Chemical Composition of PM2.5 and Its Impact on Inhalation Health Risk Evaluation in a City with Light Industry in Central China
Abstract
1. Introduction
2. Method
2.1. Sample Collection
2.2. Chemical Composition Analysis
2.3. PMF Model
2.4. Health Risk Estimation
3. Results and Discussion
3.1. Chemical Composition
3.2. Comparison of Chemical Composition on Clean and Polluted Days
3.3. Sources Apportionment of PM2.5
3.4. Health Risk Evaluation of Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shuang, Y.Y.; Liu, W.J.; Xu, Y.S.; Yi, K.; Zhou, M.; Tao, S.; Liu, W.X. Characteristics and oxidative potential of atmospheric PM2.5 in Beijing: Source apportionment and seasonal variation. Sci. Total Environ. 2019, 650, 277–287. [Google Scholar]
- Lee, C.G.; Yuan, C.S.; Chang, J.C.; Yuan, C. Effects of aerosol species on atmospheric visibility in Kaohsiung City, Taiwan. Air Waste Manag. 2005, 55, 1031–1041. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, Z.; Lau, A.K.H.; Chang, Q.L.; Lao, X.Q. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. Lancet Planet. Health 2018, 2, 114–125. [Google Scholar]
- Ai, S.; Qian, Z.M.; Guo, Y.; Yang, Y.; Rolling, C.A.; Liu, E.; Wu, F.; Lin, H. Long-term exposure to ambient fine particles associated with asthma: A cross-sectional study among older adults in six low- and middle-income countries. Environ. Res. 2019, 68, 141–145. [Google Scholar] [CrossRef]
- Agarwal, A.; Mangal, A.; Satsangi, A.; Lakhani, A.; Kumari, K.M. Characterization, sources and health risk analysis of PM2.5 bound metals during foggy and non-foggy days in sub-urban atmosphere of Agra. Atmos. Res. 2017, 197, 121–131. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B. Ambient fine particulate matter (PM2.5) in Canadian oil sands communities: Levels, sources and potential human health risk. Sci. Total Environ. 2017, 595, 828–838. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, C.; Tao, J.; Zhang, L.; Liang, X.; Ma, J.; Gao, H.; Huang, T.; Zhang, K. Chemical characterization and source apportionment of PM 2.5 in a semi-arid and petrochemical-industrialized city, Northwest China. Sci. Total Environ. 2016, 573, 1031–1040. [Google Scholar] [CrossRef]
- Frederic, L.; Adib, K.; Gilles, D.; Gilles, R.; Atallah, E.Z.; Dominique, C. Contributions of local and regional anthropogenic sources of metals in PM2.5 at an urban site in northern France. Chemosphere 2017, 181, 713–724. [Google Scholar]
- Wang, J.; Zimei, H.U.; Chen, Y.; Chen, Z.; Shiyuan, X.U. Contamination characteristics and possible sources of PM10 and PM2.5 in different functional areas of Shanghai, China. Atmos. Environ. 2013, 68, 221–229. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Yan, Q.; Wu, F.; Cheng, Y.; Zheng, S.; Wu, J.; Yang, G.; Zheng, M.; Tang, L.; et al. The impacts of pollution control measures on PM2.5 reduction: Insights of chemical composition, source variation and health risk. Atmos. Environ. 2019, 197, 103–117. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Wang, Z.; Yang, L.; Wang, J.; Wang, W. Trace elements in PM2.5 in Shandong Province: Source identification and health risk assessment. Sci. Total Environ. 2018, 621, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.T.; Pope, C.A., III; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.I.; Phalen, R.F.; Solomon, P.A. Airborne particulate matter and human health: A review. Aerosol Sci. Technol. 2005, 39, 737–749. [Google Scholar] [CrossRef]
- Roksana, K.; Shoko, K.; Sheng, N.C.F.; Masahiro, U.; Ferdosi, K.A.; Saira, T.; Chiho, W. Association between short-term exposure to fine particulate matter and daily emergency room visits at a cardiovascular hospital in Dhaka, Bangladesh. Sci. Total Environ. 2019, 646, 1030–1036. [Google Scholar]
- Yu, Y.; Yao, S.; Dong, H.; Wang, L.; Wang, C.; Ji, X.; Ji, M.; Yao, X.; Zhang, Z. Association between short-term exposure to particulate matter air pollution and cause-specific mortality in Changzhou, China. Environ. Res. 2019, 170, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Deng, J.; Du, K.; Zheng, M.; Yan, C.; Sköld, M.; Gustafsson, Ö. Regionally-varying combustion sources of the January 2013 severe haze events over eastern China. Environ. Sci. Technol. 2015, 49, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Sun, Y.; Liu, Z.R.; Ji, D.S.; Hu, B.; Liu, Q.; Wang, Y.S. Characterization of submicron aerosols during a month of serious pollution in Beijing, 2013. Atmos. Chem. Physics. 2014, 14, 1431–1432. [Google Scholar] [CrossRef]
- Zheng, S.; Pozzer, A.; Cao, C.X.; Lelieveld, J. Long-term (2001–2012) fine particulate matter (PM2.5) and the impact on human health in Beijing, China. Atmos. Chem. Phys. 2015, 14, 5715–5725. [Google Scholar] [CrossRef]
- Henan Province Bureau of Statistics; Henan Investigation Corps of National Bureau of Statistics. Henan Statistical Yearbook; China Statistics Press: Beijing, China, 2018.
