Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves
Abstract
:1. Introduction
2. Experiments
2.1. Study Area
2.2. Experimental Design, Collection of Plant Material, Bags Preparation and Exposure
2.3. Analytical Procedures
2.4. SEM Observations
2.5. Data Analysis
3. Results and Discussion
3.1. Metal Contents and Enrichment in Moss and Black Locust
3.2. Deposition fluxes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolterbeek, B. Biomonitoring of trace element air pollution: Principles, possibilities and perspectives. Environ. Pollut. 2002, 120, 11–21. [Google Scholar] [CrossRef]
- Augusto, S.; Pereira, M.J.; Maguas, C.; Branquinho, C. A step towards the use of biomonitors as estimators of atmospheric PAHs for regulatory purposes. Chemosphere 2013, 92, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Giordano, S.; Di Palma, A.; Spagnuolo, V.; De Nicola, F.; Adamo, P. Biomonitoring of atmospheric pollution by moss bags: Discriminating urban-rural structure in a fragmented landscape. Chemosphere 2016, 149, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, A.; Crespo Pardo, D.; Spagnuolo, V.; Adamo, P.; Bargagli, R.; Cafasso, D.; Capozzi, F.; Aboal, J.R.; Gonzalez, A.G.; Pokrovsky, O.; et al. Molecular and chemical characterization of a Sphagnum palustre clone: Key steps Towards a standardized and sustainable moss bag technique. Ecol. Indic. 2016, 71, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Harmens, H.; Foan, L.; Simon, V.; Mills, G. Mosses as biomonitors of atmospheric POPs pollution: A review. Environ. Pollut. 2013, 173, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Kodnik, D.; Candotto Carniel, F.; Licen, S.; Tolloi, A.; Barbieri, P.; Tretiach, M. Seasonal variations of PAHs content and distribution patterns in a mixed land use area: A case study in NE Italy with the transplanted lichen Pseudevernia furfuracea. Atmos. Environ. 2015, 113, 255–263. [Google Scholar] [CrossRef]
