Arsenic Exposure and Methylation Efficiency in Relation to Oxidative Stress in Semiconductor Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Biological Sampling
2.3. Personal Arsenic Exposure Assessment
2.4. Urinary 8-OHdG
2.5. Urinary MDA
2.6. Statistical Analysis
3. Results
3.1. As Levels in Study Subjects
3.2. As Exposure Evaluation
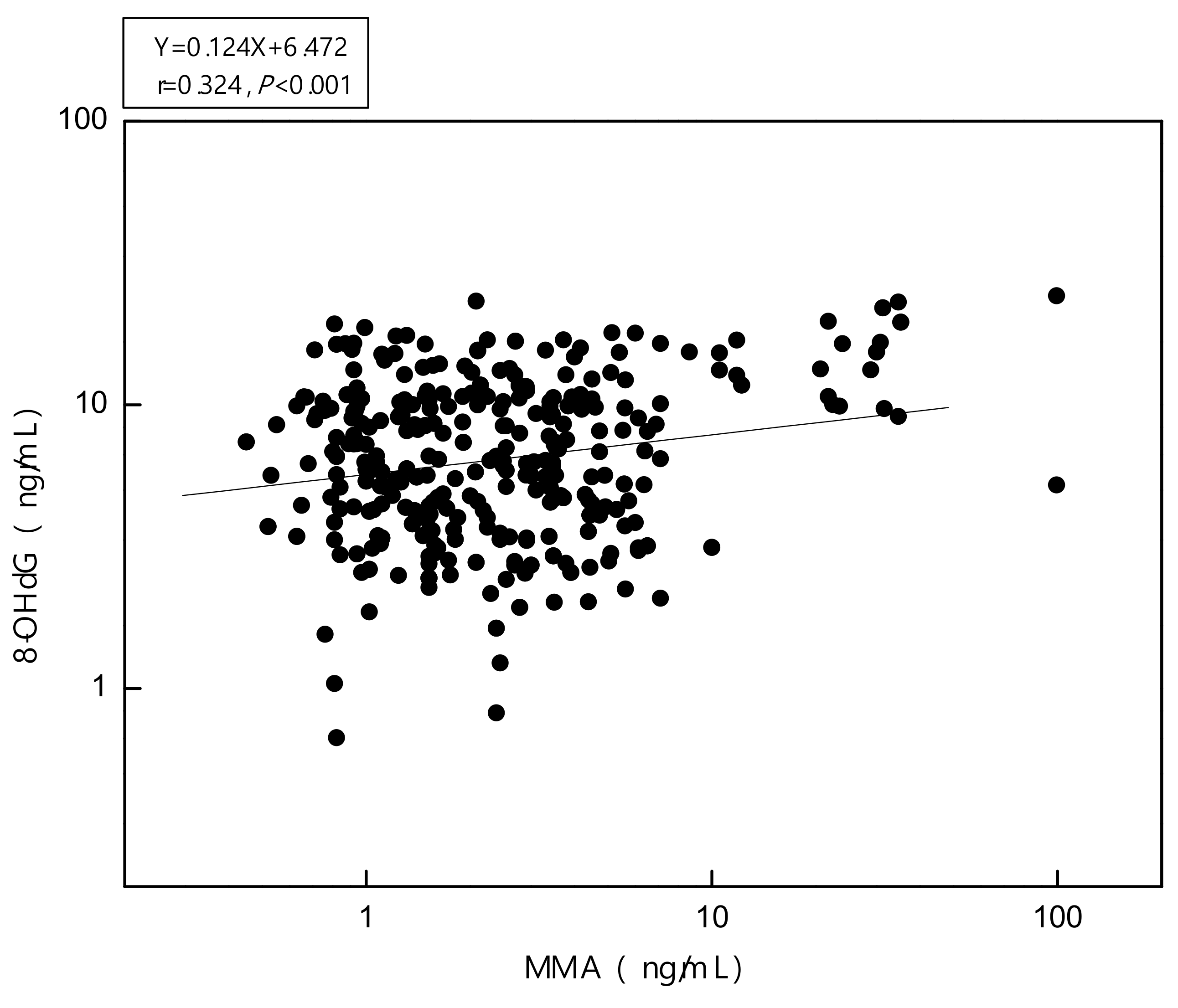
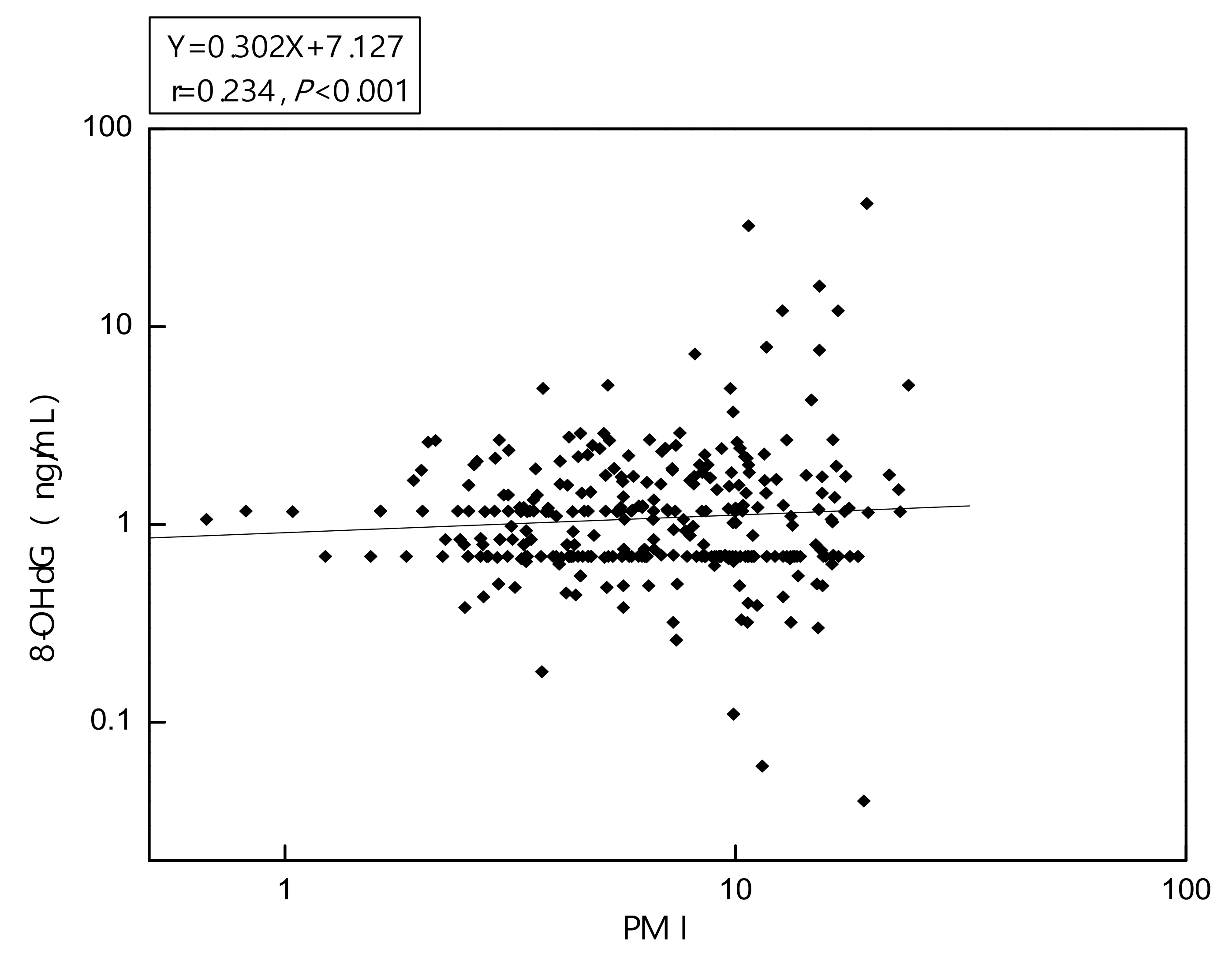
3.3. Biomarkers of Oxidative Stress Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Williams, M.E.; Baldwin, D.G. (Eds.) Semiconductor Industrial Hygiene Handbook; Noyes: Park Ridge, NJ, USA, 1994. [Google Scholar]
- De Peyster, A.; Silvers, J.A. Arsenic levels in hair of workers in a semiconductor fabrication facility. Am. Ind. Hyg. Assoc. J. 1995, 56, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Lee, Z.Y.; Wang, J.D.; Hsueh, Y.M.; Lu, I.C.; Yao, W.L. Monitoring of arsenic exposure with speciated urinary inorganic arsenic metabolites for ion implanter maintenance engineers. Environ. Res. 2002, 90, 207–216. [Google Scholar] [CrossRef]
- Chen, Y.C.; Guo, Y.L.; Su, H.J.; Hsueh, Y.M.; Smith, T.J.; Ryan, L.M.; Lee, M.S.; Chao, S.C.; Lee, J.Y.; Christiani, D.C. Arsenic methylation and skin cancer risk in southwestern Taiwan. J. Occup. Environ. Med. 2003, 45, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Su, H.J.; Guo, Y.L.; Hsueh, Y.M.; Smith, T.J.; Ryan, L.M.; Lee, M.-S.; Christiani, D. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control 2003, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Won, Y.L.; Hwang, Y.I.; Koh, D.H.; Im, H.; Kim, E.A. Assessment of arsenic exposure by measurement of urinary speciated inorganicarsenic metabolites in workers in a semiconductor manufacturing plant. Ann. Occup. Environ. Med. 2013, 25, 21. [Google Scholar] [CrossRef]
- Apostoli, P.; Bartoli, D.; Alessio, L.; Buchet, J.P. Biological monitoring of occupational exposure to inorganic arsenic. Occup. Environ. Med. 1999, 56, 825–832. [Google Scholar] [CrossRef]
- Zaprianov, Z.; Tsalev, D.; Georgieva, R.; Kaloianova, F.; Nikolova, V. New toxicokinetic exposure tests for metals based on atomic absorption analysis of the fingernails. Probl. Khigi. 1989, 14, 75–97. [Google Scholar]
- Loffredo, C.A.; Aposhian, H.V.; Cebrian, M.E.; Yamauchi, H.; Silbergeld, E.K. Variability in human metabolism of arsenic. Environ. Res. 2003, 92, 85–91. [Google Scholar] [CrossRef]
