Microbial Ecology of the Planetary Boundary Layer
Abstract
1. Introduction
2. Microbial Cell Dynamics in the Troposphere
3. Structuring Factors of Microbial Communities in the Planetary Boundary Layer
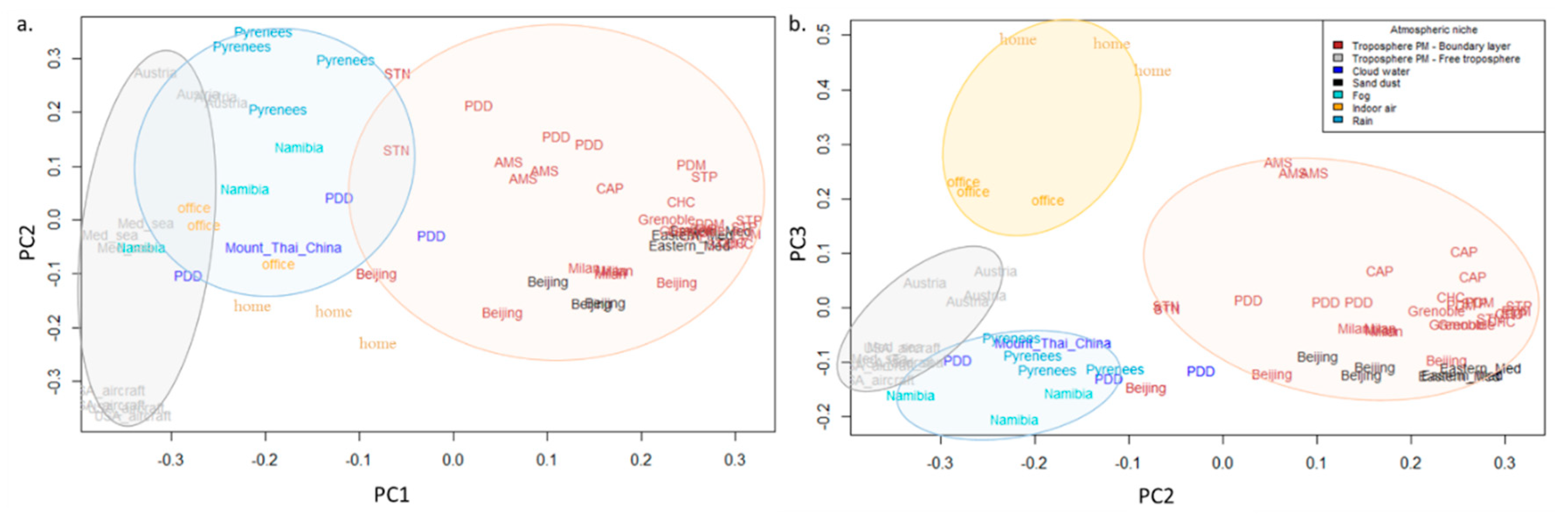
3.1. Surfaces, Aerosolization, Local Versus Distant Sources
3.2. Physical and Chemical Conditions that Might Constrain Microbial Life in the Planetary Boundary Layer
3.2.1. Physical and Chemical Conditions Characterizing the Atmosphere
3.2.2. Effects of Atmospheric Conditions on Microbial Life during Aerosolization and Aerial Transport
4. Potential Impacts of Airborne Microbial Activity on Atmospheric Chemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Després, V.R.; Nowoisky, J.F.; Klose, M.; Conrad, R.; Andreae, M.O.; Pöschl, U. Characterization of primary biogenic aerosol particles in urban, rural, and high-alpine air by DNA sequence and restriction fragment analysis of ribosomal RNA genes. Biogeosciences 2007, 4, 1127–1141. [Google Scholar] [CrossRef]
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Burrows, S.M.; Elbert, W.; Lawrence, M.G.; Pöschl, U. Bacteria in the global atmosphere—Part 1: Review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 2009, 9, 9263–9280. [Google Scholar] [CrossRef]
- Burrows, S.M.; Butler, T.; Jöckel, P.; Tost, H.; Kerkweg, A.; Pöschl, U.; Lawrence, M.G. Bacteria in the global atmosphere—Part 2: Modeling of emissions and transport between different ecosystems. Atmos. Chem. Phys. 2009, 9, 9281–9297. [Google Scholar] [CrossRef]
- Smith, D.J.; Griffin, D.W.; Jaffe, D.A. The high life: Transport of microbes in the atmosphere. Eos. Trans. AGU 2011, 92, 249–250. [Google Scholar] [CrossRef]
- Smith, D.J.; Ravichandar, J.D.; Jain, S.; Griffin, D.W.; Yu, H.; Tan, Q.; Thissen, J.; Lusby, T.; Nicoll, P.; Shedler, S.; et al. Airborne Bacteria in Earth’s Lower Stratosphere Resemble Taxa Detected in the Troposphere: Results From a New NASA Aircraft Bioaerosol Collector (ABC). Front. Microbiol 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W. Terrestrial Microorganisms at an Altitude of 20,000 m in Earth’s Atmosphere. Aerobiologia 2004, 20, 135–140. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W. Atmospheric Movement of Microorganisms in Clouds of Desert Dust and Implications for Human Health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Wickramasinghe, N.C.; Narlikar, J.V.; Rajaratnam, P. Microorganisms cultured from stratospheric air samples obtained at 41 km. FEMS Microbiol. Lett. 2003, 218, 161–165. [Google Scholar] [CrossRef]
- Imshenetsky, A.A.; Lysenko, S.V.; Kazakov, G.A. Upper boundary of the biosphere. Appl. Environ. Microbiol. 1978, 35, 1–5. [Google Scholar] [CrossRef]
- Griffin, D.W.; Gonzalez-Martin, C.; Hoose, C.; Smith, D.J. Global-Scale Atmospheric Dispersion of Microorganisms. In Microbiology of Aerosols; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 155–194. ISBN 978-1-119-13231-8. [Google Scholar]
- Smith, D.J. Aeroplankton and the Need for a Global Monitoring Network. BioScience 2013, 63, 515–516. [Google Scholar] [CrossRef][Green Version]
- Delort, A.-M.; Vaïtilingom, M.; Amato, P.; Sancelme, M.; Parazols, M.; Mailhot, G.; Laj, P.; Deguillaume, L. A short overview of the microbial population in clouds: Potential roles in atmospheric chemistry and nucleation processes. Atmos. Res. 2010, 98, 249–260. [Google Scholar] [CrossRef]
- Failor, K.C.; Schmale, D.G.; Vinatzer, B.A.; Monteil, C.L. Ice nucleation active bacteria in precipitation are genetically diverse and nucleate ice by employing different mechanisms. ISME J. 2017, 11, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Ariya, P.; Sun, J.; Eltouny, N.; Hudson, E.; Hayes, C.; Kos, G. Physical and chemical characterization of bioaerosols--Implications for nucleation processes. Int. Rev. Phys. Chem. 2009, 28, 1–32. [Google Scholar] [CrossRef]
