Abstract
Traceable determination of atmospheric mercury (Hg) represents a major analytical problem due to low environmental concentrations. Although Hg pre-concentration on activated carbon (AC) traps is a simple method for sample collection, Hg determination is difficult due to a complex matrix that cannot be easily digested using wet chemistry. Two approaches for Hg loading on iodinated AC, the purging of elemental mercury (Hg0) and the spiking a solution of standard reference material (SRM), were used to test whether spiking SRM solution on AC can be used for the traceable determination of atmospheric mercury collected as Hg0. Mercury on AC was determined using atomic absorption spectrometry after sample combustion. The detector’s response for both loading methods was identical in a wide concentration range, indicating that the spiking of SRM on AC can, indeed, be used for the calibration of analytical systems used for the determination of atmospheric mercury. This was confirmed by the determination of Hg in a real atmospheric sample collected on an iodinated AC trap and using an SRM spiking calibration. Different ACs were compared regarding their ability to quantitatively capture Hg while having the lowest breakthrough. Use of a specific impregnating solution probably converted Hg on AC to Millon’s iodide, as estimated from the fractionation thermogram.
1. Introduction
Toxic mercury (Hg) compounds can cause adverse effects on human health [1,2]. Recent modeling suggests that mercury in the atmosphere has increased three to six-fold compared to natural levels, mainly due to anthropogenic Hg emissions [3,4]. Hg emitted to the atmosphere has a long residence time (up to a year) and can travel long distances before being deposited to land or ocean surfaces [5]. One of topics in the scientific community, driven by the Minamata convention on mercury, is the quantification of the extent to which anthropogenic mercury emitted to the atmosphere is converted to inorganic mercury and subsequently methylated and incorporated in biota [6,7].
Total airborne mercury (TAM) consists of particulate bound mercury (PBM) and total gaseous mercury (TGM) [8]. TGM represents the sum of gaseous oxidized mercury (GOM, Hg(II)) and gaseous elemental mercury (GEM, Hg0) [5]. Even though Hg concentrations in the atmosphere are elevated due to anthropogenic emissions, these values all are still exceptionally low, which represents a major analytical challenge. This is especially important when performing mercury speciation in the atmospheric samples because GOM and PBM are present at pg m−3, while GEM is at ng m−3 levels [9,10]. Although there are reliable instruments capable of measuring GEM, Hg speciation still represents a major analytical challenge.
Pre-concentration of mercury is usually required for the determination of GEM with the exception of atomic absorbance spectrometers (AAS) with Zeeman background correction [11]. However, for some characteristic analysis, e.g., Hg speciation or the determination of mercury stable isotope ratios in atmospheric samples, mercury pre-concentration is an essential step [12]. The determination of mercury stable isotope ratios requires a considerable amount of mercury, usually more than 10 ng [12,13]. This requires a mercury pre-concentration system that can quantitatively collect all mercury present in the sample. Quantitative trapping is required to ensure that the fractionation of mercury stable isotopes does not occur during the pre-concentration step.
The two most commonly used methods for the pre-concentration of mercury are collection on gold-coated quartz/glass sand/beads or on impregnated activated carbon (AC) traps [12,14]. Although gold traps are commonly used for the determination of mercury in various instrumental setups, their application for prolonged collection time of mercury is questionable. Gold traps suffer from passivation of gold [15], especially under the influence of seawater aerosol due to the corrosive properties of chlorides. On the contrary, impregnated AC is rather durable and can also be used for the pre-concentration of mercury from ambient air or even from stack emissions and flue gases that are characterized by the presence of highly corrosive gases [16,17,18,19]. AC possesses a high adsorption capacity for mercury compounds and might, therefore, be used as an effective sorbent in analytical traps. Impregnated AC, particularly iodinated, brominated or chlorinated AC, has a particularly high affinity for mercury compounds [16,17]. Therefore, impregnated AC is used as an efficient adsorbent for the quantitative capturing of atmospheric mercury. The main drawback of AC is higher blanks compared to the gold traps, which requires the collection of considerable amounts of the sample to diminish the influence of these blanks on reliable Hg determination.
According to the US Environmental Protection Agency (EPA) Method 30B, AC traps are used for the collection of both GOM and GEM fractions [20]. This method is intended for use only under relatively low particulate conditions (e.g., sampling of Hg emissions from coal-fired combustion sources after all pollution control devices). Therefore, if appropriate filters are not used to remove particulate matter, PBM will also be collected on AC traps and the obtained results will represent TAM. As mercury in the atmosphere is mostly present as Hg0, this is the fraction that is mostly collected on AC traps, alongside GOM and PBM.
It is of utter importance to perform a traceable determination of mercury using appropriate calibration. Appropriate calibration for GEM would be by Hg0 so that the sample and the standard are present in the same oxidation state of mercury. Traceable calibration sources for GOM at low atmospheric concentrations are still under development [21]. The standard for Hg0 is usually obtained from a bell–jar apparatus held at a certain temperature from which a known volume of headspace vapors is taken using a gas-tight syringe. The amount of mercury taken by syringe at a certain temperature is calculated using empirical Dumarey or Huber equations [22,23,24]. The utilization of a calibration system based on bell–jar apparatus might represent an error in the determination of mercury on an impregnated AC trap due to the strong temperature dependence of these equations, and consequently cause differences compared to other calibration techniques [25,26].
The second approach to the calibration of an analytical system is the use of Hg0 produced by the reduction of standard reference material (SRM) NIST 3133 [27]. This approach is more appropriate because mercury determinations are traceable to this standard reference material. Nevertheless, this sort of calibration is somewhat tedious and time consuming as quantitative reduction of Hg(II) from NIST 3133 to elemental form (Hg0) using tin(II) chloride requires a certain amount of time. In addition, impurities in reagents can cause blanks or contamination.
