Wintertime Greenhouse Gas Fluxes in Hemiboreal Drained Peatlands
Abstract
1. Introduction
2. Materials and Methods
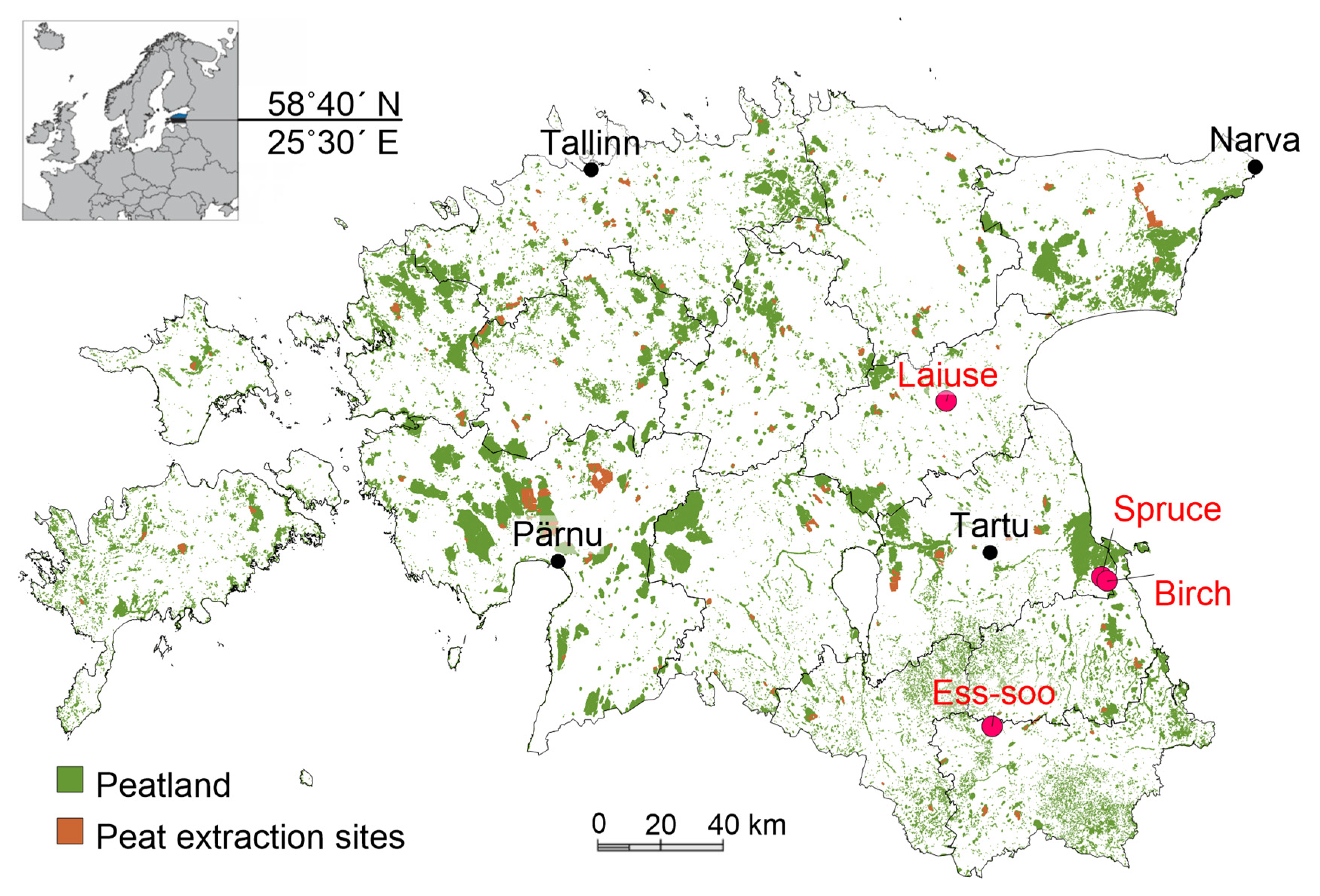
2.1. Study Sites
2.2. Meteorological Data
2.3. Field Measurements
2.4. Soil Analyses
2.5. Gas Analyses
2.6. Statistical Analyses
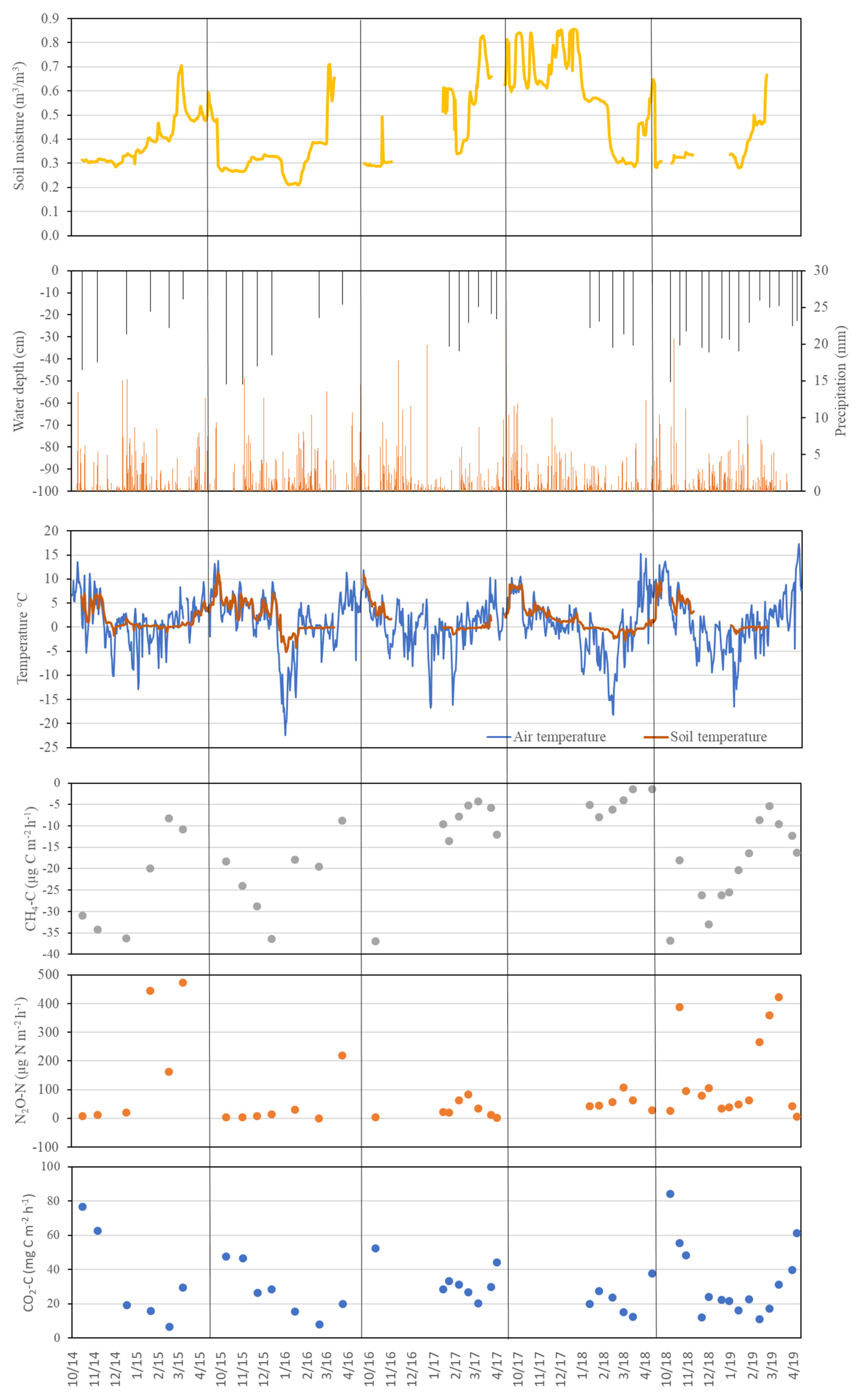
3. Results
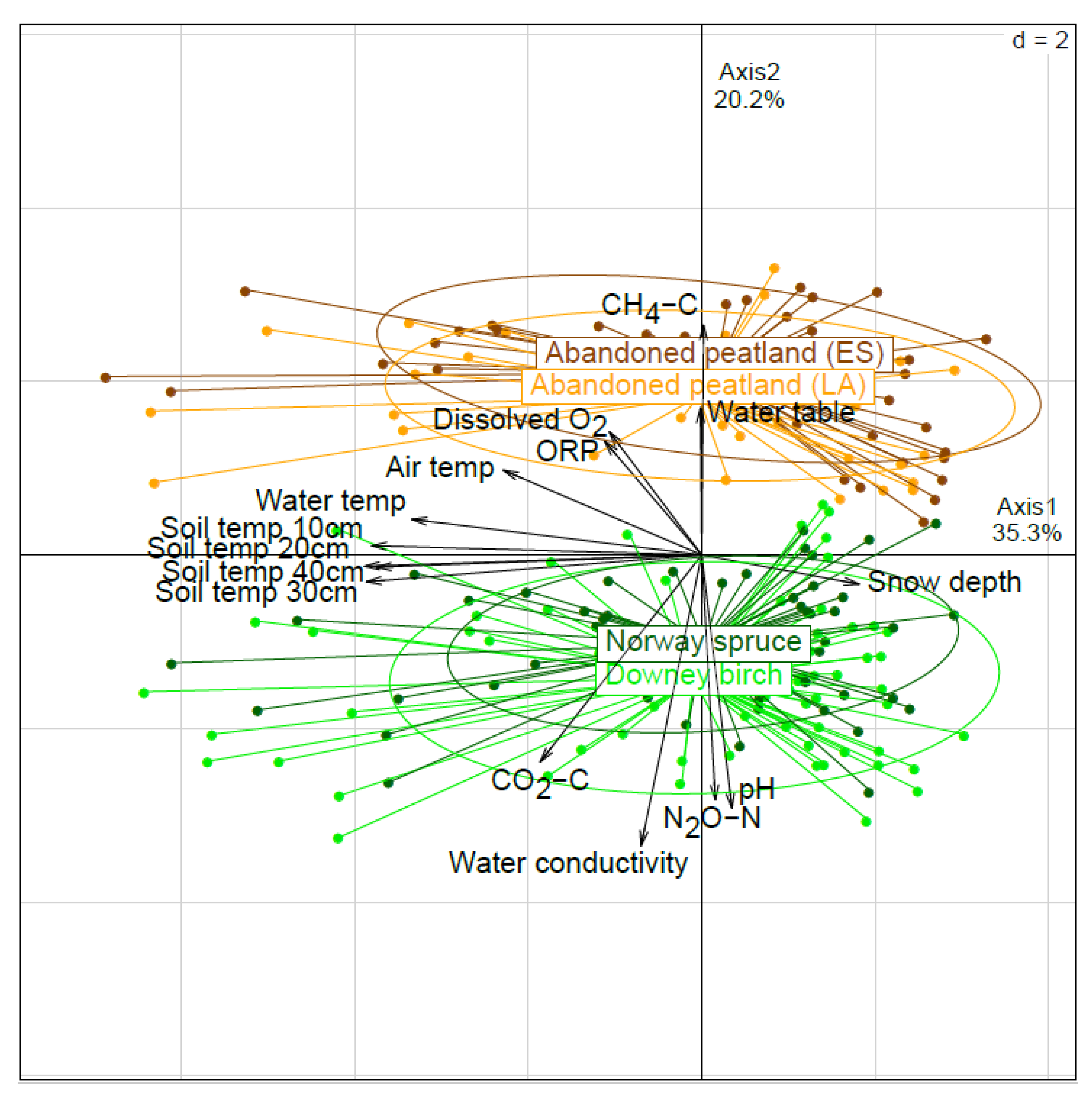
3.1. PCA Analysis
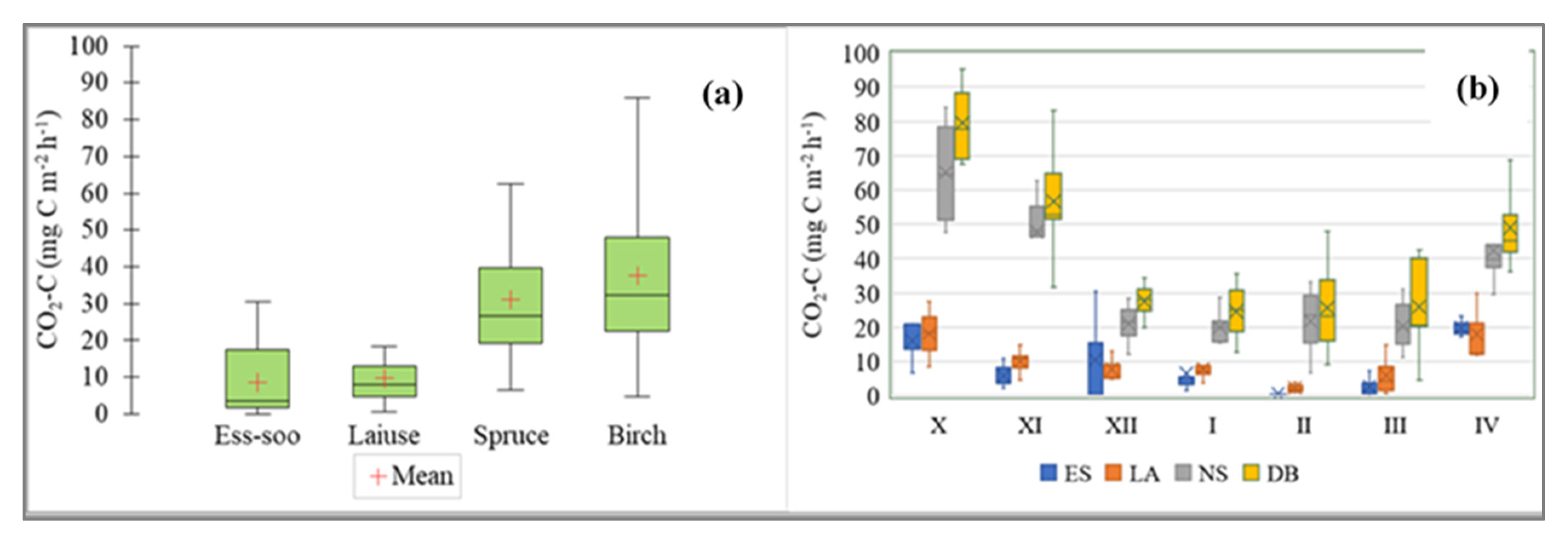
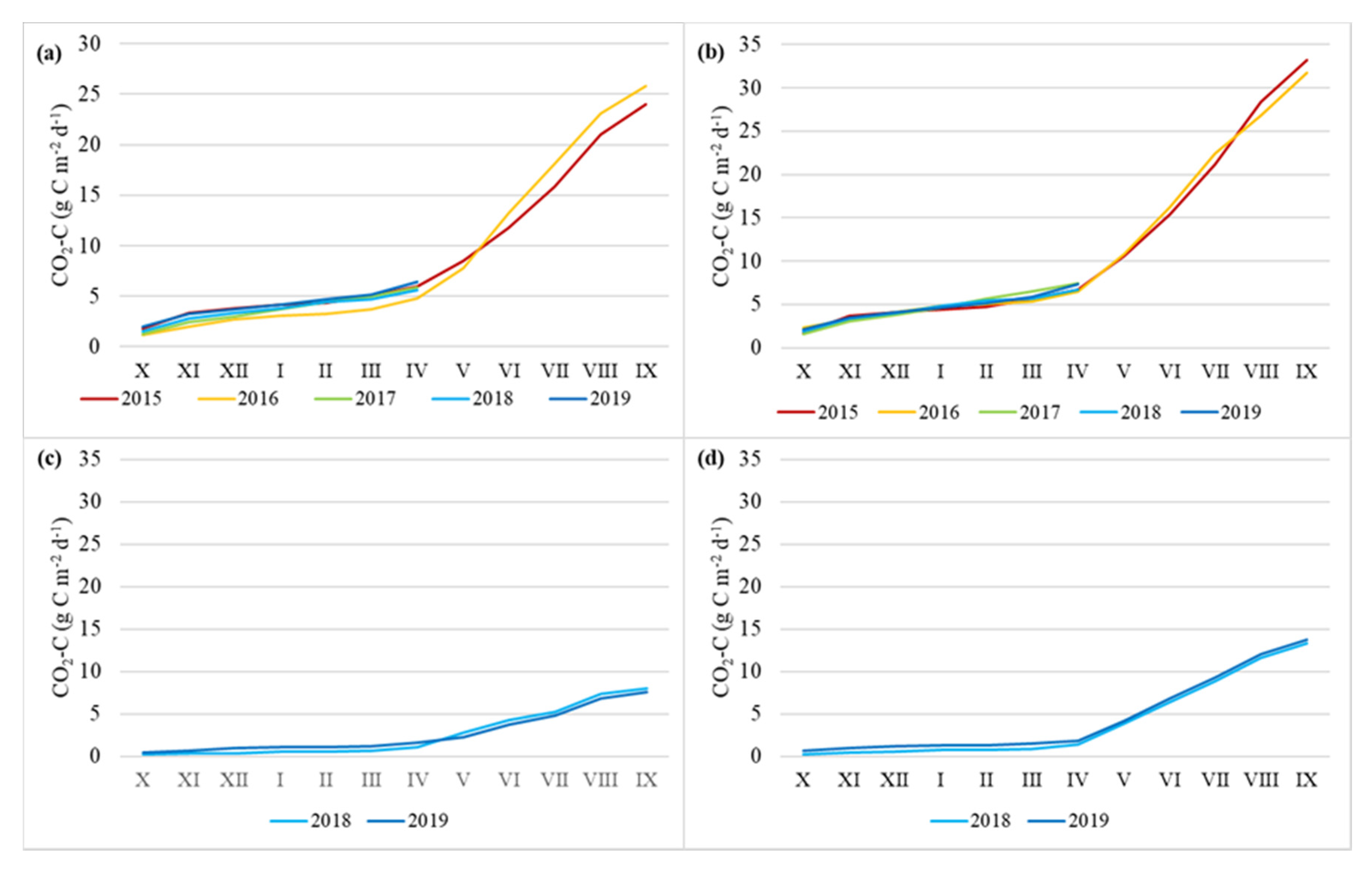
3.2. Soil CO2 Flux
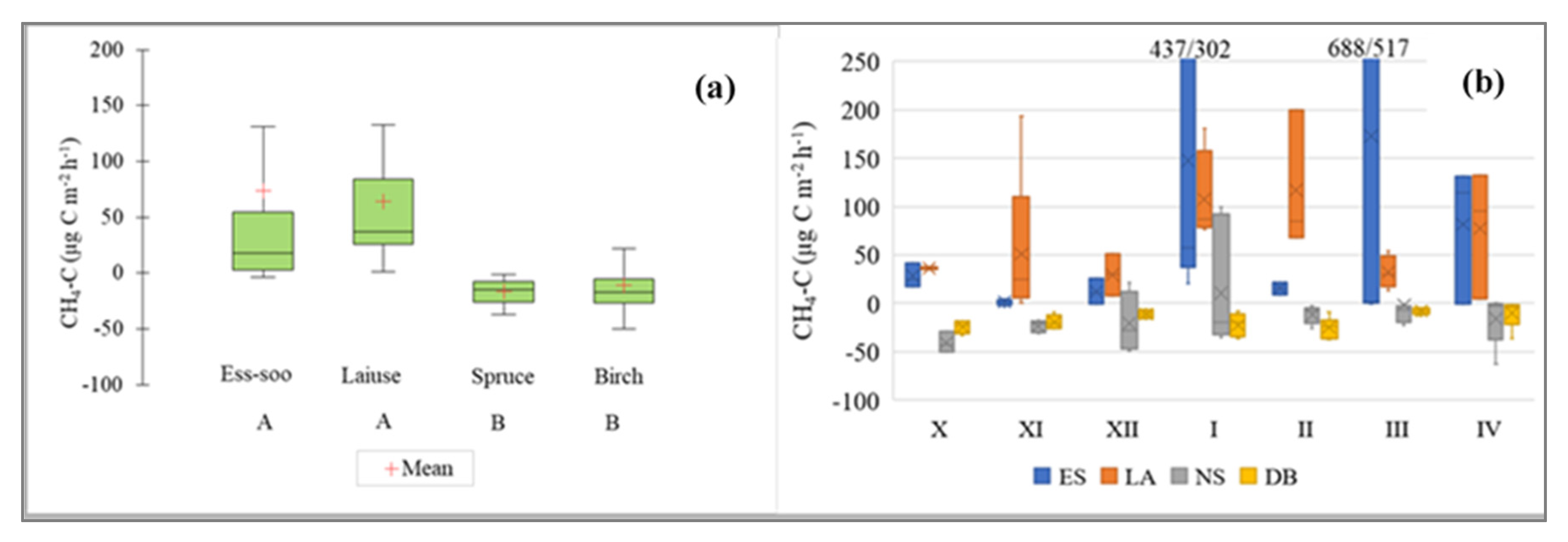
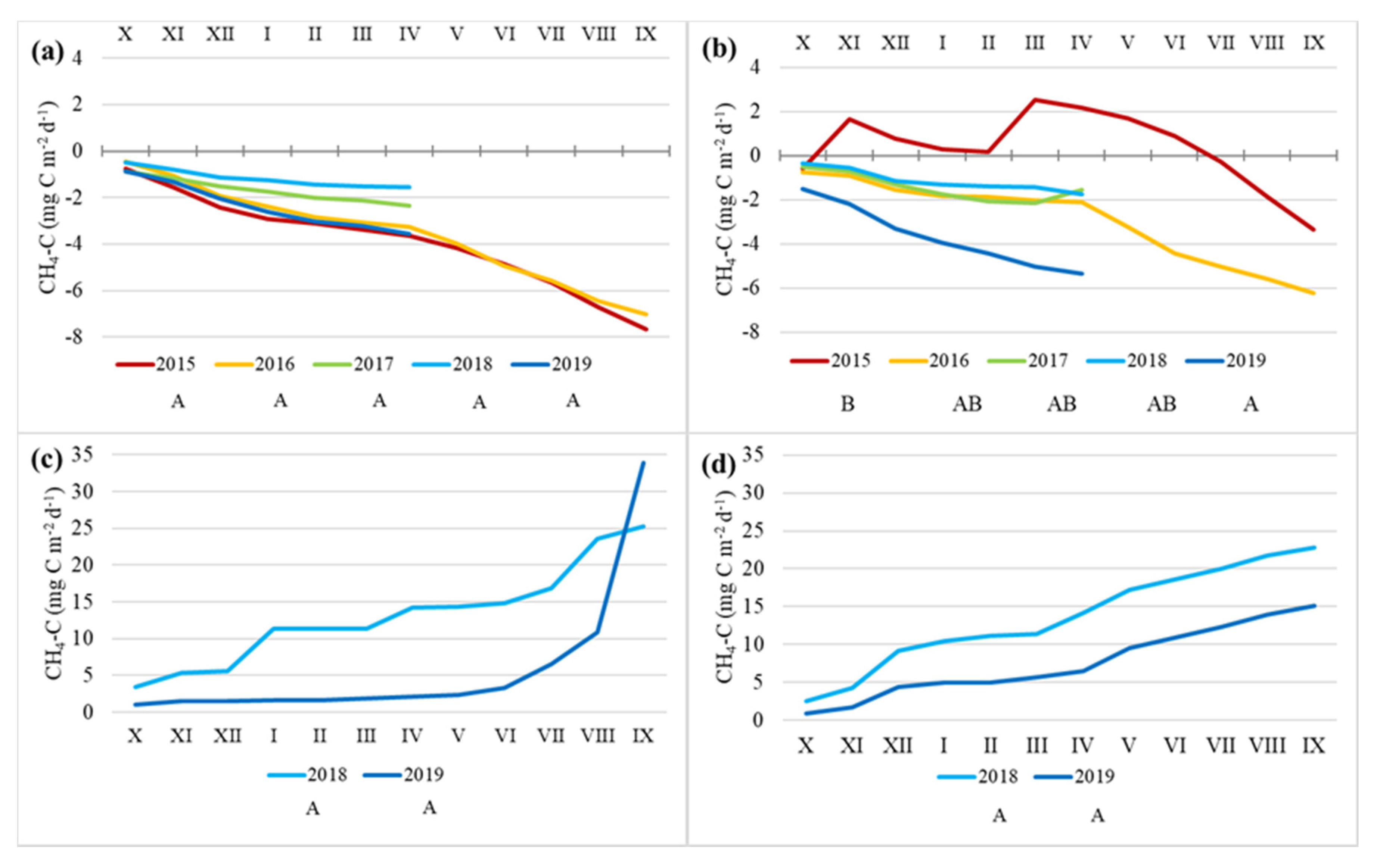
3.3. CH4 Fluxes
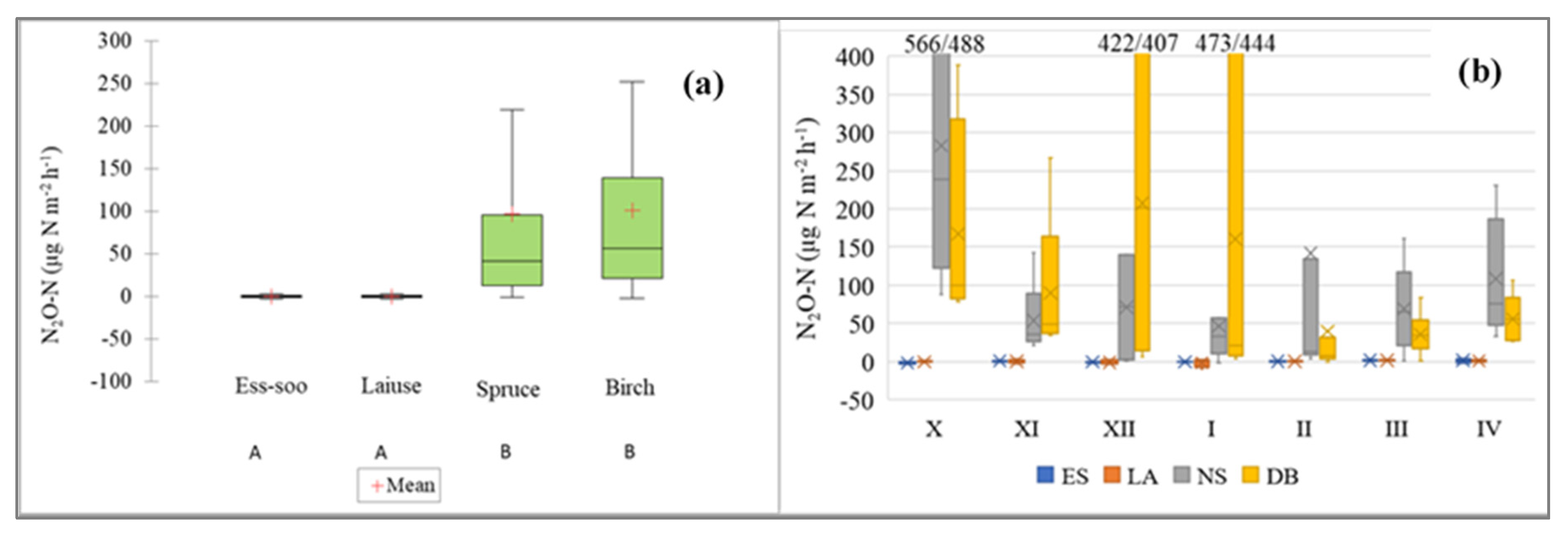
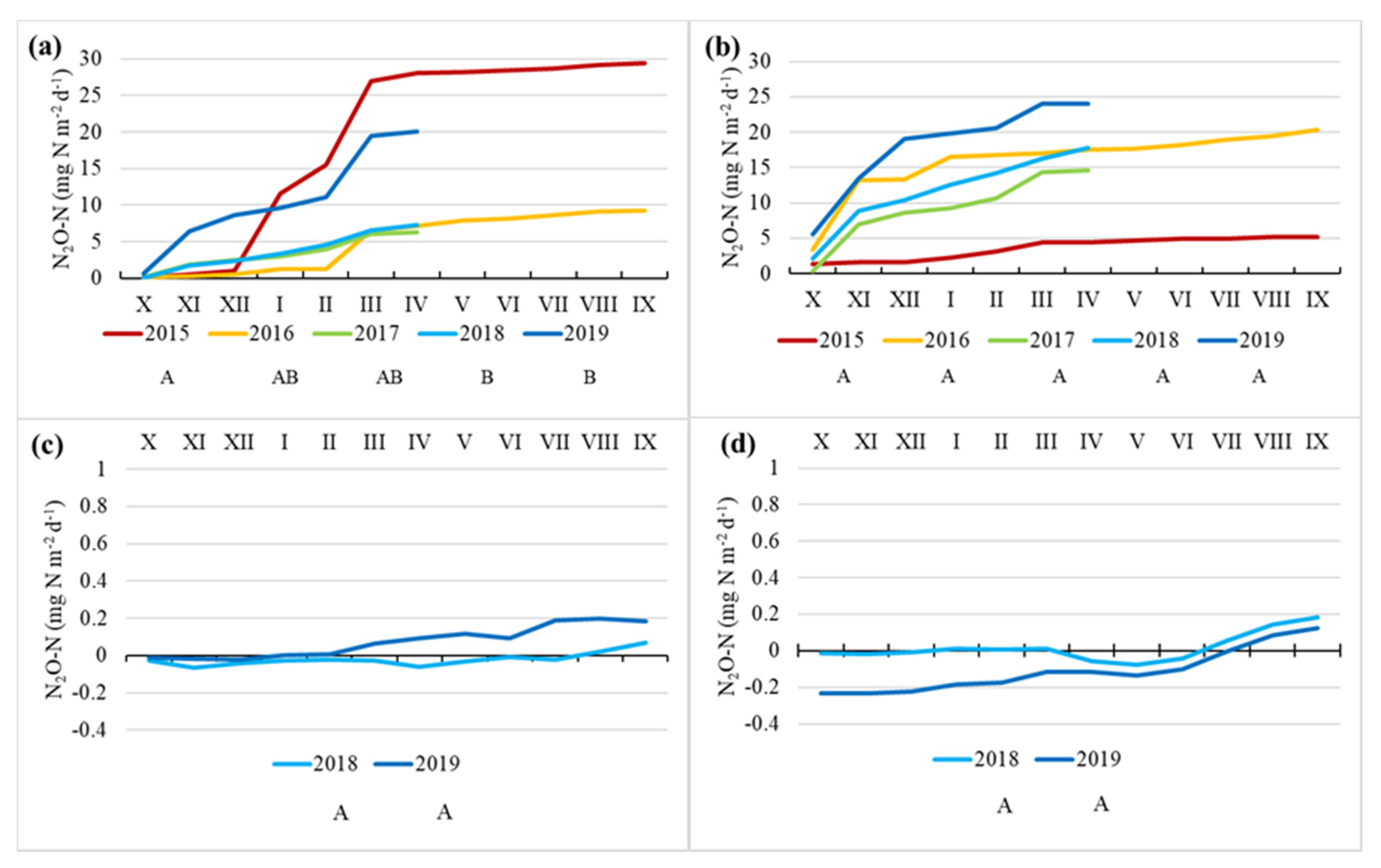
3.4. N2O Fluxes
4. Discussion
4.1. Differences in Peat Layer and Environmental Factors
4.2. Soil CO2 Fluxes
4.3. CH4 Fluxes
4.4. N2O Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- WMO Greenhouse Gas Bulletin; WMO: Geneva, Switzerland, 2019; p. 8.
- Sommerfeld, R.A.; Mosier, A.R.; Musselman, R.C. CO2, CH4 and N2O flux through a Wyoming snowpack and implications for global budgets. Nature 1993, 361, 140–142. [Google Scholar] [CrossRef]
- Winston, G.C.; Sundquist, E.T.; Stephens, B.B.; Trumbore, S.E. Winter CO2 fluxes in a boreal forest. J. Geophys. Res.-Atmos. 1997, 102, 28795–28804. [Google Scholar] [CrossRef]
- Mast, M.A.; Wickland, K.P.; Striegl, R.T.; Clow, D.W. Winter fluxes of CO2 and CH4 from subalpine soils in Rocky Mountain National Park, Colorado. Glob. Biogeochem. Cycles 1998, 12, 607–620. [Google Scholar] [CrossRef]
- Alm, J.; Saarnio, S.; Nykänen, H.; Silvola, J.; Martikainen, P. Winter CO2, CH4 and N2O fluxes on some natural and drained boreal peatlands. Biogeochemistry 1999, 44, 163–186. [Google Scholar] [CrossRef]
- Fahnestock, J.T.; Jones, M.H.; Welker, J.M. Wintertime CO2 efflux from Arctic soils: Implications for annual carbon budgets. Glob. Biogeochem. Cycles 1999, 13, 775–779. [Google Scholar] [CrossRef]
- Groffman, P.M.; Hardy, J.P.; Driscoll, C.T.; Fahey, T.J. Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Glob. Chang. Biol. 2006, 12, 1748–1760. [Google Scholar] [CrossRef]
- Maljanen, M.; Kohonen, A.-R.; Virkajärvi, P.; Martikainen, P.J. Fluxes and production of N2O, CO2 and CH4 in boreal agricultural soil during winter as affected by snow cover. Tellus Ser. B-Chem. Phys. Meteorol. 2007, 59, 853–859. [Google Scholar] [CrossRef]
- Kim, Y.; Tsunogai, S.; Tanaka, N. Winter CO2 emission and its production rate in cold temperate soils of northern Japan: 222Rn as a proxy for the validation of CO2 diffusivity. Polar Sci. 2019, 22, 100480. [Google Scholar] [CrossRef]