- Li, J.; Lin, B. Ecological total-factor energy efficiency of China’s heavy and light industries: Which performs better? Renew. Sustain. Energy Rev. 2017, 72, 83–94. [Google Scholar] [CrossRef]
- Wilson, J.G.; Kingham, S.; Pearce, J.; Sturman, A.P. A review of intraurban variations in particulate air pollution: Implications for epidemiological research. Atmos. Environ. 2005, 39, 6444–6462. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K.; Peng, R.D. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Lu, Z.; Lowenthal, D.H.; Frazier, C.A.; Solomon, P.A.; Thuillier, R.H.; Magliano, K. Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos. Environ. 1996, 30, 2079–2112. [Google Scholar] [CrossRef]
- Meng, C.C.; Wang, L.T.; Zhang, F.F.; Wei, Z.; Ma, S.M.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Wang, N.; Yin, B.H.; Wang, J.; Liu, Y.Y.; Li, W.; Geng, C.M.; Bai, Z.P. Characteristics of water-soluble ions concentration associated with PM10 and PM2.5 and source apportionment in Luohe city. Res. Environ. Sci. 2018, 31, 2073–2082. [Google Scholar]
- Kong, S.F.; Han, B.; Bai, Z.P.; Li, C.; Shi, J.W.; Xu, Z. Receptor modelling of PM2.5, PM10 and TSP in different seasons and long-range transport analysis at a coastal site of Tianjin, China. Sci. Total Environ. 2010, 408, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.F.; Li, L.; Li, X.X.; Yin, Y.; Chen, K.; Liu, D.T.; Yuan, L.; Zhang, Y.J.; Shan, Y.P.; Ji, Y.Q. The impacts of firework burning at the Chinese Spring Festival on air quality: Insights of tracers, source evolution and aging processes. Atmos. Chem. Phys. 2015, 14, 167–2184. [Google Scholar] [CrossRef]
- Terzi, E.; Argyropoulos, G.; Bougatioti, A.; Mihalopoulos, N.; Nikolaou, K.; Samara, C. Chemical composition and mass closure of ambient PM10 at urban sites. Atmos. Environ. 2010, 44, 2231–2239. [Google Scholar] [CrossRef]
- Vecchi, R.; Chiari, M.D.; Alessandro, A.; Fermo, P.; Lucarelli, F.; Mazzei, F.; Nava, S.; Piazzalunga, A.; Prati, P.; Silvani, F. A mass closure and PMF source apportionment study on the sub-micron sized aerosol fraction at urban sites in Italy. Atmos. Environ. 2008, 42, 2240–2253. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.H.H.; Tam, F.C.V.; Zhang, X.; Liu, K.M.; Yeung, C.; Feng, Y.; Cheng, Y.Y.; Wong, Y.K.; Ng, W.M. Source apportionment of fine particulate matter in Macao, China with and without organic tracers: A comparative study using positive matrix factorization. Atmos. Environ. 2019, 198, 183–193. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, S.; Wang, X.; Wei, N.; Xu, P.; Gao, X.; Chao, Y.; Wang, W. Source identification and health impact of PM2.5 in a heavily polluted urban atmosphere in China. Atmos. Environ. 2013, 75, 265–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Lang, J.; Cheng, S.; Li, S.; Zhou, Y.; Chen, D.; Zhang, H.; Wang, H. Chemical composition and sources of PM1 and PM2.5 in Beijing in autumn. Sci. Total Environ. 2018, 630, 72. [Google Scholar] [CrossRef] [PubMed]
- Reff, A.; Eberly, S.I.; Bhave, P.V. Receptor modeling of ambient particulate matter data using positive matrix factorization: Review of existing methods. J. Air Waste Manag. 2007, 57, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Masiola, M.; Hopkea, P.K.; Feltonb, H.D.; Frankb, B.P.; Rattiganb, O.V.; Wurthb, M.J.; LaDukeb, G.H. Source apportionment of PM2.5 chemically speciated mass and particle number concentrations in New York City. Atmos. Environ. 2017, 148, 215–229. [Google Scholar] [CrossRef]
- Hadley, O.L. Background PM2.5 source apportionment in the remote Northwestern United States. Atmos. Environ. 2017, 167, 298–308. [Google Scholar] [CrossRef]
- Manual RAGF. Process for Conducting Probabilistic Risk Assessment; EPA: Washington, DC, USA, 1989. [Google Scholar]
- US EPA (U.S. Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part A), 1989. EPA/540/R/1-89/002. Available online: http://www.epa.gov/swerrims/riskassessment/ragsa/index.htm (accessed on 30 March 2020).
- Ministry of Environmental Protection of People’s Republic of China. Exposure Factors Hand Book of Chinese Population (Adult); China Environment Press: Beijing, China, 2013; ISBN 978-7-5111-1592-8.
- US EPA (U.S. Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E), 2004. EPA/540/R/99/005. Available online: http://www.epa.gov/swerrims/riskassessment/ragsa/index.htm (accessed on 30 March 2020).
- US EPA (U.S. Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F), 2009. EPA-540-R-070-002. Available online: http://www.epa.gov/swerrims/riskassessment/ragsa/index.htm (accessed on 30 March 2020).
