef]
- Schiavon, N. Assessment of Building Stone Decay: A Geomorphological Approach. In Proceedings of the International Conference of Stone Cleaning and the Nature, Soiling and Decay Mechanisms of Stone, Edinburgh, UK, 14–16 April 1992; Webster, R.G.M., Ed.; Donhead Publishing: Shaftesbury, UK, 1992. [Google Scholar]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, A.; Brimblecombe, P.; Grossi, C.M.; Sabbioni, C. Carbon in black layers at the Tower of London. Environ. Sci. Technol. 2007, 41, 4199–4204. [Google Scholar] [CrossRef]
- Eyssautier-Chuine, S.; Marin, B.; Thomachot-Schneider, C.; Fronteau, G.; Schneider, A.; Gibeaux, S.; Vazquez, P. Simulation of acid rain weathering effect on natural and artificial carbonate stones. Environ. Earth Sci. 2016, 75, 748. [Google Scholar] [CrossRef]
- Hallmann, C.; Stannek, L.; Fritzlar, D.; Hause-Reitner, D.; Friedl, T.; Hoppert, M. Molecular diversity of phototrophic biofilms on building stone. FEMS Microbiol. Ecol. 2013, 84, 355–372. [Google Scholar] [CrossRef]
- Vázquez, P.; Luque, A.; Alonso, F.J.; Grossi, C.M. Surface changes on crystalline stones due to salt crystallisation. Environ. Earth Sci. 2013, 69, 1237–1248. [Google Scholar] [CrossRef]
- Shushakova, V.; Fuller, E.R., Jr.; Heidelbach, F.; Mainprice, D.; Siegesmund, S. Marble decay induced by thermal strains: Simulations and experiments. Environ. Earth Sci. 2013, 69, 1281–1297. [Google Scholar] [CrossRef]
- Fort, R.; Varas, M.J.; de Buergo, M.A.; Martin-Freire, D. Determination of anisotropy to enhance the durability of natural stone. J. Geophys. Eng. 2011, 8, S132–S144. [Google Scholar] [CrossRef]
- Goudie, A.S. Laboratory simulation of the ‘wick effect’ in salt weathering of rock. Earth Surf. Process. Landf. 1986, 11, 275–285. [Google Scholar] [CrossRef]
- Schiavon, N. Kaolinisation of granite in an urban environment. Environ. Geol. 2007, 52, 399–407. [Google Scholar] [CrossRef]
- Graue, B.; Siegesmund, S.; Oyhantcabal, P.; Naumann, R.; Licha, T.; Simon, K. The effect of air pollution on stone decay: The decay of the Drachenfels trachyte in industrial, urban, and rural environments—A case study of the Cologne, Altenberg and Xanten cathedrals. Environ. Earth Sci. 2013, 69, 1095–1124. [Google Scholar] [CrossRef]
- Perez-Monserrat, E.M.; de Buergo, M.A.; Gomez-Heras, M.; Muriel, M.J.V.; Gonzalez, R.F. An urban geomonumental route focusing on the petrological and decay features of traditional building stones used in Madrid, Spain. Environ. Earth Sci. 2013, 69, 1071–1084. [Google Scholar] [CrossRef]
- Sousa, L.M.O. The influence of the characteristics of quartz and mineral deterioration on the strength of granitic dimensional stones. Environ. Earth Sci. 2013, 69, 1333–1346. [Google Scholar] [CrossRef]
- Gomez-Heras, M.; Smith, B.J.; Fort, R. Influence of surface heterogeneities of building granite on its thermal response and its potential for the generation of thermoclasty. Environ. Geol. 2008, 56, 547–560. [Google Scholar] [CrossRef]
- Wilson, M.J. Weathering of the primary rock-forming minerals: Processes, products and rates. Clay Miner. 2004, 39, 233–266. [Google Scholar] [CrossRef]
- Gräf, V.; Jamek, M.; Rohatsch, A.; Tschegg, E. Effects of thermal-heating cycle treatment on thermal expansion behavior of different building stones. Rock Mech. Min. Sci. 2013, 64, 228–235. [Google Scholar] [CrossRef]
- Freire-Lista, D.M.; Fort, R.; Varas-Muriel, M.J. Thermal stress-induced microcracking in building granite. Eng. Geol. 2016, 206, 83–93. [Google Scholar] [CrossRef]