- Haga, D.I.; Burrows, S.M.; Iannone, R.; Wheeler, M.J.; Mason, R.H.; Chen, J.; Polishchuk, E.A.; Pöschl, U.; Bertram, A.K. Ice nucleation by fungal spores from the classes Agaricomycetes, Ustilaginomycetes, and Eurotiomycetes, and the effect on the atmospheric transport of these spores. Atmos. Chem. Phys. 2014, 14, 8611–8630. [Google Scholar] [CrossRef]
- Ariya, P.A.; Nepotchatykh, O.; Ignatova, O.; Amyot, M. Microbiological degradation of atmospheric organic compounds. Geophys. Res. Lett. 2002, 29, 34–1–34–4. [Google Scholar] [CrossRef]
- Ariya, P.A.; Amyot, M. New Directions: The role of bioaerosols in atmospheric chemistry and physics. Atmos. Environ. 2004, 38, 1231–1232. [Google Scholar] [CrossRef]
- Vaïtilingom, M.; Amato, P.; Sancelme, M.; Laj, P.; Leriche, M.; Delort, A.-M. Contribution of Microbial Activity to Carbon Chemistry in Clouds. Appl. Environ. Microbiol. 2010, 76, 23–29. [Google Scholar] [CrossRef]
- Vaïtilingom, M.; Deguillaume, L.; Vinatier, V.; Sancelme, M.; Amato, P.; Chaumerliac, N.; Delort, A.-M. Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds. Proc. Natl. Acad. Sci. USA 2013, 110, 559–564. [Google Scholar] [CrossRef]
- Aylor, D.E. Spread of Plant Disease on a Continental Scale: Role of Aerial Dispersal of Pathogens. Ecology 2003, 84, 1989–1997. [Google Scholar] [CrossRef]
- Lee, B. Life Comes from the Air: A Short Review on Bioaerosol Control. Aerosol Air Qual. Res. 2011, 11. [Google Scholar] [CrossRef]
- Srikanth, P.; Sudharsanam, S.; Steinberg, R. Bio-aerosols in indoor environment: Composition, health effects and analysis. Indian J. Med. Microbiol. 2008, 26, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef]
- Bertolini, V.; Gandolfi, I.; Ambrosini, R.; Bestetti, G.; Innocente, E.; Rampazzo, G.; Franzetti, A. Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Appl. Microbiol. Biotechnol. 2013, 97, 6561–6570. [Google Scholar] [CrossRef]
- Zhen, Q.; Deng, Y.; Wang, Y.; Wang, X.; Zhang, H.; Sun, X.; Ouyang, Z. Meteorological factors had more impact on airborne bacterial communities than air pollutants. Sci. Total Environ. 2017, 601–602, 703–712. [Google Scholar] [CrossRef]
- Genitsaris, S.; Stefanidou, N.; Katsiapi, M.; Kormas, K.A.; Sommer, U.; Moustaka-Gouni, M. Variability of airborne bacteria in an urban Mediterranean area (Thessaloniki, Greece). Atmos. Environ. 2017, 157, 101–110. [Google Scholar] [CrossRef]
- Gandolfi, I.; Bertolini, V.; Bestetti, G.; Ambrosini, R.; Innocente, E.; Rampazzo, G.; Papacchini, M.; Franzetti, A. Spatio-temporal variability of airborne bacterial communities and their correlation with particulate matter chemical composition across two urban areas. Appl. Microbiol. Biotechnol. 2015, 99, 4867–4877. [Google Scholar] [CrossRef]
- Cho, B.C.; Hwang, C.Y. Prokaryotic abundance and 16S rRNA gene sequences detected in marine aerosols on the East Sea (Korea). FEMS Microbiol. Ecol. 2011, 76, 327–341. [Google Scholar] [CrossRef]
- Park, J.; Li, P.-F.; Ichijo, T.; Nasu, M.; Yamaguchi, N. Effects of Asian dust events on atmospheric bacterial communities at different distances downwind of the source region. J. Environ. Sci. 2018, 72, 133–139. [Google Scholar] [CrossRef]
- Tanaka, D.; Sato, K.; Goto, M.; Fujiyoshi, S.; Maruyama, F.; Takato, S.; Shimada, T.; Sakatoku, A.; Aoki, K.; Nakamura, S. Airborne Microbial Communities at High-Altitude and Suburban Sites in Toyama, Japan Suggest a New Perspective for Bioprospecting. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, U.L.; Hagström, Å.; Holmfeldt, K.; Thyrhaug, R.; Geels, C.; Frohn, L.M.; Skjøth, C.A.; Karlson, U.G. High bacterial 16S rRNA gene diversity above the atmospheric boundary layer. Aerobiologia 2012, 28, 481–498. [Google Scholar] [CrossRef]
- DeLeon-Rodriguez, N. Microbiome of the Upper Troposphere: Species Composition and Prevalence, Effects of Tropical Storms, and Atmospheric Implications. Available online: http://www.pnas.org/content/110/7/2575.full (accessed on 25 July 2017).