The alternative to this approach would be directly spiking the diluted SRM NIST 3133 onto a sample matrix [28]. However, difficulties can also arise consequently. Hg species are not the same in NIST 3133 standard solution (where Hg is present as Hg(II)) and in purged Hg0 that is produced by the reduction of the former. The use of Hg(II) in solution for the calibration of system used for the determination of Hg0 on AC is questionable due to the quite different chemical and physical properties of these two Hg species. The chemistry of their corresponding adsorption onto the impregnated AC is probably represented by different physisorption/chemisorption mechanisms. Calibration using standard reference material of soils and sediments was recently compared with the calibration using the spiking of NIST 3133 standard solution onto the sample matrix [28]. Although both calibration curves were linear, the detector’s responses for the two calibration curves were not identical. This showed that the liberation of mercury from the matrix is strongly dependent on the mercury species and how well this species is bound to matrix [28]. Therefore, it is necessary to verify that this approach to calibration is, indeed, traceable to standard reference material before it can be used for the determination of mercury in atmospheric samples.
The objective of this work has been to test whether spiking of NIST 3133 standard reference material directly onto iodinated AC can be used for the calibration of system used for the traceable determination of mercury purged onto this trap as Hg0. Two approaches for loading Hg onto AC were compared; the first approach was the purging of NIST 3133-traceable Hg0 on iodinated AC traps, while the other one was the direct spiking of diluted NIST 3133 reference material onto the AC. The method was tested by the determination of a real atmospheric sample from a contaminated indoor site and calibrating using a spiking method. To estimate which AC has the best quantitative capturing of Hg0 and the lowest breakthrough, the performance of several in-house prepared iodinated ACs were compared to that of a commercially available AC. In this work, a NH4I-impregnated AC was selected because iodine forms much more stable Hg complexes compared to other halogens. In addition, ammonium ions convert Hg-iodide complexes to insoluble Millon’s iodide. We wanted to test whether the polymeric structure of Millon’s iodide is more stable than Hg-iodide complexes. Therefore, NH4I-impregnated ACs were used instead of commonly used KI-impregnated or brominated/chlorinated ACs. Peak deconvolution of fractionation thermograms was performed and results were compared to literature to estimate the stability of mercury species present on AC traps.
2. Experiments
2.1. Preparation of In-House Impregnated Activated Carbon
In-house impregnated iodinated AC was prepared using a modified method following the study by Fu et al. [12]. Virgin AC (grain size 0.5–1.0 mm; Merck, Darmstadt, Germany) was cleaned by heating at 500 °C for five hours in the stream of nitrogen gas (0.2 L min−1). After the virgin AC was cooled down to room temperature, 5.0 g was conditioned for 48 h in 0.5 L of impregnating solution containing known concentrations of impregnating salts. Used impregnating solutions were 0.10 mol L−1 NH4I, 0.03 mol L−1 NH4I, and 0.01 mol L−1 NH4I (ACS grade, Sigma Aldrich, St. Louis, MO, USA).
Following equilibration in the impregnating solution, AC was rinsed three times with Type I purified water (electrical resistivity 18.2 MΩ cm; Milli-Q water, Merck, Darmstadt, Germany) and dried in rotavapor at 60 °C under reduced pressure (50 mbar) until dry. Impregnated iodinated AC was stored in an amber glass bottle prior to use. The general scheme for the preparation of the impregnated AC is presented in Scheme 1.

Scheme 1.
General scheme for the preparation of iodinated activated carbon (AC) traps.
The amount of iodine bound on AC was estimated from the difference between the amount of iodine in the impregnating solution (0.01 mol L−1 NH4I) prior to and after the equilibration of AC. The concentration of iodine in the impregnating solution was determined using ICP-MS after filtration through a 0.2-µm filter [29,30].
2.2. Preparation of Activated Carbon Traps
AC traps were prepared by filling a known amount (150–200 mg) of commercially available iodinated AC (AIC-500; APEX Instruments: Fuquay-Varina, NC, USA) or in-house prepared impregnated iodinated AC into a pre-cleaned quartz tube and fixed using a quartz wool. Quartz tube was pre-cleaned by washing with Milli-Q water, drying at room temperature and heating at 700 °C for several minutes in an oven. Quartz wool was used as a stopper to fix the position of the impregnated AC within the quartz tube.
2.3. Loading of Mercury on Activated Carbon Traps
Mercury was loaded on the impregnated AC traps and analyzed after thermal decomposition/combustion using AAS determination. Elemental mercury (Hg0) was produced by the reduction of a known amount of the reference material NIST 3133 using tin(II) chloride solution (2 mL of 10% SnCl2 (w/v; for analysis, Merck, Darmstadt, Germany) in 10% HCl (v/v; for analysis, Merck, Darmstadt, Germany)). Produced elemental mercury was loaded onto the carbon trap (trap A, Scheme 2) by purging for 10 min using an airflow of 4 L min−1. The amount of SnCl2 in 10% HCl (v/v), the volume of this solution and the purging time were previously optimized to achieve quantitative reduction of Hg(II) from the aqueous solution. A high pumping rate (4 L min−1) was used to eliminate the possibility of water vapor condensation in the analytical cell of the AAS detector (Scheme 2) [11].
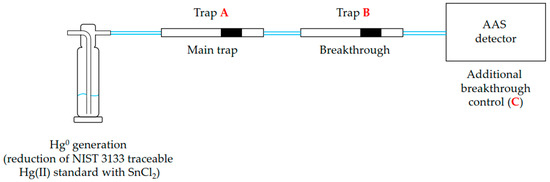
Scheme 2.