- Hao, Q.J.; Wang, Y.S.; Song, C.C.; Huang, Y. Contribution of winter fluxes to the annual CH4, CO2 and N2O emissions from freshwater marshes in the Sanjiang Plain. J. Environ. Sci. 2006, 18, 270–275. [Google Scholar]
- Kim, Y.; Ueyama, M.; Nakagawa, F.; Tsunogai, U.; Harazono, Y.; Tanaka, N. Assessment of winter fluxes of CO2 and CH4 in boreal forest soils of central Alaska estimated by the profile method and the chamber method: A diagnosis of methane emission and implications for the regional carbon budget. Tellus B Chem. Phys. Meteorol. 2007, 59, 223–233. [Google Scholar] [CrossRef]
- Dise, N. Winter Fluxes of Methane from Minnesota Peatlands. Biogeochemistry 1992, 17, 71–83. [Google Scholar] [CrossRef]
- Melloh, R.A.; Crill, P.M. Winter methane dynamics in a temperate peatland. Glob. Biogeochem. Cycles 1996, 10, 247–254. [Google Scholar] [CrossRef]
- Panikov, N.S.; Dedysh, S.N. Cold season CH4 and CO2 emission from boreal peat bogs (West Siberia): Winter fluxes and thaw activation dynamics. Glob. Biogeochem. Cycles 2000, 14, 1071–1080. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hao, Q.; Song, C. Effect of land-use change on CH4 and N2O emissions from freshwater marsh in Northeast China. Atmos. Environ. 2009, 43, 3305–3309. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Seasonal and diurnal net methane emissions from organic soils of the Eastern Alps, Austria: Effects of soil temperature, water balance, and plant biomass. Arct. Antarct. Alp. Res. 2007, 39, 438–448. [Google Scholar] [CrossRef]
- Miao, Y.; Song, C.; Wang, X.; Meng, H.; Sun, L.; Wang, J. Annual Carbon Gas Emissions from a Boreal Peatland in Continuous Permafrost Zone, Northeast China. Clean-Soil Air Water 2016, 44, 456–463. [Google Scholar] [CrossRef]
- Nykänen, H.; Alm, J.; Lång, K.; Silvola, J.; Martikainen, P.J. Emissions of CH4, N2O and CO2 from a virgin fen and a fen drained for grassland in Finland. J. Biogeogr. 1995, 22, 351–357. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Wang, G.; Chen, L.; Jin, Z.; Zhuang, Q.; He, J.-S. Methane emissions from an alpine wetland on the Tibetan Plateau: Neglected but vital contribution of the nongrowing season. J. Geophys. Res.-Biogeosci. 2015, 120, 1475–1490. [Google Scholar] [CrossRef]
- Kammann, C.; Grunhage, L.; Muller, C.; Jacobi, S.; Jager, H.J. Seasonal variability and mitigation options for N2O emissions from differently managed grasslands. Environ. Pollut. 1998, 102, 179–186. [Google Scholar] [CrossRef]
- Kim, Y.; Tanaka, N. Winter N2O emission rate and its production rate in soil underlying the snowpack in a subboreal region, Japan. J. Geophys. Res.-Atmos. 2002, 107, 4406. [Google Scholar] [CrossRef]
- Maljanen, M.; Hytönen, J.; Martikainen, P.J. Fluxes of N2O, CH4 and CO2 on afforested boreal agricultural soils. Plant Soil 2001, 231, 113–121. [Google Scholar] [CrossRef]
