Abstract
Exposure to fine particulate matter (PM2.5) has well-established systemic human health effects due in part to the chemical components associated with these exposures. Oxidative stress is a hypothesized mechanism for the health effects associated with PM2.5 exposures. The oxidative potential of PM2.5 has recently been suggested as a metric that is more indicative of human health effects than the routinely measured PM2.5 concentration. The purpose of this study was to analyze and compare the oxidative potential and elemental composition of PM2.5 collected at two locations during different seasons. PM2.5 was collected onto PTFE-coated filters (n = 16) along two highways in central Oregon, USA in the Winter (January) and Summer (July/August). PM2.5 was extracted from each filter via sonication in methanol. An aliquot of the extraction solution was used to measure oxidative potential using the dithiothreitol (DTT) assay. An additional aliquot underwent analysis via inductively coupled plasma—mass spectrometry (ICP-MS) to quantify elements (n = 20). Differences in PM2.5 elemental composition were observed between locations and seasons as well as between days in the same season. Overall, concentrations were highest in the winter samples but the contribution to total PM2.5 mass was higher for elements in the summer. Notably, the oxidative potential (nM DTT consumed/µg PM2.5/min) differed between seasons with summer samples having nearly a two-fold increase when compared to the winter. Significant negative correlations that were observed between DTT consumption and several elements as well as with PM2.5 mass but these findings were dependent on if the data was normalized by PM2.5 mass. This research adds to the growing evidence and justification for investigating the oxidative potential and composition of PM2.5 while also highlighting the seasonal variability of these factors.
1. Introduction
Fine particulate matter (PM2.5) is a component of air pollution with well-established systemic health effects. There is strong evidence linking increased concentrations of PM2.5 to cardiovascular and pulmonary comorbidities, especially amongst the elderly and those with compromised pulmonary function [1,2]. Many regulatory agencies throughout the world set standards and guidelines based on PM2.5 mass concentrations [3,4,5]. While the adverse health effects related to concentration have been well-studied, there is comparatively less known about the underlying mechanisms of the health effects, as well as connections of these effects to PM2.5 composition.
The relationship between the concentration of PM2.5 and its adverse health effects has been investigated [6,7,8], but it is also important to note that particulate matter is a heterogenous mixture with temporally and spatially varied compositions. Factors that impact PM2.5 composition include weather patterns and emission sources [9]. In coastal regions, for example, mass fractions of crustal material, trace elements, and organic matter are higher during the Fall and Winter months while sea salt particles were more prevalent in the Spring and Summer [9]. Throughout a single day, shifts in PM2.5 composition can be observed at a location. This was demonstrated in the Southeast United States where higher concentrations of carbonaceous materials were observed in the morning while higher concentrations of sulfates were present in the afternoon [10]. In addition to temporal factors, location can also impact compositional differences in PM2.5 which are strongly influenced by emission sources. Both the concentration and composition of PM2.5 in rural and urban spaces varies widely due to differing types and levels of human activity (e.g., farming, traffic, etc.) and meteorological factors [11].
Due to the established variability in PM2.5 composition, research has begun to investigate the health impacts of these differences. Epidemiology studies have shown a positive correlation between elemental concentrations (primarily C, Ni, and V) and hospital admissions for cardiovascular and respiratory issues [12,13]. Results from several studies suggest that increased toxicity is associated with PM2.5 emitted from traffic, which contains higher concentrations of carbon and specific metals than standard urban background [14,15]. It has also been noted that compositional differences in inorganic (Al, Ca, Fe, K, Mg, and Pb) and organic (polycyclic aromatic hydrocarbons) content may lead to varying mechanisms of cell death (necrosis, apoptosis, or autophagy) [16]. Establishing not only the compositional differences and resultant toxicity outcomes but also identifying the underlying mechanisms is critical for providing targeted regulations to protect human health.