- Feng, J.; Yu, H.; Su, X.; Liu, S.; Yi, L.; Pan, Y.; Sun, J.H. Chemical composition and source apportionment of PM2.5 during Chinese Spring Festival at Xinxiang, a heavily polluted city in North China: Fireworks and health risks. Atmos. Res. 2016, 182, 176–188. [Google Scholar] [CrossRef]
- Sah, D.; Verma, P.K.; Kandikonda, M.K.; Lakhani, A. Pollution characteristics, human health risk through multiple exposure pathways, and source apportionment of heavy metals in PM10 at Indo-Gangetic site. Urban Clim. 2019, 27, 149–162. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.J. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Quan, J.; Quan, L.; Xia, L.; Yang, G.; Jia, X.; Sheng, J.; Liu, Y. Effect of heterogeneous aqueous reactions on the secondary formation of inorganic aerosols during haze events. Atmos. Environ. 2015, 122, 306–312. [Google Scholar] [CrossRef]
- Turpin, B.J.; Cary, R.A.; Huntzicker, J.J. An in situ, time-resolved analyzer for aerosol organic and elemental carbon. Aerosol Sci. Technol. 1990, 12, 161–171. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J. Secondary formation of organic aerosol in the Los Angeles basin: A descriptive analysis of organic and elemental carbon concentrations. Atmos. Environ. Part A Gen. Top. 1991, 25, 207–215. [Google Scholar] [CrossRef]
- Liao, T.; Wang, S.; Ai, J.; Gui, K.; Duan, B.; Zhao, Q.; Zhang, X.; Jiang, W.; Sun, Y. Heavy pollution episodes, transport pathways and potential sources of PM2.5 during the winter of 2013 in Chengdu (China). Sci. Total Environ. 2017, 584–585, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xie, S.D.; Luo, B.; Zhai, C.Z. Particulate pollution in urban Chongqing of southwest China: Historical trends of variation, chemical characteristics and source apportionment. Sci. Total Environ. 2017, 584–585, 523–534. [Google Scholar]
- Gao, J.; Tian, H.; Ke, C.; Long, L.; Mei, Z.; Wang, S.; Hao, J.; Wang, K.; Hua, S.; Zhu, C. The variation of chemical characteristics of PM2.5 and PM10 and formation causes during two haze pollution events in urban Beijing, China. Atmos. Environ. 2015, 107, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Tian, M.; Chen, Y.; Shi, G.; Cao, X. Seasonal characteristics, formation mechanisms and source origins of PM2.5 in two megacities in Sichuan Basin, China. Atmos. Chem. Phys. 2018, 18, 865–881. [Google Scholar] [CrossRef]