- Ruedrich, J.; Kirchner, D.; Seidel, M.; Siegesmund, S. Deterioration of natural building stones induced by salt and ice crystallisation in the pore space as well as hygric expansion processes. In Geowissenschaften und Denkmalpflege. Zeitschrift Deutsche Geologische Gesellschaft; Siegesmund, S., Auras, M., Ruedrich, J., Snethlage, R., Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2005; Volume 156/1, pp. 59–73. [Google Scholar]
- Wellmann, H.W.; Wilson, A.T. Salt Weathering, a Neglected Geological Erosive Agent in Coastal and Arid Environments. Nature 1965, 205, 1097–1098. [Google Scholar] [CrossRef]
- Ruedrich, J.; Knell, C.; Enseleit, J.; Rieffel, Y.; Sigesmund, S. Stability assessment of marble statuaries of the Schlossbrucke (Berlin, Germany) based on rock strength measurements and ultrasonic wave velocities. Environ. Earth Sci. 2013, 69, 1451–1469. [Google Scholar] [CrossRef]
- Stück, H.; Koch, R.; Siegesmund, S. Petrographical and petrophysical properties of sandstones: Statistical analysis as an approach to predict material behaviour and construction suitability. Environ. Earth Sci. 2013, 69, 1299–1332. [Google Scholar] [CrossRef]
- Godts, S.; Hayen, R.; De Clercq, H. Investigating salt decay of stone materials related to the environment, a case study in the St. James church in Liège, Belgium. Stud. Conserv. 2017, 62, 329–342. [Google Scholar] [CrossRef]
- Charola, A.E.; Pühringer, J.; Steiger, M. Gypsum: A review of its role in the deterioration of building materials. Environ. Geol. 2007, 52, 339–352. [Google Scholar] [CrossRef]
- Stück, H.; Plagge, R.; Siegesmund, S. Numerical modeling of moisture transport in sandstone: The influence of pore space, fabric and clay content. Environ. Earth Sci. 2013, 69, 1161–1187. [Google Scholar] [CrossRef]
- McCabe, S.; Smith, B.J.; McAlister, J.J.; Gomez-Heras, M.; McAllister, D.; Warke, P.A.; Curran, J.M.; Basheer, P.A.M. Changing climate, changing process: Implications for salt transportation and weathering within building sandstones in the UK. Environ. Earth Sci. 2013, 69, 1225–1235. [Google Scholar] [CrossRef]
- Sebastián, E.; Cultrone, G.; Benavente, D.; Fernandez, L.L.; Elert, K.; Rodriguez-Navarro, C. Swelling damage in clay-rich sandstones used in the church of San Mateo in Tarifa (Spain). J. Cult. Herit. 2008, 9, 66–76. [Google Scholar] [CrossRef]
- Smith, B.J.; Turkington, A.V.; Warke, P.A.; Basheer, P.A.M.; McAlister, J.J.; Meneely, J.; Curran, J.M. Modelling the rapid retreat of building sandstones: A case study from a polluted maritime environment. In Natural Stone, Weathering Phenomenon, Conservation Strategies and Case Studies; Seigesmund, S., Weiss, T., Vollbrecht, A., Eds.; Geological Society: London, UK, 2002; Volume 205, pp. 347–362. [Google Scholar]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Biology in the Conservation of Works of Art; ICCROM: Roma, Italy, 1991. [Google Scholar]
- Jain, A.; Bhadauria, S.; Kumar, V.; Chauhan, R.S. Biodeterioration of sandstone under the influence of different humidity levels in laboratory conditions. Build. Environ. 2009, 44, 1276–1284. [Google Scholar] [CrossRef]
- Bjell, T.; Thorseth, I.H. Comparative studies of the lichen-rock interface of four lichens in Vingen, western Norway. Chem. Geol. 2002, 192, 81–98. [Google Scholar] [CrossRef]
- Tiennot, M.; Mertz, J.-D.; Bourgès, A. Influence of Clay Minerals Nature on the Hydromechanical and Fracture Behaviour of Stones. Rock Mech. Rock Eng. 2019, 52, 1599–1611. [Google Scholar] [CrossRef]
- Ashurst, J.; Williams, G.; Bourgès, A. Flint and the Conservation of Flint Buildings. The Building Conservation Directory. 2005. Available online: https://www.buildingconservation.com/articles/flint/flint.htm (accessed on 7 May 2020).
