1. Introduction
Boreal rivers are currently the major transporters of dissolved organic carbon (DOC) from land into the Arctic Ocean, and the connection may well become stronger under global warming [
1]. Specific functional groups along DOC backbones can affect coastal physical properties such as light attenuation, atmospheric mass transfer and surfactant adsorption. Structures including the amino acids combined as proteins, photochemically generated volatile organic compounds (VOCs) which are low in molecular weight, plus the colored or chromophoric dissolved organic matter (CDOM) are all likely to imply a biophysical impact on entry into the open sea. To the extent that freshwater chemical stability transfers into brackish coastal mixing plumes, the land-to-sea organic linkages may prove to be regional in scale. Satellite imagery already demonstrates that turbid marine zones can surpass the respective major deltas in net area.
Fresh organic compounds, which were once intracellular, such as lipids and biopolymers, tend to adsorb along the water–air interface with a strong modulation of mass and energy transfer. Heat, momentum and water vapor fluxes are all in play [
2]. Macromolecules in the monolayer enter the primary aerosol through bubble bursting [
3], and partially oxidized volatile carbon supplies organics to secondary particles, whether in the Arctic regime or globally [
4]. Proteins are biomass dominant among high molecular weight components of the cytosol, and they are not only surface active but can also serve as anti-freeze agents within sea ice [
5]. Polysaccharides contribute to gel formation and are capable of blocking brine channels within the pack. In general, polymeric and biomacromolecular organics are highly surface active whenever they are amphiphilic. Depending on local concentrations, they populate different phases or forms such as multilayers, micelles, gels and colloids [
6]. Due to this wide variety of interactions between biology and physics, it is necessary to estimate riverine fluxes mechanistically. In an era of shifting terrestrial carbon storage [
7], this will be a major means of forecasting the role of organic chemistry in Arctic oceanic change.
Our strategy for building dynamic simulations of river–coast pairing is to test a reduced kinetic model of the inputs/processes for a single system, operating at a high discharge rate. We represent an idealized Asian Arctic tributary network, based upon dissolved injection data from several continents. However, our overall configuration is set to resemble the lengthy and intensive Lena of eastern Siberia. Headwater chemistry is based on Alaskan and Asian measurements [
7,
8]. Processing modes and time constants are global averages [
9] but with initial weighting toward the coastal mode [
10]. Decay occurs through the functional sequence from soil biopolymers through oligomers to monomers, but with recondensation universally permitted in the mechanism. The latter is represented through heteropolycondensates and ultimately humics [
11,
12]. Validation is performed on Lena data [
13,
14] and other major river output studies. Final results are then superimposed on regional estimates of a functional group resolution for the polar coast and open Arctic Ocean. We end with discussions of the importance for bonding sequences in high latitude marine biogeochemistry, and further speculate on the use of reduced modeling in broader situations. Special emphasis is placed upon two main points: (1.) the potential for the incorporation of organo–chemical reaction webs in river codes, and (2.) a transition toward physical oceanographic simulations of coastal reactive flow.
Our text is structured as follows: background information is provided with regard to the global, coastal and boreal importance of dissolved organics in the aquatic system. All of the compound types protein, polysaccharide, lipid, recondensate (which we refer to as heteropolymeric), humic, biomarkers, product volatiles and chromophores are included [
11,
12,
15]. In fact, our work encompasses all macromolecules from soil sources through to open plumes, with the exclusion of structural cellulose and lignin (insoluble to the zeroth order [
9,
11]). Distinctions between functional distributions from the deep sea to surface ocean and finally freshwater systems are drawn in a section of definitions [
2,
6,
9,
10,
11,
12]. Then, we proceed through model description/results in this order: the idealized Siberian river is outlined as a low node-number channel map. Our physical means for simulating functionally resolved chemical change is Lagrangian reactive transport along the course. A conservative flow of carbon is maintained numerically, from biopolymer toward monomer with the re-bonding of intermediates. Initial DOC concentrations are established in the range of 0–6000 micromolar for the overall northern watersheds including mountain, wetland and boreal tundra [
7,
8]. Starting macromolecular levels are informed by global averages [
9].
Process rates are initially set at coastal values as an upper limit [
10] then dialed down since fresh waters tend to be less microbially active. We work from a master time scale of ten days, proportioning the various macromolecular decay types according to relative oxidation susceptibility in global systems [
11]. A representative low (percentage) fraction of several major polymer types is assigned to CDOM. This is later adjustable and absorbing groups remain nonidentified in any case [
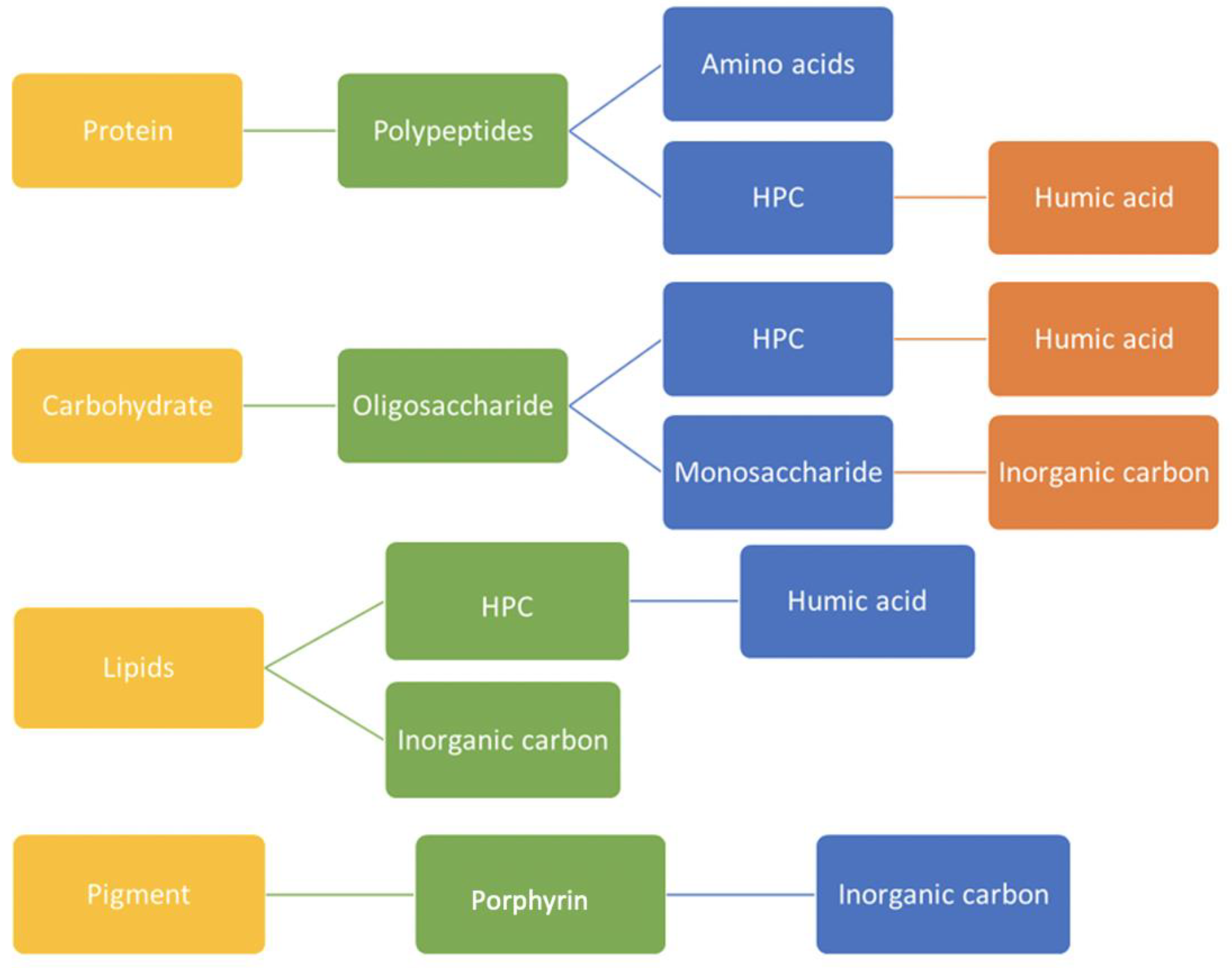
1]. Most of our organic concentrations lie in the micromolar range, expressed as a local concentration of carbon atoms. The chemical network is highly streamlined (pseudo first order), representing bacterial and photochemical degradation as a matrix of time constants. For example, chained amino acids break down into polypeptides, then the monomers with a sub-stream of recombination to heteropolycondensates (HPC) or humics. Carbohydrates become oligo/monosaccharides, then add to the HPC–humic bins. Lipids resolve themselves into specific fatty acids. Colored materials encompass a portion of aged re-condensate, plus specific pigments and porphyrins. The latter transform into inorganic and simpler carbonaceous forms.