General scheme of Hg0 generation via reduction of NIST 3133-traceable Hg(II) standard solution with SnCl2, capturing of produced Hg0 on activated carbon trap (A), and checking possible breakthrough using additional activated carbon trap (B) and additional breakthrough control (C, AAS detector).
To verify whether mercury had been quantitatively trapped on the trap A, an additional trap (trap B, Scheme 2) was placed on-line after the trap A. Furthermore, an atomic absorbance spectrometer (AAS; Lumex, Lumex Scientific, St. Petersburg, Russia) was occasionally connected to the exit of the trap B and served as an additional breakthrough control (C, Scheme 2). The purging solution was always re-purged to check the purging efficiency (i.e., whether Hg was quantitatively purged out of solution). The amount of mercury present in the purging solution was almost always below detection limit of the AAS detector (at most 0.26% of the original Hg amount).
Using the described approach, 10–2000 ng of Hg0 was purged onto AC traps. We set the upper limit to 2000 ng because for the determination of Hg in atmospheric samples from pristine and moderately contaminated areas, this amount is great enough for reliable calibration. In addition, this value corresponds to the upper end of the reported working range (2000 ng of Hg on 0.2 g of AC equals to 10,000 ng g−1). The reported working range for solid samples is from 0.5–10,000 ng g−1 (when using up to 0.5 g of sample) [11].
2.4. Determination of Mercury on Activated Carbon Traps
The determination of mercury on iodinated AC traps was performed using an AAS detector (Lumex mercury analyzer RA-915M) after thermal desorption at 700 °C in a Lumex PYRO-915+ thermal decomposition attachment (Lumex Scientific, St. Petersburg, Russia) [11]. Instrumental limit of detection (LOD) was 0.3 ng (based on three times the standard deviation of 10 system blanks).
Desorption of Hg from the AC was achieved by heating the sample in a Lumex PYRO-915+ thermal decomposition attachment after the quantitative transfer of the carbon sample and the quartz wool from the trap to an analytical quartz boat. Both iodinated AC traps (A and B; Scheme 2) were measured in the same manner. The detector’s response for mercury at different concentration levels was corrected for the signal obtained in the procedural blank, i.e., in the AC trap that was purged with 0 ng of Hg.
To determine the exact concentration or the amount of mercury present on the carbon trap, the analytical system must be properly calibrated. Therefore, we compared the instrument’s response for Hg in the carbon traps obtained by the purging of the reduced NIST 3133 standard with the instrument’s response for Hg obtained by the decomposition of the directly spiked NIST 3133 standard onto the AC sample. An aliquot (10–30 µL) of the diluted NIST 3133 standard with a known concentration was directly spiked onto the AC sample in the quartz boat, thermally decomposed/combusted at 700 °C using a Lumex PYRO-915+ thermal decomposition attachment and determined using an AAS detector Lumex mercury analyzer RA-915M. The detector’s response for mercury at each concentration level was corrected for the signal obtained in the procedural blank, i.e., in the AC trap that was spiked with a Hg-free solution.
The statistical differences between the detector’s response for Hg signal in impregnated AC, obtained by purging of Hg0 and spiking of Hg(II), were examined using paired t-test (SigmaPlot, version 12.0, Systat Software, Erkrath, Germany).
2.5. Determination of Mercury in Real Sample Using Impregnated Activated Carbon Traps—Proof of Concept
To assess the validity of the used approach in the determination of mercury, atmospheric mercury concentration in a real sample was determined using impregnated AC traps (AIC-500). An atmospheric sample with high Hg concentration was used due to the relatively high amount of mercury (minimum 50 ng) that is required for the determination of Hg with a high degree of accuracy and precision using the described method. Therefore, we collected atmospheric samples from a contaminated indoor site containing 68.2 ± 0.95 ng m−3 of Hg (average ± standard deviation of individual measurements at 10-min intervals). This concentration was determined separately using the Lumex mercury analyzer RA-915M (continuous mode) that was running during the collection of Hg on AC traps. Atmospheric mercury was loaded onto the impregnated AC trap by purging for 240 min through a soda lime trap (to remove humidity) at an airflow of 3.3 L min−1. The experiment was performed in two replicates. The amount of Hg on AC traps was determined as described above. Accuracy of the method was tested by the determination of Hg in reference material ERM EF412 (brown coal; certified value 70.0 ± 11.0 ng g−1) [31].
2.6. Thermal Fractionation of Mercury on Activated Carbon Traps
Thermal fractionation is a simple method for the estimation of individual mercury compounds released with increasing temperature. A sample of impregnated AC loaded with a known amount of Hg was transferred to a quartz boat and subjected to a heat gradient of 10 °C min−1 (from room temperature to 800 °C) under an argon flow of 0.2 L min−1. The released mercury compounds were converted to elemental mercury using a Lumex PYRO-915+ thermal decomposition attachment at 700 °C and detected using an AAS detector (Lumex mercury analyzer RA-915M). The AAS signal was obtained using Rapid software (version 1.00.442, Lumex Scientific, St. Petersburg, Russia) at 1 s resolution and was later converted to fractionation thermogram (AAS signal-temperature relation) by applying heat gradient factor [32].
2.7. Peak Deconvulation of Fractionation Thermograms
To assess how many individual Hg compounds are present in the loaded iodinated AC, peak deconvolution of the fractionation thermograms was applied. First, the obtained fractionation thermogram (at 0.17 °C resolution) was smoothened by applying a locally estimated scatterplot smoothing (LOESS) algorithm using SigmaPlot software. The obtained smoothed scatterplot was used to estimate individual constituent peaks. Peak deconvolution was performed by applying the weighted least squares method using Fityk software (version 1.3.1, M. Wojdyr, Warsaw, Poland) and assuming log-normal distribution of data [33].