- Cardenes, V.; Cnuddle, V.; Cnuddle, J.P. Petrography of roofing slate for quality assessment. In Proceedings of the 15th Euroseminar on Microscopy Applied to Building Materials, Delft, The Netherlands, 17–19 June 2015. [Google Scholar]
- Cárdenes, V.; Rubio-Ordonez, A.; Monterroso, C.; Calleja, L. Geology and geochemistry of Iberian roofing slates. Geochemistry 2013, 73, 373–382. [Google Scholar] [CrossRef]
- Gómez-Fernández, F.; Castaño, M.A.; Bauluz, B.; Ward, C.R. Optical microscope and SEM evaluation of roofing slate fissility and durability. Mater. De Construcción 2009, 59, 91–104. [Google Scholar]
- Demarco, M.M.; Oyhantcabal, P.; Stein, K.M.; Siegesmund, S. Dolomitic slates from Uruguay: Petrophysical and petromechanical characterization and deposit evaluation. Environ. Earth Sci. 2013, 69, 1361–1395. [Google Scholar] [CrossRef]
- Siedel, H. Magnesium sulphate salts on monuments in Saxony (Germany): Regional geological and environmental causes. Environ. Earth Sci. 2013, 69, 1249–1261. [Google Scholar] [CrossRef]
- Szemerey-Kiss, B.; Török, A.; Siegesmund, S. The influence of binder/aggregate ratio on the pore properties and strength of repair mortars. Environ. Earth Sci. 2013, 69, 1439–1449. [Google Scholar] [CrossRef]
- Henry, A.; McCaig, I.; Willett, C.; Godfraind, S.; Stewart, J. (Eds.) Historic England Practical Building Conservation. In Earth, Brick & Terracotta; Ashgate/Routledge: Farnham, UK, 2015. [Google Scholar]
- Bates, S.J. A Critical Evaluation of Salt Weathering Impacts on Building Materials at Jazirat al Hamra, UAE. Geoverse 2010, 1758–3411. Available online: http://geoverse.brookes.ac.uk/article_resources/batesSJ/batesSJ.htm (accessed on 9 July 2020).
- Bide, T.P.; Barron, A.J.M.; Evans, D.J. An Assessment of the Aggregate Properties of the Lower Lincolnshire Limestone in South Lincolnshire and Surrounding Areas; British Geological Survey Commissioned Report: CR/15/083; Keyworth, Nottingham British Geological Survey: Nottingham, UK, 2015; 22p. [Google Scholar]
- Palmer, T.J. Understanding the Weathering Behaviour of Caen Stone. J. Archit. Conserv. 2008, 14, 45–54. [Google Scholar] [CrossRef]
- Historic England Strategic Stone Study A Building Stone Atlas of East Sussex. Available online: https://www.southdowns.gov.uk/wp-content/uploads/2016/12/East_Sussex_Building_Stone_Atlas.pdf (accessed on 7 May 2020).