We simulated just one idealized 3000 km-long river, supposing it is typical and that in the future the Yenisey, Ob, Kolyma, Mackenzie, etc., will be representable. However, as the most easterly major system, the Lena was adopted as a first geophysical model [
12,
13,
14]. Numerically, our form is the Lagrangian box containing microbial and photochemistry and then traveling with the flow. Concentrations of the macromolecules evolve given a quasi-steady state approximation to solve the ordinary differentials (QSSA). This method dates from the early days of atmospheric chemistry, as various groups were dealing with numerical stiffness in the gas phase [
16]. However, it transposes readily to reactivity in natural waters. Several plots are presented for time-dependent, dissolved concentrations, both in terms of total carbon atoms and those associated with specific functionality. Four major initial scenarios were investigated with regard to the rates and initial concentrations. We call them fast lower, fast upper, slow lower and slow upper, referring to the choice of chemical processes and soil-to-stream initializations. The slow and fast situations are the ratio for our generic, master-constant-based time scales to one another over an order of magnitude. For best-fit contours of the total organics, multiple panels are offered for inspection including the time evolution of a proteinaceous group, the carbohydrate collection, lipids as a family and also a phenolic category along with light absorbers. Total, humic and polycondensate values are sometimes appended as reference points, since concentrations vary by orders of magnitude. Comparisons are presented with data for overall dissolved carbon along the river course, and then for key polymer types at the mouth.
In concluding sections, we review the full computational logic and our results. For each macromolecular class, it is possible to simulate time evolution along a lengthy river coordinate by accounting for mixing, with seasonal chemical transformations superimposed on fresh water transit times [
9,
12]. This suggests the possibility for incorporating more detail through networks based on realistic hydrology. Concentrations at the river mouth are compared with measurement data given standard statistical formats [
17], and through this strategy, we show that the coupled mixing and reactive processes illuminate functionality as well as total solute carbon. Concentrations at the delta mouth greatly exceed levels for the open Arctic Ocean in most cases. We thus argue that strong biophysical effects should be investigated along the coast. For example, adsorptive proteins may favor the marine–atmospheric interface with slowing to gas or momentum transfer [
2], while sugar chains co-adsorb to hydrophilic groups from below [
18]. Lipids will contribute to amphiphilicity as well, and organic chromophores extract light passing downward which might otherwise be available to support bioproduction [
1]. In general, we evaluate multiple organic classes against quantifiable thresholds for coastal physical influence.
The text ends with the development of a simple box model analysis for plume dilution into the major marine receptor, which is along-shore flow with recirculation. The relationship is configured first at a regional scale, based on salinity considerations [
19], then focused against the Arctic coastline with an estimated spreading distance derived from horizontal diffusivity [
19,
20,
21]. It is possible through just a few simple equations to bracket the potential for product concentration patterns to distribute widely depending on lifetime. However, simultaneously, it is clear that biophysical impacts must ultimately be assessed in an interactive and geographically consistent manner. Detailed mixing chemistry then reactive dynamics should be continued from headwaters through tributary–distributary systems out to the turbulent plume regime.
2. Background
Dissolved organics in natural waters are most often studied from the global perspective as a counterpoint to oxidized carbon atoms. Reduced forms may be considered a vast reservoir sequestering material from the carbonate, so that there is no participation in the sea–air interchange as CO
2 [
11]. This is just as true for terrestrial inputs as for marine products, and much effort has gone into sorting the two classes from one another [
9]. In fact, land tracers such as lipid biomarkers and lignin-derived aromatics can be used to achieve separation, and we will track some of them here in a secondary fashion as vegetation derivatives. Examples include the syringyl and vanillyl phenols, which are specific to tundra and forest, respectively [
12]. Continental sources are distinct from their marine counterparts in ways that reflect metabolism and lifestyle requirements. In addition to cytosol content such as protein, polysaccharide and lipid [
15], one must account for structural support including cellulose and lignin. We adopt global average litter proportions for the various vegetation categories, applying them as boreal averages [
9].
Structural carbohydrates and network aromatics can be set aside as solids which remain largely inert [
11]. Most proteins and lipids, on the other hand, are not only soluble but become surface active at the micromolar level (by carbon atom accounting, which we use consistently). Polysaccharides co-adsorb at or near the water–air interface. Hydrophilic groups are targeted, with divalent cations operating as electrostatic bridging agents [
18]. All of our dissolved forms are inherently partially oxidized, and furthermore they degrade near the atmosphere to generate precursors to volatile organic compounds (VOCs). Such transitions can actually be driven by photochemistry isolated to the water–air microlayer, at thicknesses as small as a monolayer or a single carbon–carbon bond [
2,
4]. Example gases include formaldehyde derivatives, as well as glyoxal and low molecular weight acids. Resins, tannins and waxes are present at trace quantities in the soil, but concentrations are typically low enough that we can avoid them preliminarily [
9]. Isoprene and terpenoids must enter flowing fresh waters continually along the banks of coniferous systems, but since some are immediately volatile in and of themselves, we anticipate rapid ventilation [
22,
23]. Only a handful of experiments are devoted to them here. Our tactic in the case of, for example, monoterpenes or related compounds is to allow fast decay toward the atmospheric Henry’s Law equilibrium. Saturation concentrations should be forced to high by regional gas phase plumes during the growing season, since northern latitudes are heavily forested.
