Study on the Sensitivity of Summer Ozone Density to the Enhanced Aerosol Loading over the Tibetan Plateau
Abstract
1. Introduction
2. Introduction of Data and the Model
2.1. Satellite Data
2.2. Model Description
2.3. Aerosol and Heterogeneous Chemical Reactions
3. Simulation and Result
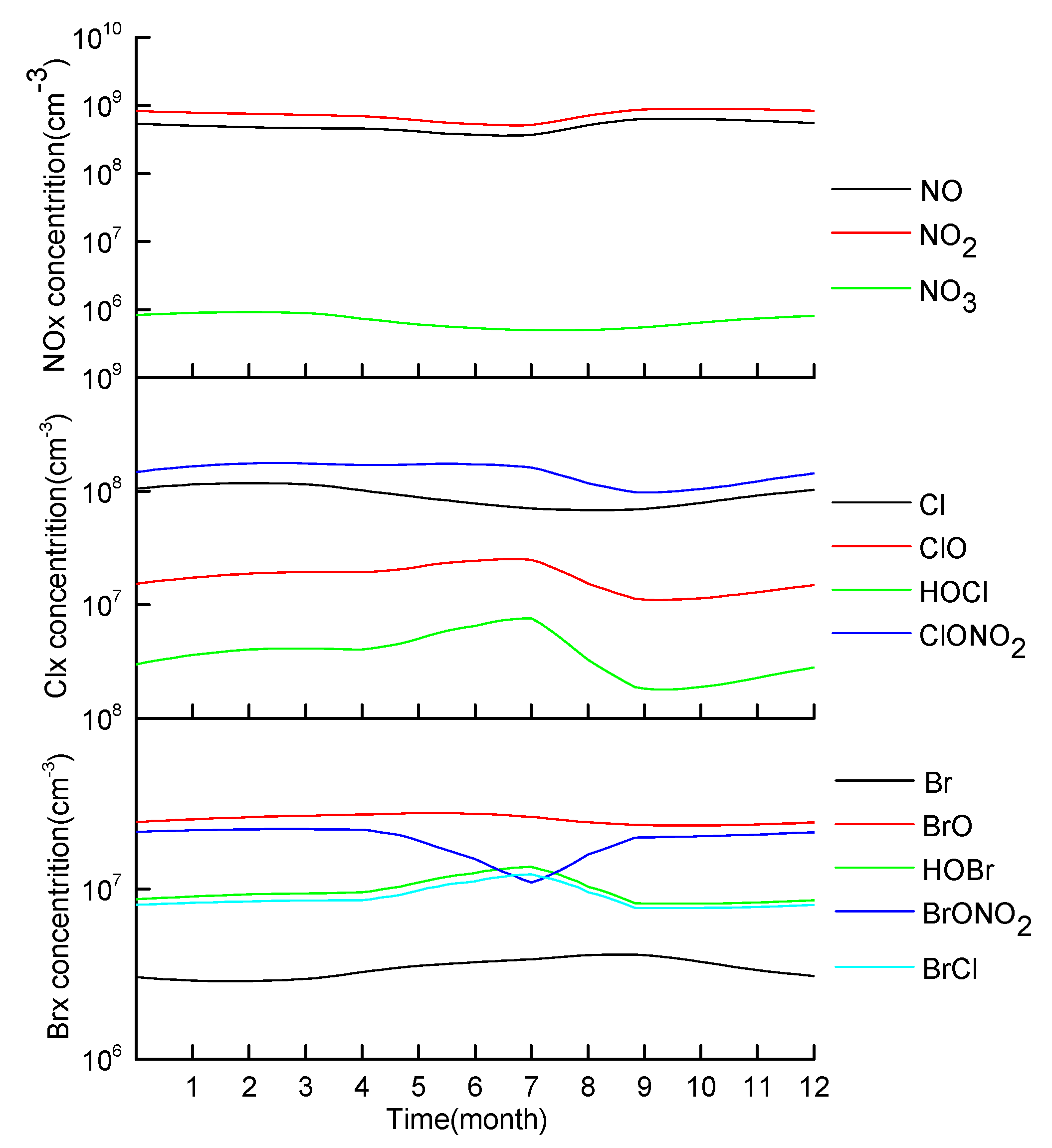
3.1. Simulation Test
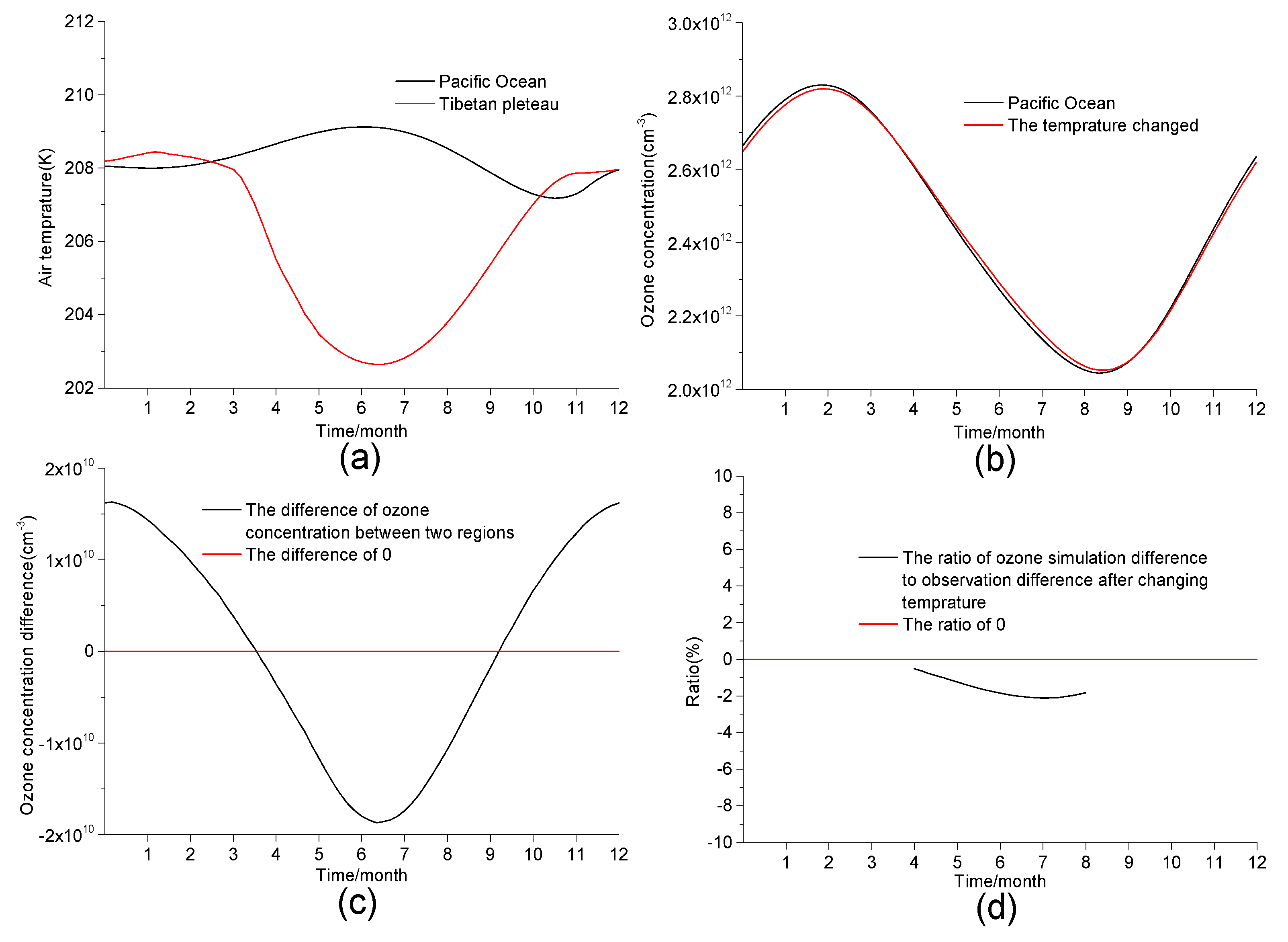
3.2. Influence of Temperature and Pressure
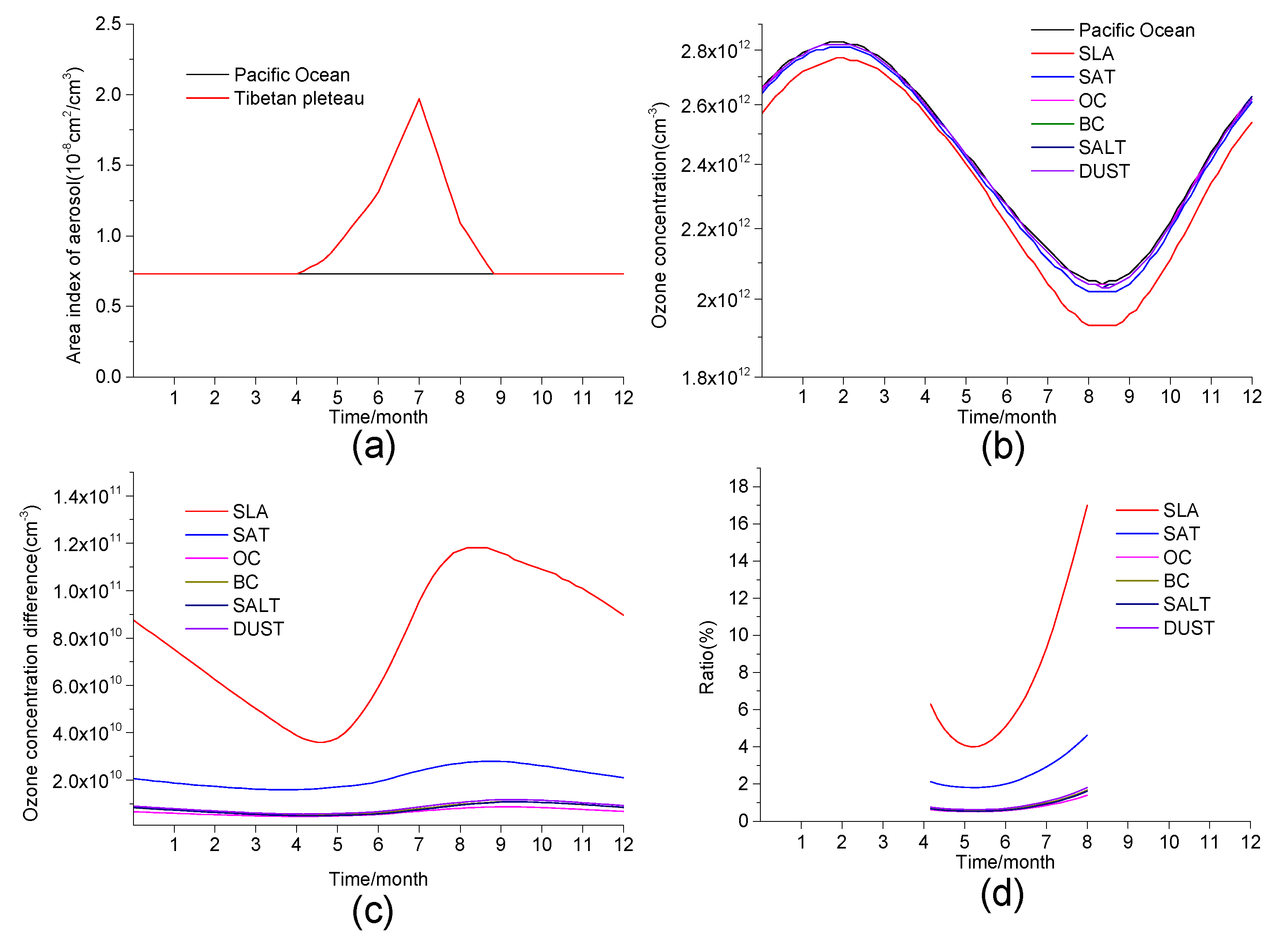
3.3. The Influence of Aerosols
4. Summary and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farman, J.C.; Gardiner, B.G.; Shanklin, J.D. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 1985, 315, 207–210. [Google Scholar] [CrossRef]
- Lefèvre, F.; Figarol, F.; Carslaw, K.S.; Peter, T. The 1997 Arctic Ozone depletion quantified from three-dimensional model simulations. Geophys. Res. Lett. 1998, 25, 2425–2428. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Li, W.; Shi, J. The changes of total ozone in China and low value ozone center of Tibetan Plateau. Chinese Sci. Bull. 1995, 40, 1396–1398. [Google Scholar]
- Fu, C.; Li, W.; Zhou, X. A simulated experiment on the formation of the ozone valley in summer over the Tibetan Plateau. In Changes of Atmospheric Ozone in China and Its Influence on Climate and Environment; China Meteorological Press: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Tian, W.; Chipperfield, M.; Huang, Q. Effects of the Tibetan Plateau on total column ozone distribution. Tellus 2008, 60B, 622–635. [Google Scholar] [CrossRef]