- Lott, G.K.; Cooper, A.H. Field guide to the building limestones of the upper Permian Cadeby formation (magnesian limestone) of Yorkshire. In Proceedings of the England’s Heritage Stone: Proceedings of a Conference, Tempest Anderson Hall, York, UK, 15–17 March 2005; pp. 80–89. [Google Scholar]
- Lott, G.K.; Richardson, C. Yorkshire stone for building the Houses of Parliament (1839–1852). Proc. Yorks. Geol. Soc. 1997, 51, 265–272. [Google Scholar] [CrossRef]
- Heritage, E. English Heritage Practical Building Conservation Stone; Odgers, D., Henry, A., Eds.; Ashgate/Routledge: Farnham, UK, 2012. [Google Scholar]
- Michette, M.; Viles, H.; Vlachou-Mogire, C.; Angus, I. Assessing the Long-term Success of Reigate Stone Conservation at Hampton Court Palace and the Tower of London. Stud. Conserv. 2020, 1–8. [Google Scholar] [CrossRef]
- Wiese, U.; Behlen, A.; Steiger, M. The influence of relative humidity on the SO2 deposition velocity to building stones: A chamber study at very low SO2 concentration. Environ. Earth Sci. 2013, 69, 1125–1134. [Google Scholar] [CrossRef]
- Fuchs, G. Allgemeine Mikrobiologie; Georg Thieme: Stuttgart, Germany, 2006; pp. 321–340. [Google Scholar]
- Colston, B.J.; Watt, D.S.; Munro, H.M. Environmentally-induced stone decay: The cumulative effects of crystallization–hydration cycles on a Lincolnshire oopelsparite limestone. J. Cult. Herit. 2001, 4, 297–307. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Grossi, C.M. Millennium-long recession of limestone facades in London. Environ. Geol. 2008, 56, 463–471. [Google Scholar]
- Snethlage, R.; Wendler, E. Moisture cycles and sandstone degradation. In Saving Our Architectural Heritage: Conservation of Historic Stone Structures; Baer, N.S., Snethlage, R., Eds.; John Wiley and Sons Ltd.: London, UK, 1997; pp. 7–24. [Google Scholar]
- McAllister, D.; McCabe, S.; Srinivasan, S.; Smith, B.J.; Warke, P.A. Moisture dynamics in building sandstone: Monitoring strategies and implications for transport and accumulation of salts. In Salt Weathering on Buildings and Stone Sculptures 2011; Ioannou, I., Theodoridou, M., Eds.; University of Cyprus: Nicosia, Cyprus, 2011; pp. 39–46. [Google Scholar]
- Cutler, N.; Viles, H. Eukaryotic microorganisms and stone biodeterioration. Geomicrobiol. J. 2010, 27, 630–646. [Google Scholar] [CrossRef]
- Ellison, R.A.; McMillan, A.A.; Lott, G.K. Ground Characterisation of the Urban Environment: A Guide to Best Practice; Research Report RR/02/05; British Geological Survey: Keyworth, Notthingham, 2002; p. 37. [Google Scholar]
- Menéndez, B. Non-Destructive Techniques Applied to Monumental Stone Conservation. Non-Destr. Test 2016. [Google Scholar] [CrossRef]
- Tambe, S.; Gauri, K.L.; Li, S.; Cobourn, W.G. Kinetic study of sulfur dioxide reaction with dolomite. Environ. Sci. Technol. 1991, 25, 2071–2075. [Google Scholar] [CrossRef]
- Watt, J.; Jarrett, D.; Hamilton, R. Dose-response functions for the soiling of heritage materials due to air pollution exposure. Sci. Total Environ. 2008, 400, 415–424. [Google Scholar] [CrossRef] [PubMed]
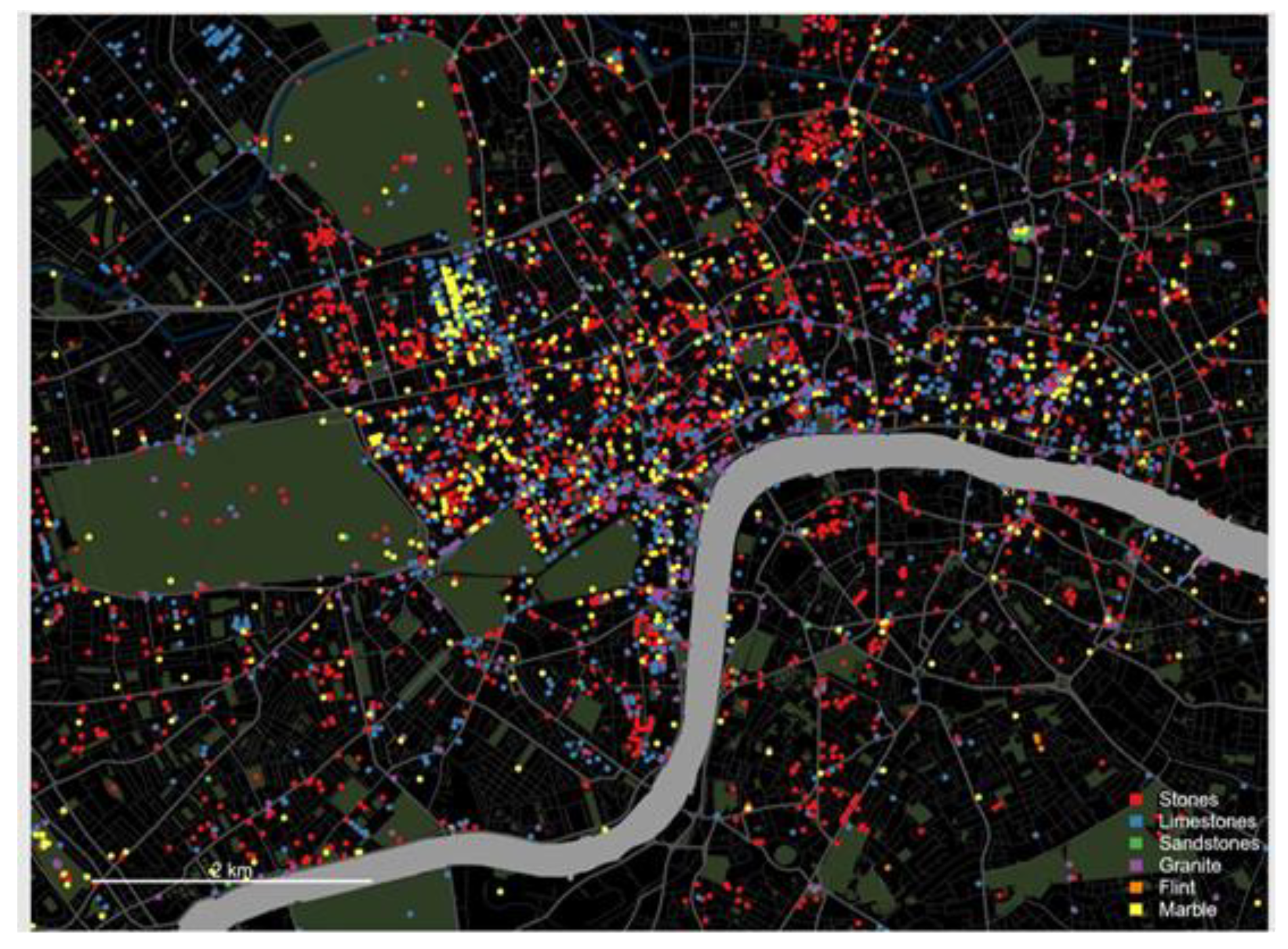

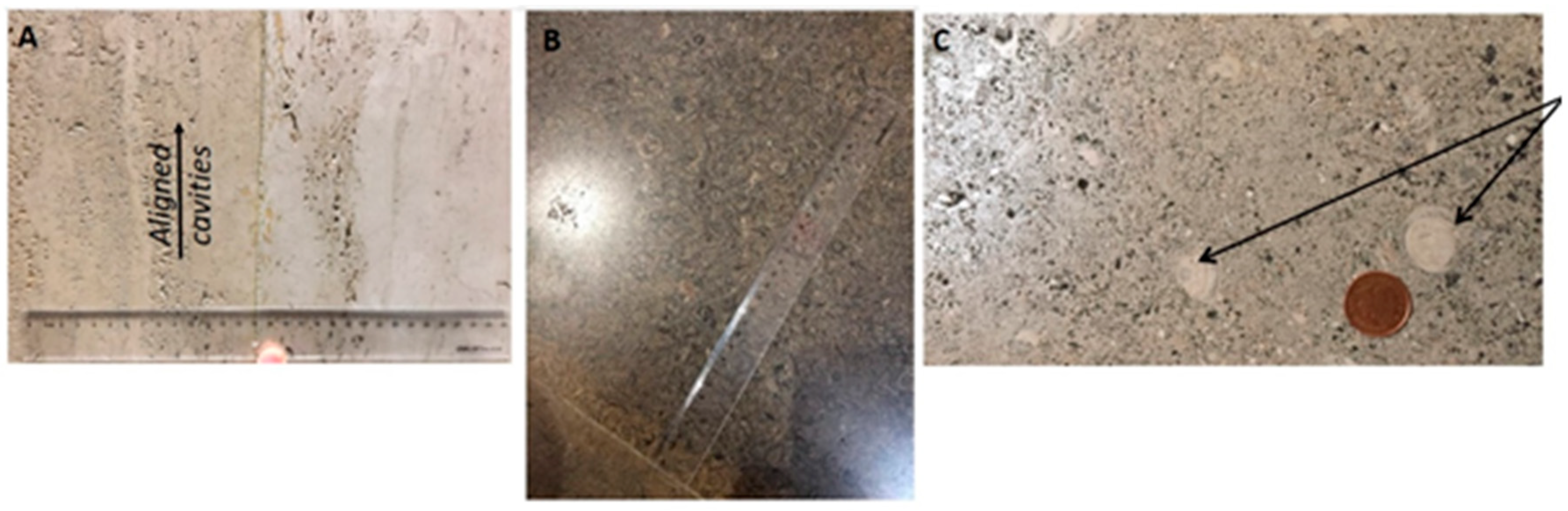

| Heritage Site | Building Stones |
|---|---|
| St Paul’s Cathedral | limestone (Portland stone) |
| Buckingham Palace | limestone (Caen); later refaced (Bath Stone) |