- Chao, C.Y.; Wong, K.K. Residential indoor PM10 and PM2.5 in Hong Kong and the elemental composition. Atmos. Environ. 2002, 36, 265–277. [Google Scholar] [CrossRef]
- Chen, X.G.; Fan, S.J. Characteristics of indoor/outdoor PM2.5 and elemental components in generic urban, roadside and industrial plant areas of Guangzhou City, China. J. Environ. Sci. 2007, 19, 35–43. [Google Scholar]
- Di Palma, A.; Capozzi, F.; Spagnuolo, V.; Adamo, P.; Giordano, S. Atmospheric particulate matter intercepted by moss-bags: Relations to moss trace element uptake and land use. Chemosphere 2017, 176, 361–368. [Google Scholar] [CrossRef]
- Spagnuolo, V.; Giordano, S.; Perez-Llamazares, A.; Ares, A.; Carballeira, A.; Fernandez, J.A.; Aboal, J.R. Distinguishing metal bioconcentration from PM in moss tissue: Testing methods of removing particles attached to the moss surface. Sci. Total Environ. 2013, 463–464, 727–733. [Google Scholar] [CrossRef]
- Capozzi, F.; Di Palma, A.; Adamo, P.; Sorrentino, M.C.; Giordano, S.; Spagnuolo, V. Indoor vs. outdoor airborne element array: A novel approach using moss bags to explore possible pollution sources. Environ. Pollut. 2019, 249, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.G.; Pokrovsky, O.S. Metal adsorption on mosses: Toward a universal adsorption model. J. Colloid Interf. Sci. 2014, 415, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.G.; Pokrovsky, O.S.; Beike, K.A.; Reski, R.; Di Palma, A.; Adamo, P.; Giordano, S.; Fernandez, J.A. Metal and proton adsorption capacities of natural and cloned Sphagnum mosses. J. Colloid Interf. Sci. 2016, 461, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Tretiach, M.; Adamo, P.; Bargagli, R.; Baruffo, L.; Carletti, L.; Crisafulli, P.; Giordano, S.; Modenesi, P.; Orlando, S.; Pittao, E. Lichen and moss bags as monitoring devices in urban areas. Part I: Influence of exposure on vitality. Environ. Pollut. 