- Bian, J. Recent advances in the study of atmospheric vertical structures in upper troposphere and lower stratosphere. Adv. Earth Sci. 2009, 24, 262–271. [Google Scholar]
- Guo, D.; Zhou, X.; Liu, Y.; Li, W.; Wang, P. The dynamic effects of the South Asian high on the ozone valley over the Tibetan Plateau. Acta Meteorol. Sinica. 2012, 70, 1302–1311. (In Chinese) [Google Scholar] [CrossRef]
- Xu, P.; Tian, W.; Zhang, J.; Luo, J.; Huang, Q.; Zhang, J. A simulation study of the transport of the stratospheric ozone to the troposphere over the northwest side of the Tibetan Plateau in spring. Acta Meteorol. Sinica 2015, 73, 529–545. (In Chinese) [Google Scholar]
- Liu, Y.; Li, W.L.; Zhou, X.J.; He, J. Mechanism of formation of the Ozone Valley over the Tibetan Plateau in summer transport and chemical process of ozone. Adv. Atmos. Sci. 2003, 20, 103–109. [Google Scholar] [CrossRef]
- Randel, W.J.; Park, M. Deep convective influence on the Asian summer monsoon anticyclone and associated tracer variability observed with Atmospheric Infrared Sounder (AIRS). J. Geophys. Res. 2006, 111, D12314. [Google Scholar] [CrossRef]
- Randel, W.J.; Park, M.; Emmons, L.; Kinnison, D.; Bernath, P.; Walker, K.A.; Boone, C.; Pumphrey, H. Asian monsoon transport of pollution to the stratosphere. Science 2010, 328, 611–613. [Google Scholar] [CrossRef]
- Park, M.; Randel, W.J.; Gettelman, A.; Massie, S.T.; Jiang, J.H. Transport above the Asian summer monsoon anticyclone inferred from Aura Microwave Limb Sounder tracers. J. Geophys. Res. 2007, 112, D16309. [Google Scholar] [CrossRef]