- Els, N.; Larose, C.; Baumann-Stanzer, K.; Tignat-Perrier, R.; Keuschnig, C.; Vogel, T.M.; Sattler, B. Microbial composition in seasonal time series of free tropospheric air and precipitation reveals community separation. Aerobiologia 2019. [Google Scholar] [CrossRef]
- Amato, P.; Ménager, M.; Sancelme, M.; Laj, P.; Mailhot, G.; Delort, A.-M. Microbial population in cloud water at the Puy de Dôme: Implications for the chemistry of clouds. Atmos. Environ. 2005, 39, 4143–4153. [Google Scholar] [CrossRef]
- Amato, P.; Besaury, L.; Joly, M.; Penaud, B.; Deguillaume, L.; Delort, A.-M. Metatranscriptomic exploration of microbial functioning in clouds. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Amato, P.; Joly, M.; Besaury, L.; Oudart, A.; Taib, N.; Moné, A.I.; Deguillaume, L.; Delort, A.-M.; Debroas, D. Active microorganisms thrive among extremely diverse communities in cloud water. PLoS ONE 2017, 12, e0182869. [Google Scholar] [CrossRef]
- Renard, P.; Canet, I.; Sancelme, M.; Wirgot, N.; Deguillaume, L.; Delort, A.-M. Screening of cloud microorganisms isolated at the Puy de Dôme (France) station for the production of biosurfactants. Atmos. Chem. Phys. 2016, 16, 12347–12358. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Bowers, R.M.; McCubbin, I.B.; Hallar, A.G.; Fierer, N. Seasonal variability in airborne bacterial communities at a high-elevation site. Atmos. Environ. 2012, 50, 41–49. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef] [PubMed]
- Mayol, E.; Arrieta, J.M.; Jiménez, M.A.; Martínez-Asensio, A.; Garcias-Bonet, N.; Dachs, J.; González-Gaya, B.; Royer, S.-J.; Benítez-Barrios, V.M.; Fraile-Nuez, E.; et al. Long-range transport of airborne microbes over the global tropical and subtropical ocean. Nat. Commun. 2017, 8, 201. [Google Scholar] [CrossRef]
- Fulton, J.D. Microorganisms of the Upper Atmosphere: III. Relationship between Altitude and Micropopulation. Appl. Environ. Microbiol. 1966, 14, 237–240. [Google Scholar] [CrossRef]
- Els, N.; Baumann-Stanzer, K.; Larose, C.; Vogel, T.M.; Sattler, B. Beyond the planetary boundary layer: Bacterial and fungal vertical biogeography at Mount Sonnblick, Austria. Geo Geogr. Environ. 2019, 6, e00069. [Google Scholar] [CrossRef]
- Griffin, D.W.; Kellogg, C.A.; Garrison, V.H.; Lisle, J.T.; Borden, T.C.; Shinn, E.A. Atmospheric microbiology in the northern Caribbean during African dust events. Aerobiologia 2003, 19, 143–157. [Google Scholar] [CrossRef]
- Li, Y.; Lu, R.; Li, W.; Xie, Z.; Song, Y. Concentrations and size distributions of viable bioaerosols under various weather conditions in a typical semi-arid city of Northwest China. J. Aerosol Sci. 2017, 106, 83–92. [Google Scholar] [CrossRef]
- Weil, T.; De Filippo, C.; Albanese, D.; Donati, C.; Pindo, M.; Pavarini, L.; Carotenuto, F.; Pasqui, M.; Poto, L.; Gabrieli, J.; et al. Legal immigrants: Invasion of alien microbial communities during winter occurring desert dust storms. Microbiome 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Baba, T.; Ichijo, T.; Himezawa, Y.; Enoki, K.; Saraya, M.; Li, P.-F.; Nasu, M. Abundance and Community Structure of Bacteria on Asian Dust Particles Collected in Beijing, China, during the Asian Dust Season. Biol. Pharm. Bull. 2016, 39, 68–77. [Google Scholar] [CrossRef]
- Dong, L.; Qi, J.; Shao, C.; Zhong, X.; Gao, D.; Cao, W.; Gao, J.; Bai, R.; Long, G.; Chu, C. Concentration and size distribution of total airborne microbes in hazy and foggy weather. Sci. Total Environ. 2016, 541, 1011–1018. [Google Scholar] [CrossRef]
- Clauss, M. Particle size distribution of airborne micro-organisms in the environment-A review. Landbauforsch. Volkenrode 2015, 65, 77–100. [Google Scholar] [CrossRef]
- Amato, P.; Joly, M.; Schaupp, C.; Attard, E.; Möhler, O.; Morris, C.E.; Brunet, Y.; Delort, A.-M. Survival and ice nucleation activity of bacteria as aerosols in a cloud simulation chamber. Atmos. Chem. Phys. 2015, 15, 6455–6465. [Google Scholar] [CrossRef]