Climate change significance of the DOC will be strongly amplified in the Arctic, for a variety of reasons, that will prove relevant to our modeling. These are nicely summarized in the introduction to one of our more important open water references [
1], and further with respect to terrestrial ecotone shifts by Frey and McClelland [
7]. Seasonal discharge will naturally follow patterns of the regional hydrological cycle for a given water shed. Permafrost degradation and the migration of peat/boglands may either pulse-in or reduce organic injections during the melt, depending on local geochemical circumstances. Trends are likely to be complex on a decadal scale. However, of course, whatever the differential, it should maximize each spring. Early ice loss will increase photochemical and biological activity along much of the several thousand kilometer stream length, but especially in deltas and along the coast. Altered organic levels necessarily entail CDOM variation [
1], and so also fluctuations in oceanic radiation attenuation. This effect is readily seen in satellite images to be a strong one. As aqueous concentrations adjust to regional warming and ecosystem change, they will alter a variety of fluxes into the polar marine atmosphere. At the sea–air boundary, evolving surfactant adsorption plays into both primary aerosol formation and the calming of capillarity, such that all manner of mass-energy transfer is in play [
2,
3,
6,
18]. Even metal-binding ligand levels could be tuned, as increased light availability and mixing drive primary production toward the pole. Enhanced nutrient utilization will expand small patches of iron limitation. In the present research, we attempt to lay the groundwork for the dynamic simulation of riverine DOC flow change, by introducing organic chemistry at a functional level. Moreover, we test for physical significance by exploring extensions into coastline plumes and peripheral seas.
3. Definitions
Biologically driven carbon chemistry is extremely complex—second only to biology itself—so that careful definitions are demanded when entering our discussions. We adopted a marine perspective as the foundation, since this reflects our recent research background. However, we then worked back toward central Asia, since eventual goals are cross cutting and multidisciplinary relative to the Arctic Ocean biophysics. A conceptual starting point has been our experience with biosurfactants, acquired lately at the scale of the global sea–air interface [
2,
6,
10,
18]. By now, we have delved deeply enough into this topic to consider separately such specialized biopolymers as peptidoglycan, chitin, chitosan and the molecules of information [
2,
15]. However, within a few publications, earlier effort led us to focus on larger contributors to phytoplanktonic biomass—the broad classes protein, polysaccharide and lipid. Of these particular biomacromolecular types, the first and third turn out to be by orders of magnitude the most surface active [
2,
6]. Such behavior is a direct consequence of their metabolic responsibilities within the cell.
Moving upstream, one must naturally superimpose an understanding of unique terrestrial compounds and most especially, the ring structures of cellulose and lignin [
9,
11,
12]. In the freshwater context, their pure forms are mainly subtractable because they are less soluble. There is, however, an important subtlety. Rigid, cyclic biopolymers can act as sources of aromaticity during the synthesis of humics, which are determined mainly operationally as a laboratory precipitate (or adsorbate) under acid conditions. Benner goes so far as to distinguish marine and terrestrial humic categories by this criterion [
15]. The former are of modest molecular weight and are largely condensed from fresh phytoplanktonic cytosol, while the latter carry an extended double bonded signature from land. Aromaticity is a litmus test for diagnosing terrestrial fractions in the bulk ocean [
9]. However, fragments of the fresh carbon linger as a backdrop. A key point in all cases is that active biopolymeric imprints from the inside of living cells must transition through concerted microbial–photochemical attack toward refractory forms [
11]. The latter may well boast geologic lifetimes [
15].
A kinetic formula found to be useful and realistic is just that of fresh degradation funneled through mixed polymeric intermediates [
2,
6,
10,
15]. Heterogeneous chains built up from random monomeric and substituent units exhibit aquatic residence times between those of fresh release and growing resistance. This sequence is common to research on the full spectrum of natural waters, from high altitude fresh systems to the deep sea [
11]. We originally adopted it with regard to remote open ocean plumes flowing away from typical phytoplankton blooms. A central composition bin was referred to generically as processed carbon in this instance [
6]. It was then further divided, into subsets specific to peptides, sugars and crossovers. We considered the family of lipids to constitute a short lived or labile fraction, based on direct coastal observations. However, in more mature surfactant studies, we were forced to deal with global cycling to and from the deep sea, and it proved preferable to define the intermediate pool in terms of mixed structures spreading across all surface waters. These were referred to as heteropolycondensates, partly for obvious etymological reasons but also following oceanographic community tradition [
9,
10,
11,
12,
15].
Clearly, our group has been treating the notion of bioorganic intermediacy somewhat liberally, as emphasis shifts over regions of the total sea. Time constants and meanings have been varied as a matter of convenience, depending on the specific environmental situation. However, degradation consists ubiquitously of background enzymatic action augmented by photochemical breakdown given sunlight [
9,
12,
15]. Microbial catalytic and high energy solar processes are the only means available to advance oxidation working against substantial activation energies for carbon bond rearrangement. The stabilization of diverse chains is merely an inescapable logical outcome, leading to intractable arrangements. In the present work, transformation steps of interest are those lying between the entry of soil carbon into headwaters, then operating during downhill flow along tributary–distributary networks, and finally blending as a kinetic continuum into polar seawater. Chemical intermediates are unavoidable and turn out to be instructive. In the present work, the term heteropolycondensate is invoked yet again but transferred upstream (and abbreviated HPC). This refers to the potential for riverine transition structures bridging toward further terrestrial humic accumulation.
Other entries in our interdisciplinary lexicon will perhaps be more familiar to the global aquatic chemist. We stipulate that stabilizing, acid-precipitable carbon dominates in the soil subsurface, well ahead of the river scheme [
7,
11]. This is just the standard humic mass, with its inherent aromaticity. The reader may wish to note that the term is itself operational, since it derives from the Latin root for “coming from the earth” (it is also thus technically a misnomer when applied to the sea). Our idealized boreal soil substrate is also infused with the fresh biomaterial of litter [
9,
11,
12], and necessarily in between earth and sea we postulate HPC-style intermediates. These are permitted to decay toward their natural sinks in all reservoirs. From the river terminus, the language of chemical oceanography once again prevails, as we consider brackish media, coastal turbidity, sedimentation, the microfilm coating, an intense microbial food web, and the interaction of chromophore groups with incoming solar [
1,
9,
10]. Regional photosynthesis, mixed layer heating and the field of pack ice become relevant. The overall framework is seen to be integrative of Earth system components that are most often modeled in isolation.
4. Early Geometry and Initial DOC Concentrations
Our model structure consists of one primary (first or longest) river starting from some mountainous area where gradients are steep and biogeochemical activity relatively low. We introduce two nodes or tributaries along the journey to the sea, one at a distance of 1000 km from the source and another at 2000 km. These inflows are simulated independently using the same kinetics. The reader should note a similarity to major Russian (Siberian) systems in general [
12] and more specifically with regard to the Lena as in the experiments of Lara and others [
13,
14]. Plus, the resemblance to specific alternate networks such as the Yenisey and Ob is obvious since there will always be drainage of the central Asian plateau. Flow rates are set at the very rough round value of one meter per second following the extensive older USGS data from North America but consistent with modern satellite retrievals [
24,
25]. Thus, the total transit is made by any one Lagrangian chemistry box in about one month, in agreement with global hydrological averaging [
11].
The plotted initial flow is usually taken to emanate from high elevation [
8]. More concentrated solutes are added to the alpine headland value first at the one thousand milestone, and they are characteristic of familiar wetlands including both woodland and peat ecosystems [
7]. This injection occurring early in our coarse network can be thought of as corresponding with the entry of the Vilyuy and its organics [
13,
14]. Then, an inflow is appended as filtered tundra vegetation [
8]. Depending on the wetland type around primary streams, their initial concentrations are varied between round figures as discussed in the introduction. According to the group Shogren et al. [
8], total dissolved organic concentrations for alpine and tundra watersheds fall respectively in the ranges of 30–300 versus 100–1000 micromolar. In the latter case, the most common measurements lie between 300 and 500. Seasonality is handled through local averaging. The Frey/McClelland data provide us with estimates for high solute forests or bogs, and the values may be compared with historical tabulations in, for example, Stumm and Morgan [
11]. Roughly speaking, woodland, bog and peat fall in the interval from 500 to 6000 micromolar with peaks around 3000 [
7].