- Park, M.; Randel, W.J.; Emmons, L.K.; Livesey, N.J. Transport pathways of carbon monoxide in the Asian summer monsoon diagnosed from model of ozone and related tracers (MOZART). J. Geophys. Res. 2009, 114, D08303. [Google Scholar] [CrossRef]
- Bian, J.; Yan, R.; Chen, H. Tropospheric pollutant transport to the stratosphere by Asian summer monsoon. Chinese J. Atmos. Sci. 2011, 35, 897–902. (In Chinese) [Google Scholar]
- Solomon, S. Stratospheric ozone depletion: A review of concepts and history. Rev. Geophys. 1999, 37, 275–316. [Google Scholar] [CrossRef]
- Vernier, J.P.; Thomason, L.W.; Kar, J. CALIPSO detection of an Asian tropopause aerosol layer. Geophys. Res. Lett. 2011, 38, 1451–1453. [Google Scholar] [CrossRef]
- Thomason, L.W.; Vernier, J.P. Improved SAGE II cloud/aerosol categorization and observations of the Asian tropopause aerosol layer: 1989–2005. Atmos. Chem. Phys. 2013, 13, 4605–4616. [Google Scholar] [CrossRef]
- Junge, C.E.; Chagnon, C.W.; Manson, J.E. Stratospheric aerosols. J. Meteorol. 1961, 18, 81–108. [Google Scholar] [CrossRef]
- Li, W.; Yu, S. A numerical simulation of the Spatial-temporal distribution of aerosols over the Tibetan Plateau and their radiative forcing and climatic effects. Sci. China (D Ser.) 2001, B12, 300–307. (In Chinese) [Google Scholar]
- Zhou, R.; Chen, Y.; Bi, Y.; Yi, M. Aerosol distribution over the Tibetan Plateau and its relationship with ozone. Plateau Meteorol. 2008, 27, 500–508. (In Chinese) [Google Scholar]
- Liu, Y.; Li, W.; Zhou, X. A possible effect of heterogeneous reactions on the formation of the ozone valley over the Tibetan Plateau. Acta Meteorol. Sinica 2010, 68, 836–846. (In Chinese) [Google Scholar]