3. Results and Discussion
3.1. Comparison of the Detectors Response for Mercury on Activated Carbon Traps Using Two Methods
To estimate whether direct spiking of NIST 3133-treaceable Hg standards on AC traps can be used for the calibration of the system, we compared the detector’s response for mercury that was spiked on a trap against the detector’s response for mercury purged on carbon traps (sum of Hg amount present on traps A and B; Scheme 2). The results shown in Figure 1 clearly indicate a linear response between these two methods of loading mercury on iodinated AC traps (AIC-500) at different concentration levels. Furthermore, the slope of the linear regression line is 1.0111 with the R2 value of 0.9986 indicating an almost identical detector’s response for both methods. There were no statistically significant differences in the detector’s response for the Hg signal in impregnated AC obtained by the purging of Hg0 and the spiking of Hg(II) (p = 0.724; paired t-test).

Figure 1.
Linear dependence of the detector’s signal for Hg on activated carbon traps (AIC-500) obtained by two loading methods (purging onto carbon traps and spiking on activated carbon). a.u.—arbitrary units.
These results indicate that mercury that is purged from the atmospheric sample on the AC trap can be easily determined using combustion, while the analytical system can be calibrated by the simple spiking of the AC using the appropriate dilution of the reference standard material NIST 3133. Direct spiking of NIST 3133-treaceable mercury standard solution on carbon can, therefore, be used for the traceable calibration of analytical systems for the determination of mercury purged on carbon traps.
3.2. Comparison of Hg Adsorption on Different Impregnated Activated Carbon Traps
To test the robustness and versatility of the method, we tested different iodinated AC traps. The results from the adsorption and breakthrough on AIC-500 carbon traps are presented alongside the results from other iodinated AC traps to facilitate data comparison (Table 1). To test whether other impregnated AC traps behave in the similar manner, we prepared several in-house impregnated AC traps. The detector’s response for mercury purged to different impregnated AC traps agrees with the signal obtained by the direct spiking of the standard reference material at 1000 ng level. We determined which traps and corresponding impregnating solutions are the best for achieving quantitative mercury trapping and which impregnated AC traps show the lowest breakthrough of mercury at the 1000 ng level. The comparison of the results for different impregnated AC traps is presented in Table 1.

Table 1.
Performance of commercially available and in-house prepared activated carbon traps. All results refer to 1000 ng of purged/spiked Hg.
As seen in Table 1, commercially available AC (AIC-500) and in-house prepared AC impregnated with 0.01 mol L−1 NH4I solution show the best performance. Both traps show quantitative capturing of mercury at 1000 ng level and exceptionally low breakthrough, as seen by the amount of mercury adsorbed on trap B. It is important to note that the additional breakthrough control (C) was always within the noise of the AAS detector. In addition, the amount of mercury present in the purging solution was always up to 0.26% of the original Hg amount present in the purging vessel (impinger) indicating that mercury was completely purged out onto the AC trap. Due to the quantitative recovery of the Hg purged to the carbon traps relative to spiking, we presume that spiking can be used as a reliable calibration method for the determination of Hg on impregnated AC traps.
We decided to use only AIC-500 traps for the determination of mercury in the real atmospheric sample and for the assessment of linearity (Figure 1) due to slightly better results compared to in-house prepared AC traps (better repeatability on trap A and the lowest breakthrough on trap B (Table 1)). We presume that the calibration is also linear up to 1000 ng when using in-house prepared AC treated with 0.01 mol L−1 NH4I and 0.03 mol L−1 NH4I. This assumption is based on the fact that AAS signals for AIC-500 and mentioned AC traps are similar at the 1000 ng level (within ±3.33% of the average signal for AIC-500). However, these linearities were not tested, as explained above.
High amounts of iodine seem to affect Hg determinations, as seen by low Hg recoveries on AC treated with 0.10 mol L−1 (Table 1). This might be due to interferences between mercury and iodine absorption lines (254 and 256 nm, respectively) [34]. In cases when complex matrixes are analyzed, strong background absorption arises due to the production of large amounts of smoke and interference radicals. The observed strong background absorption cannot be corrected using the inherent selectivity of the Zeeman atomic absorption spectrometer [11]. Low iodine amounts show quantitative trapping and do not considerably affect Hg determinations by the AAS detector. The amount of iodine bound on AC treated with impregnating solution with the lowest iodine content (0.01 mol L−1 NH4I) was estimated from the difference between the amount of iodine in this solution prior to and after the equilibration of AC. The concentration of iodine in the impregnating solution was lowered from 0.01 mol L−1 prior to equilibration to 0.0078 mol L−1 after equilibration. This change corresponds to the adsorption of 0.21 mmol of iodide per gram of AC (i.e., mass fraction of iodide 2.69%). Comparison of this value with the literature is rather difficult: commercial AC traps do not disclose the amount of the bound halogen of activated carbon, while scientific articles usually do not report the content. The most comprehensive review of sorbents for mercury removal from flue gas [16] reported iodinated AC that contained 3.5% of iodine, which is slightly higher than the iodine content in in-house prepared iodinated AC. Higher iodine content on AC might be useful for the removal of mercury from flue gas, but it is not appropriate for the determination of low atmospheric Hg concentrations, as described above.
Even though iodine in iodinated AC might cause problems during Hg determinations, it also creates much more stable Hg complexes compared to other halogens. Therefore, it is important to balance between analytical performance and the stability of Hg complexes when using iodinated AC traps. AC treated with impregnating solution containing the lowest iodine content (0.01 mol L−1 NH4I) seems to be right at the balance, as demonstrated by good analytical performance at 1000 ng level (Table 1). Even though this balancing would not be required when using chlorinated or brominated ACs, we intentionally wanted to test this approach using NH4I due to the specific composition of the reaction product (Millon’s iodide).