- Huang, Y.L.; Hsueh, Y.M.; Huang, Y.K.; Yip, P.K.; Yang, M.H.; Chen, C.J. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci. Total Environ. 2009, 407, 2608–2614. [Google Scholar] [CrossRef]
- Park, D.; Yang, H.; Jeong, J.; Ha, K.; Choi, S.; Kim, C.; Yoon, C.; Park, D.; Paek, D. A comprehensive review of arsenic levels in the semiconductor manufacturing industry. Ann. Occup. Hyg. 2010, 54, 869–879. [Google Scholar]
- Kitchin, K.T. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001, 172, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.W.; Hicks, J.B.; Fabianova, E. Airborne arsenic and urinary excretion of arsenic metabolites during boiler cleaning operations in a Slovak coal-fired power plant. Environ. Health Perspect. 1997, 105, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, F.I.; Akan, J.C.; Chellube, Z.M.; Waziri, M. Levels of Heavy Metals in Human Hair and Nail Samples from Maiduguri Metropolis, Borno State, Nigeria. World Environ. 2012, 2, 81–89. [Google Scholar] [CrossRef]
- Hu, C.W.; Wang, C.J.; Chang, L.W.; Chao, M.R. Clinical-scale high-throughput analysis of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine by isotope-dilution liquid chromatography-tandem mass spectrometry with on-line solid phase extraction. Clin. Chem. 2006, 52, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.; Carrizales, L.; Yáñez, L.; Mejia, J.; Batres, L.; Ortiz, D.; Diaz-Barriga, F. Arsenic increased lipid peroxidation in rat tissues by a mechanism independent of glutathione levels. Environ. Health Perspect. 1995, 103 (Suppl. 1), 85–88. [Google Scholar] [PubMed]
- Ghatak, S.; Biswas, A.; Dhali, G.K.; Chowdhury, A.; Boyer, J.L.; Santra, A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol. Appl. Pharmacol. 2011, 251, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kulawiec, M.; Owens, K.M.; Singh, A.; Singh, K.K. Sustained Early Disruption of Mitochondrial Function Contributes to Arsenic-Induced Prostate Tumorigenesis. Biochemistry (Mosc.) 2016, 81, 1089–1100. [Google Scholar] [CrossRef]
- Erhola, M.; Toyokuni, S.; Okada, K.; Tanaka, T.; Hiai, H.; Ochi, H.; Uchida, K.; Osawa, T.; Nieminen, M.M.; Alho, H.; et al. Biomarker evidence of DNA oxidation in lung cancer patients: Association of urinary 8-hydroxy-2’-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997, 409, 287–291. [Google Scholar] [CrossRef]