- Thomason, L.W.; Bedka, K.M. Increase in upper tropospheric and lower stratospheric aerosol levels and its potential connection with Asian pollution. J. Geophys. Res. Atmos. 2015, 120, 1608–1619. [Google Scholar]
- Margaret, A.T. Polar clouds and Sulfate Aerosols. Science 1996, 272, 1597. [Google Scholar]
- Lowe, D.; Mackenzie, A.R. Polar stratospheric cloud microphysics and chemistry. J. Atmos. Sol.-Terr. Phy. 2008, 70, 13–40. [Google Scholar] [CrossRef]
- Mauldin, L.E.; Zaun, N.H.; McCormick, M.P.; Guy, J.H.; Vaughn, W.R. Stratospheric aerosol and gase experiment II instrument: A functional description. Opt. Eng. 1985, 24, 242307. [Google Scholar] [CrossRef]
- Chu, W.P.; McCormick, M.P.; Lenoble, J.; Brogniez, C.; Pruvost, P. SAGE II inversion algorithm. J. Geophys. Res. 1989, 94, 8339–8351. [Google Scholar] [CrossRef]
- Thomason, L.W.; Poole, L.R.; Randall, C.E. SAGE III aerosol extinction validation in the Arctic winter: Comparisons with SAGE II and POAM III. Atmos. Chem. Phys. 2007, 7, 1423–1433. [Google Scholar] [CrossRef]
- Steinbrecht, W.; Claude, H.; Köhler, U.; Hoinka, K.P. Correlations between tropopause height and total ozone: Implications for long-term changes. J. Geophys. Res. 1998, 103, 19183–19192. [Google Scholar] [CrossRef]
- Yang, P.; Brasseur, G. Dynamics of the oxygen-hydrogen system in the mesosphere: 1. Photochemical equilibria and catastrophe. J. Geophys. Res. 1994, 99, 20955–20965. [Google Scholar] [CrossRef]
- Wang, G.; Yang, P. On the Nonlinear response of lower stratosphere ozone to NOX and ClOX perturbations. Chinese J. Geophy. 2007, 50, 51–57. (In Chinese) [Google Scholar] [CrossRef]