We translate these results into a table defining major scenario types. Several critical assumptions then enter into choices for the initial concentrations assigned to individual organic classes. The proportion of humic substance should be greater than a majority based on global and Arctic observations reviewed by Hedges et al. [
9] then later in Dittmar and Kattner [
12]. The vegetative structural materials consisting mainly of cellulose and lignin are presumed insoluble and so are set aside. Fresh cellular biomacromolecules are then fractionated based on global estimates for overall ground level and soil plant matter, whether living or having transitioned to litter.
Subsurface organo-functionality becomes complex on land long before the river is reached. Ecosystem-level nutrient and water limitations dictate the columnar buildup of biomass, litter and soil reservoirs with the eventual output to the atmospheric and aqueous phases. We parameterized details here using selected observational constraints, aiming for coupling with land models as the calculations gain acceptance. About half of all above-ground carbon exists as the engineering cellulose or lignin, but several percent of the remainder can be assigned to the fresh biomacromolecular classes of protein, polysaccharide and lipid [
9,
11]. Cutins, tannins and specialty substances (molecules of communication, protection, information) are present at low levels as well. Of the latter, we deal in the present work only with the release of volatile organics. It is postulated that cellulose and lignin are somewhat inactive, while fresh amphiphiles and polymers are by contrast very mobile. Note that measurements historically indicate that riverine dissolved carbon is two thirds heterogeneous [
9,
11,
12]. Hence, we initialize with protein–carbohydrate-lipid all rounded to 5 percent, which is conservation consistent. This logic implies at least a factor of uncertainty of two, but we feel it constitutes a reasonable starting point.
In some runs, the individual fresh concentrations were doubled or tripled without changing the total dissolved carbon, but agreement at the generic river outlet was sacrificed relative to measurements [
12]. Preferred round values for all species are given in
Table 1, below the summed or total concentrations. In the preliminary soil reactor, which is arbitrarily set to flow at 0.1 m/s over 100 km, the equilibration is permitted into oligomeric, monomeric and recondensate forms. Intermediate molecular weights are thus monitored explicitly and their curves will be obvious in our images. Note that the cytosol macromolecules are set equal to one another. This differs from global marine carbon and fresh biopolymer cycling, for which sixty–twenty–twenty ratios can usually be assumed [
15]. Terrestrial material contains hemicellulose and waxes that tend to even out the macromolecular fractionation. Finally, we fill decrements up to 100% with heteropolycondensate which then decays to humic chains. All of this closely follows diagenetic evolution schemes outlined in the classic aqueous chemistry text Stumm and Morgan [
11]. The vanillyl and syringyl phenols are superimposed at 1% concentration as biomarker examples, for exploration of taiga and tundra inputs, respectively.
5. Model Description and Mechanism
The model itself is a Lagrangian box or aqueous chemistry volume moving toward the sea at one meter per second. Exceptions occur at the start of the simulation and its end, where the flow is reduced to 0.1 m per second for 100 km. The slowing represents a soil reactor/processor and a matching (terminal) deltaic counterbalance. These artifices are intended for later refinement. We are now seeking interactions with land or delta model groups to provide accurate, regional time scales as constraints. The hope is to reciprocate by returning concentration relationships along the much longer intermediate web. Our chemical mechanism is described in the extensive network
Table 2. An arithmetic shorthand condenses branching across what is in fact a strictly unimolecular process set. For example, the term 3(10/13) means a slight speed-up relative to the faster of two loss channels given the coupling of 3 days with 10 days. This and any related factors apply in several cases.
Within our table entries, the reader will find a matrix of biomacromolecular types prominent in riverine chemical evolution, their sources as we define them, a master list of baseline time constants, and products. Timescales are temperature-independent open ocean values for coastlines hinging on the central figure ten days [
10,
15]. These are likely to prove faster than any along the boreal river path. Rates were therefore downgraded as sensitivity tests, and one becomes the preferred scenario. Microbial consumption tends to be the fastest removal, but it should nonetheless remain weak over the course [
9,
12]. Referencing is as follows: for cellulose, lignin and humic chains plus related physical properties, see the review by Hedges et al. [
9]; for protein, polysaccharide, lipid and heteropolycondensate (HPC) Ogunro and colleagues are fairly thorough [
10]; for polypeptides, amino acids, oligosaccharidic and monosaccharidic substances, the Benner overview is informative [
15]; for vanillyl and syringyl phenols, Dittmar and Kattner discuss the background ecology [
12]; for CDOM, pigments and porphyrin consult Stedmon et al. [
1] plus the aquatic chemistry texts; and for VOC
II, Mungall and company [
4] give examples of small species released into the Arctic atmosphere by irradiation.
The purpose of the table is really to provide a mental image of carbon flow, through the redox switching of degradation–stabilization. It actually constitutes an idealized set of fates, for overall biomacromolecular mass. For example, protein loss logically leads first to peptides then amino acid. Such conclusions are readily verified for boreal rivers per the detailed organic perspective of Dittmar and Kattner [
12]. Routings are displayed schematically in our
Figure 1 [
11]. Pseudo-first order competitions occur at the branch points, leading to time scales given in days in the third table column. Some are semi-arbitrary, and derivations are available from the authors.
As Lagrangian integrations proceed, functionally resolved species are treated as independent over a time step in an exponential-form Euler, embodied by the quasi-steady state approximation or QSSA [
16]. A summary of the equation set is offered just below. Here, the
Ci are concentrations for some biomacromolecule of interest, while
Cj(k) are those of others in the network, P and L represent the production term and loss constant, respectively, and the symbolic delta t is a short interval following the volume downstream. Since concentrations are updated one by one, mass conservation is only strictly maintained as the step size is reduced. A conclusion here will be that photochemistry and microbial action are relatively slow, so that stiffness is not a concern and we avoid linear algebraic inversion. In the present work, tests were conducted down to one second over the month-long simulations, but most runs advanced at one hundred seconds. Concentrations are always carried in micromolar of pure carbon, equivalent to elemental millimole/m
3:
Several special cases need to be mentioned relating to the table kinetics. We postulated that cellulose and lignin are insoluble. This is supported by a history of observations [
11], and aqueous equilibrium values are only of the nanomolar order. The polysaccharides are sometimes termed starch and they represent high molecular weight sugar chains. Specific monomer identities are given in Dittmar and Kattner [
12] but none are tracked here—examples include glucose and mannose. The lipids are modeled as long chain saturated or double bonded fatty acids rather than triglycerides or phosphorus structures. Therefore, small amphiphiles are not considered to be polymeric and there is no need for an intermediate. Several biomarkers are included, e.g., the lignin-derived phenols which are vanillyl and syringyl aromatics. They are capable of representing upstream taiga and tundra ecosystems, and we set their initial concentrations at arbitrary unit percentages. The concept is to demonstrate the potential for organics to track terrestrial ecodynamics. We could consider adding marker lipids along with waxes such as cutin, but this will be reserved for later [
9,
12]. We also performed offline computations for two types of volatile—those formed early in the process within a soil reactor environment, plus late-comers attributable to photolysis in the river plume and extending out into the open sea. Mechanisms are described schematically in the table columns.