- Toraason, M.; Hayden, C.; Marlow, D.; Rinehart, R.; Mathias, P.; Werren, D.; DeBord, D.G.; Reid, T.M. DNA strand breaks, oxidative damage, and 1-OH pyrene in roofers with coal-tar pitch dust and/or asphalt fume exposure. Int. Arch. Occup. Environ. Health 2001, 74, 396–2404. [Google Scholar] [CrossRef]
- Kasai, H.; Crain, P.F.; Kuchino, Y.; Nishimura, S.; Ootsuyama, A.; Tanooka, H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis 1986, 7, 849–851. [Google Scholar] [CrossRef]
- Kosugi, H.; Enomoto, H.; Ishizuka, Y.; Kikugawa, K. Variations in the level of thiobarbituric acid reactant in health humans under different physiological conditions. Biol. Pharm. Bull. 1994, 17, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Pan, C.H.; Huang, Y.L.; Wu, M.T.; Chang, L.W.; Wang, C.J.; Chao, M.R. Effects of arsenic exposure among semiconductor workers: A cautionary note on urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine. Free Radic. Biol. Med. 2006, 40, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.; Leese, E. Arsenic speciation in clinical samples: Urine analysis using fast micro-liquid chromatography ICP-MS. Anal. Bioanal. Chem. 2011, 399, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.M.; Hsu, M.K.; Chiou, H.Y.; Yang, M.H.; Huang, C.C.; Chen, C.J. Urinary arsenic speciation in subjects with or without restriction from seafood dietary intake. Toxicol. Lett. 2002, 133, 83–91. [Google Scholar] [CrossRef]
- Pan, C.H.; Chan, C.C.; Wu, K.Y. Effects on Chinese restaurant workers of exposure to cooking oil fumes: A cautionary note on urinary 8-Hydroxy-2’-Deoxyguanosine. Cancer Epidemiol. Biomark. Prev. 2008, 17, 335–3357. [Google Scholar] [CrossRef]
- Chao, M.R.; Wang, C.J.; Wu, M.T.; Pan, C.H.; Kuo, C.Y.; Yang, H.J.; Chang, L.W.; Hu, C.-W. Repeated measurements of urinary methylated/oxidative DNA lesions, acute toxicity, and mutagenicity in coke oven workers. Cancer Epidemiol Biomark. Prev. 2008, 17, 3381–3389. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Gurzau, E.S.; Le, X.C.; Gurzau, A.; Healy, S.M.; Lu, X.; Ma, M.; Yip, L.; Zakharyan, R.A.; Maiorino, R.M.; et al. Occurrence of monomethylarsonous acid in urine of human exposed to inorganic arsenic. Chem. Res. Toxicol. 2002, 13, 693–697. [Google Scholar] [CrossRef]