| Westminster Abbey | sandstone (Reigate stone), limestone (Kentish Rag with Purbeck as decorative columns; refaced with Portland and Yellow Bath stones) and chalk |
| White Tower of London | limestone (Kentish Rag rubblestone, Caen and Quarr) with sandstone (Reigate stone as upper dressings) |
| British Library | limestone (Hauteville as paving stone; Portland and Purbeck for flooring; travertine as indoor decorative slabs), granite (Royken granite: facade and steps), sandstone (outdoor, mounted decorative slab), red bricks (Figure 3 and Figure 4) |
| Marble Arch | marble (Carrara) |
| Burlington House, Tower Bridge British Museum, Somerset House, Bank of England and Mansion House | limestone (Portland) and granite (Cornish) |
| New quay and docksides | granite (Cornish and Scottish) and sandstone (Yorkshire) |
| The Palace of Westminster; New Houses of Parliament | limestone (originally magnesian limestone, Cadeby Formation, from quarries at Bolsover Moor and Mansfield; later substituted from the quarries at Anston for most of the upper fabric; ultimately replaced by Clipsham stone |
| St. Pancras Grand Hotel | red bricks (made from clay), sandstones (Derbyshire) limestones (Lincolnshire) roofing slates (Leicestershire) and granites (Cumbria) |
| Southwark Cathedral | flint cobbles in dark mortar with pale stone quoins |
| Trafalgar Square | granite (Dartmoor: Nelson’s column and base; Aberdeen granite: bollards, walls and statue plinths; Cornish: inlaid strips); limestone (paving); sandstone (red Mansfield: paving) |
| Albert Memorial | granite (Cornish: walling, steps and lower platform); slates (paving slabs); limestone (fossiliferous: paving slabs; sandstone (Red Mansfield: paving slabs); marble (Campanella: statue) |
| Big Ben | bricks, limestone (Caen and Anston, in addition to Clipsham for restoration), granite (Cornish) |
| Building Stone | Provenance and Age of Formation | Description | Distinguishing Properties Related to Usage | Deterioration that can be Exacerbated by * Climate Change |
|---|---|---|---|---|
| Limestone | ||||
| Portland stone [21] | Dorset (UK) Upper Jurassic (152 to 145 Ma) | Formed of “oolites” cemented by calcitic cement. The oolites are formed when tiny sand grains act as nuclei for deposition of concentric layers of carbonate material around it, physically evenly rounded by the action of waves and water currents. Some calcareous algal pellets as well as shell fragments can be present. | The well-cemented nature of Portland Stone contributes to the compactness and strength. The oolites behave like well-distributed ball bearings, and consequently the rocks lack any directional properties and can be cut with equal ease in all directions. The microporous oolites that often bear traces of borings are infilled by diagenetic calcitic cement, thereby reducing the microporosity and adding further strength. Large volumes of primary pore spaces remain uncemented. These interconnected, intergranular macropores offer pathways for drying out after wetting, making the stones more resistant to the impact of cyclic wetting and drying. | Generally resistant, but once already weathered, the added pore spaces can provide increased surface area for dissolution and, water and moisture retention. |