- Bo, Z.; Qiang, Z.; Yang, Z.; He, K.B.; Kai, W.; Zheng, G.; Duan, F.K.; Ma, Y.L.; Kimoto, T. Heterogeneous chemistry: A mechanism missing in current models to explain secondary inorganic aerosol formation during the January 2013 haze episode in North China. Atmos. Chem. Phys. 2015, 14, 2031–2049. [Google Scholar]
- Zheng, G.J.; Duan, F.K.; Su, H.; Ma, Y.L.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Zhang, Q.; Chen, C.; Wang, L.; Wei, Z.; Zhou, S.; Parworth, C.; Zheng, B.; Canonaco, F. Wintertime aerosol chemistry and haze evolution in an extremely polluted city of the North China Plain: Significant contribution from coal and biomass combustion. Atmos. Chem. Phys. 2017, 17, 4751–4768. [Google Scholar] [CrossRef]
- Fu, Q.; Zhuang, G.; Wang, J.; Xu, C.; Huang, K.; Li, J.; Hou, B.; Lu, T.; Streets, D.G. Mechanism of formation of the heaviest pollution episode ever recorded in the Yangtze River Delta, China. Atmos. Environ. 2008, 42, 2023–2036. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Tang, A.; Yuan, H.; Sun, Y.; Chen, S.; Zheng, A. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Ohta, S.; Okita, T. A chemical characterization of atmospheric aerosol in Sapporo. Atmos. Environ. Part A Gen. Top. 1990, 24, 815–822. [Google Scholar] [CrossRef]
- Pekney, N.J.; Davidson, C.I.; Robinson, A.; Zhou, L.; Hopke, P.; Eatough, D.; Rogge, W.F. Major source categories for PM2.5 in pittsburgh using PMF and UNMIX. Aerosol Sci. Technol. 2006, 40, 910–924. [Google Scholar] [CrossRef]
- Hasheminassab, S.; Daher, N.; Saffari, A.; Wang, D.; Ostro, B.D.; Sioutas, C. Spatial and temporal variability of sources of ambient fine particulate matter (PM2.5) in California. Atmos. Chem. Phys. 2014, 14, 20045–20081. [Google Scholar] [CrossRef]
- Feng, J.; Yu, H.; Liu, S.; Su, X.; Li, Y.; Pan, Y.; Sun, J. PM2.5 levels, chemical composition and health risk assessment in Xinxiang, a seriously air-polluted city in North China. Environ. Geochem. Health 2016, 39, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Sternbeck, J.; Din, K.S.; Andréasson, K. Metal emissions from road traffic and the influence of resuspension—Results from two tunnel studies. Atmos. Environ. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Yao, L.; Yang, L.; Yuan, Q.; Yan, C.; Dong, C.; Meng, C.; Sui, X.; Yang, F.; Lu, Y.; Wang, W. Sources apportionment of PM2.5 in a background site in the North China Plain. Sci. Total Environ. 2016, 541, 590–598. [Google Scholar] [CrossRef]