- Wen, J.; Wen, W.; Li, L.; Liu, H. Methylation capacity of arsenic and skin lesions in smelter plant workers. Environ. Toxicol. Pharmacol. 2012, 34, 624–630. [Google Scholar] [CrossRef]
- Vahter, M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci. Prog. 1999, 821, 69–88. [Google Scholar] [CrossRef]
- Hsueh, Y.M.; Chiou, H.Y.; Huang, Y.L.; Wu, W.L.; Huang, C.C.; Yang, M.H.; Lue, L.C.; Chen, G.S.; Chen, C.J. Serum β-carotene level arsenic methylation capability and incidence of skin cancer. Cancer Epidemol. Biomark. Prev. 1997, 6, 589–596. [Google Scholar]
- Kumar, A.; Kaundal, R.K.; Iyer, S.; Sharma, S.S. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007, 80, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Cooke, M.S.; Tsai, Y.H.; Chao, M.R. 8-Oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine concentrations in various human body fluids: Implications for their measurement and interpretation. Arch. Toxicol. 2015, 89, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Belling, D.; Poulsen, H.E. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Res. 2002, 30, e7. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Matsugo, S.; Morimoto, K. Mutagenicity of oxidative DNA damage in Chinese hamster V79 cells. Carcinogenesis 1997, 18, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Langård, S.; Gundersen, N.; Tsalev, D.L.; Gylseth, B. Whole blood chromium level and chromium excretion in the rat after zinc chromate inhalation. Acta Pharmacol. Toxicol. 1978, 42, 142–149. [Google Scholar] [CrossRef]
- Li, X.; Pi, J.; Li, B.; Xu, Y.; Jin, Y.; Sun, G. Urinary arsenic speciation and its correlation with 8-OHdG in Chinese residents exposed to arsenic through coal burning. Bull. Environ. Contam. Toxicol. 2008, 81, 406–411. [Google Scholar] [CrossRef]
- Fujino, Y.; Guo, X.; Liu, J.; Matthews, I.P.; Shirane, K.; Wu, K.; Kasai, H.; Miyatake, M.; Tanabe, K.; Kusuda, T.; et al. Chronic arsenic exposure and urinary 8-hydroxy-2’-deoxyguanosine in an arsenic-affected area in inner Mongolia, China. Expo. Annal. Environ. Epidemiol. 2005, 15, 147–152. [Google Scholar] [CrossRef]
| Variable | Control Subjects (n = 91) | Production Staff (n = 278) | Maintenance Staff (n = 149) |
|---|---|---|---|
| Age (years) | 36.2 ± 5.8 | 34.5 ± 4.9 | 34.1 ± 3.6 |
| Height (cm) | 170.0 ± 5.6 | 169.5 ± 4.6 | 170.2 ± 6.4 |