| Purbeck | Dorset (UK) Upper Jurassic (~145 Ma) | Highly fossiliferous and dark with clay minerals and organic matter and pyrite. Deposited under shallow freshwater conditions. Well cemented cracks are present. | Because of its dark colour and hardness, it can be polished and forms a good substitute for the more expensive marbles. | The expansion and contraction of the clay minerals related to the wetting/drying cycles is facilitated by the condensed moisture on the dense surface of the stone. This results in solubility of part of the surface matrix and the staining of pyrites that leads to the deterioration of the stone. They tend to delaminate along the bedding planes. The role of polishing in preservation is not understood. |
| Lincolnshire limestone (Clipsham stone) [79] | From Dorset to Yorkshire (Limestone Belt); commonly obtained from the large quarries at Clipsham Middle Jurassic (~165 Ma) | Typically, medium to coarse grained, shelly and/or oolitic. Subordinate silty, sandy or muddy beds with silicate grains of terrigenous origin may be present. The shell fragments and other skeletal remains well cemented by calcitic spar. Post depositional diagenetic alteration and consequent recrystallisation leads to further variability, where it may be shelly or oolitic. | The oolites internally cemented with radial calcite crystals that give them strength and reduce the microporosity. Lack of calcitic cement leaves the primary pore space open facilitating rapid drying. Calcite cement fills up the primary and the secondary pore spaces in the shelly varieties, the latter generated on the dissolution of the aragonitic shells, obscuring any lines of weaknesses that otherwise existed along the boundaries of the bioclasts. Clipsham stone is moderately strong and massively bedded. Performs well in sulphur-polluted atmospheres. | High to moderate porosity as intergranular macropores and micropores associated with the ooliths, sparite cement and micritic intraclasts. |
| Caen stone [80] | France (Normandy) Mid Jurassic (~167 Ma) | Pelleted and bioclastic fine limesands deposited in the seabed, that pass to shallow deposits of lagoonal sediments and muds fringed with oolites. They are underlain by the deeper water deposits of sponge-rich marls. Large shell fragments present at times with minute pyrite crystals. Overgrowth cement filling up intergranular pore spaces, interlocking the nucleus and the overgrowth for the bioclastic fragments. | Uniform texture that can be attributed to the compact and uniform faecal/psudofaecal pellets, with easy carvability and no obvious sedimentary laminations. Dense structure and low porosity when dominated by the bioclasts. | Severe decay due to gypsum formation facilitated by the presence of micropores in the pellets. Damp conduction when used with impermeable bricks. Oxidation halo around pyrite. |
| Quarr [81] | Isle of Wight Palaeogene | Bioclastic, freshwater with two contrasting lithologies. Fine-grained with bioclasts of thin-walled bivalve fragments in a micritised matrix present. This contrasts with coarsely bioclastic, porous limestone with fragmented mollusc shells replaced by calcite cement. The layers of these broken and abraded fossil shells result in a characteristic laminar texture. | The framework of fossil fragment and cement where present, gives it strength. | Decay due to gypsum formation facilitated by the presence of the highly porous framework of the coarsely bioclastic limestone where the fragmented mollusc shells are replaced with calcite cement. |