- Maljanen, M.; Komulainen, V.M.; Hytönen, J.; Martikainen, P.; Laine, J. Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil characteristics. Soil Biol. Biochem. 2004, 36, 1801–1808. [Google Scholar] [CrossRef]
- Papen, H.; Butterbach-Bahl, K. A 3-year continuous record of nitrogen trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany: 1. N2O emissions. J. Geophys. Res. Atmos. 1999, 104, 18487–18503. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Congreves, K.A.; Abalos, D.; Berg, A.A.; Brown, S.E.; Ambadan, J.T.; Gao, X.; Tenuta, M. Globally important nitrous oxide emissions from croplands induced by freeze-thaw cycles. Nat. Geosci. 2017, 10, 279–283. [Google Scholar] [CrossRef]
- Kull, A.; Jaagus, J.; Kuusemets, V.; Mander, Ü. The effects of fluctuating climatic and weather events on nutrient dynamics in a narrow mosaic riparian peatland. Boreal Environ. Res. 2008, 13, 243–263. [Google Scholar]
- Groffman, P.M.; Driscoll, C.T.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Tierney, G.L. Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 2001, 56, 135–150. [Google Scholar] [CrossRef]
- Zimov, S.; Semiletov, I.; Daviodov, S.; Voropaev, Y.; Prosyannikov, S.; Wong, C.; Chan, Y. Wintertime CO2 Emission from Soils of Northeastern Siberia. Arctic 1993, 46, 197–204. [Google Scholar] [CrossRef][Green Version]
- Zimov, S.; Zimova, G.; Daviodov, S.; Daviodova, A.; Voropaev, Y.; Voropaeva, Z.; Prosiannikov, S.; Prosiannikova, O.; Semiletova, I.; Semiletov, I. Winter Biotic Activity and Production of CO2 in Siberian Soils-a Factor in the Greenhouse-Effect. J. Geophys. Res.-Atmos. 1993, 98, 5017–5023. [Google Scholar] [CrossRef]
- Zhang, J.B.; Song, C.C.; Yang, W.Y. Cold season CH4, CO2 and N2O fluxes from freshwater marshes in northeast China. Chemosphere 2005, 59, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kodama, Y. Environmental factors regulating winter CO2 flux in snow-covered boreal forest soil, interior Alaska. Biogeosci. Discuss. 2012, 9, 1129–1159. [Google Scholar] [CrossRef]
- Aurela, M.; Laurila, T.; Tuovinen, J.P. Annual CO2 balance of a subarctic fen in northern Europe: Importance of the wintertime efflux. J. Geophys. Res.-Atmos. 2002, 107, 4607. [Google Scholar] [CrossRef]
- Oechel, W.C.; Vourlitis, G.; Hastings, S.J. Cold season CO2 emission from Arctic soils. Glob. Biogeochem. Cycles 1997, 11, 163–172. [Google Scholar] [CrossRef]
- Monson, R.K.; Lipson, D.L.; Burns, S.P.; Turnipseed, A.A.; Delany, A.C.; Williams, M.W.; Schmidt, S.K. Winter forest soil respiration controlled by climate and microbial community composition. Nature 2006, 439, 711–714. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Jones, S.E.; Schoolmaster, D.R.; Fierer, N.; Lennon, J.T. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 2013, 57, 217–227. [Google Scholar] [CrossRef]