2007, 146, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Adamo, P.; Giordano, S.; Sforza, A.; Bargagli, R. Implementation of airborne trace element monitoring with devitalised transplants of Hypnum cupressiforme Hedw.: Assessment of temporal trends and element contribution by vehicular traffic in Naples city. Environ. Pollut. 2011, 159, 1620–1628. [Google Scholar] [CrossRef]
- Beike, A.K.; Spagnuolo, V.; Lüth, V.; Steinhart, F.; Ramos-Gomez, J.; Krebs, M.; Adamo, P.; Rey-Asensio, A.I.; Fernàndez, J.A.; Giordano, S.; et al. Clonal in vitro propagation of peat mosses (Sphagnum, L.) as novel green resources for basic and applied research. Plant Cell Tissue Organ. Cult. 2015, 120, 1037–1049. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, F.; Giordano, S.; Aboal, J.R.; Adamo, P.; Bargagli, R.; Boquete, T.; Di Palma, A.; Real, C.; Reski, R.; Spagnuolo, V.; et al. Best options for the exposure of traditional and innovative moss bags: A systematic evaluation in three European countries. Environ. Pollut. 2016, 214, 362–373. [Google Scholar] [CrossRef]
- De Nicola, F.; Murena, F.; Costagliola, M.A.; Alfani, A.; Baldantoni, D.; Prati, M.V.; Sessa, L.; Spagnuolo, V.; Giordano, S. A multi-approach monitoring of particulate matter, metals and PAHs in an urban street canyon. Environ. Sci. Pollut. Res. 2013, 20, 4969–4979. [Google Scholar] [CrossRef] [Green Version]
- Ares, A.; Aboal, J.R.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernandez, J.A. Moss bag biomonitoring: A methodological review. Sci. Total Environ. 2012, 432, 143–158. [Google Scholar] [CrossRef]
- Tzvetkova, N.; Petkova, K. Bioaccumulation of heavy metals by leaves of R. pseudoacacia as a bioindicator tree in industrial zones. J. Environ. Biol. 2015, 36, 59–63. [Google Scholar]
