1. Introduction
Ambient air pollution, especially particulate matter, is among the biggest environmental health risks globally [
1,
2,
3,
4]. Exposure to air pollution is linked with non-communicable diseases such as cardiovascular and respiratory diseases, and cancers [
5]. While different assessments recognize the importance of ambient air pollution as a health risk, there are substantial differences in the estimated deaths. For instance in the Global Burden of Disease (GBD) study [
2] it was estimated that 2.9 million deaths were related to fine particle (PM
2.5) exposure in 2017, while Burnett et al. (2018) estimated 8.9 million deaths in 2015 [
2]. These disparities are mainly due to methodological differences [
6].
A review by Papadogeorgou et al. (2019) [
7] related to health effects of fine particles at low exposure levels report statistically significant associations between long-term fine particle exposure and total mortality as well as mortality due to cardiovascular diseases and respiratory diseases. In the Nordic countries, annual ambient air pollution levels are relatively low, and generally meet the EU guideline values. Regardless of the low exposures, ambient air pollution remains a significant environmental risk factor in Nordic countries. For instance, in Sweden and Finland, 7600 and 2000 premature deaths, respectively, were attributable to air pollution in 2015 [
8,
9]. Premature deaths attributable to air pollution are leading to considerable economic costs. In four Nordic countries (Denmark, Sweden, Finland and Norway), air pollution related deaths were estimated to lead to a total cost of 7 billion euros in 2015 [
10].
In the burden of disease assessments, population exposures are often linked with health outcomes using relative risks (RR) from epidemiological studies. The relation between increase in exposure and associated increase in risk at low levels of air pollution has often been assumed to be linear [
11]. However, over the past decade supra-linear shapes with steeper risk increase at lower exposure levels have been suggested [
7,
12,
13,
14]. Burnett et al. 2014 [
14] presented integrated exposure-response (IER) functions which combine relative risks from different air pollution sources into non-linear functions. The IER functions include theoretical thresholds (Theoretical Minimum Risk Exposure Level, TMREL) which are assigned as “a uniform distribution with lower/ upper bounds given by the average of the minimum and 5th percentile of outdoor air pollution cohort studies exposure distributions conducted in North America” [
15]. The IER functions have become the main concentration-response (C-R) functions used in the GBD studies for fine particle exposure.
Several tools are developed for estimation of air pollution related health burden. Anenberg et al. (2016) [
16] conducted the first systematic survey of air pollution health risk assessment tools, where they reviewed 17 tools. They concluded that these tools had several common characteristics like similar sources for C-R functions, population data and baseline mortality rates. However, several important differences were reported e.g., related to exposure data sources, format and technical properties.
While Anenberg et al. (2016) [
16] focused more on qualitative comparison of health risk assessment tools; Malmqvist et al. (2018) [
17] also quantified disparities in different scenarios of air pollution levels (cut-off points), compared two different coefficients for mortality and looked at the impact of the study population on the results. The differences were remarkable, for instance, the use of the Beelen et al. (2014) [
18] coefficient for natural-cause mortality (hazard ratio (HR) 1.14 per 10 µg/m
3) instead of Pope et al. (2002) [
19] coefficient for all-cause mortality (HR 1.06 per 10 µg/m
3) doubled the mortality estimates.
In another follow-up study, Beelen et al. (2014) [
18] results were analyzed for sub-regional differences in the C-R relationships between South European, Central and Nordic cohorts [
20]. Even though not statistically significant, the results were consistent with the hypothesis that in the more northern climates buildings are tighter, better insulated, and protect occupants also partly from ambient air pollution.
Recent reports [
21,
22,
23] have increasingly pointed to the need for reviewing the impacts of air pollution in the Nordic countries due to the large uncertainties in estimating the effects at low exposure levels. Currently there is no consensus on which shape of the concentration-response function should be applied and whether a threshold should be used.
The objective of this work is to quantify the disparities in air pollution-related death estimates. We concentrate on low exposure areas where the concentration-response models with thresholds raise questions of their suitability to these areas. The aim of the first part of the work is compare three existing health risk assessment tools to estimate premature deaths attributable to annual ambient PM2.5 and O3 concentrations in 2015 using the same exposure data source in the tools (part 1). The aim of the second part is to quantify the disparities in the attributable death estimates due to concentration-response functions (part 2). In the third part we evaluate the impact of spatial resolution on exposure modelling and further on the estimated deaths (part 3).
4. Discussion
We identified substantial disparities between the attributable death estimates in the Nordic countries. The biggest differences were found in the comparison of five commonly used concentration-response functions, while smaller differences were found between the compared tools as these tools use the same source of concentration-response functions. Additionally, Malmqvist et al. (2018) [
17] found that selection of exposure-response function was causing substantial differences between the nine health impact assessments included in their study.