Oxidative stress occurs when the accumulation of reactive oxygen species (ROS) overwhelms the body’s mechanisms for neutralizing them. This state can contribute to a number of adverse health effects including cardiovascular, neurological, respiratory, reproductive and kidney diseases, as well as cancer [17]. PM2.5 is known to cause oxidative stress via the generation of ROS such as hydrogen peroxide (H2O2) or hydroxyl radicals (•OH) [18]. The tendency of a chemical species to oxidize a target molecule is known as its oxidative potential [19]. Analysis of oxidative potential has been conducted for a number of PM2.5 studies using various assays including the dithiothreitol (DTT) assay [20,21]. Understanding the role of PM2.5 composition and source contributions to oxidative potential is an area of growing interest particularly due to the recent hypothesis that oxidative potential and composition are more health relevant metrics of PM2.5 than mass [22]. Previous research has observed differences in oxidative potential based on source contributions with increased oxidative potential for PM2.5 from traffic and underground railway stations as compared to rural and lower traffic locations [22]. Seasonal variations have also been explored with several studies identifying increased oxidative potential in the summer compared to the winter [23,24,25,26]. Other studies have investigated the connection between oxidative potential and fine particulate matter composition and identified significant positive correlations between oxidative potential measured by DTT assays and the concentration of transition metals present [27]. While there is growing research in this field there is a need to further establish the trends of oxidative potential based on PM2.5 source contributions, seasonal differences, and chemical composition particularly in understudied areas of the world like the state of Oregon in the United States.
In this study we explore the connection between PM2.5 composition and oxidative potential at two sampling locations across seasons. PM2.5 filter-based samples were extracted and aliquots of each sample were used for chemical composition analysis and oxidative potential analysis. We hypothesized that differences would be observed in chemical composition and oxidative potential between the sampling locations and seasons. The study also was designed to explore correlations between oxidative potential and composition of PM2.5 and analyze how these factors may change between seasons.
2. Experiments
2.1. Reagents
Solvents and reagents included methanol, potassium phosphate monobasic (KH2PO4), and 1,4-Dithiothreitol (DTT) (Thermo Fisher Scientific, Waltham, MA, USA), as well as 5,5′-Dithiobis (2′-nitrobenzoic acid) (DTNB) (Sigma Aldrich, St. Louis, MO, USA).
2.2. PM2.5 Samples
Sampling sites were established monitoring locations of the Lane Regional Air Protection Agency that had similar potential traffic sources as both were adjacent to state highways in central Oregon, USA in residential areas (Site A: OR Route 58 near Oakridge, Oregon and Site B: OR Route 99 in Eugene, Oregon). All sampling procedures and gravimetric procedures were according to United States Environmental Protection Agency (EPA) standards with federal reference methods employed [28,29]. PM2.5 samples were collected on 47 mm PTFE-coated filters during 2016 in the winter (January) and summer (July and August). Sampling occurred at both Sites A and B on 4 days for each season (winter—1, 2, 3, and 4 January; summer—14, 17, 20 July and 1 August). Samples were collected for 24 h at 16.7 L per minute (LPM) flowrate. A blank 47 mm PTFE-coated filter, without collected PM2.5, underwent all analyses to serve as a methods control. Samples underwent gravimetric analysis in a temperature and humidity-controlled chamber to determine PM2.5 masses by pre- and post-sampling filter weights and were then stored away from light at −20 °C.
2.3. Extraction
Each filter and control were sonicated (60 Hz, Branson Ultrasonics Corporation, Brookfield, CT) for 60 min in approximately 8 mL of methanol. Each filter was then removed from the tube and rinsed with additional methanol to collect any residual PM2.5 remaining on the filter [21]. The resulting PM2.5 solution was used for subsequent analyses and represents compounds that were methanol-soluble.
2.4. Analysis of Oxidative Potential
A 96-well plate version of the DTT assay was used for all analyses as previously described [21] with minor modifications. Briefly, each PM2.5 sample in methanol and controls ((a) blank filter, (b) vehicle (phosphate buffer), and (c) all reagents but the quenching reagent) underwent the DTT assay. All samples and controls were mixed with 100 µL of phosphate buffer and 5 µL of 0.5 mM DTT solution prior to incubation at 37 °C for 20 min. After incubation, 10 µL of 1 mM DTNB was added to quench the reaction. The plate was then read on a plate reader at 412 nm. DTT consumption was determined based on a DTT calibration curve (0, 0.2, 0.4, 0.6, 0.8, and 1.0 mM) prepared with stock DTT and methanol to adjust for sample volume. All samples, controls, and calibration standards were run in triplicate.