- Capozzi, F.; Di Palma, A.; Adamo, P.; Spagnuolo, V.; Giordano, S. Monitoring chronic and acute PAH atmospheric pollution using transplants of the moss Hypnum cupressiforme and Robinia pseudoacacia leaves. Atmos. Environ. 2017, 150, 45–54. [Google Scholar] [CrossRef]
- Steinnes, E.; Rühling, Å.; Lippo, H.; Makinen, A. Reference materials for large scale metal deposition surveys. Accred. Qual. Assur. 1997, 2, 243–249. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biometrics Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.C.; Di Palma, A.; Mele, F.; Arena, C.; Adamo, P.; Spagnuolo, V.; Giordano, S. Implication of vitality, seasonality and specific leaf area on PAH uptake in moss and lichen transplanted in bags. Ecol. Indic. 2020, 108, 105727. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of leaf trait relationships: A test across six biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Bussotti, F.; Grossoni, P. European and Mediterranean oaks (Quercus, L.; Fagaceae): SEM characterization of the micromorphology of the abaxial leaf surface. Bot. J. Linn. Soc. 1997, 124, 183–199. [Google Scholar] [CrossRef]
- Hueglin, C.; Gehrig, R.; Baltensperger, U.; Gysel, M.; Monn, C.; Vonmont, H. Chemical characterisation of PM2.5, PM10 and coarse particles at urban, nearcity and rural sites in Switzerland. Atmos. Environ. 2005, 39, 637–651. [Google Scholar] [CrossRef]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Hejwowski, T.; Weronski, A. The effect of thermal barrier coating on diesel engine performance. Vacuum 2002, 65, 427–432. [Google Scholar] [CrossRef]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef]
- Arpadjan, S.; Celik, G.; Taskesen, S.; Gucer, S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem. Toxicol. 2008, 46, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G.; Bastos de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, D.; Boutin, C.; Allison, J.E.; Parsons, J.L.; Ellis, D.M. Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS ONE 2015, 10, 0129936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 534. [Google Scholar]