- Yan, J.; Jin, L.; Wang, G. A Study of the mechanism of nonlinear responses in stratospheric ozone. Climat. Env. Res. 2012, 17, 639–645. (In Chinese) [Google Scholar]
- Yu, P.; Toon, O.B.; Neely, R.R.; Martinsson, B.G.; Brenninkmeijer, C.A.M. Composition and physical properties of the Asian tropopause aerosol layer and the North American tropospheric aerosol layer. Geophys. Res. Lett. 2015, 42, 2540–2546. [Google Scholar] [CrossRef]
- Gu, Y.; Liao, H.; Bian, J. Summertime nitrate aerosol in the upper troposphere and lower stratosphere over the Tibetan Plateau and the South Asian summer monsoon region. Atmos. Chem. Phys. 2016, 15, 32049–32099. [Google Scholar] [CrossRef]
- Carslaw, K.S.; Luo, B.P.; Clegg, S.L.; Peter, T.; Brimblecombe, P.; Crutzen, P.J. Stratospheric aerosol growth and HNO3 gas phase depletion from coupled HNO3 and water uptake by liquid particles. Geophys. Res. Lett. 1994, 21, 2479–2482. [Google Scholar] [CrossRef]
- Carslaw, K.S.; Luo, B.P.; Peter, T. An analytic expression for the composition of aqueous HNO3-H2SO4 stratospheric aerosols including gas phase removal of HNO3. Geophys. Res. Lett. 1995, 22, 1877–1880. [Google Scholar] [CrossRef]
- Carslaw, K.S.; Peter, T.; Clegg, S.L. Modeling the composition of liquid stratospheric aerosols. Rev. Geophys. 1997, 35, 125–154. [Google Scholar] [CrossRef]
- Yu, P.; Rosenlof, K.H.; Liu, S.; Telg, H.; Thornberry, T.D.; Rollins, A.W.; Portmann, R.W.; Bai, Z.; Ray, E.A.; Duan, Y.; et al. Efficient transport of tropospheric aerosol into the stratosphere via the Asian summer monsoon anticyclone. P. Natl. Acad. Sci. 2017, 114, 6972–6977. [Google Scholar] [CrossRef]