2.5. Chemical Analysis by ICP-MS
Aliquots of each extracted PM2.5 and control solution were blown to dryness with N2, re-suspended in 10 mL of milli-Q water, and sonicated for 5 min. Samples were then analyzed for trace metal content by Inductively Coupled Plasma—Mass Spectrometry (Thermo Fisher Element XR ICP-MS). Quantitation consisted of three runs and three passes per sample to ensure a representative average of element concentrations. Quantitative data in parts per billion (ppb) was collected for Ag, Ba, Ca, Cd, Ce, Co, Cr, Cs, Cu, Fe, Ga, Mn, Ni, P, Pb, Sr, Tl, U, V, and Zn with calibration curves generated using Multielement Calibration Standard Solution 2A (Spex Certiprep, Metuchen, NJ, USA). ICP-MS instrumental parameters are displayed in Supplemental Table S1. Reagent and laboratory blank filter controls were analyzed alongside samples to facilitate background subtraction and to evaluate instrumental drift during analysis. Method accuracy was evaluated by analyzing NIST certified standard reference material 1640a “Trace Elements in Water”.
2.6. Statistical Analysis
Statistical analysis for all data was performed with Sigmaplot 14.0 (Systat Software, Inc., San Jose, CA, USA) and Excel 16.0 (Microsoft Corporation, Redmond, WA, USA). All data were reported as a mean ± standard deviation (SD) and corrected with blank filters or other appropriate controls. Data was analyzed using a one- or two-way analysis of variance (ANOVA) or Student’s t-test to determine differences between PM2.5 samples. Differences with p values ≤ 0.05 were considered significant, unless otherwise noted. Pearson correlation coefficients were calculated and corrected for multiple tests using the Bonferroni correction to determine the p-value that was statistically significant.
3. Results and Discussion
3.1. Chemical Constituents of PM2.5
Elements normalized by PM2.5 mass were determined for each sampling site and day (Figure 1). These values represent the summed total of all quantifiable elements that were soluble in methanol. Significant differences were observed between the two Sites on the sampling days in both the winter and summer; however, there was not a consistent pattern on which Site had elevated concentrations. In the winter, there was over a 2.8-fold increase in the average summed total element concentration at Site B compared to Site A. This pattern was not observed in the summer. Comparing between seasons, elemental concentrations were nearly 4-fold higher in the summer samples across both Sites. These findings are consistent with the 5 highest total element days all being observed in the summer. Significant differences were also observed between Sites on the same days (indicated by * in Figure 1) and a significant difference across all sampling dates was observed between Sites A and B. Specific factors that may impact the location and seasonal differences are discussed in detail below. Overall, variability in elemental concentrations across seasons, locations, and even days was observed in this research, highlighting the importance of routine monitoring to understand the chemical composition of PM2.5.
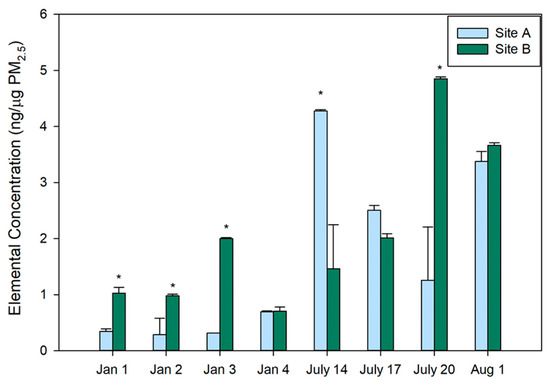
Figure 1.