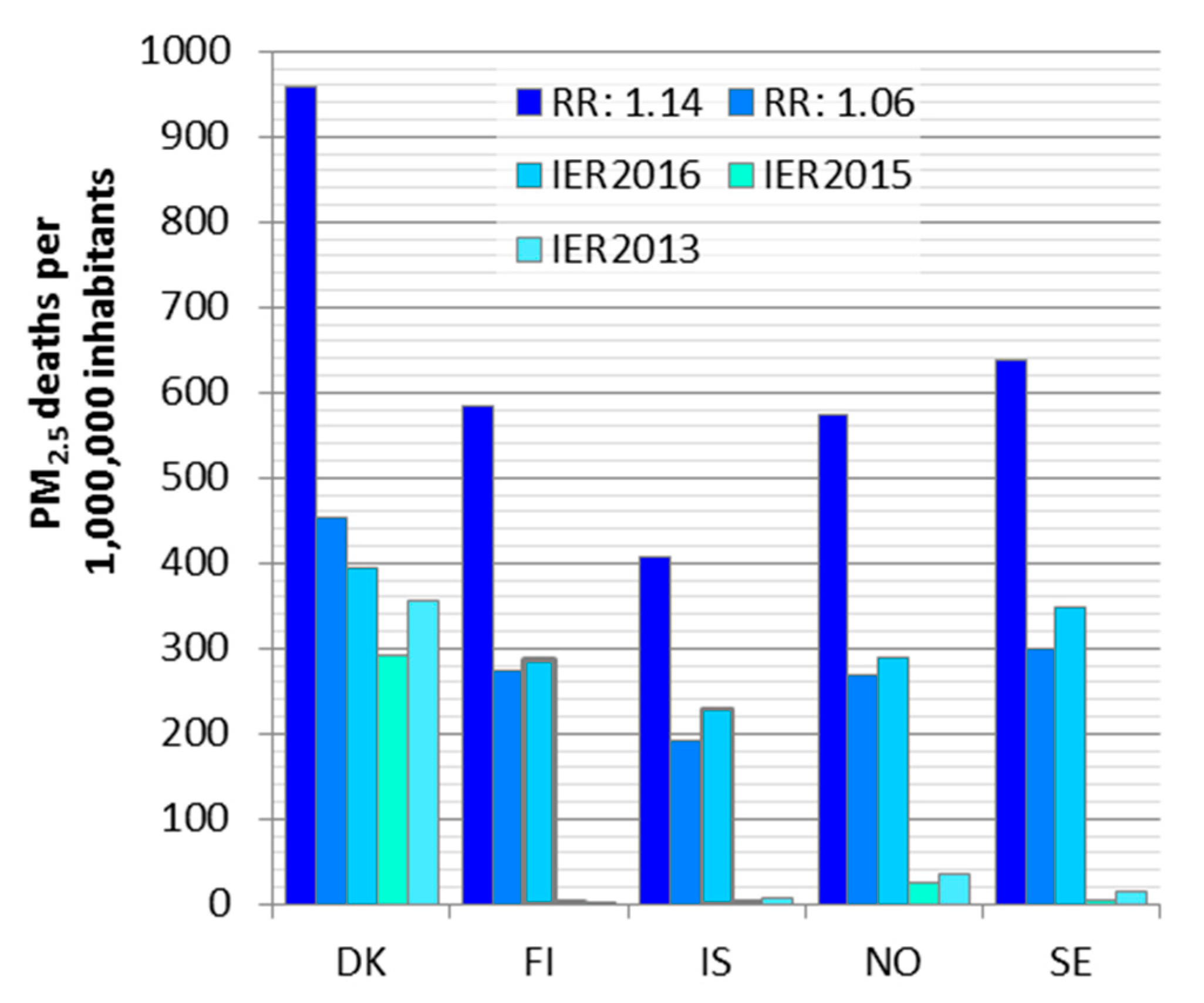
We compared two log-linear functions (RR:1.06 [
47] and RR:1.14 [
18]) and three sets of IER functions (GBD2013, GBD2015 and GBD2016 [
48,
49,
50]). The log-linear functions had over twofold differences in the relative risks and consequently in the estimated deaths. The earlier IER versions seem to underestimate the health impacts in low exposure countries due to the high theoretical threshold. The closer to the theoretical threshold value the exposure is, the larger the differences between the IER versions are. The latest IER version (IER2016, [
50]) included in this comparison led to attributable deaths close to the ones estimated, with a relative risk of 1.062 used in all the tools we compared in this work (ARP, EVA and ISTE). In this version the theoretical threshold was lower than in the previous IER versions.