- Mayol, E.; Jiménez, M.A.; Herndl, G.J.; Duarte, C.M.; Arrieta, J.M. Resolving the abundance and air-sea fluxes of airborne microorganisms in the North Atlantic Ocean. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Aller, J.Y.; Kuznetsova, M.R.; Jahns, C.J.; Kemp, P.F. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 2005, 36, 801–812. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Syzdek, L.D. Water-to-Air Transfer and Enrichment of Bacteria in Drops from Bursting Bubbles. Appl. Environ. Microbiol. 1982, 43, 1001–1005. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Environmental Chemistry—Chemistry—Subjects—Wiley. In Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 1998. [Google Scholar]
- Jaenicke, R. Atmospheric aerosols and global climate. J. Aerosol Sci. 1980, 11, 577–588. [Google Scholar] [CrossRef]
- Tignat-Perrier, R.; Dommergue, A.; Thollot, A.; Keuschnig, C.; Magand, O.; Vogel, T.M.; Larose, C. Global airborne microbial communities controlled by surrounding landscapes and wind conditions. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Michaud, J.M.; Thompson, L.R.; Kaul, D.; Espinoza, J.L.; Richter, R.A.; Xu, Z.Z.; Lee, C.; Pham, K.M.; Beall, C.M.; Malfatti, F.; et al. Taxon-specific aerosolization of bacteria and viruses in an experimental ocean-atmosphere mesocosm. Nat. Commun. 2018, 9, 2017. [Google Scholar] [CrossRef]
- Fahlgren, C.; Gómez-Consarnau, L.; Zábori, J.; Lindh, M.V.; Krejci, R.; Mårtensson, E.M.; Nilsson, D.; Pinhassi, J. Seawater mesocosm experiments in the Arctic uncover differential transfer of marine bacteria to aerosols. Environ. Microbiol. Rep. 2015, 7, 460–470. [Google Scholar] [CrossRef]
- Amato, P.; Demeer, F.; Melaouhi, A.; Fontanella, S.; Martin-Biesse, A.-S.; Sancelme, M.; Laj, P.; Delort, A.-M. A fate for organic acids, formaldehyde and methanol in cloud water: Their biotransformation by micro-organisms. Atmos. Chem. Phys. 2007, 7, 4159–4169. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Shamy, M.; Redal, M.A.; Khoder, M.; Awad, A.H.; Elserougy, S. Microorganisms associated particulate matter: A preliminary study. Sci. Total Environ. 2014, 479–480, 109–116. [Google Scholar] [CrossRef]
- Womack, A.M.; Artaxo, P.E.; Ishida, F.Y.; Mueller, R.C.; Saleska, S.R.; Wiedemann, K.T.; Bohannan, B.J.M.; Green, J.L. Characterization of active and total fungal communities in the atmosphere over the Amazon rainforest. Biogeosciences 2015, 12, 6337–6349. [Google Scholar] [CrossRef]
- Sesartic, A.; Lohmann, U.; Storelvmo, T. Bacteria in the ECHAM5-HAM global climate model. Atmos. Chem. Phys. 2012, 12, 8645–8661. [Google Scholar] [CrossRef]
- Elbert, W.; Taylor, P.E.; Andreae, M.O.; Pöschl, U. Contribution of fungi to primary biogenic aerosols in the atmosphere: Wet and dry discharged spores, carbohydrates, and inorganic ions. Atmos. Chem. Phys. 2007, 7, 4569–4588. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Górny, R.; Lawniczek-Walczyk, A. Effect of two aerosolization methods on the release of fungal propagules from a contaminated agar surface. Ann. Agric. Environ. Med. AAEM 2012, 19, 279–284. [Google Scholar]
- Joung, Y.S.; Ge, Z.; Buie, C.R. Bioaerosol generation by raindrops on soil. Nat. Commun. 2017, 8, 14668. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef]
- Bowers, R.M.; McLetchie, S.; Knight, R.; Fierer, N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 2011, 5, 601–612. [Google Scholar] [CrossRef]
- Innocente, E.; Squizzato, S.; Visin, F.; Facca, C.; Rampazzo, G.; Bertolini, V.; Gandolfi, I.; Franzetti, A.; Ambrosini, R.; Bestetti, G. Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Sci. Total Environ. 2017, 677–687. [Google Scholar] [CrossRef]
- Uetake, J.; Tobo, Y.; Uji, Y.; Hill, T.C.J.; DeMott, P.J.; Kreidenweis, S.; Misumi, R. Seasonal changes of airborne bacterial communities over Tokyo and influence of local meteorology. arXiv 2019, arXiv:Bio/542001. [Google Scholar] [CrossRef]