- Barceloux, D.G.; Barceloux, D. Molybdenum. J. Toxicol. Clin. Tox. 1999, 37, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, R. Trace Elements in Terrestrial Plants. An Ecophysiological Approach to Biomonitoring and Biorecovery; Springer-Verlag: Berlin, Germany, 1998; p. 324. [Google Scholar]
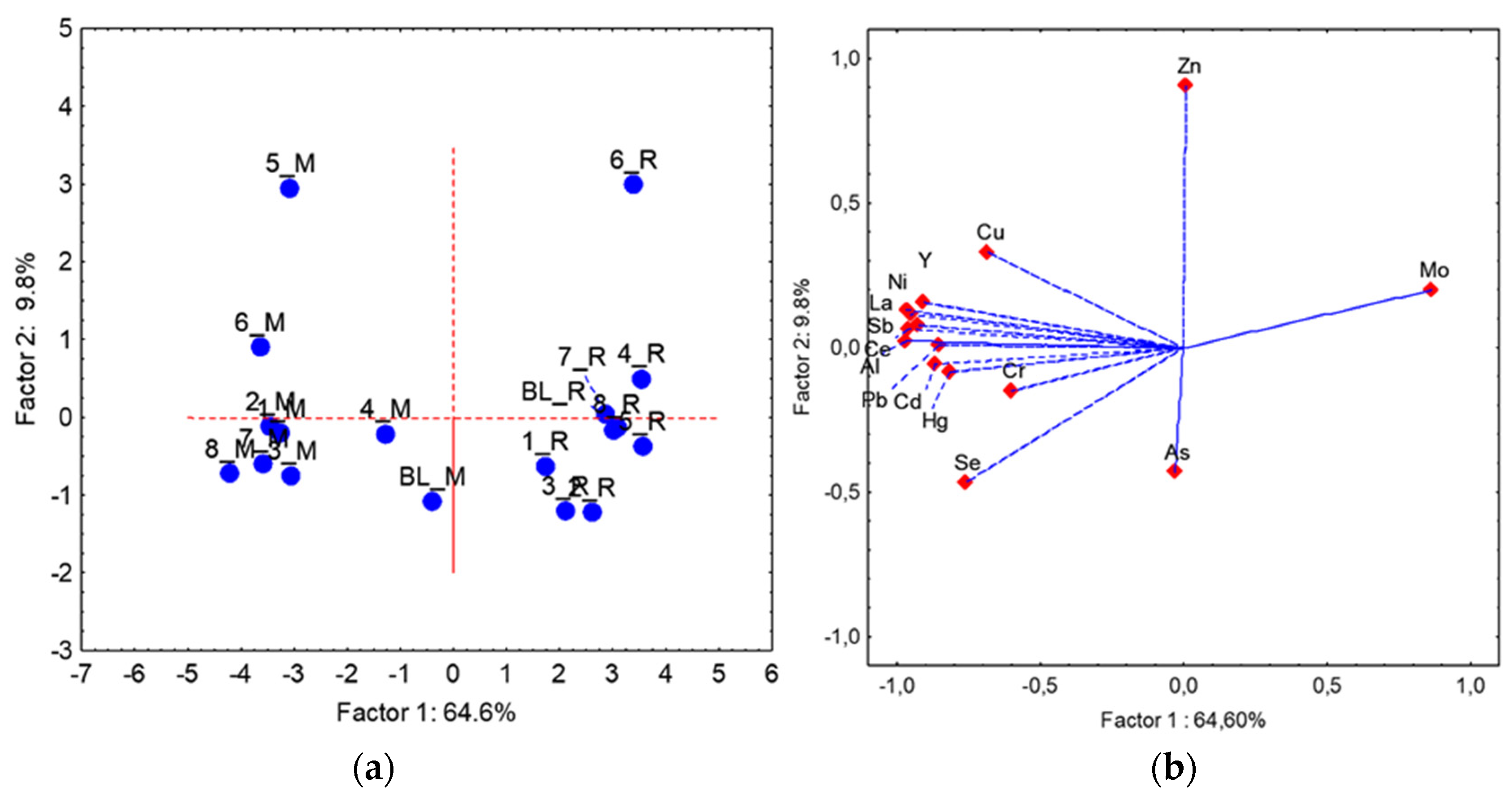
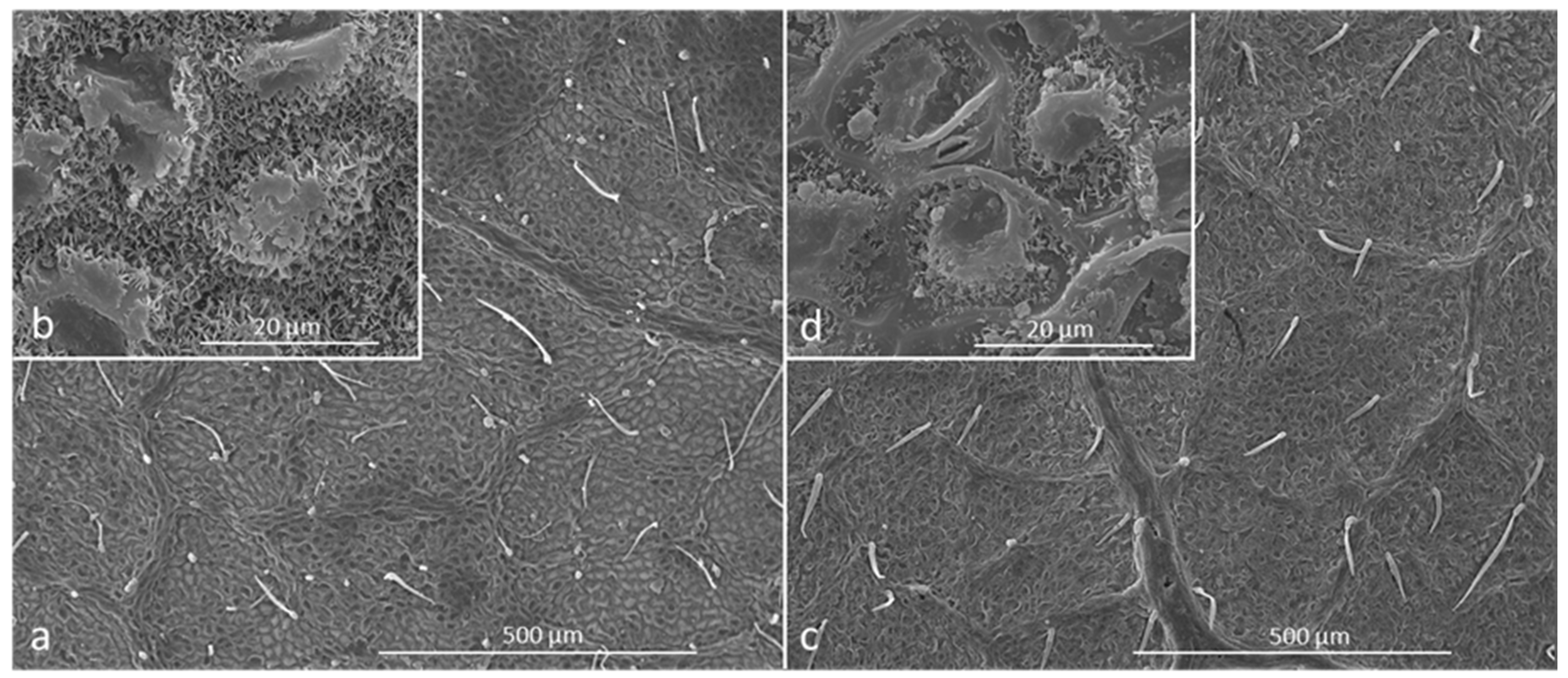

| Al ** | As | Cd ** | Ce ** | Cr ** | Cu * | Hg ** | La ** | Mo ** | Ni ** | Pb ** | Sb ** | Se * | Y ** | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1_R | 300 ± 20 | 0.2 ± 0.1 | 0.02 ± 0.00 | 0.7 ± 0.2 | 2.2 ± 0.1 | 8.6 ± 0.1 | 0.05 ± 0.01 | 0.5 ± 0.1 | 1.3 ± 0.2 | 0.3 ± 0.1 | 1.8 ± 0.2 | 0.09 ± 0.02 | 0.52 ± 0.02 | 0.15 ± 0.01 | 28 ± 0.9 |
| 2_R | 200 ± 10 | 0.5 ± 0.4 | 0.01 ± 0.00 | 0.4 ± 0.1 | 3.7 ± 1.5 | 5.2 ± 0.6 | 0.06 ± 0.00 | 0.2 ± 0.0 | 2.5 ± 0.2 | 0.3 ± 0.2 | 1.2 ± 0.1 | 0.12 ± 0.00 | 0.33 ± 0.11 | 0.08 ± 0.00 | 24 ± 1.2 |
| 3_R | 433 ± 57 | 0.4 ± 0.2 | 0.01 ± 0.00 | 0.9 ± 0.2 | 3.1 ± 0.4 | 5.9 ± 0.3 | 0.04 ± 0.02 | 0.4 ± 0.2 | 1.7 ± 0.1 | 0.4 ± 0.1 | 1.9 ± 0.5 | 0.12 ± 0.01 | 0.40 ± 0.02 | 0.17 ± 0.02 | 19 ± 0.1 |
| 4_R | 200 ± 27 | 0.3 ± 0.1 | 0.01 ± 0.00 | 0.2 ± 0.0 | 3.1 ± 0.3 | 9.1 ± 0.4 | 0.01 ± 0.00 | 0.07 ± 0.01 | 2.4 ± 0.1 | 0.7 ± 0.0 | 0.1 ± 0.0 | 0.04 ± 0.01 | 0.23 ± 0.01 | 0.02 ± 0.00 | 35 ± 1.9 |
| 5_R | 430 ± 10 | 0.3 ± 0.1 | 0.01 ± 0.00 | 0.1 ± 0.0 | 2.8 ± 0.0 | 6.6 ± 0.1 | 0.02 ± 0.00 | 0.05 ± 0.01 | 2.5 ± 0.1 | 0.4 ± 6.7 | 0.6 ± 0.1 | 0.04 ± 0.01 | 0.33 ± 0.03 | 0.03 ± 0.00 | 25 ± 0.8 |
| 6_R | 180 ± 54 | 0.1 ± 0.0 | 0.01 ± 0.00 | 0.5 ± 0.2 | 2.6 ± 0.1 | 8.6 ± 0.4 | 0.03 ± 0.00 | 0.5 ± 0.1 | 3.5 ± 0.5 | 0.7 ± 0.1 | 0.5 ± 0.3 | 0.15 ± 0.01 | 0.10 ± 0.00 | 0.14 ± 0.02 | 63 ± 4.5 |
| 7_R | 166 ± 57 | 0.3 ± 0.2 | 0.01 ± 0.00 | 0.4 ± 0.1 | 2.7 ± 0.2 | 6.9 ± 0.1 | 0.03 ± 0.01 | 0.2 ± 0.0 | 2.3 ± 0.2 | 0.8 ± 1.3 | 0.8 ± 0.2 | 0.24 ± 0.08 | 0.27 ± 0.01 | 0.12 ± 0.01 | 31 ± 1.5 |