A major goal is to dynamically assess any biophysical influence the macromolecules may exert once they enter a generic marine mixed layer. Hence, we took special care to chemically characterize light absorbers. Pigments are represented first by standard chlorophyll, also given a round low value signifying that it must be present from the outset. We hypothesized that chlorophyll decays to its porphyrin ring, which remains optically active. Finally, we took a low percentage from each of the proteinaceous, humic and heteropolycondensate pools along with other pi-electron rings and defined the total to be chromophoric. CDOM is an especially apparent and readily monitored fraction of freshwater organics [
1]. In fact, since its concentrations are not directly measured as a rule, we will deal in relative concentrations per absorption properties at the reference wavelength of 375 nanometers—normalizing to local DOC. Specifically, we associated the coefficient of 10 m
−1 with the total level of 1000 micromolar. It is possible to calculate a physical optical cross section from these figures (0.01 m
2/millimole), and furthermore, to verify that only a small portion of the available carbon atoms need enter into the quantum mechanics of light extraction. The specific groups involved remain poorly understood.
Ultimately, radiation integrations should be performed along the wavelength coordinate, but for the moment, it is sufficient to note the following: Stedmon-type spectral slopes S as in [
1] suggest little proportional change moving to 400 nm and perhaps an order of magnitude less in attenuation in the mid-PAR (blue photosynthetically available radiation, 500 nm). Control over penetration depth therefore remains strong relative to the reference point, as one moves toward the infrared. Stedmon et al. explored the details from an empirical standpoint with respect to the primary production, photochemistry, heating, ice retreat and more. Before moving on to the results, we conclude this section by reiterating a crucial simplification. Please bear in mind that all concentrations are documented here in units of micromolar-dissolved carbon atoms, regardless of the length of a polymeric or aliphatic chain. A kilodalton strand thus enhances the elemental molarity accordingly.
7. Aqueous Volatile Organics as a Set of Special Cases
Volatile compounds which are the theme of the present issue deserve special mention at this point. They are closely related to our biomacromolecular families in that higher molecular weight carbon serves as a synthetic basis in many cases. Organic gases must have biochemical precursors, whether intra- or extracellular [
4,
26]. Marine phytoplankton produce isoprene as a byproduct during photosynthesis, and fluxes are often proportional to primary production [
27]. Analog terrestrial processes are energized through ATP driving 3-PGA generation (glycerate 3-phosphate, a metabolic intermediate) [
28]. With respect to river headwaters, we simulate VOC evolution as Henry’s Law equilibration, moving either upward or downward toward the dissolved steady state. Initially, degassing is prevented by physical capping since the atmosphere is not in intimate contact with the porous soil subsurface. There is no headspace for an aqueous phase tracer to enter. In several offline calculations, however, such barriers were removed. Analytical and Lagrangian boxes were uncapped then released for short excursions downstream, as loss occurs in minutes to hours so that full river lengths were unnecessary. Gas dynamics were portrayed as ventilation across the liquid laminar layer at the top of the turbulent upstream fluid. Isoprene served as the main model compound. In this sub-investigation, we focused first on the woodland tributary type identified at node one in
Table 1, where for example conifer-derived terpenoids are likely to enter the aqueous soil environment [
23,
28]. The early input segment, however, also encompasses northern peats and bog-lands [
7], so that volatile production has to be viewed as hypothetical. To address a broad spectrum of VOC concentrations, we studied the evolution for the entire scenario range low to upper, with local aqueous isoprene varied as a proportion of the total DOC. This provided a demonstration for variability that may be inherent to terrestrial aquatic trace gas chemistry.
We began by assuming that forest litter contains common terpenoids at parts per thousand of the total dissolved organic mass [
23,
28]. The figure is high and somewhat arbitrary. After an initial period of confinement, a head space was opened imagining that aqueous flow emerges from soil substrata. Ventilation rates were modeled via the standard laminar layer interface parameterization [
22]—but with diffusivities and Henry’s Law constant values appropriate for isoprene [
26]. We postulated a sharp rise in micromolarity in the spring as temperatures warmed, and related detritus underwent early diagenetic processing. However, further in the exercise, atmospheric mole fractions were varied upward from zero to those expected in the wake of a photosynthesizing forest [
28]. The basic result was just this: because organic gases trapped in soil are volatile by definition and headwaters are shoal, the equilibration of the aqueous flow medium can be fast. We assumed a nominal baseline depth of one meter with sensitivity testing, and instantaneous turbulent mixing below the thin barrier. Ventilation into pristine air masses and equilibration with forest haze plumes were both extremely rapid. Time scales in fact were of the order of hours or less, so it is obvious that control is established locally along the course. The calculations were repeated for our other ecosystem choices of mountain and far northern tundra. Though concentrations differed widely, conclusions were similar.
To address the organic volatility of polar ocean systems, we estimated a mixed layer buildup for carbon volatile material released according to the photosynthetic rate, into the pool of water column DOC [
26]. Refer to the mechanism table, near the bottom of our schematic list. Computational methods most often employed for large scale mapping follow either algorithms applied about a decade ago to dimethyl sulfide [
29] or else to the full spectrum of dissolved organic functionality leading to surfactant buildup [
10]. Levels sufficient for outflow from the sea surface are readily supported. Isoprene and the monoterpenes were taken as examples—they are rudimentary in molecular structure, containing only carbon and hydrogen atoms, plus high latitude sea–air flux determinations have been reviewed by the Shaw group [
26] among others. We note especially that adsorbed organic monolayers are now thought to contribute independently to volatilization. As reported by Mungall et al. [
4], the Arctic sea surface film is demonstrably an atmospheric source for low molecular weight carbon. This is true in particular for small oxygenated species such as acids, aldehydes, ketones, diols or glyoxal. Amplification must be anticipated in riverine coastal plumes [
12].
9. Summary and Discussion
Aqueous organic chemistry transformations along an Arctic river are driven from their inception by terrestrial biota, but the oxidized group distributions are necessarily complex. Functionality will be conserved to some extent heading downstream, with release into coastal waters where solutes impact regional biophysics of the marine system. Examples include surfactant influence on aerosol and sea air transfer [
2], and light penetration because biomacromolecules contain chromophores [
1]. Thus, there is a potential for land ecosystems to communicate at a distance with oceanic analogs—by modulating mixing behaviors, composition and primary production in peripheral seas. To sort out this complex environmental chemical situation (simulate the effects), we developed a first-generation kinetic model arrayed along an idealized Siberian river. We investigated the dissolved organic composition in an obvious but unique manner, by insisting on functional resolution and then tracking the interplay between highly divergent structures. Protein, lipid, carbohydrate, heterogeneous recondensate, humic and fulvic substances are all treated. Furthermore, we subcategorized and carried both oligomeric and monomeric units where appropriate, while allowing for not only photochemical/microbial decay but also the random rebuilding of intermediate polymers [
9,
11].