- Tignat-Perrier, R.; Dommergue, A.; Thollot, A.; Magand, O.; Amato, P.; Joly, M.; Sellegri, K.; Vogel, T.M.; Larose, C. Seasonal shift in airborne microbial communities. Sci. Total Environ. 2020, 716, 137129. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Hara, K.; Kobayashi, F.; Kurosaki, Y.; Kakikawa, M.; Matsuki, A.; Chen, B.; Shi, G.; Hasegawa, H.; Iwasaka, Y. Vertical distribution of airborne bacterial communities in an Asian-dust downwind area, Noto Peninsula. Atmos. Environ. 2015, 119, 282–293. [Google Scholar] [CrossRef]
- Gat, D.; Mazar, Y.; Cytryn, E.; Rudich, Y. Origin-Dependent Variations in the Atmospheric Microbiome Community in Eastern Mediterranean Dust Storms. Environ. Sci. Technol. 2017, 51, 6709–6718. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Hara, K.; Iwata, A.; Lee, K.C.; Kawai, K.; Kai, K.; Kobayashi, F.; Pointing, S.B.; Archer, S.; Hasegawa, H.; et al. Variations in airborne bacterial communities at high altitudes over the Noto Peninsula (Japan) in response to Asian dust events. Atmos. Chem. Phys. 2017, 17, 11877–11897. [Google Scholar] [CrossRef]
- Reche, I.; Ortega-Retuerta, E.; Romera, O.; Villena, E.P.; Baquero, R.M.; Casamayor, E.O. Effect of Saharan dust inputs on bacterial activity and community composition in Mediterranean lakes and reservoirs. Limnol. Oceanogr. 2009, 54, 869–879. [Google Scholar] [CrossRef]
- Prospero, J.M.; Blades, E.; Mathison, G.; Naidu, R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 2005, 21, 1–19. [Google Scholar] [CrossRef]
- Creamean, J.M.; Suski, K.J.; Rosenfeld, D.; Cazorla, A.; DeMott, P.J.; Sullivan, R.C.; White, A.B.; Ralph, F.M.; Minnis, P.; Comstock, J.M.; et al. Dust and Biological Aerosols from the Sahara and Asia Influence Precipitation in the Western U.S. Science 2013, 339, 1572–1578. [Google Scholar] [CrossRef]
- Itani, G.N.; Smith, C.A. Dust Rains Deliver Diverse Assemblages of Microorganisms to the Eastern Mediterranean. Sci. Rep. 2016, 6, 22657. [Google Scholar] [CrossRef]
- Maki, T.; Susuki, S.; Kobayashi, F.; Kakikawa, M.; Tobo, Y.; Yamada, M.; Higashi, T.; Matsuki, A.; Hong, C.; Hasegawa, H.; et al. Phylogenetic analysis of atmospheric halotolerant bacterial communities at high altitude in an Asian dust (KOSA) arrival region, Suzu City. Sci. Total Environ. 2010, 408, 4556–4562. [Google Scholar] [CrossRef]
- Griffin, D.W.; Garrison, V.H.; Herman, J.R.; Shinn, E.A. African desert dust in the Caribbean atmosphere: Microbiology and public health. Aerobiologia 2001, 17, 203–213. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W.; Garrison, V.H.; Peak, K.K.; Royall, N.; Smith, R.R.; Shinn, E.A. Characterization of Aerosolized Bacteria and Fungi From Desert Dust Events in Mali, West Africa. Aerobiologia 2004, 20, 99–110. [Google Scholar] [CrossRef]
- Maki, T.; Lee, K.C.; Kawai, K.; Onishi, K.; Hong, C.S.; Kurosaki, Y.; Shinoda, M.; Kai, K.; Iwasaka, Y.; Archer, S.D.J.; et al. Aeolian Dispersal of Bacteria Associated With Desert Dust and Anthropogenic Particles Over Continental and Oceanic Surfaces. J. Geophys. Res. Atmos. 2019, 124, 5579–5588. [Google Scholar] [CrossRef]
- Katra, I.; Arotsker, L.; Krasnov, H.; Zaritsky, A.; Kushmaro, A.; Ben-Dov, E. Richness and Diversity in Dust Stormborne Biomes at the Southeast Mediterranean. Sci. Rep. 2014, 4, 5265. [Google Scholar] [CrossRef] [PubMed]
- Delmont, T.O.; Malandain, C.; Prestat, E.; Larose, C.; Monier, J.-M.; Simonet, P.; Vogel, T.M. Metagenomic mining for microbiologists. ISME J. 2011, 5, 1837–1843. [Google Scholar] [CrossRef]
- Xie, W.; Wang, F.; Guo, L.; Chen, Z.; Sievert, S.M.; Meng, J.; Huang, G.; Li, Y.; Yan, Q.; Wu, S.; et al. Comparative metagenomics of microbial communities inhabiting deep-sea hydrothermal vent chimneys with contrasting chemistries. ISME J. 2011, 5, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, L.; Zhang, Y.; Liu, H.; Jing, H. Comparative metagenomics study reveals pollution induced changes of microbial genes in mangrove sediments. Sci. Rep. 2019, 9, 5739. [Google Scholar] [CrossRef] [PubMed]
- Tringe, S.G.; von Mering, C.; Kobayashi, A.; Salamov, A.A.; Chen, K.; Chang, H.W.; Podar, M.; Short, J.M.; Mathur, E.J.; Detter, J.C.; et al. Comparative metagenomics of microbial communities. Science 2005, 308, 554–557. [Google Scholar] [CrossRef]