- Lohila, A.; Aurela, M.; Regina, K.; Tuovinen, J.-P.; Laurila, T. Wintertime CO2 exchange in a boreal agricultural peat soil. Tellus Ser. B-Chem. Phys. Meteorol. 2007, 59, 860–873. [Google Scholar] [CrossRef]
- Van Bochove, E.; Theriault, G.; Rochette, P.; Jones, H.G.; Pomeroy, J.W. Thick ice layers in snow and frozen soil affecting gas emissions from agricultural soils during winter. J. Geophys. Res.-Atmos. 2001, 106, 23061–23071. [Google Scholar] [CrossRef]
- Mørkved, P.T.; Dörsch, P.; Henriksen, T.M.; Bakken, L.R. N2O emissions and product ratios of nitrification and denitrification as affected by freezing and thawing. Soil Biol. Biochem. 2006, 38, 3411–3420. [Google Scholar] [CrossRef]
- Öquist, M.G.; Petrone, K.; Nilsson, M.; Klemedtsson, L. Nitrification controls N2O production rates in a frozen boreal forest soil. Soil Biol. Biochem. 2007, 39, 1809–1811. [Google Scholar] [CrossRef]
- Flessa, H.; Dörsch, P.; Beese, F. Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J. Geophys. Res. Atmos. 1995, 100, 23115–23124. [Google Scholar] [CrossRef]
- Maljanen, M.; Virkajärvi, P.-J.Y.; Hytönen, J.; Öquist, M.; Sparrman, T.; Martikainen, P.J. Nitrous oxide production in boreal soils with variable organic matter content at low temperature–snow manipulation experiment. Biogeosciences 2009, 6, 2461–2473. [Google Scholar] [CrossRef]
- Maljanen, M.; Alm, J.; Martikainen, P.J.; Repo, T. Prolongation of soil frost resulting from reduced snow cover increases nitrous oxide emissions from boreal forest soil. Boreal Environ. Res. 2010, 15, 34–42. [Google Scholar]
- Goldberg, S.D.; Borken, W.; Gebauer, G. N2O emission in a Norway spruce forest due to soil frost; concentration and isotope profiles shed a new light on an old story. Biogeochem. Dordr. 2010, 97, 21–30. [Google Scholar] [CrossRef]
- Mastepanov, M.; Sigsgaard, C.; Dlugokencky, E.J.; Houweling, S.; Ström, L.; Tamstorf, M.P.; Christensen, T.R. Large tundra methane burst during onset of freezing. Nature 2008, 456, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xu, X.; Sun, X.; Tian, H.; Sun, L.; Miao, Y.; Wang, X.; Guo, Y. Large methane emission upon spring thaw from natural wetlands in the northern permafrost region. Environ. Res. Lett. 2012, 7, 034009. [Google Scholar] [CrossRef]
- Cris, R.; Buckmaster, S.; Bain, C.; Reed, M. Global Peatland Restoration Demonstrating SUCCESS; IUCN UK National Committee Peatland Programme: Edinburgh, Scotland, 2014. [Google Scholar]