Total elements/PM2.5 mass (ng/µg) for Site A and B in winter and summer. Elements (ng) per PM2.5 mass (µg) for each sampling day for Sites A and B are reported for January (Jan.) and July. Element concentrations represent the summed totals of all elements that were quantified for a given sample following blank correction. The legend represents Site A and Site B with their corresponding colors. Mean concentrations with standard deviation, represented by error bars, are reported based on triplicate measurements. A two-way ANOVA was used to determine significant differences (p ≤ 0.05) between the sampling sites and days denoted by *.
Individual element concentrations at Sites A and B (Figure 2) demonstrate the variability in concentrations across days, sampling sites, and seasons. The most abundant element across all sites and seasons was Ca, however this element was excluded from the figure as it was 5 times higher than all other individual elemental concentrations. The second highest element by concentration was Fe in both the winter (Figure 2A, average concentration across sites/days of 4.7 ng/m3) and in the summer (Figure 2B, average concentration across sites/days of 3.5 ng/m3). One exception to this was the PM2.5 collected at site B on 1 January which had a higher Sr concentration (6.6 ng/m3). Sr concentrations in the winter were variable (0.21–6.6 ng/m3, 2 samples below quantification limits) and were below detection limits in the summer for all samples. Pb concentrations in the winter were variable (0.02–1.4 ng/m3, 1 sample below quantification limits) but similar to Sr were much less prevalent in the summer (0.01–0.3 ng/m3, 4 samples below quantification limits). Pb concentrations are of particular concern due to the established health effects, including cardiovascular-related hospital admissions, following increased exposure to Pb in PM2.5 [12]. All individual element concentrations with standard deviations are reported in SI Tables S2–S4.
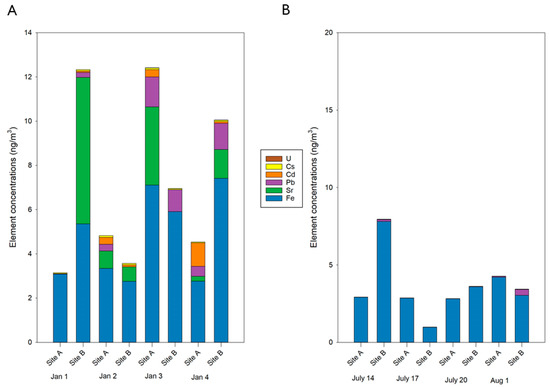
Figure 2.
Concentrations (ng) of elements in PM2.5 collected at Sites A and B. Bar graphs detailing the elemental composition of PM2.5 collected at Sites A and B in the winter (A) and summer (B). Concentrations are reported for each individual element, represented by differing colors, in ng. Ca was excluded from this figure due to its elevated concentrations in comparison to the other elements quantified. Ca concentrations are presented in Table S2.
Concentrations of all quantified elements in the winter ranged from 11.9–54.3 ng/m3 and from 7.6–38.0 ng/m3 in the summer. When excluding the two most abundant elements, Ca and Fe, these ranges were 0.09–5.4 ng/m3 for the winter and 0.007–0.02 ng/m3 for the summer. Several elements were present only in the winter (Ag, Cd and Sr), demonstrating the reduced concentration and variety of elements in the summer. These findings are consistent with previous studies that observed higher concentrations and more variety in elements in the winter compared to other seasons [30,31,32]. One rationale for these findings may be inversion events, which are more common in the winter months particularly in low lying areas like basins [33,34]. Sites A and B are both in the Willamette Basin [35], making them in an inversion-prone area and thus the potential for trapping of air pollutants during these events. Unfortunately, additional data, including ozone concentrations, was not available at these locations, making it difficult to confirm if these events occurred. Additional contributing factors to the increased total elements in the winter at both sites may be source dependent, but a likely influence is the overall increased mass loadings collected in the winter (Table S5). This indicates that while a greater concentration of elements was present in the winter, on a per µg of PM2.5 basis, the elemental concentrations were higher in the summer. And thus, in the summer elements contributed more to the total PM2.5 mass compared to the winter suggesting that components, outside of the elements measured, are impacting PM2.5 mass in the winter.