- Moan, J.; Peak, M.J. Effects of UV radiation of cells. J. Photochem. Photobiol. B Biol. 1989, 4, 21–34. [Google Scholar] [CrossRef]
- Santos, A.L.; Oliveira, V.; Baptista, I.; Henriques, I.; Gomes, N.C.M.; Almeida, A.; Correia, A.; Cunha, Â. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch. Microbiol. 2013, 195, 63–74. [Google Scholar] [CrossRef]
- DasSarma, P.; DasSarma, S. Survival of microbes in Earth’s stratosphere. Curr. Opin. Microbiol. 2018, 43, 24–30. [Google Scholar] [CrossRef]
- Smith, D.J.; Griffin, D.W.; McPeters, R.D.; Ward, P.D.; Schuerger, A.C. Microbial survival in the stratosphere and implications for global dispersal. Aerobiologia 2011, 27, 319–332. [Google Scholar] [CrossRef]
- Yang, Y.; Yokobori, S.; Yamagishi, A. UV-resistant bacteria isolated from upper troposphere and lower stratosphere. Biol. Sci. Space 2008, 22, 18–25. [Google Scholar] [CrossRef]
- Bryan, N.C.; Christner, B.C.; Guzik, T.G.; Granger, D.J.; Stewart, M.F. Abundance and survival of microbial aerosols in the troposphere and stratosphere. ISME J. 2019, 13, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.N.; Braga, G.U.L.; Fernandes, É.K.K.; Keyser, C.A.; Hallsworth, J.E.; Roberts, D.W. Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Curr. Genet. 2015, 61, 383–404. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Anderson, A.J.; Roberts, D.W. Evaluating physical and nutritional stress during mycelial growth as inducers of tolerance to heat and UV-B radiation in Metarhizium anisopliae conidia. Mycol. Res. 2008, 112, 1362–1372. [Google Scholar] [CrossRef]
- Hagiwara, D.; Sakai, K.; Suzuki, S.; Umemura, M.; Nogawa, T.; Kato, N.; Osada, H.; Watanabe, A.; Kawamoto, S.; Gonoi, T.; et al. Temperature during conidiation affects stress tolerance, pigmentation, and trypacidin accumulation in the conidia of the airborne pathogen Aspergillus fumigatus. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Vorob’eva, L.I. Stressors, Stress Reactions, and Survival of Bacteria: A Review. Appl. Biochem. Microbiol. 2004, 40, 217–224. [Google Scholar] [CrossRef]
- D’Amico, S.; Collins, T.; Marx, J.-C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef]
- Nedwell, D.B. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microbiol. Ecol. 1999, 30, 101–111. [Google Scholar] [CrossRef]
- Berry, E.D.; Foegeding, P.M. Cold Temperature Adaptation and Growth of Microorganisms. J. Food Prot. 1997, 60, 1583–1594. [Google Scholar] [CrossRef]
- Joly, M.; Amato, P.; Sancelme, M.; Vinatier, V.; Abrantes, M.; Deguillaume, L.; Delort, A.-M. Survival of microbial isolates from clouds toward simulated atmospheric stress factors. Atmos. Environ. 2015, 117, 92–98. [Google Scholar] [CrossRef]
- Ehrlich, R.; Miller, S.; Walker, R.L. Effects of Atmospheric Humidity and Temperature on the Survival of Airborne Flavobacterium. Appl. Environ. Microbiol. 1970, 20, 884–887. [Google Scholar] [CrossRef]
- Wright, D.N.; Bailey, G.D.; Hatch, M.T. Survival of airborne Mycoplasma as affected by relative humidity. J. Bacteriol. 1968, 95, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Mattimore, V.; Battista, J.R. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 1996, 178, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Dose, K.; Bieger-Dose, A.; Labusch, M.; Gill, M. Survival in extreme dryness and DNA-single-strand breaks. Adv. Space Res. 1992, 12, 221–229. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Li, S.W.; Gaidamakova, E.K.; Matrosova, V.Y.; Zhai, M.; Sulloway, H.M.; Scholten, J.C.; Brown, M.G.; Balkwill, D.L.; Daly, M.J. Protein oxidation: Key to bacterial desiccation resistance? ISME J. 2008, 2, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.K.; Zachara, J.M.; Balkwill, D.L.; Kennedy, D.; Li, S.W.; Kostandarithes, H.M.; Daly, M.J.; Romine, M.F.; Brockman, F.J. Geomicrobiology of High-Level Nuclear Waste-Contaminated Vadose Sediments at the Hanford Site, Washington State. Appl. Environ. Microbiol. 2004, 70, 4230–4241. [Google Scholar] [CrossRef]