| Bath stone [21] | Bath region, England, UK Upper Middle Jurassic (~195 to 135 Ma) | Oolitic, with diameter of ooliths ranging from 0.2 to 0.8 mm, along with a smaller proportion of larger, mm-sized shell fragments in the cemented, calcitic matrix. The bioclastic fragments are distributed along laminations. The secondary pore spaces along dissolved bioclasts are well cemented with calcite. | Strong, rigid and resistant due to the low porosity crystalline, calcitic matrix and the cemented bioclasts. The overall mechanical strength is not compromised by the weak ooliths. | Individual ooliths, being soft and crumbly are preferentially weathered as compared to the more resistant shell fragments and the calcite cement. Holes formed by preferential dissolution of the microporous ooliths, leaving behind the shell fragments and the encasing calcite cement, contribute to enhanced salt dissolution and water/moisture retention during intense rainfall. |
| Magnesian limestone [82,83] | Quarries at Bolsover Moor (Derbyshire, Mansfield (Nottinghamshire) and Anston (South Yorkshire) | Diagenetically altered—the original bioclastic fabric remains intact when the alteration is minimal otherwise a coarse crystalline fabric develops with no relict of its primary depositional structure or fabric. | An interlocking, porous framework with a high proportion of fine sand quartz grains. The crystalline texture along with the highly resistant quartz grains contributes to the durability. The common occurrence of large open or carbonate crystal-lined cavities or vugs as a consequence of recrystallisation, can favour drying. | In urban settings, magnesium salts are more soluble than calcium rich varieties. Once affected by acid rain, the derived magnesium sulphate by-products within the pore spaces have a greater volumetric expansion as compared to gypsum. For the Anston stones, the original bioclastic texture has been preserved despite the olomitization, with the framework consisting of accumulations of abraded, dolomitised, bioclastic fragments. This bioclastic framework with the interconnected pore network compromises the durability of the stones. Affected by surface discoloration, efflorescence, blistering and ultimately severe surface exfoliation. |
| Kentish ragstone (Lower Greensand Bed) [19] | Kent (~115 to 110 Ma) | Grey in colour, consisting of rounded detrital grains of quartz and the green iron silicate mineral glauconite, with associated bioclasts, cemented by diagenetic calcite. | Hard due to cementing that contributes to the overall strength. | Numerous fractures, identified under optical microscope, distributed throughout the rock contribute towards a high secondary porosity that weakens the rock structure. An inhomogeneous texture and high microporosity that can be attributed to diagenetic recrystallisation contributes to the degradation of the stone. |
| Sandstone | ||||