The framework for our study is a numerical network mimicking the Russian Lena drainage [
13,
14], and extending to a length of 3000 km. We included two distinct input tributaries as a matter of demonstration, connecting with the main flow at 1000 and 2000 km from a hypothetical highland source. Transport and chemical reactivity are represented in a Lagrangian mode. Along the primary stretch and also tributaries, the processing of various organic molecules is simulated by analogy with the coastal ocean, which provided baseline rates [
10]. However, these have been slowed on a sensitivity test basis to match total dissolved carbon measurements, and the best fit with actual data was achieved for the rates low enough that mixing becomes a dominant factor. The riverine organic mechanism we derived was perhaps the most detailed yet applied to boreal aqueous carbon cycling, but it is nonetheless compact and amenable to insertion into hydraulic codes. Major dissolved forms permitted to enter the river extend well beyond the macromolecules mentioned above to specific markers including (1.) terrestrial lignin phenols capable of distinguishing e.g., tundra, (2.) analogs for the volatile organics and most especially terpenoids plus small intermediate oxidation states, as well as (3.) CDOM apportioned from all dissolved organic matter [
1,
12,
15]. Biotracers decompose to inorganics in our scheme, and absorbers become porphyrins in part since extinction is contributed by chlorophyll.
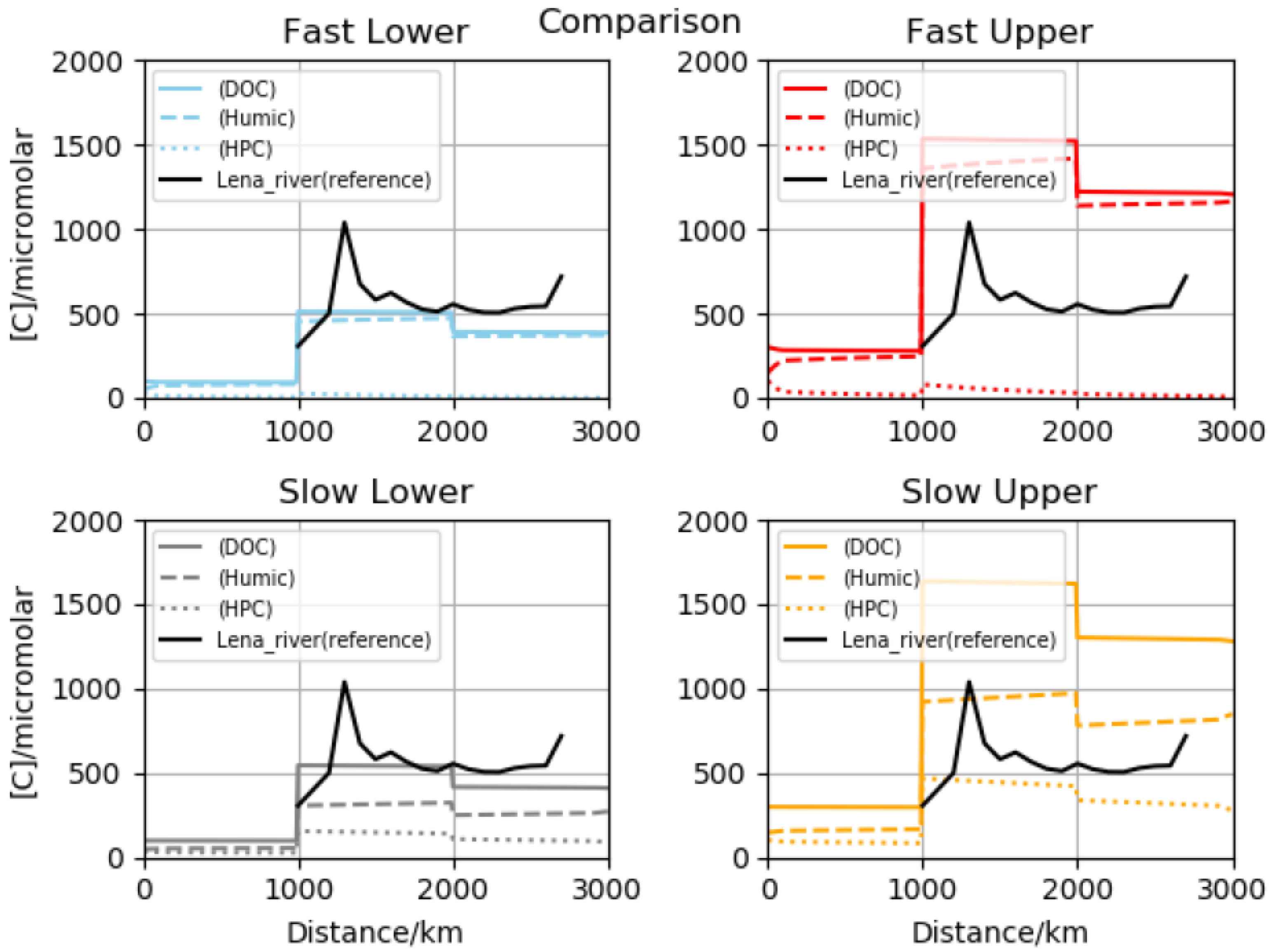
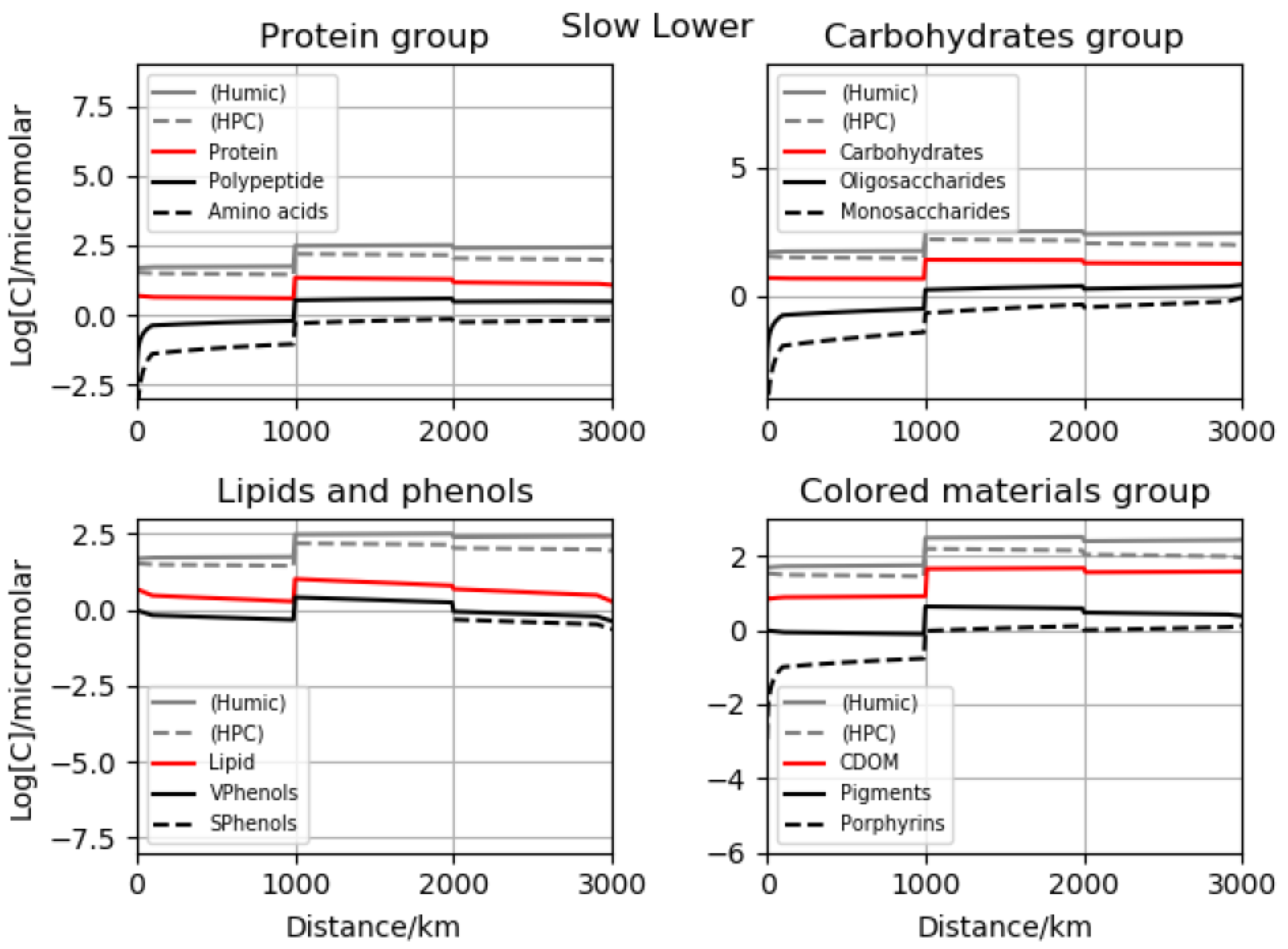
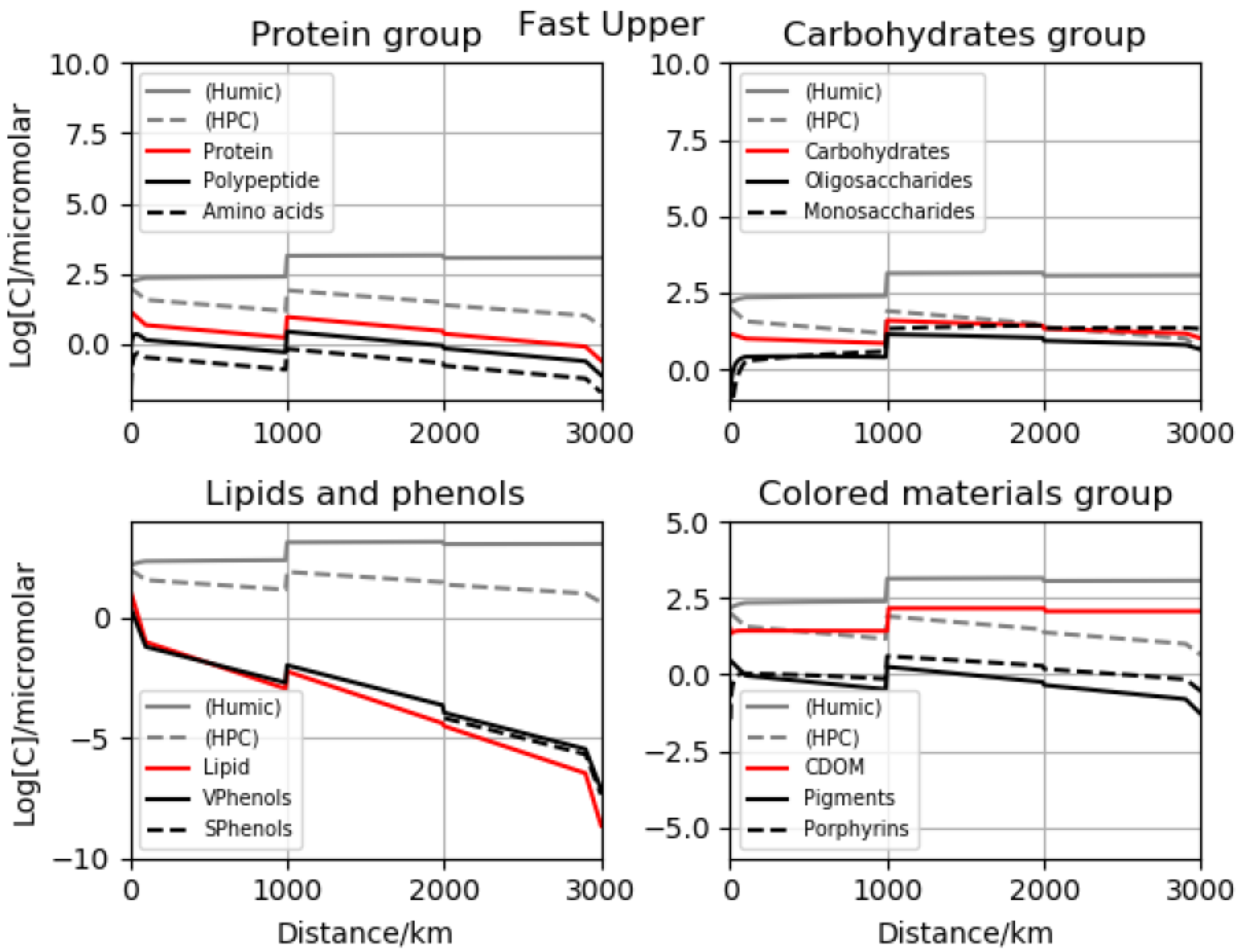
Four main lines of numerical experiment were developed, by varying initial soil-injected concentrations [
7,
8] as well as boreal aqueous turnover rates for the various species [
9,
10]. In several sets of runs, we applied the maximum possible soil levels from observation, and for two further groups, much lower startup values were assumed. These broad calculation families were labeled upper and lower versions, respectively. In sub-simulations, we adopted either reactivities equal to coastal estimates or reduced by a factor of ten. Excluding stable classes such as conserved organic carbon, temporal variation was significant. Comparison with the Lena data obtained downstream from Yakutsk [