- Police, S.; Sahu, S.K.; Tiwari, M.; Pandit, G.G. Chemical composition and source apportionment of PM2.5 and PM2.5–10 in Trombay (Mumbai, India), a coastal industrial area. Particuology 2018, 37, 143–153. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Xie, S.; Zeng, L.; Zheng, M.; Salmon, L.G.; Shao, M.; Slanina, S. Source apportionment of PM2.5 in Beijing by positive matrix factorization. Atmos. Environ. 2006, 40, 1526–1537. [Google Scholar]
- Liang, X.X.; Huang, T.; Lin, S.Y.; Wang, J.X.; Mo, J.Y.; Gao, H.; Wang, Z.X.; Li, J.X.; Lian, L.L.; Ma, J.M. Chemical composition and source apportionment of PM1 and PM2.5 in a national coal chemical industrial base of the Golden Energy Triangle, Northwest China. Sci. Total Environ. 2019, 188–199. [Google Scholar] [CrossRef]
- Chuang, M.T.; Chen, Y.; Lee, C.; Cheng, C.; Tsai, Y.; Chang, S.; Su, Z. Apportionment of the sources of high fine particulate matter concentration events in a developing aerotropolis in Taoyuan, Taiwan. Environ. Pollut. 2016, 214, 273–281. [Google Scholar] [CrossRef]
- Jiang, N.; Yin, S.; Guo, Y.; Li, J.; Kang, P.; Zhang, R.; Tang, X. Characteristics of mass concentration, chemical composition, source apportionment of PM2.5 and PM10 and health risk assessment in the emerging megacity in China. Atmos. Pollut. Res. 2018, 9, 309–321. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, L.; Zhou, X.; Duan, J.; Li, Y.; Hu, J.; He, K. Chemical characteristics and source apportionment of PM2.5 in Lanzhou, China. Sci. Total Environ. 2017, 601–602, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, J.; Chow, J.C.; Huang, R.J.; Shen, Z.; Chen, L.W.A.; Ho, K.F.; Watson, J.G. Inter-annual variability of wintertime PM2.5 chemical composition in Xi’an, China: Evidences of changing source emissions. SCI Total Environ. 2016, 545–546, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xiao, Z.; He, L.; Shi, Z.; Cao, Y.; Tian, Z. Chemical composition and source apportionment of PM2.5 in urban areas of Xiangtan, Central South China. Int. J. Environ. Res. Public Health 2019, 16, 539. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Song, N.; Dai, O.; Mei, R.; Sui, B.; Bi, X. Chemical composition and source apportionment of ambient PM2.5 during the non-heating period in Taian, China. Atmos. Res. 2016, 170, 23–33. [Google Scholar] [CrossRef]
- Zou, B.B.; Huang, X.; Zhang, B.; Dai, J.; Zeng, L.; Feng, N.; He, L. Source apportionment of PM2.5 pollution in an industrial city in southern China. Atmos. Pollut. Res. 2017, 8, 1193–1202. [Google Scholar] [CrossRef]