| 8_R | 100 ± 24 | 0.5 ± 0.3 | 0.05 ± 0.01 | 0.3 ± 0.1 | 3.1 ± 0.1 | 6.7 ± 0.1 | 0.03 ± 0.01 | 0.2 ± 0.0 | 2.5 ± 0.3 | 0.3 ± 0.1 | 0.8 ± 0.1 | 0.30 ± 0.02 | 0.23 ± 0.01 | 0.07 ± 0.00 | 39 ± 1.5 |
| BL_R | 102 ± 20 | 0.1 ± 0.0 | BDL | 0.1 ± 0.0 | 2.5 ± 0.1 | 5.8 ± 0.0 | 0.05 ± 0.02 | 0.07 ± 0.01 | 0.7 ± 0.2 | 2.6 ± 0.1 | 0.2 ± 0.0 | 0.01 ± 0.00 | 0.24 ± 0.02 | 0.02 ± 0.00 | 18 ± 0.7 |
| 1_M | 2385 ± 100 | 0.35 ± 0.07 | 0.13 ± 0.02 | 3.95 ± 0.09 | 3.93 ± 0.20 | 11.9 ± 1.95 | 0.05 ± 0.03 | 1.94 ± 0.03 | 0.23 ± 0.03 | 1.7 ± 0.26 | 5.34 ± 0.31 | 0.82 ± 0.16 | 0.46 ± 0.05 | 0.76 ± 0.02 | 20.6 ± 1.56 |
| 2_M | 2366 ± 100 | 0.35 ± 0.21 | 0.13 ± 0.01 | 3.82 ± 0.28 | 3.56 ± 0.05 | 13.0 ± 2.64 | 0.05 ± 0.05 | 1.91 ± 0.07 | 0.28 ± 0.02 | 1.66 ± 0.05 | 6.61 ± 0.52 | 0.89 ± 0.07 | 0.46 ± 0.11 | 0.79 ± 0.04 | 17.9 ± 0.83 |
| 3_M | 2166 ± 200 | 0.5 ± 0.36 | 0.12 ± 0.02 | 3.83 ± 0.22 | 3.81 ± 0.09 | 11.2 ± 0.37 | 0.05 ± 0.04 | 1.9 ± 0.02 | 0.27 ± 0.01 | 1.56 ± 0.11 | 7.11 ± 0.73 | 0.89 ± 0.07 | 0.46 ± 0.05 | 0.71 ± 0.03 | 16.9 ± 0.56 |
| 4_M | 1666 ± 50 | 0.2 ± 0.0 | 0.1 ± 0.00 | 2.72 ± 0.09 | 3.66 ± 0.15 | 7.8 ± 0.57 | 0.06 ± 0.04 | 1.26 ± 0.07 | 0.21 ± 0.02 | 1.6 ± 0.22 | 3.11 ± 0.17 | 0.65 ± 0.09 | 0.36 ± 0.11 | 0.54 ± 0.03 | 20.3 ± 1.5 |
| 5_M | 2100 ± 100 | 0.3 ± 0.14 | 0.15 ± 0.01 | 3.41 ± 0.18 | 3.90 ± 0.02 | 11.0 ± 0.28 | 0.06 ± 0.03 | 1.65 ± 0.03 | 0.26 ± 0.02 | 2.33 ± 0.11 | 6.59 ± 2.73 | 0.77 ± 0.08 | 0.36 ± 0.05 | 0.79 ± 0.05 | 57.8 ± 8.25 |
| 6_M | 2600 ± 200 | 0.2 ± 0.1 | 0.12 ± 0.01 | 4.65 ± 0.21 | 3.73 ± 0.11 | 8.91 ± 0.35 | 0.06 ± 0.02 | 2.22 ± 0.05 | 0.24 ± 0.02 | 1.7 ± 0.11 | 4.42 ± 0.11 | 1.15 ± 0.13 | 0.46 ± 0.05 | 0.98 ± 0.02 | 46.4 ± 3.33 |
| 7_M | 1900 ± 324 | 0.5 ± 0.2 | 0.13 ± 0.03 | 3.36 ± 0.42 | 3.66 ± 0.15 | 9.68 ± 1.11 | 0.05 ± 0.03 | 1.59 ± 0.19 | 0.18 ± 0.01 | 2.23 ± 0.57 | 10.9 ± 4.03 | 1.32 ± 0.16 | 0.43 ± 0.11 | 0.74 ± 0.05 | 20.4 ± 1.12 |