| Weight (kg) | 65.3 ± 9.3 | 64.8 ± 7.7 | 69.4 ± 9.8 *† |
| Body mass index (kg/m2) | 22.5 ± 2.2 | 22.5 ± 2.5 | 23.9 ± 2.8 *† |
| Work years (years) | 9.5 ± 2.6 | 10.0 ± 2.5 | 10.1 ± 1.9 |
| Workdays per week (days) | 4.8 ± 1.0 | 4.5 ± 0.8 | 4.8 ± 0.6 |
| Work hours per day (hours) | 8.9 ± 2.3 | 10.1 ± 1.9 # | 9.6 ± 1.5 * |
| Alcohol consumption | 27 (29.6%) | 82 (29.4%) | 61 (40.9%) |
| Potential chemical exposure & | 25 (27.5%) | 196 (70.5%) # | 90 (60.4%) * |
| Variable | Control Subjects (n = 91) | Production Staff (n = 278) | Maintenance Staff (n = 149) |
|---|---|---|---|
| Geometric mean (geometric standard deviation) | |||
| 8-OHdG (µg/L creatinine) | 3.9 (2.1) | 5.2 (1.8) * | 7.6 (1.9) *† |
| MDA (µg/L creatinine) | 119.7 (2.1) | 161.8 (1.9) * | 171.9 (2.3) * |
| As(III) (µg/L) | 0.7 (2.3) | 0.9 (2.4) | 2.4 (3.2) * |
| As(V) (µg/L) | 0.9 (2.1) | 1.0 (2.1) | 2.7 (2.6) *† |
| MMA (µg/L) | 1.7 (2.1) | 1.9 (2.1) | 3.6 (2.9) * † |
| DMA (µg/L) | 11.2 (2.0) | 16.2 (1.9) * | 24.1 (2.1) *† |
| iAs (µg/L) a | 1.8 (1.8) | 2.3 (2.0) | 4.3 (2.2) *† |
| Total As (µg/L) b | 15.8 (1.8) | 21.1 (1.8) * | 34.5 (2.1) *† |
| PMI (MMA/iAs) | 1.2 (1.7) | 1.4 (1.9) | 2.3 (2.1) *† |
| SMI (DMA/MMA) | 7.9 (4.5) | 12.5 (5.1) | 14.8 (4.9) *† |
| Hair As (ng/g) | 20.1 (8.9) | 48.2 (3.2) * | 82.8 (7.7) * |
| Fingernail As (µg/g) | 94.2 (2.5) | 218.1 (3.4) * | 354.9 (3.6) * |
| Variables | Log10 Urinary Total As (µg/L) | Log10 Hair As (µg/g) | Log10 Fingernail As (µg/g) | |||
|---|---|---|---|---|---|---|
| Regression Coefficient | 95% CI | Regression Coefficient | 95% CI | Regression Coefficient | 95% CI | |
| Work site | ||||||
| Production staff vs. control subjects | 0.189 | 0.297–0.478 * | 0.240 | 0.112–0.368 * | 0.419 | 0.222–0.617 * |
| Maintenance staff vs. control subjects | 0.387 | 0.102–0.275 * | 0.469 | 0.208–0.730 * | 0.641 | 0.427–0.856 * |
| Alcohol consumption (y/n) | −0.004 | −0.059–0.052 | 0.033 | −0.132–0.198 | 0.048 | −0.085–0.180 |
| Potential chemical exposure (y/n) † | −0.022 | −0.079–0.036 | 0.048 | −0.123–0.219 | −0.046 | −0.184–0.092 |
| Work years (years) | 0.010 | −0.003–0.022 | 0.029 | 0.012–0.046 * | 0.070 | 0.007–0.133 * |
| Workdays per week (days) | 0.054 | −0.004–0.111 | 0.124 | −0.035–0.284 | 0.038 | −0.092–0.169 |
| Work hours per day (hours) | 0.039 | 0.001–0.045 * | 0.040 | −0.023–0.103 | 0.011 | −0.042–0.063 |