- Kirner, O.; Ruhnke, R.; Buchholz-Dietsch, J.; Jökel, P.; Brühl, C.; Steil, B. Simulation of polar stratospheric clouds in the chemistry-climate-model EMAC via the submodel PSC. Geosci. Model Dev. Discuss. 3 2010, 2071–2108. [Google Scholar] [CrossRef]
- Burkholder, J.B.; Sander, S.P.; Abbatt, J.; Barker, J.R.; Huie, R.E.; Kolb, C.E.; KuryloM, J.; Orkin, V.L.; Wilmouth, D.M.; Wine, P.H. Chemical kinetics and photochemical data for use in atmospheric studies; Evaluation No.18, JPL Publication 15-10; Jet Propulsion Laboratory: Pasadena, CA, USA, 2015. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, Y. Ozone variations over the Tibetan and Iranian Plateaus and their relationship with the South Asia High. J. Univ. Sci. Tech. Chin. 2005, 35, 899–908. (In Chinese) [Google Scholar]
- Vernier, J.P.; Pommereau, J.P.; Garnier, A.; Pelon, J.; Larsen, N.; Nielsen, J.; Christensen, T.; Cairo, F.; Thomason, L.W.; Leblanc, T.; et al. Tropical stratospheric aerosol layer from CALIPSO lidar observations. J. Geophys. Res. 2009, 114, D00H10. [Google Scholar] [CrossRef]
- Bourgeois, Q.; Bey, I.; Stier, P. A permanent aerosol layer at the tropical tropopause layer driven by the intertropical convergence zone. Atmos. Chem. Phys. Discuss. 2012, 12, 2863–2889. [Google Scholar] [CrossRef]
- He, Q.; Li, C.; Ma, J.; Wang, H.; Yan, X.; Lu, J.; Liang, Z.; Qi, G. Lidar-observed enhancement of aerosols in the upper troposphere and lower stratosphere over the Tibetan Plateau induced by the Nabro volcano eruption. Atmos. Chem. Phys. 2014, 14, 11687–11696. [Google Scholar] [CrossRef]