- Wu, C.F.; Wu, S.; Wu, Y.; Cullen, A.C.; Larson, T.V.; Williamson, J.; Liu, L.J.S. Cancer risk assessment of selected hazardous air pollutants in Seattle. Environ. Int. 2009, 35, 516–522. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yuan, Z.B.; Li, Y.; Lau, A.K.H.; Louie, P.K.K. Characterization and source apportionment of health risks from ambient PM10 in Hong Kong over 2000–2011. Atmos. Environ. 2015, 122, 892–899. [Google Scholar] [CrossRef]

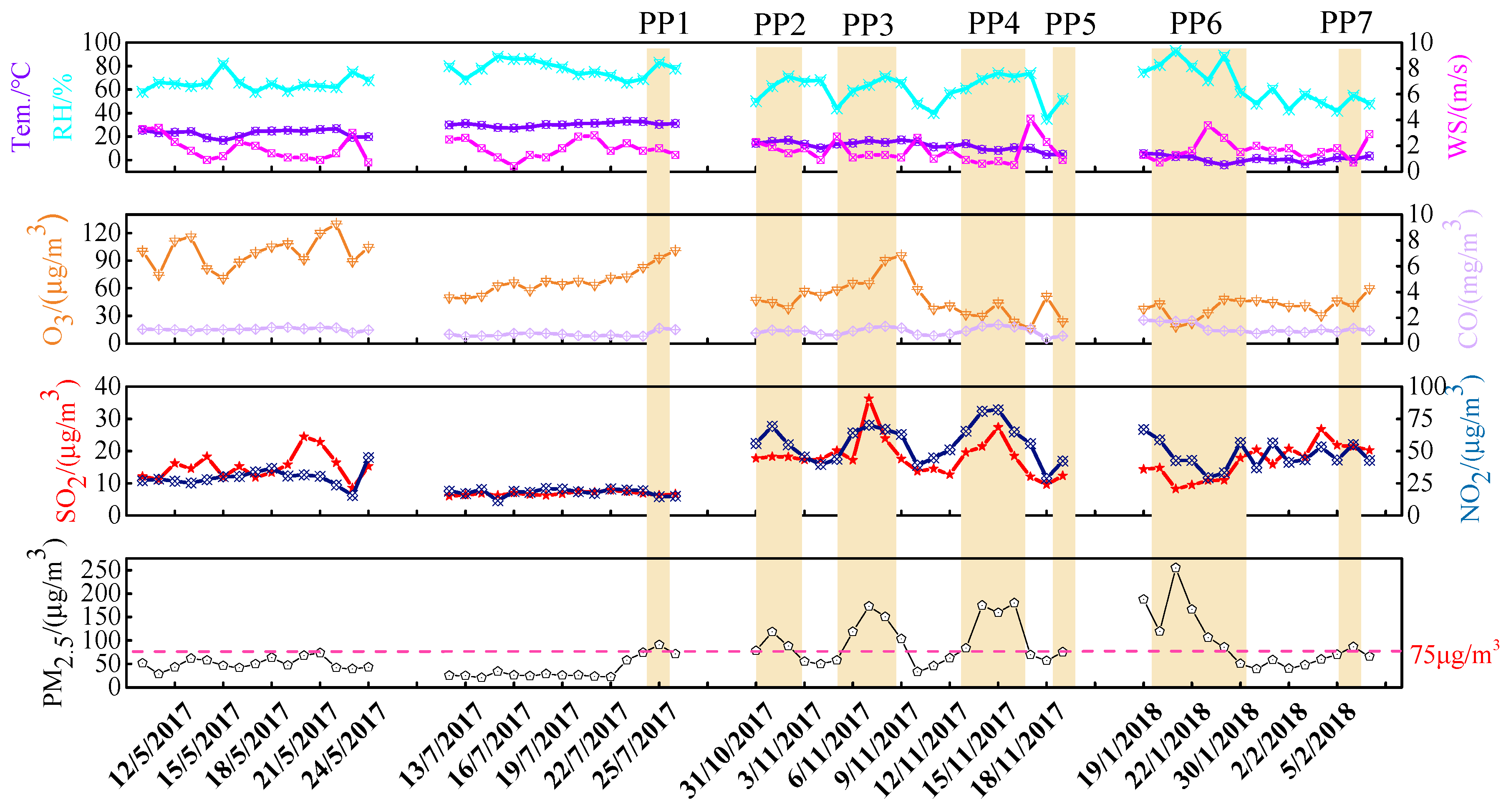

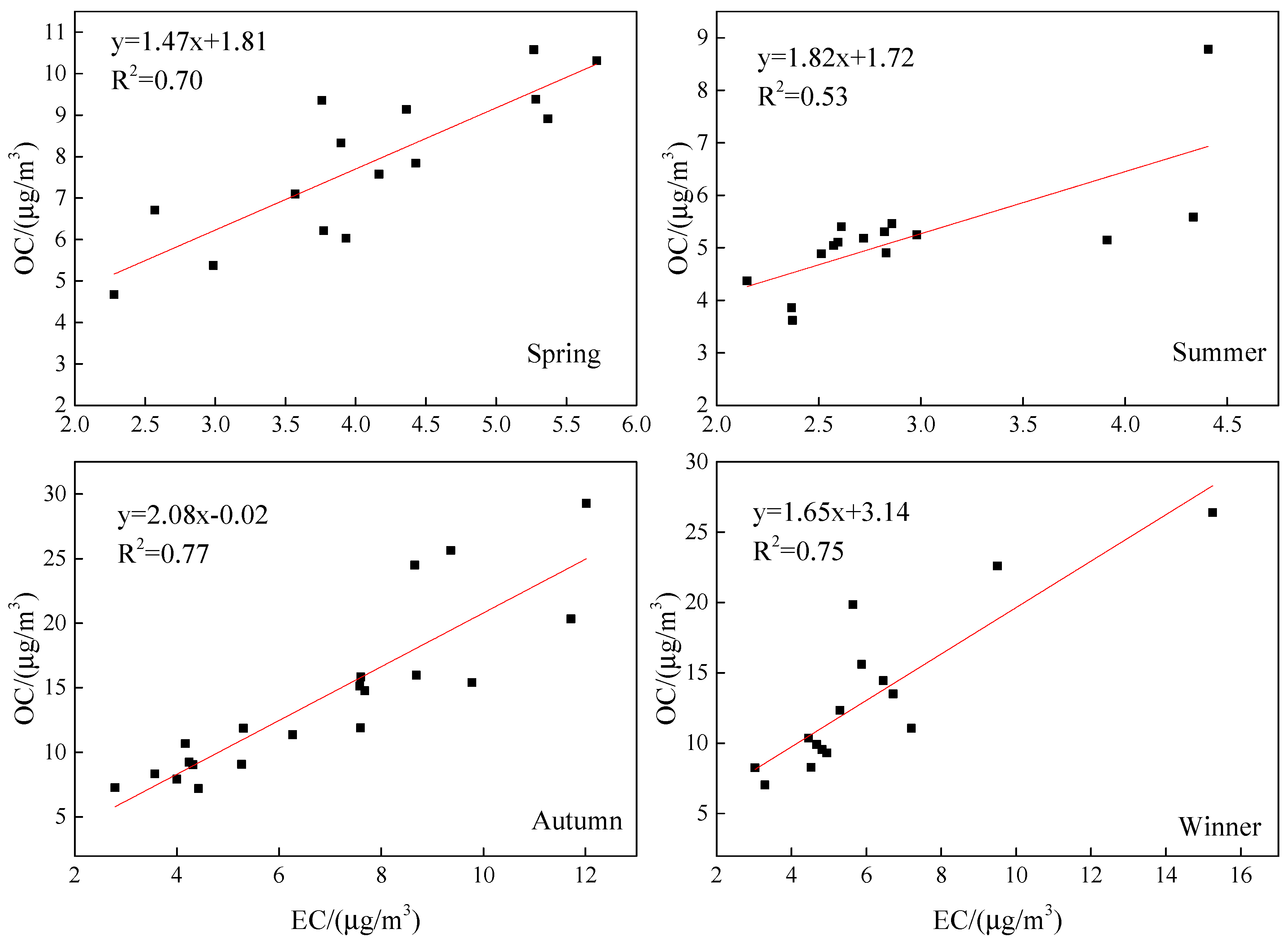
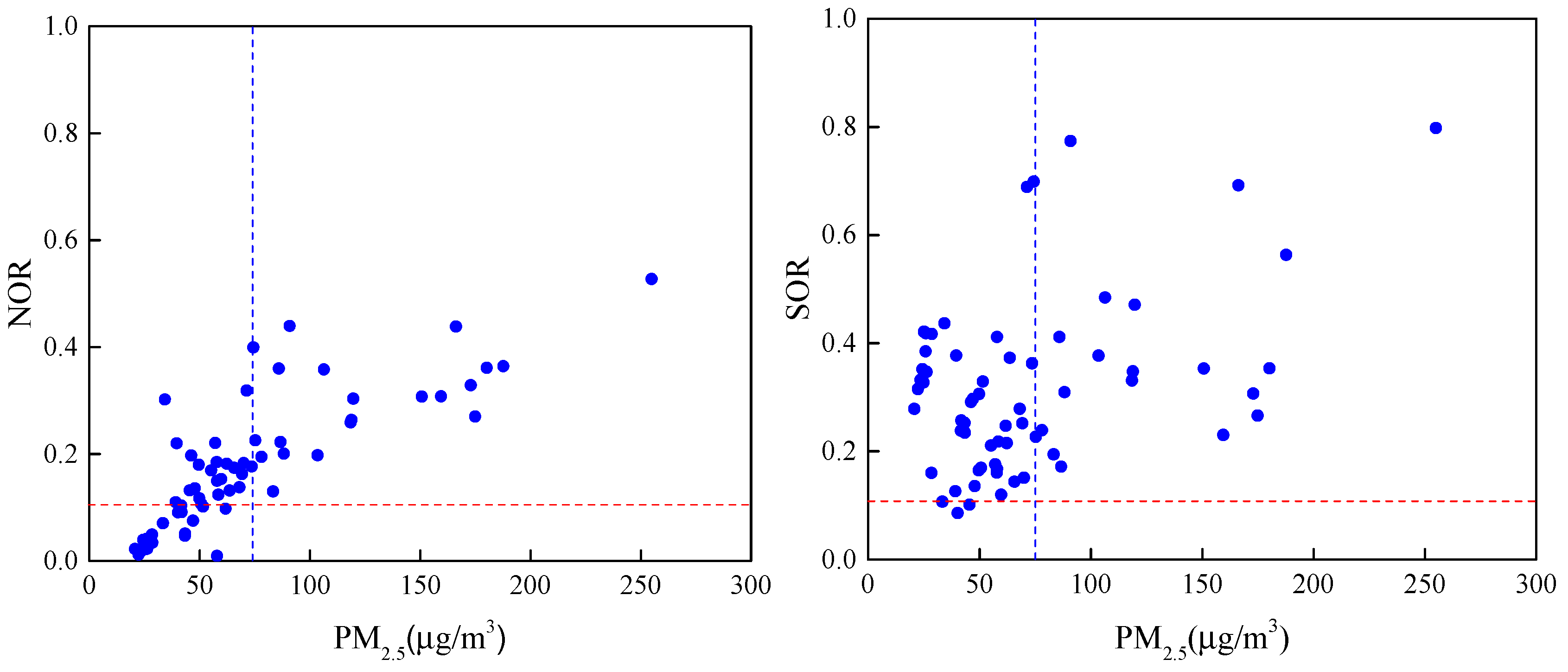
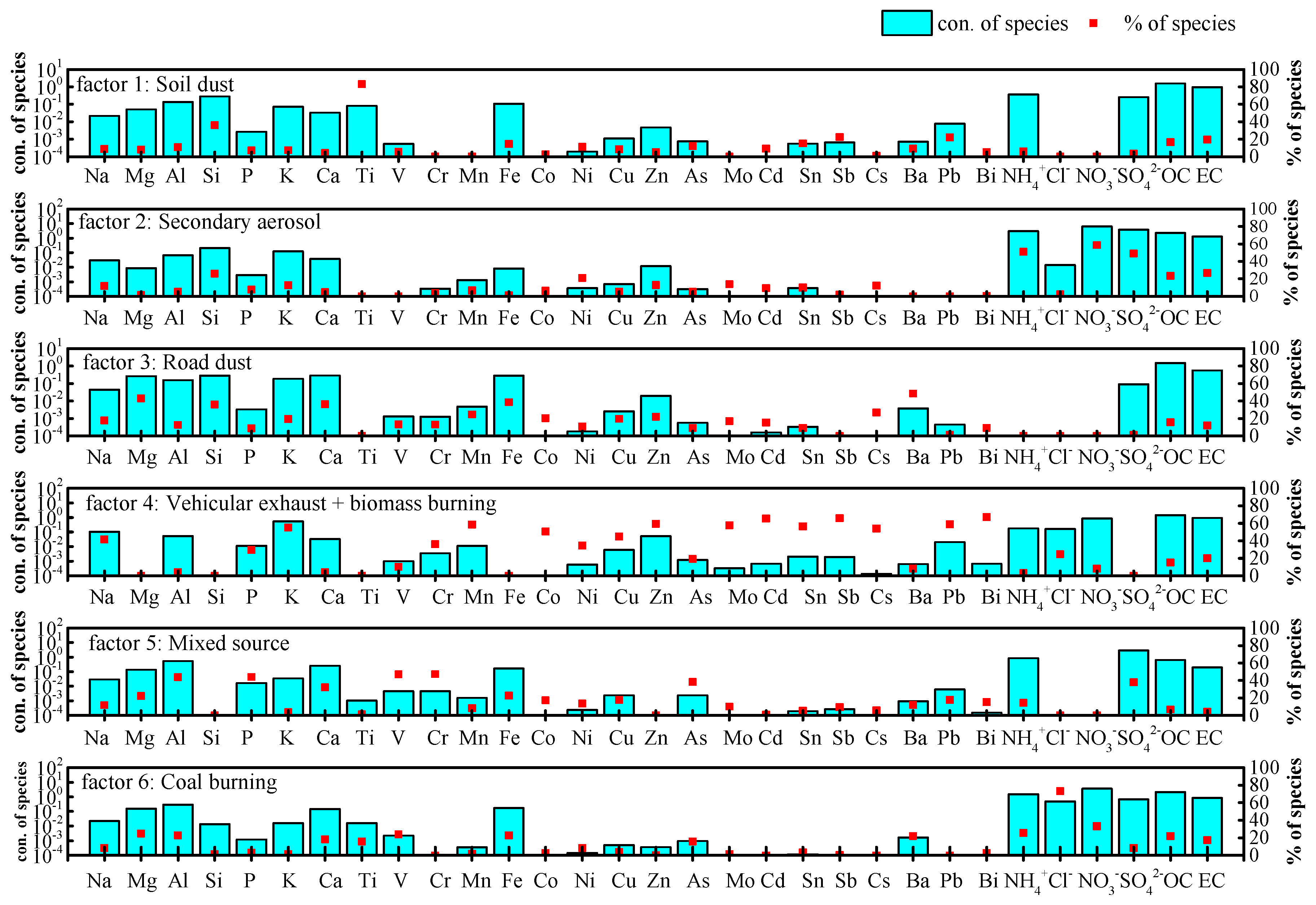

| Species | Spring | Summer | Autumn | Winter | Annual |
|---|---|---|---|---|---|
| Meteorological parameters | |||||
| Tem./(°C) | 22.8 ± 3 | 30.3 ± 1.7 | 12.2 ± 3.6 | −0.1 ± 2.2 | 16.2 ± 11.1 |
| RH/% | 65.3 ± 6.1 | 77.6 ± 6.6 | 60.2 ± 11.4 | 58.1 ± 13.9 | 66.0 ± 12.8 |
| WS/(m/s) | 1.8 ± 0.9 | 1.8 ± 0.7 | 1.6 ± 0.9 | 1.9 ± 0.8 | 1.7 ± 0.8 |
| Concentrations of gaseous pollutants (µg/m3) | |||||
| O3 | 99 ± 16 | 68 ± 14 | 48 ± 20 | 42 ± 9 | 63 ± 27 |
| SO2 | 15 ± 4 | 7 ± 1 | 18 ± 6 | 18 ± 5 | 15 ± 6 |
| NO2 | 30 ± 6 | 18 ± 3 | 56 ± 14 | 44 ± 9 | 39 ± 18 |
| Concentrations of PM2.5 and chemical compositions (µg/m3) | |||||
| PM2.5 | 51 ± 13 | 38 ± 22 | 97 ± 47 | 96 ± 61 | 73 ± 49 |
| SO42− | 8.6 ± 3.7 | 9 ± 7.8 | 9.3 ± 5.7 | 12.3 ± 11.2 | 9.8 ± 7.7 |
| NO3− | 5.2 ± 2.4 | 3.6 ± 5.3 | 22.9 ± 14.1 | 22.4 ± 17 | 14.4 ± 14.9 |
| NH4+ | 4.4 ± 1.9 | 4.6 ± 4.3 | 9.5 ± 5.3 | 11.2 ± 8.5 | 7.7 ± 6.3 |
| Cl− | 0.2 ± 0.1 | 0.2 ± 0.2 | 1.8 ± 1.6 | 2.8 ± 1.5 | 1.3 ± 1.6 |
| K+ | 0.9 ± 0.5 | 0.3 ± 0.1 | 1.1 ± 0.6 | 1.3 ± 0.7 | 0.9 ± 0.6 |
| OC | 7.9 ± 2.0 | 5.2 ± 1.2 | 14 ± 6.6 | 13.2 ± 5.5 | 10.4 ± 6 |
| EC | 4.1 ± 1.2 | 2.9 ± 0.7 | 6.8 ± 2.8 | 6.1 ± 3 | 5.1 ± 2.7 |
| MD | 10.7 ± 2.4 | 8.1 ± 0.8 | 11.3 ± 2.7 | 8.6 ± 2.2 | 9.8 ± 2.6 |
| TE | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| Period | Concentration | PM2.5 | SO42− | NO3− | NH4+ | Cl | K | OM | EC | MD | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clean day | Mass concentration (μg/m3) | 46 | 7.0 | 5.5 | 4.2 | 0.8 | 0.6 | 10.5 | 4.0 | 10.2 | 3.2 |
| Proportion (%) | / | 15 | 12 | 9 | 2 | 1 | 23 | 9 | 22 | 7 | |
| Polluted day | Mass concentration (μg/m3) | 130 | 16.5 | 31.4 | 14.7 | 2.5 | 1.5 | 23.7 | 8.3 | 12.1 | 19.3 |
| Proportion (%) | / | 13 | 24 | 11 | 2 | 1 | 18 | 6 | 9 | 15 |