13] indicated that one particular combination may be somewhat superior—that termed slow lower. We performed comparisons with both total organics and outlet biomacromolecular determinations, and satisfactory agreement was obtained. Results were also placed in the context of pan-arctic data sets, and all preferred results fell inside the standard deviation envelopes. Stepping back to the full-length vantage point (highland-to-sea), we showed that in most cases concentrations are enriched across the delta–coast boundary. This is despite the complex input, mixing, dilution and processing. In fact, we argued that outlet levels may be biophysically significant, since known thresholds are surpassed for adsorbed coverage (surfactants in the Langmuir–Freundlich sense), primary organic aerosol emission mechanisms including the co-association of carbohydrates through metal cations [
4,
18], surface tension effects on sea–air transfer [
2,
6], light absorption [
1,
15] and more. Cutoffs documented in our reviews for marine film chemistry are roughly 10 and 0.1–1 micromolar bulk carbon for proteins and lipids, respectively. Moreover, we now have reason to believe that remote sensing will demonstrate the suppression of high latitude capillary waves in both hemispheres [
32].
Although simulated and measured riverine concentrations are higher than those of Arctic seawater for any of the organic forms considered, questions remain regarding the areal extent of the plumes generated. Moreover, it is their (bio-)geographic area which will serve as an early indicator of ecological or economic importance. Indeed in the immediate vicinity of a deltaic or estuarine exit, dissolved carbon concentrations are necessarily at maximum. However, overall it is now necessary to begin mapping potential zones of oceanic physical influence. Dilution factors will exert themselves rapidly in the along-shore environment, and ultimately turbulent horizontal mixing drives communication with the free background. We anticipate that these issues must soon be addressed in coupled river input-general circulation models. To conclude, a preview is offered for river tracer distribution patterns. We constructed and estimated these in an analytical fashion. Coastal mixing calculations are now put forward at a back of the envelope level, and we propose to improve them as legitimately linked land–river–delta–plume simulations become available.