| Age (years) | 0.003 | −0.003–0.009 | 0.002 | −0.034–0.039 | 0.003 | −0.011–0.180 |
| BMI (kg/m2) | −0.002 | −0.011–0.007 | −0.010 | −0.037–0.016 | 0.005 | −0.016–0.199 |
| Parameters | Log10 8-OHdG (µg/g Creatinine) | Log10 MDA (µg/g Creatinine) |
|---|---|---|
| Predictors | Regression Coefficient (95% Confidence Interval) | Regression Coefficient (95% Confidence Interval) |
| Worksite | ||
| Production staff vs. control subjects | 0.124(0.018–0.230) * | 0.178(0.035–0.322) * |
| Maintenance staff vs. control subjects | 0.210(0.087–0.332) * | 0.246(0.121–0.370) * |
| Alcohol consumption (y/n) | 0.002(−0.068–0.072) | 0.026(–0.056–0.108) |
| Potential chemical exposure (y/n) | 0.039(−0.033–0.110) | 0.004(–0.080–0.088) |
| Work years (years) | 0.008(−0.011–0.026) | 0.010(–0.011–0.031) |
| Workdays per week (days) | 0.009(−0.023–0.041) | 0.060(–0.031–0.043 |
| Work hours per day (hours) | 0.018(0.001–0.035) * | 0.001(−0.021–0.022) |
| Age (years) | –0.003(−0.011–0.005) | 0.004(–0.005–0.013) |
| BMI (kg/m2) | 0.007(−0.004–0.018) | −0.009(–0.022–0.004) |
| Log10 iAs (µg/L) | 0.002(−0.007–0.010) | 0.009(–0.001–0.019) |
| Log10 Urinary total As (µg/L) | 0.167(0.010–0.3325) * | 0.004(–0.181–0.188) |
| Log10 Hair As (µg/g) | 0.018(−0.040–0.075) | 0.044(–0.024–0.112) |
| Log10 Fingernail As (µg/g) | 0.065(−0.007–0.137) | 0.003(–0.081–0.088) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.-H.; Lin, C.-Y.; Lai, C.-H.; Jeng, H.A. Arsenic Exposure and Methylation Efficiency in Relation to Oxidative Stress in Semiconductor Workers. Atmosphere 2020, 11, 464. https://doi.org/10.3390/atmos11050464
Pan C-H, Lin C-Y, Lai C-H, Jeng HA. Arsenic Exposure and Methylation Efficiency in Relation to Oxidative Stress in Semiconductor Workers. Atmosphere. 2020; 11(5):464. https://doi.org/10.3390/atmos11050464
Chicago/Turabian StylePan, Chih-Hong, Ching-Yu Lin, Ching-Huang Lai, and Hueiwang Anna Jeng. 2020. "Arsenic Exposure and Methylation Efficiency in Relation to Oxidative Stress in Semiconductor Workers" Atmosphere 11, no. 5: 464. https://doi.org/10.3390/atmos11050464
APA StylePan, C.-H., Lin, C.-Y., Lai, C.-H., & Jeng, H. A. (2020). Arsenic Exposure and Methylation Efficiency in Relation to Oxidative Stress in Semiconductor Workers. Atmosphere, 11(5), 464. https://doi.org/10.3390/atmos11050464