Gaseous Hg(II) can be captured on AC traps [20], but it cannot be separately determined as the whole AC from the trap is quantitatively transferred to an analytical quartz boat and subjected to combustion. Hg(II) can be only determined using speciation traps (solid KCl + AC). In addition, the loading of traceable amounts of gaseous Hg(II) at low atmospheric concentrations is still under development [21].
3.3. Determination of Mercury in Real Atmospheric Sample—Proof of Concept
An atmospheric sample from contaminated indoor site containing 68.2 ± 0.95 ng m−3 of Hg was collected on the AIC-500 activated carbon trap. Based on this concentration, purging time and airflow through the trap, it was calculated that 54.0 ± 0.99 ng of Hg was collected on the AC trap (average ± standard deviation of two parallel measurements). The response of the AAS detector was compared with the corresponding response for the NIST 3133 spike onto AC (50 ng of Hg). Following the subtraction of corresponding blanks, the AC trap contained 53.2 ± 3.05 ng of Hg. The accuracy of Hg determinations was tested using reference material ERM EF412 (brown coal). The obtained value (69.7 ± 2.86 ng g−1; n = 4) was within the uncertainty of its certified value (70.0 ± 11.0 ng g−1).
Although the obtained average value of Hg content in the real atmospheric sample is slightly lower than the calculated amount of Hg, it is still within the standard deviation of measurements. The possible reason for this observation is Hg adsorption on soda lime. Although normally this never represents an issue, long sampling time (240 min) might have caused the adsorption of a significant amount of water vapor from the air, and possibly some Hg might have been dissolved in this water. This is especially important for gaseous oxidized mercury, which is readily soluble in water [15]. The relatively high standard deviation of the determined Hg on the AC trap can be attributed to the variability in the spiking volume (10 µL), which should be as smallest as possible.
The determination of Hg using AC traps can be readily used for atmospheric samples containing elevated Hg concentrations with a high degree of accuracy and precision using the described method. However, for samples with ambient atmospheric concentrations (about 1.5 ng m−3) [35,36,37,38], longer purging time or higher airflows are required. This might cause unwanted Hg transformations during sample collection or the breakthrough of mercury (especially in case of high airflow). These issues can be overcome by sampling lower amounts of mercury and/or using a more sensitive detector (e.g., atomic fluorescence spectrometer). However, the level cannot be extremely low, due to the relatively high blanks of impregnated AC (Table 1).
In principle, this method could be applied for the characterization of Hg transformations in atmospheric samples using the stable isotope fractionation approach. The method is limited by the relatively high LOD; for reliable determination of stable isotope ratios, blanks should ideally account for less than 2% of the collected amount of atmospheric Hg [39,40]. Furthermore, desorption of mercury from the AC traps must be quantitative to avoid Hg fractionation on the AC. This is readily achieved using combustion at a high temperature. Released Hg must be quantitatively trapped in a solution that can completely oxidize Hg0 to Hg(II) (e.g., solution of potassium permanganate or mixture of nitric and hydrochloric acid) [12]. Exit from the Lumex PYRO-915+ thermal decomposition attachment could be connected to this trapping solution to quantitatively collect combustion products. As the calibration is performed immediately after the determination of Hg in samples, a combusted NIST 3133 standard could be trapped in this solution and used directly as a bracketing standard.
3.4. Thermal Fractionation of Hg on Impregnated Activated Carbon
Impregnation of AC using NH4I solutions and not with commonly used KI solutions was performed because the former readily reacts with Hg and forms the insoluble iodide of the Millon’s base, [Hg2N]I·H2O. Its literature decomposition temperature of 350–400 °C is close to the decomposition temperature (about 370 °C) obtained using thermal fractionation [41]. The obtained thermograms for AIC-500 and in-house prepared iodinated AC (0.01 mol L−1 NH4I) are presented in Figure 2. In-house prepared iodinated AC has a higher maximum temperature for the highest peak. However, it also starts to release mercury at a lower temperature (about 150 °C) compared to AIC-500.
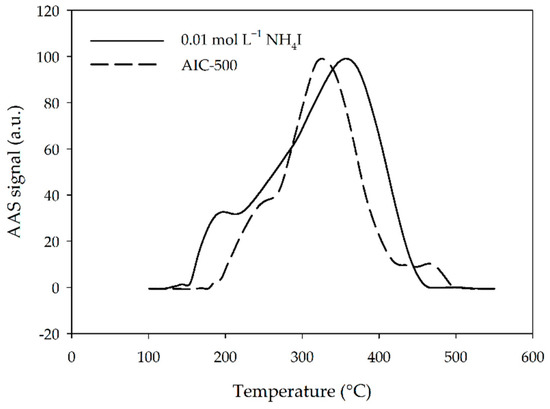
Figure 2.
Fractionation thermograms for AIC-500 and in-house prepared iodinated activated carbon (0.01 mol L−1 NH4I). Parts of the thermogram below 100 °C and above 550 °C were removed for clarity as the values for AAS signal were about zero. a.u.—arbitrary units.
To assess how many peaks (i.e., Hg compounds) are present under each curve in Figure 2, peak deconvolution was performed using the weighted least squares method [33]. Assuming log-normal distribution of the constituent peaks, we identified that each thermogram is comprised of three individual peaks (Figure 3). However, without direct comparison with pure Hg standards on impregnated AC, it is not possible to exactly determine the exact composition of the three individual mercury compounds (not in the scope of this work).

Figure 3.
Peak deconvolution of fractionation thermograms for (a) AIC-500 and (b) in-house prepared iodinated activated carbon (0.01 mol L−1 NH4I) assuming log-normal distribution of constituent peaks. Black dots represent smoothed experimental points (weighted least squares method), red curves deconvoluted peaks and the black curve the summarized model (sum of red curves). Parts of the thermograms below 100 °C and above 550 °C were removed for clarity as the values for AAS signal were about zero. a.u.—arbitrary units.