| Reigate stone (Upper Greensand Bed) [84] | Surrey Lower Cretaceous (105 Ma) | Calcareous sandstone/sandy limestone. Sandy and glauconitic, well cemented with silica and calcite cement. Bioturbation features are common. Beside glauconite, bioclastic debris also comprises the framework, with dominant fine to medium grained quartz. | Variation in the proportion of quartz grains, glauconite and carbonate cement/matrix. Though, if well-cemented, it can be highly durable and requires low maintenance over its life [85]. Additionally, it has a high thermal expansion capacity. | Often soft and weakly compacted and porous, and therefore swells when wet. Exhibits pronounced contour scaling and flaking when exposed (Figure 5) and surface powdering in sheltered areas. |
| Red and White Mansfield | Nottingham Permian (299–251 Ma) | Dolostone transiting to dolomitic sandstone | Durable due to high quartz sand content and as the sand grains are cemented by the dolomite. | Poor weathering quality because of the calcareous constituents that rapidly destroys the structure of the stone. |
| Granite | ||||
| Aberdeen Scottish Cornish: (Dartmoor, Bodmin Moor (Cheesewring); Cumbria Shap Granite | Variable | Many varieties. Coarsely crystalline with quartz, feldspar and biotite mica with various accessory minerals. Large phenocrysts of K-feldspar may be set in the groundmass (porphyritic texture) but in some cases may be relatively finer grained. | The interlocking crystals provide cohesion which adds strength and makes them suitable for polishing without plucking of the grains. The predominance of silica and other relatively stable minerals make it particularly strong and durable. | Incipient kaolinisation causing the feldspars to become cloudy; deferruginisation resulting in the release of iron from the biotite and its dispersal throughout the rock body, thereby discolouring the rock to some rusty shade due to iron oxide staining. Water leakage through joints when of inferior quality comprising smaller block size. Smearing by white lime from the mortar between such blocks. |
| Royken | (Norway, ~250 Ma) | An interlocking texture of prominent coarse, feldspar laths seen, set against a background groundmass of finer grey-to-dark-grey quartz, with flakes of black biotite mica with some silvery muscovite mica also present Figure 3C. Textures show some contrasts, with variable size of the feldspar laths from very coarse to slender. | The crystalline texture of the rock with the interlocking mineral grains give it compactness and strength. The reddish colour of the rock can be attributed to the presence of potassium feldspar that adds to the aesthetic. The granite surface is roughened underfoot by the tougher crystals of feldspar, a perfect non-slip surface on a wet day. | As above |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, S.; Orr, S.A.; Aktas, Y.D. A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management. Atmosphere 2020, 11, 788. https://doi.org/10.3390/atmos11080788
Basu S, Orr SA, Aktas YD. A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management. Atmosphere. 2020; 11(8):788. https://doi.org/10.3390/atmos11080788
Chicago/Turabian StyleBasu, Sudeshna, Scott Allan Orr, and Yasemin D. Aktas. 2020. "A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management" Atmosphere 11, no. 8: 788. https://doi.org/10.3390/atmos11080788
APA StyleBasu, S., Orr, S. A., & Aktas, Y. D. (2020). A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management. Atmosphere, 11(8), 788. https://doi.org/10.3390/atmos11080788