One limitation of our study is that we used only a point value for population mean exposure without taking into account the exposure distribution in any other tools than EVA. While this might not lead to a significant difference for linear functions without threshold, it can cause remarkable underestimation when using supra-linear IER functions. This can be the case especially when the mean exposure is close to the threshold and exposure distribution is not taken into account.
The differences in the estimated deaths for individual counties were up to 24% between the three tools when compared to tool averages. We identified that the main causes of the differences were baseline mortality data used in the tools. The disparities in the baseline mortality in comparison to national statistics were ranging from −6% to 23%. These differences reflected on the estimation of attributable deaths, and seemed to mostly explain the differences between ISTE and ARP. Another major cause for disparities among the tools was inclusion of acute deaths in addition to chronic ones in EVA. Double counting of chronic and acute mortality is avoided in EVA by subtracting years of life lost (YLL) due to acute deaths from the YLL due to chronic deaths. For PM2.5 EVA had the highest attributable death estimates for all countries, from which acute deaths comprise about 17%. In overall, ISTE had the lowest attributable death estimates as well as the lowest baseline mortality.
All the tools included in this work are suitable for estimating the health risks of air pollution at low exposure levels. EVA modelling tool has one advantage of being closely linked to air quality models and it has a high spatial resolution. In the ISTE tool it is possible to take into account exposure distributions. EVA and ARP tools enable estimation of economic costs in addition to health impacts. AirQ+ tool is readily available for the public and includes non-linear C-R functions in addition to linear ones. The results obtained in this work can be utilized in the tool updating.
The source of exposure data can greatly affect the health impact results, and therefore we aimed to control its impacts by using the same source of exposures for all tools. For example, surface concentrations from different air pollution models, even when they use the same input for the emissions, can lead to a difference in the mortality estimates of up to a factor of three [