Based on the literature data, we assume that the Hg bound to in-house impregnated iodinated AC is in form of Millon’s base due to the similar theoretical decomposition temperature. The iodide of Millon’s base decomposes on heating; decomposition starts at a temperature of 160 °C [41], which is the temperature at which the first peak starts to appear during the thermal fractionation of in-house prepared AC (Figure 3b). The second deconvoluted peak at 280 °C might be attributed to the decomposition of an unidentified intermediate degradation compound, as the structure of Millon’s base is polymeric in nature and the chemical formula [Hg2N]+ does not represent an exact ionic species. Millon’s base is composed of a silica-like network of N and Hg a in four- and two-coordination, respectively, with anion and water (if present) in the interstitial spaces [42]. Furthermore, the decomposition is not rapid until about 350–400 °C [41], where we have observed the third peak. Slow decomposition towards the maximum is one of the reasons why we applied the log-normal distribution of the constituent peaks during the peak deconvolution analysis.
Most binary transition-metal–nitrogen compounds are highly endothermic compounds, including Hg–N compounds [43]. The possible explosive decomposition of these nitrogen-rich compounds is due to their extremely low energy barriers [44]. The decomposition of the Millon’s iodide is similar to the thermal decomposition of other salts of Millon’s base. [Hg2N]NO3 has a similar decomposition temperature (380 °C) [45], while [Hg2N]N3 exhibits a smooth decomposition accompanied by the release of molecular nitrogen and Hg0 [43]. Nevertheless, the decomposition temperature of [Hg2N]I on activated carbon should be taken with a high degree of reservation, because it was recently shown that matrix effect greatly influences the decomposition of various Hg compounds by shifting the temperature of Hg released from the sample [32].
Although Hg is purged onto iodinated AC traps as Hg0, it is most probably oxidized to Hg(II) on the surface. The dynamic adsorption of mercury on iodinated AC traps suggests complex mechanisms for the adsorption [46]. Physical adsorption is probably the first step in the removal of mercury by both virgin and impregnated ACs [16]. Recently, it was demonstrated that purged Hg0 is readily oxidized to Hg(II) on the brominated AC surface at 30 °C and 140 °C, indicating that chemisorption is the likely adsorption mechanism of Hg [47]. As Hg0 removal efficiency increases with adsorption temperature, it is assumed that Hg0 vapors chemically react with impregnated iodine on the AC surface (rates of chemisorption increase with the rise in temperature) [17]. Desorption of mercury from spent ACs, impregnated with both iodine and KI, suggests that Hg0 is oxidized by elemental iodine and trapped in the form of K2HgI4 and KHgI3 [48]. We presume that iodine also acts as an oxidant in in-house impregnated iodinated AC, as iodide has a low standard reduction potential (+0.54 V) and might be oxidized to elemental iodine by oxygen from the moist air. Consequently, Hg0 is probably oxidized to HgI42− and HgI3−, but in contrast to KI, the presence of ammonium ion converts these Hg-iodide complexes to Millon’s iodide, [Hg2N]I. Gaseous Hg(II) can also be trapped on iodinated AC and is probably first converted to HgI2, then to Hg-iodide complexes, and finally to [Hg2N]I. The formation of HgI2 enables impregnated AC to remove even more elemental mercury [16]. We presume that GOM and GEM are readily converted to Hg-iodide complexes on the AC surface and later fixed in the form of Millon’s iodide, thus providing additional active sites for further adsorption of Hg on activated carbon.
4. Conclusions
AC can be used for quantitative pre-collection of atmospheric mercury. The determination of mercury bound to AC can be easily achieved using combustion coupled with atomic absorption spectrometry. Comparison of different ACs demonstrated that commercial iodinated AC and activated carbon treated with 0.01 mol L−1 NH4I show quantitative capturing of Hg0 (99.3–100%) while having the lowest breakthrough (<LOD − 0.27%). AC containing a greater amount of iodine shows lower Hg recoveries, probably due to interferences during analysis using atomic absorption. The identical response of the AAS detector for two Hg loading methods (purging of Hg0 and spiking SRM solution) in a wide concentration range (10–2000 ng) indicated that the spiking of SRM on AC can be readily used for the calibration of the analytical system used for the determination of atmospheric mercury. The concentration of Hg in a real atmospheric sample collected on an iodinated AC trap was determined using spiking calibration and was traceable to SRM NIST 3133. The accuracy of the method was confirmed by a good agreement between the measured Hg content in a reference material ERM EF412 and its certified value. The use of ammonium iodide in impregnating solutions, probably converted Hg on AC to the iodide of Millon’s base, as the positions of deconvoluted peaks from fractionation thermogram agreed well with the literature values.
Author Contributions
Conceptualization, I.Ž. and M.H.; methodology, I.Ž. and M.J.; validation, I.Ž., S.B., M.J. and J.K.; formal analysis, I.Ž., M.J. and S.B.; data curation, I.Ž. and J.K.; writing—original draft preparation, I.Ž.; writing—review and editing, S.B., J.K. and M.H.; visualization, I.Ž.; supervision, M.H.; project administration, M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EMPIR, grant number 16ENV01 (MercOx); Slovenian Research Agency, grant number P1-0143.