- Salm, J.-O.; Maddison, M.; Tammik, S.; Soosaar, K.; Truu, J.; Mander, U. Emissions of CO2, CH4 and N2O from undisturbed, drained and mined peatlands in Estonia. Hydrobiologia 2012, 692, 41–55. [Google Scholar] [CrossRef]
- Mäkiranta, P.; Hytönen, J.; Aro, L.; Maljanen, M.; Pihlatie, M.; Potila, H.; Shurpali, N.J.; Laine, J.; Lohila, A.; Martikainen, P.J.; et al. Soil greenhouse gas emissions from afforested organic soil croplands and cutaway peatlands. Boreal Environ. Res. 2007, 12, 159–175. [Google Scholar]
- Raudsaar, M.; Pärt, E.; Adermann, V. Forest resources. In Yearbook Forest 2013; Estonian Environment Agency: Tartu, Estonia, 2013; pp. 1–42. [Google Scholar]
- Milakovsky, B.; Frey, B.; James, T. Carbon Dynamics in the Boreal Forest. In Managing Forest Carbon in a Changing Climate; Ashton, M.S., Tyrrell, M.L., Spalding, D., Gentry, B., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 109–135. ISBN 978-94-007-2232-3. [Google Scholar]
- Kotta, J.; Herkül, K.; Jaagus, J.; Kaasik, A.; Raudsepp, U.; Alari, V.; Arula, T.; Haberman, J.; Järvet, A.; Kangur, K.; et al. Linking atmospheric, terrestrial and aquatic environments: Regime shifts in the Estonian climate over the past 50 years. PLoS ONE 2018, 13, e0209568. [Google Scholar] [CrossRef]
- Stielstra, C.M.; Lohse, K.A.; Chorover, J.; McIntosh, J.C.; Barron-Gafford, G.A.; Perdrial, J.N.; Litvak, M.; Barnard, H.R.; Brooks, P.D. Climatic and landscape influences on soil moisture are primary determinants of soil carbon fluxes in seasonally snow-covered forest ecosystems. Biogeochemistry 2015, 123, 447–465. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.A.; Savage, K.; Sundquist, E.T.; Steudler, P. Effect of summer throughfall exclusion, summer drought, and winter snow cover on methane fluxes in a temperate forest soil. Soil Biol. Biochem. 2006, 38, 1388–1395. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Livingston, G.P. Use of Chamber Systems to Measure Trace Gas Fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; ASA Special Publications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1993; Volume 55, pp. 63–78. ISBN 978-0-89118-321-1. [Google Scholar]
- Soosaar, K.; Mander, Ü.; Maddison, M.; Kanal, A.; Kull, A.; Lõhmus, K.; Truu, J.; Augustin, J. Dynamics of gaseous nitrogen and carbon fluxes in riparian alder forests. Ecol. Eng. 2011, 37, 40–53. [Google Scholar] [CrossRef]
- Christensen, T.R.; Jonasson, S.; Callaghan, T.V.; Havström, M. Spatial variation in high-latitude methane flux along a transect across Siberian and European tundra environments. J. Geophys. Res. Atmos. 1995, 100, 21035–21045. [Google Scholar] [CrossRef]