31]. This is due to the differences in the models themselves; in particular the representation of gas-phase chemistry and the aerosol schemes, as well as the native spatial resolutions of the models and the meteorological input they use to drive the chemistry. However, the validity of the air pollution models used for health impact assessments should be tested against measurements in order to quantify the uncertainty using the particular model.
The C-R functions applied refer to annual mean concentrations; however, the exposure period which is leading to the health outcomes can be even decades. We assume that the annual concentration reflects the long-term exposure. This approach, however, cannot take into account for instance moving history but the concentrations are based on the home addresses in 2015. In this work the population weighted concentrations apply to the total population and we did not consider different age groups separately. However, it can be assumed that some differences in concentrations between age groups are possible. Urbanization is one trend which can reflect on exposure of different age groups as older people tend to stay in the country-side, while younger move to urban areas. Personal exposure between age groups can also differ due to time activity patterns. However, in this work time activity patterns were not taken into account as the calculations are based on ambient concentrations.
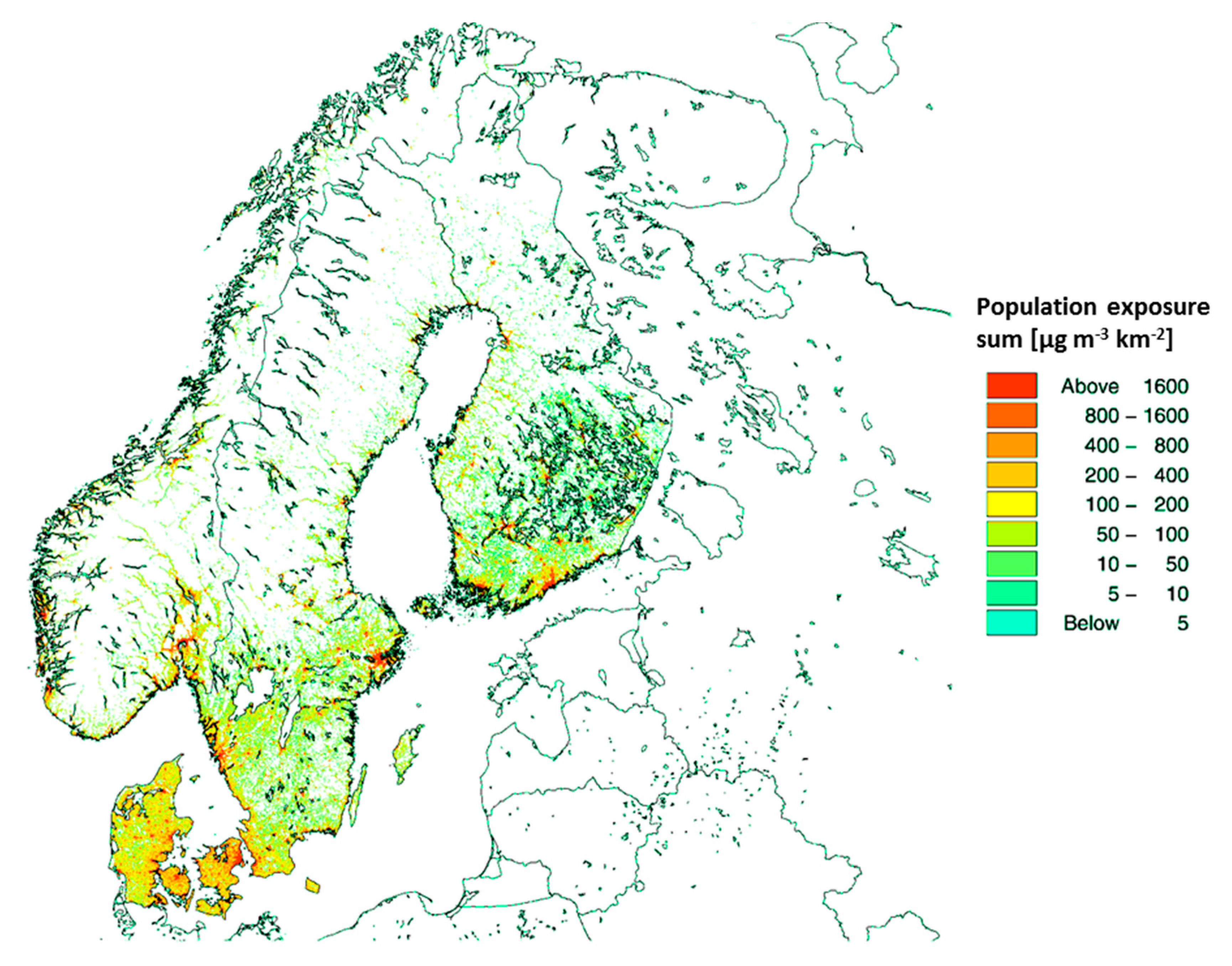
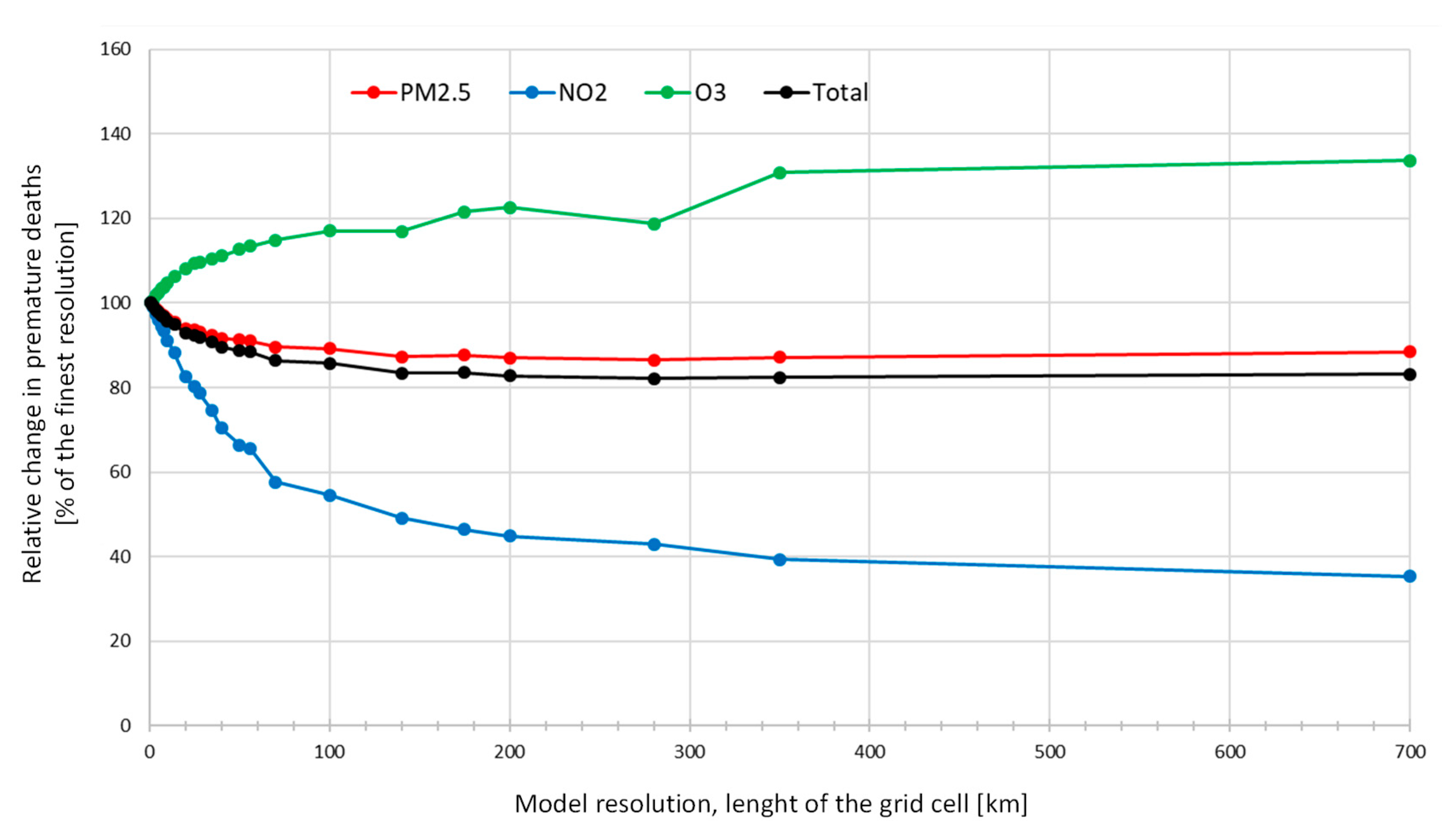
In the sensitivity analysis of exposure resolution, we found that lower spatial resolution leads to a substantial decrease in the estimated attributable deaths for PM2.5 and NO2. For O3 the impact was the reverse. A high spatial resolution is especially important for the locally emitted chemical species such as primary emitted particulate matter and for NO2 and O3 (reverse impact). These pollutants are controlled by relatively fast chemistry (e.g., compared to the chemistry of secondary formed particles) and therefore are characterized by higher local spatial variability. Typically, for these species, higher concentrations are found close to the more densely populated areas, since emissions are located close to the population. For example, particle and NOx emissions from traffic and particles from residential heating typically occur within the more densely populated areas.
Our experiment shows that the spatial resolution has a significant impact on the health impact assessment related to air pollution and that high-resolution modelling is especially important, when the results are used for policy development as an underestimation of 34% of the attributable deaths and related external costs can change political priorities.
Korhonen et al. (2019) [