| 8_M | 1833 ± 542 | 0.15 ± 0.07 | 0.33 ± 0.04 | 2.75 ± 0.82 | 8.43 ± 1.44 | 9.97 ± 0.97 | 0.07 ± 0.04 | 1.34 ± 0.33 | 0.29 ± 0.02 | 2.96 ± 0.11 | 3.7 ± 0.74 | 0.85 ± 0.11 | 0.53 ± 0.11 | 0.6 ± 0.16 | 20.4 ± 1.91 |
| BL_M | 1810 ± 73 | 0.2 ± 0.1 | 0.12 ± 0.01 | 2.64 ± 0.07 | 3.81 ± 0.16 | 4.92 ± 0.5 | 0.04 ± 0.02 | 1.24 ± 0.03 | 0.17 ± 0.01 | 1.19 ± 0.07 | 2.05 ± 1.41 | 0.22 ± 0.01 | 0.39 ± 0.07 | 0.5 ± 0.02 | 11.4 ± 0.59 |
| Al | As | Cd | Ce | Cr | Cu | Hg | La | Mo | Ni | Pb | Sb | Se | Y | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1_R | 25.25 | 0.0224 | 0.0017 | 0.0640 | 0.1852 | 0.7239 | 0.0039 | 0.0457 | 0.1167 | 0.0281 | 0.1554 | 0.0073 | 0.0421 | 0.0123 | 2.37 |
| 2_R | 16.83 | 0.0421 | 0.0008 | 0.0396 | 0.3143 | 0.4442 | 0.0048 | 0.0171 | 0.2144 | 0.0295 | 0.1041 | 0.0098 | 0.0281 | 0.0064 | 2.07 |
| 3_R | 36.47 | 0.0337 | 0.0008 | 0.0794 | 0.2525 | 0.5011 | 0.0036 | 0.0382 | 0.1504 | 0.0393 | 0.1658 | 0.0104 | 0.0337 | 0.0143 | 1.61 |
| 4_R | 16.85 | 0.0309 | 0.0008 | 0.0076 | 0.2666 | 0.7694 | 0.0008 | 0.0059 | 0.2034 | 0.0617 | 0.0157 | 0.0031 | 0.0196 | 0.0019 | 2.96 |
| 5_R | 36.20 | 0.0253 | 0.0008 | 0.0115 | 0.2385 | 0.5578 | 0.0017 | 0.0051 | 0.2183 | 0.0337 | 0.0544 | 0.0036 | 0.0281 | 0.0024 | 2.11 |
| 6_R | 15.19 | 0.0084 | 0.0008 | 0.0476 | 0.2231 | 0.7269 | 0.0028 | 0.0463 | 0.3026 | 0.0589 | 0.0463 | 0.0126 | 0.0084 | 0.0114 | 5.34 |
| 7_R | 14.02 | 0.0309 | 0.0008 | 0.0415 | 0.2273 | 0.5825 | 0.0028 | 0.0244 | 0.2006 | 0.0673 | 0.0730 | 0.0199 | 0.0224 | 0.0098 | 2.64 |
| 8_R | 8.41 | 0.0463 | 0.0045 | 0.0272 | 0.2525 | 0.5710 | 0.0023 | 0.0185 | 0.2149 | 0.0281 | 0.0755 | 0.0253 | 0.0196 | 0.0061 | 3.29 |
| BL_R | 8.61 | 0.0084 | - | 0.0072 | 0.2151 | 0.6086 | 0.0041 | 0.0055 | 0.0635 | 0.0168 | 0.0183 | 0.0006 | 0.0206 | 0.0019 | 1.54 |
| 1_M | 98.17 | 0.0265 | 0.0024 | 0.2295 | 0.0218 | 1.2339 | 0.0017 | 0.1233 | 0.0106 | 0.0899 | 0.5802 | 0.1061 | 0.0135 | 0.0449 | 1.62 |