- Mander, Ü.; Uri, V.; Ostonen-Märtin, I.; Truu, M.; Maddison, M.; Soosaar, K.; Teemusk, A.; Hansen, R.; Järveoja, J.; Aosaar, J.; et al. Carbon and Nitrogen Cycling in Forests with Altered Water Regime. (In Estonian: Muudetud Veerežiimiga Metsade Süsiniku- ja Lämmastikuringe). Report of the Research Project of the Estonian State Forest Management Centre (RMK) 2013–2016. 2016. Available online: https://media.rmk.ee/files/Rakendusuuring%20lopparuanne:Kodusoometsad.pdf (accessed on 9 July 2020).
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://www.mcglinnlab.org/publication/2019-01-01_oksanen_vegan_2019/ (accessed on 9 July 2020).
- Huth, V.; Jurasinski, G.; Glatzel, S. Winter emissions of carbon dioxide, methane and nitrous oxide from a minerotrophic fen under nature conservation management in north-east Germany. Mires Peat 2012, 10, 1–13. [Google Scholar]
- Järveoja, J.; Peichl, M.; Maddison, M.; Soosaar, K.; Vellak, K.; Karofeld, E.; Teemusk, A.; Mander, Ü. Impact of water table level on annual carbon and greenhouse gas balances of a restored peat extraction area. Biogeosciences 2016, 13, 2637–2651. [Google Scholar] [CrossRef]
- Waddington, J.M.; Rotenberg, P.A.; Warren, F.J. Peat CO2 production in a natural and cutover peatland: Implications for restoration. Biogeochemistry 2001, 54, 115–130. [Google Scholar] [CrossRef]
- Hilasvuori, E.; Akujärvi, A.; Fritze, H.; Karhu, K.; Laiho, R.; Mäkiranta, P.; Oinonen, M.; Palonen, V.; Vanhala, P.; Liski, J. Temperature sensitivity of decomposition in a peat profile. Soil Biol. Biochem. 2013, 67, 47–54. [Google Scholar] [CrossRef]
- Mastný, J.; Urbanová, Z.; Kaštovská, E.; Straková, P.; Šantrůčková, H.; Edwards, K.R.; Picek, T. Soil organic matter quality and microbial activities in spruce swamp forests affected by drainage and water regime restoration. Soil Use Manag. 2016, 32, 200–209. [Google Scholar] [CrossRef]
- Natali, S.M.; Watts, J.D.; Rogers, B.M.; Potter, S.; Ludwig, S.M.; Selbmann, A.-K.; Sullivan, P.F.; Abbott, B.W.; Arndt, K.A.; Birch, L.; et al. Large loss of CO2 in winter observed across the northern permafrost region. Nat. Clim. Chang. 2019, 9, 852–857. [Google Scholar] [CrossRef]
- Pihlatie, M.K.; Kiese, R.; Brüggemann, N.; Butterbach-Bahl, K.; Kieloaho, A.-J.; Laurila, T.; Lohila, A.; Mammarella, I.; Minkkinen, K.; Penttilä, T.; et al. Greenhouse gas fluxes in a drained peatland forest during spring frost-thaw event. Biogeosciences 2010, 7, 1715–1727. [Google Scholar] [CrossRef]
- Bubier, J.; Crill, P.; Mosedale, A. Net ecosystem CO2 exchange measured by autochambers during the snow-covered season at a temperate peatland. Hydrol. Process. 2002, 16, 3667–3682. [Google Scholar] [CrossRef]