60] also studied the impacts of spatial resolution of exposure model on deaths attributable to PM
2.5. They estimated the differences caused by spatial resolution on deaths attributable to PM
2.5 exposure by averaging exposure from 1 km × 1 km to 50 km × 50 km resolution. They found that lowering the resolution from the highest to the coarsest led to a decrease in the attributable deaths by 14 % and nearly 90 % for log-linear and IER functions, respectively. Korhonen et al. (2019) applied the IER2013 functions [
14] which have a theoretical threshold close to the Finnish exposure levels leading to large impacts for resolution changes.
5. Conclusions
We compared three available tools to estimate deaths attributable to PM2.5 and O3 exposures in five Nordic countries. Between the tools, the estimated deaths ranged from 8500 to 11,400 for PM2.5, and from 230 to 260 for O3 exposure in the Nordic area. Death rates per million inhabitants were the highest in Denmark and the lowest in Iceland. All the tools were based on concentration-response functions recommended by WHO working group. The variation among the tools was mainly due to differences in the baseline mortality inputs.
Substantially larger differences were found in the comparison of concentration-response functions. At maximum, a ten-fold difference was calculated for the whole Nordic region (1800–18,000 deaths). Differences between estimates for individual countries were even larger, likely due to concentration-response function shapes and in particular, the applied thresholds in combination with point value exposures. The Nordic area with low exposures is especially sensitive to thresholds.
When spatial exposure models are used, sufficiently high resolution is necessary to avoid underestimation of exposures and health effects. This is especially important for air pollutants which are strongly influenced by local emissions and atmospheric chemistry like NO2 and primary particulate matter. For O3 the impact of smaller resolution is reversed due to lower exposures at highly populated urban areas.
On-going epidemiological studies assessing the relationship between air pollution and health effects in the Nordic area will provide valuable inputs for the understanding of the health burden of air pollution in low exposure areas. Nevertheless, due to challenges in statistical power it is unlikely that definitive answers will be reached in the near future. Therefore, the methodological differences between assessments need to be addressed when interpreting results. When choosing tools, the user should be aware of the methodological choices and their possible impacts on the results.