Acknowledgments
The authors would like to thank Warren T. Corns for donation in kind of iodinated activated carbon.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Azevedo, B.; Barros Furieri, L.; Peçanha, F.M.; Wiggers, G.A.; Frizera Vassallo, P.; Ronacher Simões, M.; Fiorim, J.; Rossi de Batista, P.; Fioresi, M.; Rossoni, L.; et al. Toxic Effects of Mercury on the Cardiovascular and Central Nervous Systems. J. Biomed. Biotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Amos, H.M.; Jacob, D.J.; Streets, D.G.; Sunderland, E.M. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Biogeochem. Cycles 2013, 27, 410–421. [Google Scholar] [CrossRef]
- Mason, R.P.; Choi, A.L.; Fitzgerald, W.F.; Hammerschmidt, C.R.; Lamborg, C.H.; Soerensen, A.L.; Sunderland, E.M. Mercury biogeochemical cycling in the ocean and policy implications. Environ. Res. 2012, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Ariya, P.A.; Amyot, M.; Dastoor, A.; Deeds, D.; Feinberg, A.; Kos, G.; Poulain, A.; Ryjkov, A.; Semeniuk, K.; Subir, M.; et al. Mercury Physicochemical and Biogeochemical Transformation in the Atmosphere and at Atmospheric Interfaces: A Review and Future Directions. Chem. Rev. 2015, 115, 3760–3802. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.S.; Evers, D.C.; Bank, M.S.; Hammerschmidt, C.R.; Pierce, A.; Basu, N.; Blum, J.; Bustamante, P.; Chen, C.; Driscoll, C.T.; et al. Importance of Integration and Implementation of Emerging and Future Mercury Research into the Minamata Convention. Environ. Sci. Technol. 2016, 50, 2767–2770. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Bank, M.S. Mercury in the Environment: Pattern and Process; University of California Press: Los Angeles, CA, USA, 2012; ISBN 9780520271630. [Google Scholar]
- Ren, X.; Luke, W.T.; Kelley, P.; Cohen, M.D.; Olson, M.L.; Walker, J.; Cole, R.; Archer, M.; Artz, R.; Stein, A.A. Long-Term Observations of Atmospheric Speciated Mercury at a Coastal Site in the Northern Gulf of Mexico during 2007–2018. Atmosphere 2020, 11, 268. [Google Scholar] [CrossRef]
- Sprovieri, F.; Hedgecock, I.M.; Pirrone, N. An investigation of the origins of reactive gaseous mercury in the Mediterranean marine boundary layer. Atmos. Chem. Phys. 2010, 10, 3985–3997. [Google Scholar] [CrossRef]
- Sholupov, S.; Pogarev, S.; Ryzhov, V.; Mashyanov, N.; Stroganov, A. Zeeman atomic absorption spectrometer RA-915+ for direct determination of mercury in air and complex matrix samples. Fuel Process. Technol. 2004, 85, 473–485. [Google Scholar] [CrossRef]
- Fu, X.; Heimbürger, L.E.; Sonke, J.E. Collection of atmospheric gaseous mercury for stable isotope analysis using iodine-and chlorine-impregnated activated carbon traps. J. Anal. At. Spectrom. 2014, 29, 841–852. [Google Scholar] [CrossRef]
- Bérail, S.; Cavalheiro, J.; Tessier, E.; Barre, J.P.G.; Pedrero, Z.; Donard, O.F.X.; Amouroux, D. Determination of total Hg isotopic composition at ultra-trace levels by on line cold vapor generation and dual gold-amalgamation coupled to MC-ICP-MS. J. Anal. At. Spectrom. 2017, 32, 373–384. [Google Scholar] [CrossRef]
- Horvat, M. Determination of Mercury and its Compounds in Water, Sediment, Soil and Biological Samples. In Dynamics of Mercury Pollution on Regional and Global Scales; Springer: New York, NY, USA, 2005; pp. 153–190. [Google Scholar]
- Huang, J.; Lyman, S.N.; Hartman, J.S.; Gustin, M.S. A review of passive sampling systems for ambient air mercury measurements. Environ. Sci. Process. Impacts 2014, 16, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Novel Sorbents for Mercury Removal from Flue Gas. Ind. Eng. Chem. Res. 2000, 39, 1020–1029. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, Y.-C.; Jurng, J.; Lee, T.G. Removal of gas-phase elemental mercury by iodine- and chlorine-impregnated activated carbons. Atmos. Environ. 2004, 38, 4887–4893. [Google Scholar] [CrossRef]
- Musmarra, D.; Karatza, D.; Lancia, A.; Prisciandaro, M.; Di Celso, G.M. A comparison among different sorbents for mercury adsorption from flue gas. Chem. Eng. Trans. 2015, 43, 2461–2466. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yue, B.-Y.; Wu, X.-W.; Liu, Q.; Jiao, F.-P.; Jiang, X.-Y.; Chen, X.-Q. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. 2016, 23, 5056–5076. [Google Scholar] [CrossRef]
- US EPA. Method 30B—Mercury Sorbent Trap Procedure. Available online: https://www.epa.gov/sites/production/files/2017-08/documents/method_30b.pdf (accessed on 3 May 2018).
- Saxholm, S.; Rajamäki, T.; Hämäläinen, J.; Hildén, P. Dynamic calibration method for reactive gases. Meas. Sci. Technol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Dumarey, R.; Temmerman, E.; Adams, R.; Hoste, J. The accuracy of the vapour-injection calibration method for the determination of mercury by amalgamation/cold-vapour atomic absorption spectrometry. Anal. Chim. Acta 1985, 170, 337–340. [Google Scholar] [CrossRef]
- Dumarey, R.; Brown, R.J.C.; Corns, W.T.; Brown, A.S.; Stockwell, P.B. Elemental mercury vapour in air: The origins and validation of the ‘Dumarey equation’ describing the mass concentration at saturation. Accredit. Qual. Assur. 2010, 15, 409–414. [Google Scholar] [CrossRef]
- Huber, M.L.; Laesecke, A.; Friend, D.G. Correlation for the Vapor Pressure of Mercury. Ind. Eng. Chem. Res. 2006, 45, 7351–7361. [Google Scholar] [CrossRef]
- Gustin, M.S.; Huang, J.; Miller, M.B.; Peterson, C.; Jaffe, D.A.; Ambrose, J.; Finley, B.D.; Lyman, S.N.; Call, K.; Talbot, R.; et al. Do we understand what the mercury speciation instruments are actually measuring? Results of RAMIX. Environ. Sci. Technol. 2013, 47, 7295–7306. [Google Scholar] [CrossRef] [PubMed]
- Quétel, C.R.; Zampella, M.; Brown, R.J.C. Temperature dependence of Hg vapour mass concentration at saturation in air: New SI traceable results between 15 and 30°C. TrAC—Trends Anal. Chem. 2016, 85, 81–88. [Google Scholar] [CrossRef]
- US EPA. Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. Available online: https://www.epa.gov/sites/production/files/2015-08/documents/method_1631e_2002.pdf (accessed on 2 February 2020).