- Sanders, S.W.; Maxcy, R.B. Isolation of radiation-resistant bacteria without exposure to irradiation. Appl. Environ. Microbiol. 1979, 38, 436–439. [Google Scholar] [CrossRef]
- Rainey, F.A.; Ray, K.; Ferreira, M.; Gatz, B.Z.; Nobre, M.F.; Bagaley, D.; Rash, B.A.; Park, M.-J.; Earl, A.M.; Shank, N.C.; et al. Extensive Diversity of Ionizing-Radiation-Resistant Bacteria Recovered from Sonoran Desert Soil and Description of Nine New Species of the Genus Deinococcus Obtained from a Single Soil Sample. Appl. Environ. Microbiol. 2005, 71, 5225–5235. [Google Scholar] [CrossRef]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef]
- Wood, J.M. Bacterial responses to osmotic challenges. J. Gen. Physiol. 2015, 145, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Alsved, M.; Holm, S.; Christiansen, S.; Smidt, M.; Ling, M.; Boesen, T.; Finster, K.; Bilde, M.; Löndahl, J.; Šantl-Temkiv, T. Effect of Aerosolization and Drying on the Viability of Pseudomonas syringae Cells. Front. Microbiol. 2018, 9, 3086. [Google Scholar] [CrossRef] [PubMed]
- Deguillaume, L.; Charbouillot, T.; Joly, M.; Vaïtilingom, M.; Parazols, M.; Marinoni, A.; Amato, P.; Delort, A.-M.; Vinatier, V.; Flossmann, A.; et al. Classification of clouds sampled at the puy de Dôme (France) based on 10 yr of monitoring of their physicochemical properties. Atmos. Chem. Phys. 2014, 14, 1485–1506. [Google Scholar] [CrossRef]
- Thomas, R.J.; Webber, D.; Hopkins, R.; Frost, A.; Laws, T.; Jayasekera, P.N.; Atkins, T. The Cell Membrane as a Major Site of Damage during Aerosolization of Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Han, T.; Fennell, D.E.; Mainelis, G. Release of Free DNA by Membrane-Impaired Bacterial Aerosols Due to Aerosolization and Air Sampling. Appl. Environ. Microbiol. 2013, 79, 7780–7789. [Google Scholar] [CrossRef]
- Ng, T.W.; Chan, W.L.; Lai, K.M. Importance of stress-response genes to the survival of airborne Escherichia coli under different levels of relative humidity. AMB Express 2017, 7. [Google Scholar] [CrossRef]
- Ng, T.W.; Ip, M.; Chao, C.Y.H.; Tang, J.W.; Lai, K.P.; Fu, S.C.; Leung, W.T.; Lai, K.M. Differential gene expression in Escherichia coli during aerosolization from liquid suspension. Appl. Microbiol. Biotechnol. 2018, 102, 6257–6267. [Google Scholar] [CrossRef]
- Mainelis, G.; Górny, R.L.; Reponen, T.; Trunov, M.; Grinshpun, S.A.; Baron, P.; Yadav, J.; Willeke, K. Effect of electrical charges and fields on injury and viability of airborne bacteria. Biotechnol. Bioeng. 2002, 79, 229–241. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Samson, R.A. Food Mycology: A Multifaceted Approach to Fungi and Food; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-2098-4. [Google Scholar]
- Martiny, A.C.; Tai, A.P.K.; Veneziano, D.; Primeau, F.; Chisholm, S.W. Taxonomic resolution, ecotypes and the biogeography of Prochlorococcus. Environ. Microbiol. 2009, 11, 823–832. [Google Scholar] [CrossRef]
- Hellweger, F.L.; van Sebille, E.; Fredrick, N.D. Biogeographic patterns in ocean microbes emerge in a neutral agent-based model. Science 2014, 345, 1346–1349. [Google Scholar] [CrossRef]
- Hernández, K.L.; Quiñones, R.A.; Daneri, G.; Farias, M.E.; Helbling, E.W. Solar UV radiation modulates daily production and DNA damage of marine bacterioplankton from a productive upwelling zone (36° S), Chile. J. Exp. Mar. Biol. Ecol. 2007, 343, 82–95. [Google Scholar] [CrossRef]
- Herndl, G.J.; Müller-Niklas, G.; Frick, J. Major role of ultraviolet-B in controlling bacterioplankton growth in the surface layer of the ocean. Nature 1993, 361, 717–719. [Google Scholar] [CrossRef]