| City | Method | Source Contribution (%) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling | Model | Coal Combustion | Industrial Emission | Secondary Aerosol | Dust | Vehicle Exhaust | Biomass Burning | Other Sources | ||
| Luohe, China | 2017–2018, Urban, clean days | PMF | 11 | 28 | Soil dust: 12 Road dust: 13 | 16 | Mixed source (husbandry and food procession industry): 21 | This study | ||
| 2017–2018, Urban, pollution days | PMF | 2 | 49 | Soil dust: 6 Road dust: 5 | 17 | Mixed source (husbandry and food procession industry): 21 | This study | |||
| Zhengzhou China | 2013–2015 Pollution days | CMB | 14 | 8 | Nitrate: 13 Sulfate: 16 | 8 | 7 | 12 | carbon + refractory material: 2 | [66] |
| 2013–2015 Other days | CMB | 27 | 9 | Nitrate: 20 Sulfate: 18 | 14 | 15 | 9 | carbon + refractory material: 2 | ||
| Xiangtan China | 2016–2017 urban | PMF | 6–9 | Secondary inorganic aerosols: 25–27 | 16–18 | 21–22 | coal combustion + secondary aerosols: 19–21 steel industry: 8–9 | [69] | ||
| Lanzhou China | 2012 winter urban | PMF | 28.7 | 33.0 | 13.3 | 8.8 | Steel industry: 7.1 Power plant: 3.12 Smelting industry: 6.0 | [67] | ||
| 2013 summer urban | PMF | 3.1 | 14.8 | 11.6 | 25.2 | Steel industry: 6.7 Power plant: 3.4 Smelting industry: 35.2 | ||||
| Xian China | 1.1.–2.28. 2006. urban | PMF | 31.2 | 9.8 | 20.9 | 12.8 | 19.3 | 6.0 | [68] | |
| 1.1.–2.28. 2008. urban | PMF | 27.6 | 11.5 | 23.2 | 11.7 | 20.9 | 5.1 | |||
| 1.1.–2.28. 2010. urban | PMF | 24.1 | 12.6 | 17.5 | 19.4 | 21.3 | 5.1 | |||
| Taian China | 8–9.11. 2014. urban | PMF | 17.94 | 9.41 | 27.47 | 16.65 | Metal manufacturing: 19.06 other: 9.47 | [70] | ||
| New York, USA | June–July 2009, 2010, urban | PMF | sulfate:35 nitrate:14 | 14 | 16 | Aged sea-salt: 9 residual oil: <5 fresh sea-salt: <5 | [34] | |||
| Dongguan China | 2014 Suburb | PMF | 5–8 | 5–8 | Nitrate:5–8 Sulfate:20 secondary organic:10 | 5–8 | 21 | 11 | Ship emission: 5–8 | [71] |
| Fort McKay, Canada | March 2009–January 2011 suburb | PMF | Secondary sulfate: 30.77 | Fugitive dust: 32.4 | Secondary nitrate/biomass burning: 26.4 Mining/mobile: 10.1 Other: 0.4 | [6] | ||||
| Element | Child Intake Dinh(Child) mg kg−1 day−1 | Adult Intake Dinh(Adult) mg kg−1 day−1 | Lifetime Intake LADD mg kg−1 day−1 | Child Risk Index HI | Adult Risk Index HI | Carcinogenic Risk mg kg−1 day−1 |
|---|---|---|---|---|---|---|
| V | 7.31 × 10−7 | 3.65 × 10−7 | 1.04 × 10−4 | 5.21 × 10−5 | ||