| 2_M | 98.17 | 0.0265 | 0.0029 | 0.2077 | - | 1.4291 | 0.0016 | 0.1186 | 0.0189 | 0.0841 | 0.8030 | 0.1190 | 0.0135 | 0.0512 | 1.15 |
| 3_M | 62.90 | 0.0529 | 0.0006 | 0.2089 | - | 1.1075 | 0.0004 | 0.1162 | 0.0183 | 0.0664 | 0.8924 | 0.1184 | 0.0135 | 0.0373 | 0.97 |
| 4_M | - | 0.0000 | - | 0.0137 | - | 0.5155 | 0.0019 | 0.0039 | 0.0077 | 0.0723 | 0.1863 | 0.0755 | - | 0.0077 | 1.57 |
| 5_M | 51.14 | 0.0176 | 0.0053 | 0.1330 | 0.0159 | 1.0858 | 0.0023 | 0.0721 | 0.0165 | 0.2016 | 0.8001 | 0.0978 | - | 0.0507 | 15.23 |
| 6_M | 139.32 | 0.0000 | 0.0006 | 0.3529 | - | 0.7036 | 0.0027 | 0.1738 | 0.0124 | 0.0899 | 0.4179 | 0.1643 | 0.0135 | 0.0851 | 6.17 |
| 7_M | 15.87 | 0.0529 | 0.0029 | 0.1254 | - | 0.8383 | 0.0014 | 0.0627 | 0.0024 | 0.1840 | 1.5602 | 0.1948 | 0.0076 | 0.0419 | 1.58 |
| 8_M | 4.11 | - | 0.0376 | 0.0184 | 0.8154 | 0.8900 | 0.0037 | 0.0186 | 0.0218 | 0.3133 | 0.2904 | 0.1113 | 0.0253 | 0.0180 | 1.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, F.; Di Palma, A.; Sorrentino, M.C.; Adamo, P.; Giordano, S.; Spagnuolo, V. Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves. Atmosphere 2020, 11, 148. https://doi.org/10.3390/atmos11020148
Capozzi F, Di Palma A, Sorrentino MC, Adamo P, Giordano S, Spagnuolo V. Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves. Atmosphere. 2020; 11(2):148. https://doi.org/10.3390/atmos11020148
Chicago/Turabian StyleCapozzi, Fiore, Anna Di Palma, Maria Cristina Sorrentino, Paola Adamo, Simonetta Giordano, and Valeria Spagnuolo. 2020. "Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves" Atmosphere 11, no. 2: 148. https://doi.org/10.3390/atmos11020148
APA StyleCapozzi, F., Di Palma, A., Sorrentino, M. C., Adamo, P., Giordano, S., & Spagnuolo, V. (2020). Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves. Atmosphere, 11(2), 148. https://doi.org/10.3390/atmos11020148