The elemental profiles were assessed at each of the locations in the winter (Figure 3A) and summer (Figure 3B). In general, across seasons Ca was the largest contributor (>65% in the winter and >75% in the summer) followed by Fe (≥18% in both seasons) for all but one sample (Site B on 2 January). The higher percentage of Ca in the summer may be due in part to the reduced variety and concentrations of detected elements in the summer compared to the winter. Ca and Fe are markers of brake dust [36,37]. Since both sites were located along highways, this likely explains the substantial contributions of these two elements in all samples, independent of season. Additionally, Fe is released during steel production [38] and the proximity of several steel corporations in the sampling area may contribute to the concentration of Fe in the samples. Our findings of high contributions of Ca and Fe in PM2.5 samples are in alignment with previous research [39]. There was an anomaly from the observed compositional profile trends at Site B on 20 July when an increased contribution of Fe (46.3% compared to the average of 18.1%) was observed. Subsequently this resulted in a decreased contribution of Ca relative to the other samples. Meteorological factors including wind speed/direction, temperature, and humidity may have also contributed to the observed differences. Average PM2.5 mass concentrations for the summer sampling sites were within 0.25 µg/m3 of the seasonal averages but the representativeness of this data for the entire winter and summer seasons for elemental concentrations is unknown. However, the daily variations observed in this research suggests that assessing daily samples is important as seasonal averages may not fully display daily differences in PM2.5 composition.
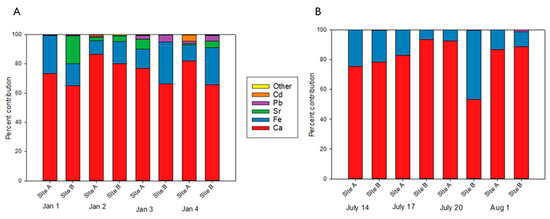
Figure 3.
Elemental compositional profiles. Percent contributions of elements for each day and site are represented for winter (A) and summer (B) in January (Jan) and July. All values are reported out of 100% of the total elemental concentration for individual sampling days/sites. The legend represents elements with their corresponding colors. “Other” represents the combined contribution of Cs, Tl, U, and Ag.
The variety of quantified elements in the winter was greater than the summer. Several elements including Ag, Cd, Cs, Pb, Sr, and Tl were quantifiable in the winter while the only element outside of Ca and Fe quantified in the summer was Pb on a single day at a single location (Site B on 1 August). A potential rationale for the increased contributions of elements outside of Ca and Fe in the winter may be the use of rock salt for treatment of snow and ice. Meteorological data collected from the region during the sampling periods reported light precipitation before and during the winter sampling dates with minimum temperatures consistently below freezing. Commercial rock salt can potentially contain trace amounts of Ag, Cd, and Sr [40], which all contributed to the compositional profile in the winter but not the summer. When considering the summer compositional profiles, it is important to note that these only include elements that were quantifiable based on instrument limits of quantification. Furthermore, the concentrations of elements not quantified in this research (i.e., Ni, V, Zn) may make a substantial contribution to the summer elemental profile but this is unknown for our study.
3.2. Oxidative Potential of PM2.5
The oxidative potential of each sample was assessed by measuring the DTT consumption based on a standard DTT curve (Figure 4). Significant differences were observed between Sites A and B on both 2 and 3 January. This suggests that spatial differences in the PM2.5, even in relatively close proximity with similar source contributions, can result in differences in oxidative potential measurements. This emphasizes the need for further research into a variety of locations and daily, not just seasonal, impacts.
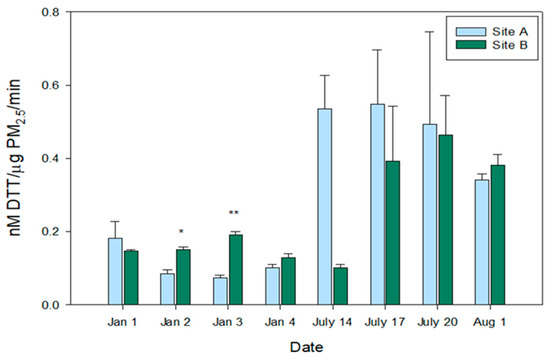
Figure 4.