- Winter, C.; Moeseneder, M.M.; Herndl, G.J. Impact of UV Radiation on Bacterioplankton Community Composition. Appl. Environ. Microbiol. 2001, 67, 665–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alonso-Sáez, L.; Gasol, J.M.; Lefort, T.; Hofer, J.; Sommaruga, R. Effect of natural sunlight on bacterial activity and differential sensitivity of natural bacterioplankton groups in northwestern Mediterranean coastal waters. Appl. Environ. Microbiol. 2006, 72, 5806–5813. [Google Scholar] [CrossRef]
- Ruiz-González, C.; Lefort, T.; Galí, M.; Montserrat Sala, M.; Sommaruga, R.; Simó, R.; Gasol, J.M. Seasonal patterns in the sunlight sensitivity of bacterioplankton from Mediterranean surface coastal waters. FEMS Microbiol. Ecol. 2012, 79, 661–674. [Google Scholar] [CrossRef]
- Ruiz Gonzalez, C.; Simó, R.; Sommaruga, R.; Gasol, J.M. Away from darkness: A review on the effects of solar radiation on heterotrophic bacterioplankton activity. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Klein, A.M.; Bohannan, B.J.M.; Jaffe, D.A.; Levin, D.A.; Green, J.L. Molecular Evidence for Metabolically Active Bacteria in the Atmosphere. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Womack, A.M. UV-resistant bacteria isolated from upper troposphere and lower stratosphere (PDF Download Available). ResearchGate 2010. [Google Scholar] [CrossRef]
- Sattler, B.; Puxbaum, H.; Psenner, R. Bacterial growth in supercooled cloud droplets. Geophys. Res. Lett. 2001, 28, 239–242. [Google Scholar] [CrossRef]
- Falkowski, P.; Barber, R.; Smetacek, V. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 1998, 281, 200–207. [Google Scholar] [CrossRef]
- Yooseph, S.; Andrews-Pfannkoch, C.; Tenney, A.; McQuaid, J.; Williamson, S.; Thiagarajan, M.; Brami, D.; Zeigler-Allen, L.; Hoffman, J.; Goll, J.B.; et al. A Metagenomic Framework for the Study of Airborne Microbial Communities. PLoS ONE 2013, 8, e81862. [Google Scholar] [CrossRef] [PubMed]
- Aalismail, N.A.; Ngugi, D.K.; Díaz-Rúa, R.; Alam, I.; Cusack, M.; Duarte, C.M. Functional metagenomic analysis of dust-associated microbiomes above the Red Sea. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bøifot, K.O.; Gohli, J.; Moen, L.V.; Dybwad, M. Performance evaluation of a new custom, multi-component DNA isolation method optimized for use in shotgun metagenomic sequencing-based aerosol microbiome research. Environ. Microbiome 2020, 15, 1. [Google Scholar] [CrossRef]
- Dommergue, A.; Amato, P.; Tignat-Perrier, R.; Magand, O.; Thollot, A.; Joly, M.; Bouvier, L.; Sellegri, K.; Vogel, T.; Sonke, J.E.; et al. Methods to investigate the global atmospheric microbiome. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Mbareche, H.; Veillette, M.; Bilodeau, G.J.; Duchaine, C. Bioaerosol Sampler Choice Should Consider Efficiency and Ability of Samplers To Cover Microbial Diversity. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Karlsson, E.; Johansson, A.-M.; Ahlinder, J.; Lundkvist, M.J.; Singh, N.J.; Brodin, T.; Forsman, M.; Stenberg, P. Airborne microbial biodiversity and seasonality in Northern and Southern Sweden. PeerJ 2020, 8, e8424. [Google Scholar] [CrossRef]
- Tipton, L.; Zahn, G.; Datlof, E.; Kivlin, S.N.; Sheridan, P.; Amend, A.S.; Hynson, N.A. Fungal aerobiota are not affected by time nor environment over a 13-y time series at the Mauna Loa Observatory. Proc. Natl. Acad. Sci. USA 2019, 116, 25728–25733. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tignat-Perrier, R.; Dommergue, A.; Vogel, T.M.; Larose, C. Microbial Ecology of the Planetary Boundary Layer. Atmosphere 2020, 11, 1296. https://doi.org/10.3390/atmos11121296
Tignat-Perrier R, Dommergue A, Vogel TM, Larose C. Microbial Ecology of the Planetary Boundary Layer. Atmosphere. 2020; 11(12):1296. https://doi.org/10.3390/atmos11121296
Chicago/Turabian StyleTignat-Perrier, Romie, Aurélien Dommergue, Timothy M. Vogel, and Catherine Larose. 2020. "Microbial Ecology of the Planetary Boundary Layer" Atmosphere 11, no. 12: 1296. https://doi.org/10.3390/atmos11121296
APA StyleTignat-Perrier, R., Dommergue, A., Vogel, T. M., & Larose, C. (2020). Microbial Ecology of the Planetary Boundary Layer. Atmosphere, 11(12), 1296. https://doi.org/10.3390/atmos11121296