| Cr | 2.11 × 10−6 | 1.05 × 10−6 | 7.42 × 10−6 | 7.38 × 10−2 | 3.68 × 10−2 | 3.11 × 10−4 |
| Mn | 3.65 × 10−6 | 1.82 × 10−6 | 2.61 × 10−1 | 1.30 × 10−1 | ||
| Co | 4.12 × 10−8 | 2.05 × 10−8 | 1.45 × 10−7 | 7.21 × 10−3 | 3.60 × 10−3 | 1.42 × 10−6 |
| Ni | 3.82 × 10−7 | 1.91 × 10−7 | 1.34 × 10−6 | 1.86 × 10−5 | 9.26 × 10−6 | 1.13 × 10−6 |
| Zn | 2.61 × 10−5 | 1.30 × 10−5 | 8.66 × 10−5 | 4.32 × 10−5 | ||
| As | 4.98 × 10−7 | 2.48 × 10−7 | 1.75 × 10−6 | 1.65 × 10−3 | 8.25 × 10−4 | 2.64 × 10−5 |
| Cd | 4.46 × 10−7 | 2.23 × 10−7 | 1.57 × 10−6 | 4.46 × 10−4 | 2.23 × 10−4 | 1.00 × 10−5 |
| Pb | 7.68 × 10−6 | 3.83 × 10−6 | 2.18 × 10−3 | 1.09 × 10−3 | ||
| ∑ | 4.16 × 10−5 | 2.08 × 10−5 | 1.22 × 10−5 | 3.46 × 10−1 | 1.73 × 10−1 | 3.50 × 10−4 |
| Sources | Soil Dust | Secondary Aerosol | Road Dust | Vehicle Exhaust | Mixed Source | Coal Combustion |
|---|---|---|---|---|---|---|
| Mass concentration (μg/m3) | 5.75 | 23.33 | 5.81 | 10.03 | 4.42 | 12.97 |
| HI Child | 9.25 × 10−4 | 2.08 × 10−2 | 7.54 × 10−2 | 1.85 × 10−1 | 5.86 × 10−2 | 5.35 × 10−3 |
| HI Adult | 4.62 × 10−4 | 1.04 × 10−2 | 3.76 × 10−2 | 9.23 × 10−2 | 2.92 × 10−2 | 2.67 × 10−3 |
| Rt | 4.29 × 10−6 | 1.35 × 10−5 | 4.50 × 10−5 | 1.26 × 10−4 | 1.58 × 10−4 | 4.35 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Zhao, X.; Wang, J.; Yin, B.; Geng, C.; Niu, D.; Yang, W.; Yu, H.; Li, W. Chemical Composition of PM2.5 and Its Impact on Inhalation Health Risk Evaluation in a City with Light Industry in Central China. Atmosphere 2020, 11, 340. https://doi.org/10.3390/atmos11040340
Wang N, Zhao X, Wang J, Yin B, Geng C, Niu D, Yang W, Yu H, Li W. Chemical Composition of PM2.5 and Its Impact on Inhalation Health Risk Evaluation in a City with Light Industry in Central China. Atmosphere. 2020; 11(4):340. https://doi.org/10.3390/atmos11040340
Chicago/Turabian StyleWang, Na, Xueyan Zhao, Jing Wang, Baohui Yin, Chunmei Geng, Dawei Niu, Wen Yang, Hao Yu, and Wei Li. 2020. "Chemical Composition of PM2.5 and Its Impact on Inhalation Health Risk Evaluation in a City with Light Industry in Central China" Atmosphere 11, no. 4: 340. https://doi.org/10.3390/atmos11040340
APA StyleWang, N., Zhao, X., Wang, J., Yin, B., Geng, C., Niu, D., Yang, W., Yu, H., & Li, W. (2020). Chemical Composition of PM2.5 and Its Impact on Inhalation Health Risk Evaluation in a City with Light Industry in Central China. Atmosphere, 11(4), 340. https://doi.org/10.3390/atmos11040340