DTT Consumption per PM2.5 mass per minute. DTT (dithiothreitol) consumption (nM) per PM2.5 mass (µg) per minute of reaction incubation for each sampling day for Sites A and B are reported following blank filter corrections in January (Jan) and July. The legend represents Site A and Site B with their corresponding colors. Mean DTT consumption values with standard deviation, represented by error bars, are reported based on triplicate measurements. Students t-tests were run to determine significant differences (p ≤ 0.05) between the two sampling sites for each sampling day, denoted by * or ** for p < 0.001.
Overall, the summer samples consumed more DTT per µg of methanol-soluble PM2.5 at both Sites when compared to the winter samples, except for 14 July at Site B. The differences between seasons were not statistically significant, due largely in part to the increased variability within the triplicate measurements for the summer samples. Significant differences on the same day of sampling were observed between Sites in the winter, suggesting that compositional differences between Sites may be driving DTT consumption. These findings are consistent with those observed in the total element concentrations (ng/µg PM2.5, Figure 1) where summer PM2.5 had an increased concentration of elements compared to the winter. Beyond the higher per mass contribution of elements in the summer, there were potentially other compounds including DTT active compounds (i.e., Cu, quinones) that may have been present in high concentrations in the summer but not the winter [41,42]. Further exploration into the connections between DTT consumption and chemical constituents of PM2.5 are detailed below.
Our results are consistent with previous work in Fresno, CA, that observed a higher average of DTT consumption during the summer [43]. Conversely, a study from China found higher concentrations of PM2.5 and DTT consumption during the winter [23]. One potential rationale for these differences may be regional variability as our study findings aligned with another study in the western United States. Additional factors to consider are variation in the meteorology, sampling design, and source contributions. Finally, PM2.5 mass may again play a role in the interpretation of this data. For consistency across previous studies DTT consumption is reported as consumption/µg PM2.5/min. However, if not considering PM2.5 mass, the amount of DTT consumed was not significantly different between Sites (SI Figure S1), indicating that the filter mass loadings may be driving the oxidative potential responses. This is discussed further in the section below.
3.3. Connections between Chemical Components and Oxidative Potential of PM2.5
The relationship between DTT consumption, elemental concentrations, and PM2.5 mass was assessed (Table 1 and Table 2). We found multiple significant Pearson correlation coefficients (R) between the concentrations of elements, the total sum of elements, and PM2.5. Several elements had significant positive correlations with the total sum of elements (in ng), including Ca (0.968), Fe (0.557), Sr (0.490), and Tl (0.472); data reported as (element (R)). These correlations are consistent with the increased concentrations of these elements relative to the others quantified. When considering the element concentrations normalized by PM2.5 mass (ng/µg, Table 2) similar trends were seen for Ca (0.995) and Fe (0.730). Significant positive correlations were also observed between individual elements including Pb with 5 other elements (Ag, Cs, Fe, Tl, and U). Previous research has observed significant relationships between Ca and Fe, Ca and Sr, and Fe and Sr [44]. Our study found no significant correlations between these elements when considering concentrations in ng. There are a number of reasons for this including the locations of the studies as well as sources and meteorology present.

Table 1.
Pearson’s Correlations for DTT, elements in ng, and PM2.5.

Table 2.
Pearson’s Correlations for DTT, elements in ng/µg PM2.5, and PM2.5.
PM2.5 mass and elements showed significant positive correlations for several individual elements (ng) that were only quantified in the winter samples (Cd, Sr, Tl, U) or at concentrations over 2-fold larger in the winter (Cs, Pb). This is consistent with previous work that has observed significant correlations between elements and PM2.5 mass [45]. These correlations were not observed when normalizing the elements (ng/µg PM2.5) which is consistent with our findings that the summer had elevated normalized total concentrations but a reduced number of quantifiable elements.