- Berisha, S.; Živković, I.; Kotnik, J.; Mlakar, T.L.; Horvat, M. Quantification of total mercury in samples from cement production processing with thermal decomposition coupled with AAS. Accredit. Qual. Assur. 2020, 25, 233–242. [Google Scholar] [CrossRef]
- Takaku, Y.; Shimamura, T.; Masuda, K.; Igarashi, Y. Iodine Determination in Natural and Tap Water Using Inductively Coupled Plasma Mass Spectrometry. Anal. Sci. 1995, 11, 823–827. [Google Scholar] [CrossRef][Green Version]
- Jerše, A.; Jaćimović, R.; Maršić, N.K.; Germ, M.; Šircelj, H.; Stibilj, V. Determination of iodine in plants by ICP-MS after alkaline microwave extraction. Microchem. J. 2018, 137, 355–362. [Google Scholar] [CrossRef]
- ERM-EF412 Brown Coal (GCV, Ash, Volatile Matter, Elements). Available online: https://crm.jrc.ec.europa.eu/p/40455/40467/By-material-matrix/Fuels/ERM-EF412-BROWN-COAL-GCV-Ash-Volatile-Matter-Elements/ERM-EF412 (accessed on 1 May 2020).
- Sedlar, M.; Pavlin, M.; Popovič, A.; Horvat, M. Temperature stability of mercury compounds in solid substrates. Open Chem. 2014, 13, 404–419. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Barnett, N.W.; Kirkbright, G.F. Electrothermal vaporisation sample introduction into an atmospheric pressure helium microwave-induced plasma for the determination of iodine in hydrochloric acid. J. Anal. At. Spectrom. 1986, 1, 337. [Google Scholar] [CrossRef]
- Cizdziel, J.; Jiang, Y.; Nallamothu, D.; Brewer, J.; Gao, Z. Air/Surface Exchange of Gaseous Elemental Mercury at Different Landscapes in Mississippi, USA. Atmosphere 2019, 10, 538. [Google Scholar] [CrossRef]
- Gratz, L.; Eckley, C.; Schwantes, S.; Mattson, E. Ambient Mercury Observations near a Coal-Fired Power Plant in a Western U.S. Urban Area. Atmosphere 2019, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Slemr, F.; Brunke, E.-G.; Ebinghaus, R.; Temme, C.; Munthe, J.; Wängberg, I.; Schroeder, W.; Steffen, A.; Berg, T. Worldwide trend of atmospheric mercury since 1977. Geophys. Res. Lett. 2003, 30, 1–4. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Ebinghaus, R.; Kock, H.; Dommergue, A. A review of worldwide atmospheric mercury measurements. Atmos. Chem. Phys. 2010, 10, 8245–8265. [Google Scholar] [CrossRef]
- Liu, H.; Yu, B.; Yang, L.; Wang, L.; Fu, J.; Liang, Y.; Bu, D.; Yin, Y.; Hu, L.; Shi, J.; et al. Terrestrial mercury transformation in the Tibetan Plateau: New evidence from stable isotopes in upland buzzards. J. Hazard. Mater. 2020, 400, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Reinfelder, J.R.; Fu, P.; Huang, W. Variation in the mercury concentration and stable isotope composition of atmospheric total suspended particles in Beijing, China. J. Hazard. Mater. 2020, 383, 1–7. [Google Scholar] [CrossRef]
- Weiser, H.B. The Luminescence of the Iodide of Millon’s Base. J. Phys. Chem. 1917, 21, 37–47. [Google Scholar] [CrossRef]
- Urben, P.G. Bretherick’s Handbook of Reactive Chemical Hazards, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Lund, H.; Oeckler, O.; Schröder, T.; Schulz, A.; Villinger, A. Mercury Azides and the Azide of Millon’s Base. Angew. Chemie Int. Ed. 2013, 52, 10900–10904. [Google Scholar] [CrossRef]
- Tornieporth-Oetting, I.C.; Klapötke, T.M. Covalent Inorganic Azides. Angew. Chemie Int. Ed. 1995, 34, 511–520. [Google Scholar] [CrossRef]
- Nockemann, P.; Meyer, G. Formation of NH4[Hg3(NH)2](NO3)3 and Transformation to [Hg2N](NO3). Z. Anorg. Allg. Chem. 2002, 628, 2709–2714. [Google Scholar] [CrossRef]
- Matsumura, Y. Adsorption of mercury vapor on the surface of activated carbons modified by oxidation or iodization. Atmos. Environ. 1974, 8, 1321–1327. [Google Scholar] [CrossRef]
- Sasmaz, E.; Kirchofer, A.; Jew, A.D.; Saha, A.; Abram, D.; Jaramillo, T.F.; Wilcox, J. Mercury chemistry on brominated activated carbon. Fuel 2012, 99, 188–196. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Sorbents for Mercury Removal from Flue Gas; U.S. Department of Energy: Pittsburgh, PA, USA, 1998.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).