- Song, Y.; Wang, G.; Yu, X.; Zou, Y. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Sulkava, P.; Huhta, V. Effects of hard frost and freeze-thaw cycles on decomposer communities and N mineralisation in boreal forest soil. Appl. Soil Ecol. 2003, 22, 225–239. [Google Scholar] [CrossRef]
- Comerford, D.P.; Schaberg, P.G.; Templer, P.H.; Socci, A.M.; Campbell, J.L.; Wallin, K.F. Influence of experimental snow removal on root and canopy physiology of sugar maple trees in a northern hardwood forest. Oecologia 2013, 171, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, Y.; Chen, J.; Wang, X.; Zhang, Q.; Bond-Lamberty, B. Seasonality of soil CO2 efflux in a temperate forest: Biophysical effects of snowpack and spring freeze–thaw cycles. Agric. For. Meteorol. 2013, 177, 83–92. [Google Scholar] [CrossRef]
- Åström, H.; Metsovuori, E.; Saarinen, T.; Lundell, R.; Hänninen, H. Morphological characteristics and photosynthetic capacity of Fragaria vesca L. winter and summer leaves. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 215, 33–39. [Google Scholar] [CrossRef]
- Lundell, R.; Saarinen, T.; Åström, H.; Hänninen, H. The boreal dwarf shrub Vaccinium vitis-idaea retains its capacity for photosynthesis through the winter. Botany 2008, 86, 491–500. [Google Scholar] [CrossRef]
- Saarinen, T.; Rasmus, S.; Lundell, R.; Kauppinen, O.-K.; Hänninen, H. Photosynthetic and phenological responses of dwarf shrubs to the depth and properties of snow. Oikos 2016, 125. [Google Scholar] [CrossRef]
- Minkkinen, K.; Penttilä, T.; Laine, J. Tree stand volume as a scalar for methane fluxes in forestry-drained peatlands in Finland. Boreal Environ. Res. 2007, 12, 127–132. [Google Scholar]
- Ojanen, P.; Minkkinen, K.; Alm, J.; Penttilä, T. Soil–atmosphere CO2, CH4 and N2O fluxes in boreal forestry-drained peatlands. For. Ecol. Manag. 2010, 260, 411–421. [Google Scholar] [CrossRef]
- Sarkkola, S.; Hökkä, H.; Koivusalo, H.; Nieminen, M.; Ahti, E.; Päivänen, J.; Laine, J. Role of tree stand evapotranspiration in maintaining satisfactory drainage conditions in drained peatlands. Can. J. For. Res. 2010, 40, 1485–1496. [Google Scholar] [CrossRef]
- Brooks, P.D.; Schmidt, S.K.; Williams, M.W. Winter production of CO2 and N2O from alpine tundra: Environmental controls and relationship to inter-system C and N fluxes. Oecologia 1997, 110, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Pärn, J.; Verhoeven, J.T.A.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Järveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, S.; Barba, J.; Bond-Lamberty, B.; Vargas, R. Using greenhouse gas fluxes to define soil functional types. Plant Soil 2018, 423, 285–294. [Google Scholar] [CrossRef]


| Year | Precipitation (mm) | Mean Air Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| Jõgeva | Tartu | Võru | Jõgeva | Tartu | Võru | |