A flexible box model was designed for the quick assessment of plume dilution within an unspecified peripheral sea. For example, the Laptev can be seen as a receptacle constrained by Severnaya Zemlya and the New Siberian Islands, while the complete eastern Arctic Shelf is intensely fueled by multiple discharges [
12,
19]. In the equations just below, V are the volume flow rates associated with either assemblages of ocean currents which may be recycling (subscript
o) or else river inputs (subscript
r). Additional identifiers
i/f signify initial and final states for transiting seawater. Lower case v subscripted “as” is restricted to the current velocity oriented along shore, and depth z always refers to the average mixed layer (ML). The K are just horizontal diffusion coefficients as often tabulated for coastal sites around the globe [
20,
33]. Moreover, letter t is a flexible time period capable of taking on several meanings, but here mainly defining an advection scale. Applying mass conservation and then minimal rearrangements, we obtain symmetrical relationships for the dilution of either marine or terrestrial inputs to aggregate pipe flow:
The first entry of this set is just a river-to-ocean tracer concentration balance in the simplest possible form. The next two summarize the linear relationships among concentration ratios. Quick tests such as Cr = 0 versus infinite, Co,i = Cr can be used to check that the derived dilution ratios are sensible. In the last line, we point out that for rapid processing, it is more appropriate to consider local mixing.
One scheme for applying such relationships is to first calibrate against well understood stable substances. For example, minerals and salt constitute only minor components of Arctic river water [
12]. Salinity values therefore drop dramatically along the brackish Asian coastline, from order 35 to 25 psu [
19]. The ratio 5/7 falls out of the
Co,f/
Co,i expression as an approximation and leads to
Vr/
Vo = 2/5. One can use well known discharge rates for the Russian sector (several times 10
4 m
3/s [
12]) and a scale volume of (1000 km)
2 by 30 m
zML to verify a reasonable sub-basin refill/purge rate of ten years. Thus, we feel confident in moving on to assess the dissolved organics since the decay constant is long and comparable [
19]. For shorter lived tracers it is necessary to use
Vo computed along shore in the final expression. The limiting round figure inputs are 1 m/s, 30 m mixed layer depth, the central global (horizontal) eddy diffusivity of 10
3 m
2/s and the duration of one day implying a one hundred kilometer stretch of coastline, giving receptor flows of about 3 × 10
5 m
3/s. An individual spring river discharge peak reaches 3 × 10
4 [
12] so that
Vr/
Vo = 1/10. Final oceanic dilution can then be computed for both stable and transient tracers. Results are sampled in
Table 5, which explores many of the equation terms. References for abundance are [
1,
12,
19].
Fractions and ratios in the table have what are sometimes obvious values, but the aim here is clarity. Note that in order to avoid clutter, we assume that blank cells repeat from neighbors above them. To the extent that our linear approximations reflect current and upcoming reality, salinity allows the calibration for the integrated dissolved organics. Agreement is excellent for full systems modeling and MIT data quoted in support [
19]. For a world with global warming and permafrost/peat/bog destabilization, we assign an arbitrary increase in river DOC of a factor of three [
7]. Coastal dissolved carbon jumps dramatically and a zeroth order guess would be that biophysical effects may scale. Chromophores are not adequately represented by the salinity model because they are relatively short lived—as little as one year [
1]. So we compute an independent along-shore flow with updated local volume ratios. It applies to a single one hundred kilometer stretch downstream, and in some cases the major discharges are closely spaced so that there will be additivity. Even in the present day, light absorbers only dilute to about one per meter (375 nm) and given the ecosystem disruption, shorter scales are not inconceivable.
In conclusion, we now organize a set of broader factors arising, with respect to river-sourced organics as the Arctic thaws in coming decades. Our Lena model estimates likely apply to other prominent watersheds—Kolyma, Yenisey, Ob, Mackenzie, etc., with smaller systems intervening. The issues we have come across are presented here in bullet form, since they are numerous and interactive/overlapping. Our considered opinion is that it would be well worth the community asking:
- -
What portion of ancient litter in permafrost remains in active, biomacromolecular forms? Are functions somehow preserved? Perhaps contemporary initial protein/lipid proportionalities do not translate to the millennial scale.
- -
What portion of ancient carbon assigned to humics still actually behaves in sub-functional form, perhaps retaining absorptive or adsorbing capabilities? Humic acids are known to be oligomerically heterogenous. Relict segments could wield undue influence.
- -
Can we faithfully simulate the chemical composition of future boreal soils? Is it necessary or even possible to portray millennial organic microbial processing?
- -
Similar issues apply within the delta regime, as organics passing into the ocean to become pelagic tracers/contributors. High concentrations require that we take all nonlinearities seriously.
- -
Will proportions of light absorbing groups evolve and redistribute? It seems unlikely that they would remain fixed as source ecosystems far upstream reconfigure.
- -
Will sufficient organic mass be released from the high north to monolayer-cover the entire Arctic Ocean? More likely in variable interannual pulses, but perhaps at some heightened steady state?
- -
Will mixed layer and film carbon reservoirs act as regional ultra-sources of critical atmospheric products: primary organic aerosol, low molecular weight (oxidized) carbon as VOC, or the eventual secondary organic aerosol (SOA)?
It is by now widely accepted that an ice-free Arctic lies only a few decades ahead [
1,
7]. While basic drivers of marine primary production are already under evaluation under the new world order (nutrients fertilizing phytoplankton), dissolved organic chemistry of the northern ocean will steadily intensify and is receiving but little model attention. It appears that the spreading of basin scale films/slicks and the potential darkening of the summer column cannot be precluded. Thus, the evolution of not only carbon dioxide fluxes but also those of other elemental carrier gases, organic aerosol, momentum, heat, and water vapor are all in play. The integrated effects would constitute a hemisphere-scale communication, from warming ice age ecosystems on the continents down to the Anthropocene northern sea. Information would be transmitted by detailed molecular structure, given a chained carbon alphabet. Thus, we propose that a little recognized need for the Arctic systems modeling community will soon be an incorporation of dynamic aqueous organic tracers well beyond the traditional DOC.
Our own family of boreal system simulators now includes several regionally and finely gridded ocean models, with competitive marine ecodynamics and seawater biogeochemistry mechanisms. However, the classification of the dissolved organic forms is just beginning to enter, and only at the level of gross stability (for example, semi-lability as in [
10]). Both labile and refractory macromolecules are omitted, and this really means most forms except perhaps pure polysaccharides and a poorly defined small fragment of the open marine heteropolycondensate [
6,
10]. Model river inputs currently range from none at all in some versions of our Arctic code suite, leaving pelagic ecosystems to fend for themselves in fueling reduced carbon, through annual averages for inorganic mass, with some attention to the spring freshet. However, inconsistency is the rule, and we anticipate that Arctic ocean validation exercises will soon underscore a strong need for coupling land–river inputs.
The preferred organo–chemical kinetics scheme we developed, consisting of about a dozen mildly interacting structures, should blend smoothly into tributary, deltaic distributary, plume interface and coastal algorithms. Furthermore, the Asian river network appears to be mixing-dominated, and so it must also be closely defined by initialization working from the top. Functionally resolved models of boreal soil and permafrost composition must be consulted. Moreover, the continental in-flux of organics must finally be rectified with global marine biogeochemistry, which remains coarse at the pelagic entry point. Our own open-water projects have recently moved in this direction through the addition of independent DOC tracers. Our resolved biomacromolecules can be adjusted, for example, to represent the suite of surfactants, aerosol or trace gas sources plus even the chromophores.