- Orlando, J.; Brasseur, G.; Tyndall, G.S. Atmospheric Chemistry and Global Change; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Kilifarska, N.A. Hemispherical asymmetry of the lower stratospheric O3 response to galactic cosmic rays forcing. ACS Earth Space Chem. 2017, 1, 80–88. [Google Scholar] [CrossRef]
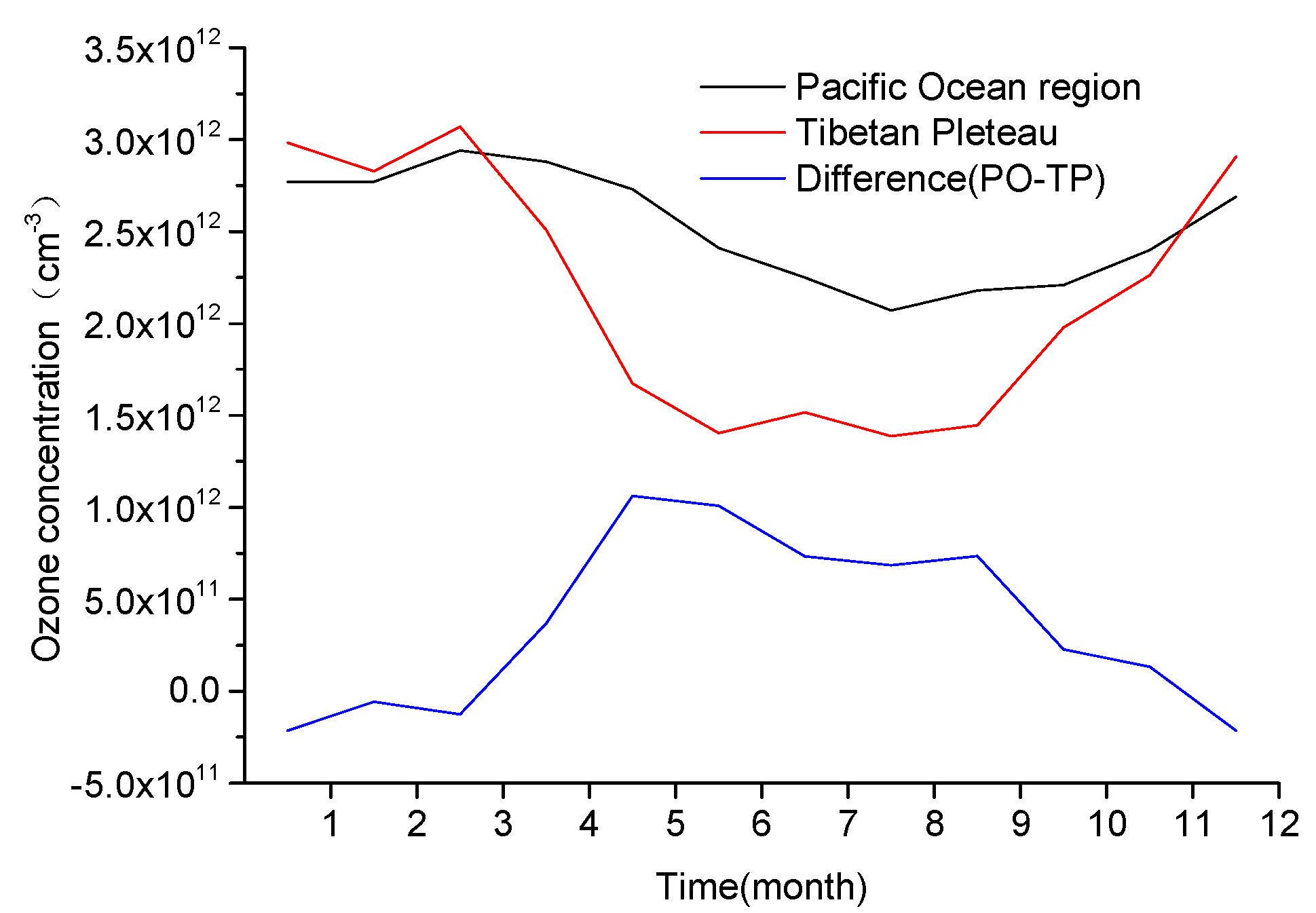
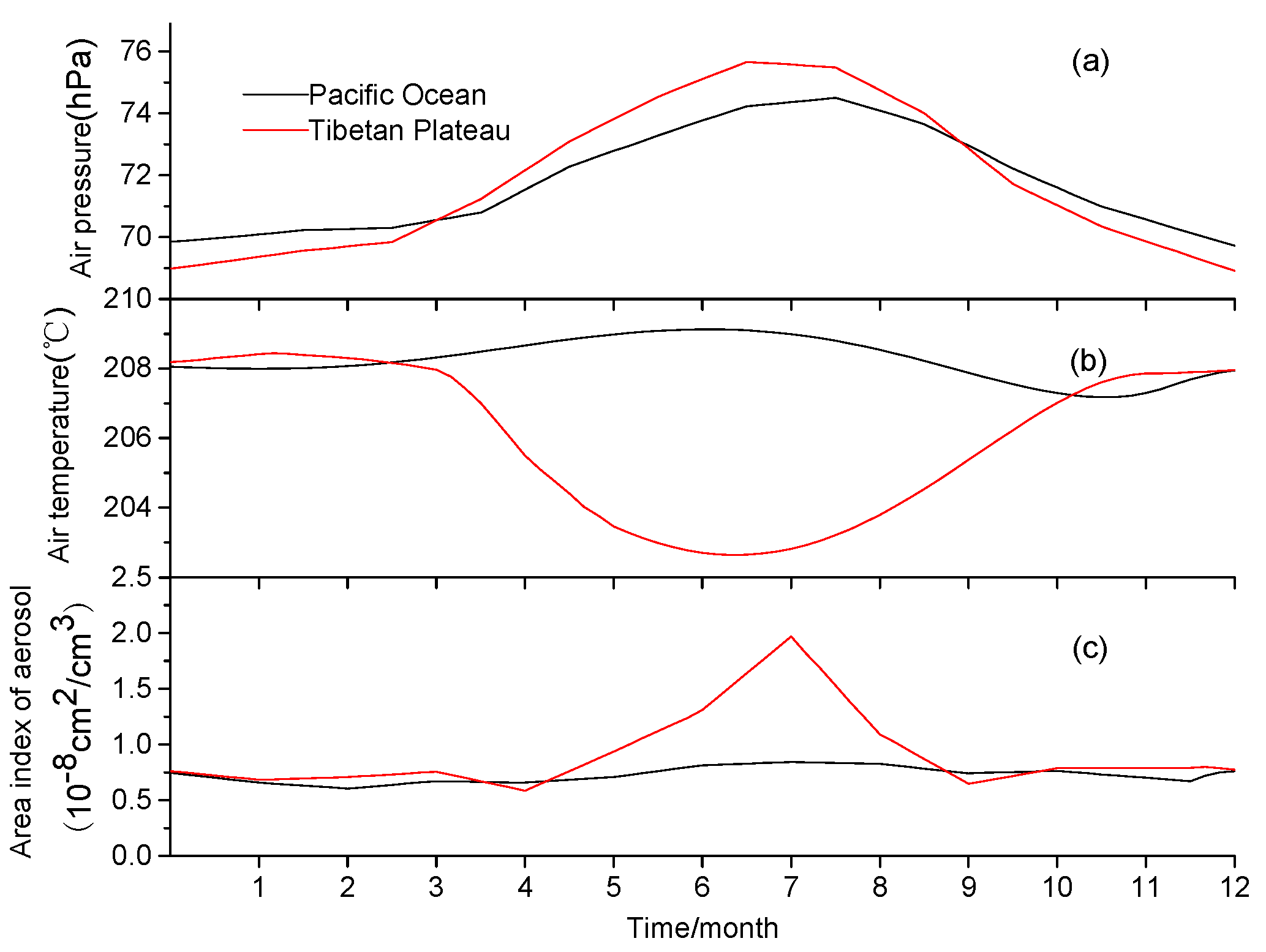
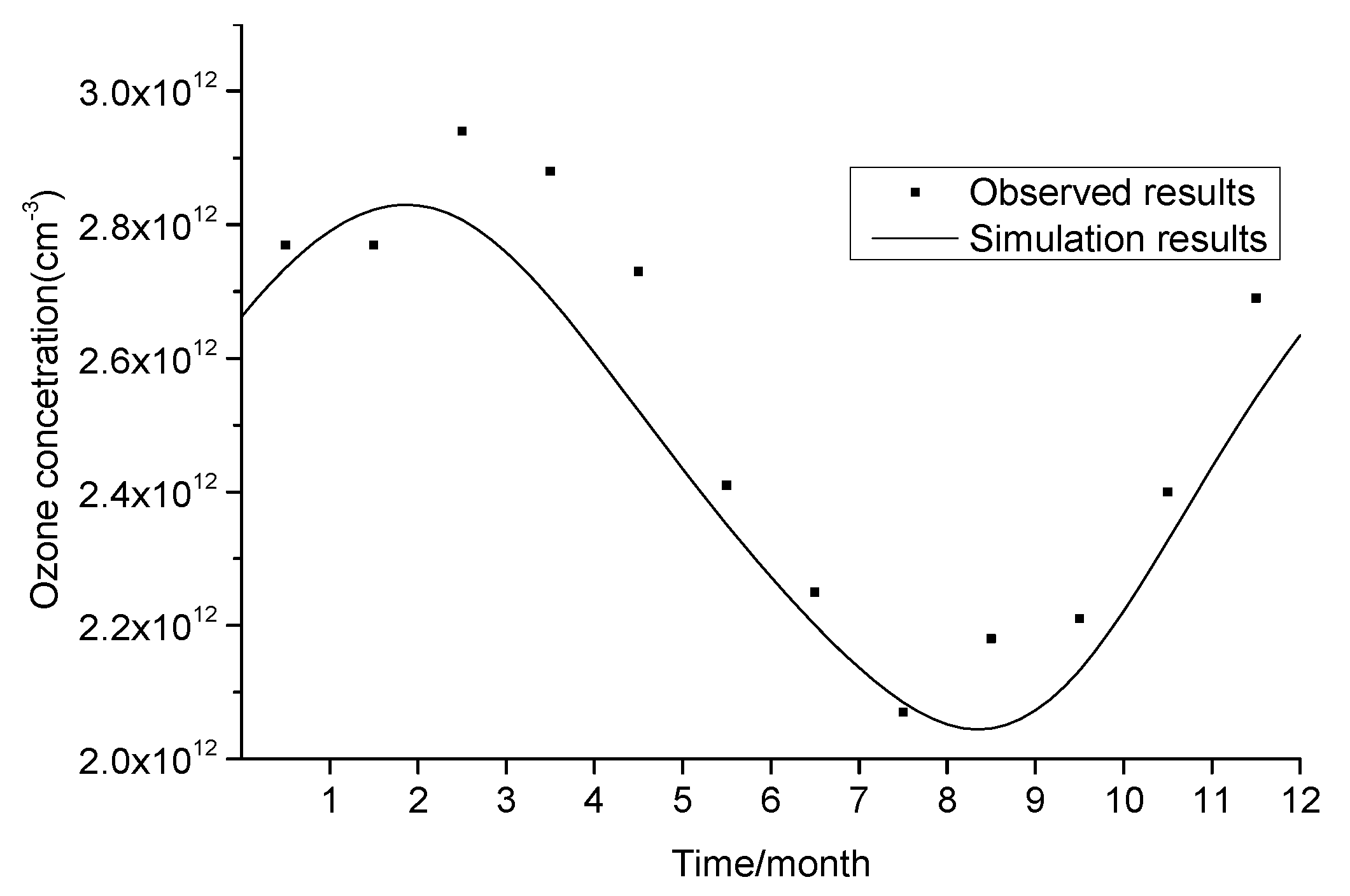
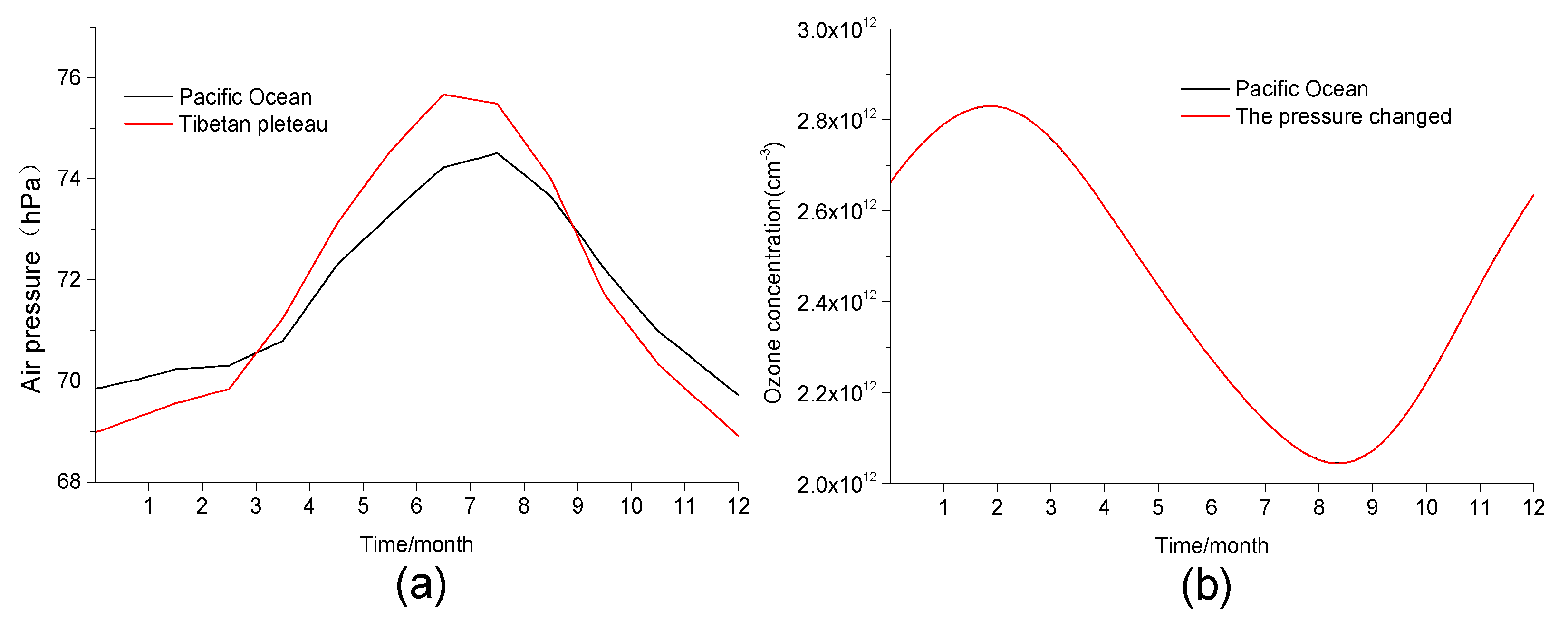
| Chemical Reaction | Sulfuric Acid Droplets (gam) | Solid Sulfuric Acid Salt (Stkcf) | OC (Stkcf) | BC (Stkcf) | Sea Salt (Stkcf) | Mineral Dust (Stkcf) |
|---|---|---|---|---|---|---|
| NO2 + 0.5 × H2O→0.5 HNO3 + 0.5 HNO2 | 1× 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 |
| NO3 + H2O→HNO3 + OH | 1× 10−1 | 1 × 10−1 | 1 × 10−1 | 1 × 10−1 | 1 × 10−1 | 1 × 10−1 |
| N2O5 + H2O→2 HNO3 | f(p, t) | 4 × 10−3 | 2.6 × 10−4 | 5 × 10−3 | 5 × 10−3 | 1 × 10−2 |
| HOCl + HCl→Cl2 + H2O | f(p, t) | 8 × 10−1 | ||||
| HOCl + HBr→BrCl + H2O | 8 × 10−1 | |||||
| ClONO2 + H2O→HOCl + HNO3 | f(p, t) | 1 × 10−4 | ||||
| ClONO2 + HCl→Cl2 + HNO3 | f(p, t) | 1 × 10−5 | ||||
| HOBr + HCl→BrCl + H2O | f(p, t) | 8 × 10−1 | ||||
| HOBr + HBr→Br2 + H2O | 2 × 10−1 | 2 × 10−1 | ||||
| BrONO2 + H2O→HOBr + HNO3 | f(p, t) | 8 × 10−1 | 3 × 10−1 | |||
| BrONO2 + HCl→BrCl + HNO3 | 9 × 10−1 | 9 × 10−1 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| SNOx | 15 cm−3 s−1 | DHNO3 | 0.55 × 10−8 s−1 |
| SClOx | 55 cm−3 s−1 | DHCl | 0.40 × 10−7 s−1 |