| 2015/16 | 283 | 253 | 223 | −5.3 | −2.6 | −2.3 |
| 2016/17 | 199 | 190 | 223 | −7.5 | −6.0 | −6.2 |
| 2017/18 | 206 | 154 | 185 | −13.1 | −11.0 | −11.2 |
| 2018/19 | 185 | 199 | 153 | −7.2 | −4.6 | −4.5 |
| Study Site | Laiuse APEA | Ess-soo APEA | Downy Birch DPF | Norway Spruce DPF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Greenhouse Gas | CO2 | CH4 | N2O | CO2 | CH4 | N2O | CO2 | CH4 | N2O | CO2 | CH4 | N2O |
| Snow cover depth | −0.58 *** | −0.47 ** | 0.34 * | −0.59 *** | −0.48 *** | |||||||
| Water table | 0.46 ** | −0.40 * | −0.28 * | 0.52 *** | 0.37 ** | |||||||
| Soil water content | ||||||||||||
| Air temperature | 0.47 ** | −0.38 * | 0.45 ** | 0.48 ** | −0.44 ** | 0.60 *** | 0.67 *** | |||||
| Ground surface temperature | 0.71 *** | 0.56 ** | 0.47 * | −0.40 * | 0.67 *** | 0.72 *** | ||||||
| Soil temperature—10 cm | 0.51 ** | 0.43 ** | 0.55 *** | 0.53 ** | −0.62 *** | 0.57 *** | −0.38 ** | 0.72 *** | −0.49 *** | −0.33 * | ||
| Soil temperature—20 cm | 0.37 * | 0.36 * | 0.41 * | −0.62 *** | 0.53 *** | −0.57 *** | 0.63 *** | −0.65 *** | ||||
| Soil temperature—30 cm | 0.39 * | 0.36 * | −0.57 *** | 0.59 *** | −0.59 *** | 0.57 *** | −0.73 *** | −0.30 * | ||||
| Soil temperature—40 cm | 0.41 * | −0.57 *** | 0.56 *** | −0.57 *** | 0.53 *** | −0.67 *** | ||||||
| Water temperature | 0.36 * | −0.38 * | 0.37 ** | −0.51 *** | 0.30 * | −0.83 *** | ||||||
| Water pH | 0.29 * | |||||||||||
| Water O2 content | ||||||||||||
| Water ORP | 0.37 * | −0.44 ** | 0.27 * | |||||||||
| Water electrical conductivity | −0.50 *** | 0.29 * | ||||||||||
| Soil electrical conductivity | 0.43 * | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viru, B.; Veber, G.; Jaagus, J.; Kull, A.; Maddison, M.; Muhel, M.; Espenberg, M.; Teemusk, A.; Mander, Ü. Wintertime Greenhouse Gas Fluxes in Hemiboreal Drained Peatlands. Atmosphere 2020, 11, 731. https://doi.org/10.3390/atmos11070731
Viru B, Veber G, Jaagus J, Kull A, Maddison M, Muhel M, Espenberg M, Teemusk A, Mander Ü. Wintertime Greenhouse Gas Fluxes in Hemiboreal Drained Peatlands. Atmosphere. 2020; 11(7):731. https://doi.org/10.3390/atmos11070731
Chicago/Turabian StyleViru, Birgit, Gert Veber, Jaak Jaagus, Ain Kull, Martin Maddison, Mart Muhel, Mikk Espenberg, Alar Teemusk, and Ülo Mander. 2020. "Wintertime Greenhouse Gas Fluxes in Hemiboreal Drained Peatlands" Atmosphere 11, no. 7: 731. https://doi.org/10.3390/atmos11070731
APA StyleViru, B., Veber, G., Jaagus, J., Kull, A., Maddison, M., Muhel, M., Espenberg, M., Teemusk, A., & Mander, Ü. (2020). Wintertime Greenhouse Gas Fluxes in Hemiboreal Drained Peatlands. Atmosphere, 11(7), 731. https://doi.org/10.3390/atmos11070731