| SBrOx | 13 cm−3 s−1 | DHBr | 0.50 × 10−7 s−1 |
| SCH4 | 2000 cm−3 s−1 | DCO2 | 0.40 × 10−7 s−1 |
| O3 | HOX | NOX | ClOX | BrOX | HNO3 | ClONO2 | BrONO2 | CH4 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) | 2.55 × 1012 | 7.43 × 106 | 2.66 × 109 | 1.57 × 106 | — | 9.08 × 109 | 9.3 × 107 | — | 4.29 × 1012 |
| (2) | 3.48 × 1012 | 1.87 × 106 | 2.76 × 109 | 1.25 × 106 | — | 2.21 × 1010 | 9.01 × 107 | — | 3.02 × 1012 |
| (3) | 2.96 × 1012 | 6.15 × 106 | 5.83 × 108 | 1.48 × 107 | 4.27 × 107 | 4.49 × 109 | 2.12 × 108 | 5.93 × 106 | 4.30 × 1012 |
| (4) | 2.32 × 1012 | 3.24 × 106 | 8.09 × 108 | 2.46 × 107 | 2.90 × 107 | 3.91 × 109 | 1.48 × 108 | 1.33 × 107 | 2.43 × 1012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Wang, G.; Yang, P. Study on the Sensitivity of Summer Ozone Density to the Enhanced Aerosol Loading over the Tibetan Plateau. Atmosphere 2020, 11, 138. https://doi.org/10.3390/atmos11020138
Yan J, Wang G, Yang P. Study on the Sensitivity of Summer Ozone Density to the Enhanced Aerosol Loading over the Tibetan Plateau. Atmosphere. 2020; 11(2):138. https://doi.org/10.3390/atmos11020138
Chicago/Turabian StyleYan, Jianjun, Geli Wang, and Peicai Yang. 2020. "Study on the Sensitivity of Summer Ozone Density to the Enhanced Aerosol Loading over the Tibetan Plateau" Atmosphere 11, no. 2: 138. https://doi.org/10.3390/atmos11020138
APA StyleYan, J., Wang, G., & Yang, P. (2020). Study on the Sensitivity of Summer Ozone Density to the Enhanced Aerosol Loading over the Tibetan Plateau. Atmosphere, 11(2), 138. https://doi.org/10.3390/atmos11020138