Correlations between DTT consumption and PM2.5 mass and composition were made with PM2.5 mass normalized and non-normalized values for element concentrations (Table 1 and Table 2, respectively). For DTT consumption (nM/min) we found significant positive correlations with several elements (Cs, Tl) and PM2.5 mass (Table 1). This was consistent with previous research identifying similar positive correlations with DTT consumption [46]. However, when using normalized DTT consumption (nM DTT/µg PM2.5/min), we observed that PM2.5 mass had a significant negative correlation with DTT consumed. This was not surprising, as the summer samples had lower PM2.5 mass but higher DTT values per µg PM2.5. Since the inverse was found when not normalizing the data (nM DTT/min or ng), these findings highlight the need to interpret data from various studies that use both normalized [46,47] and non-normalized [48] values for DTT or elements as the results can significantly differ. This is of particular concern if the PM2.5 masses between samples have stark differences, as was observed in our study (Table S5), leading to different interpretations of the data. For example, the sum total of elements compared to normalized DTT, if using the non-normalized elemental concentration (ng), shows a slightly negative not statistically significant correlation to DTT. However if using the normalized elemental concentration (ng/µg) there is a significant positive correlation to DTT consumption. Similar findings were observed with Ca, where using non-normalized elemental concentrations had a slightly non-significant negative correlation with DTT consumption but when normalizing the Ca concentration, a significant positive correlation was observed with DTT. This emphasizes the need to consider the effects of reported units in studies looking at chemical composition and oxidative potential.
While the total and individual element concentrations were elevated in the winter sampling period for PM2.5 this was not reflected in the PM2.5 mass normalized element concentrations or the oxidative potential assessment. Several elements and total PM2.5 mass concentration had significant negative correlations with normalized DTT consumption, suggesting that components of PM2.5, other than elements, play a role in DTT consumption.
One limitation of our work is the absence of additional constituents of PM2.5. Elements, particularly transition metals, previously shown to be associated with oxidative potential, including Al, Cu, Mn, Ni, V and Zn [15,49], were excluded due to calibration curves that exceeded our quality standards. Of note, we did not analyze for organic compounds, which have previously been shown to induce oxidative potential [41,42,50]. Organic compounds including polycyclic aromatic hydrocarbons and alkanes have been observed to be elevated at some locations in the summer however this is variable based on the locations sampled [51]. Thus, we cannot definitively determine if the organic compounds played a role in the increased oxidative potential observed but it is one likely rationale for these findings. Inclusion of these constituents may have identified positive correlations with DTT however that does not impact the observed findings which demonstrate the inability to consistently attribute oxidative potential to some elements, including Fe and Pb. Additionally, only a subset of days in two seasons were studied, so little is known about the chemical composition and oxidative potential during the fall and spring which is of importance since oxidative potential has previously been observed to be highest in the fall [23]. Finally, while the DTT assay is one method for the measurement of oxidative potential, there are alternative methods that can be utilized [22]. The various methods of oxidative potential assessment have limitations, including elevated affinity for transition metals compared to other components of PM2.5 for the DTT assay [42]. These factors should be considered when interpreting results. Future studies investigating daily differences in these factors across all seasons in addition to paired in vitro and in vivo research would greatly support our findings and identify trends beyond those observed in our selective sampling periods.
4. Conclusions
We observed significant differences in elemental composition between sampling locations along highways in central Oregon, USA with trends suggesting increased concentrations during the winter but increased contributions to PM2.5 mass from elements in the summer. These trends were driven by increased PM2.5 mass concentrations and the variety of quantifiable elements in the winter. Interestingly, we observed increases in oxidative potential of the PM2.5 samples in the summer suggesting that despite the elevated PM2.5 mass in the winter, the components of PM2.5 in the summer resulted in increased oxidative potential. This study demonstrates the influence of PM2.5 composition in oxidative potential responses and highlights the importance of comparisons between PM2.5 mass normalized and non-normalized data. Further research is needed to understand the variation within seasons of PM2.5 composition and oxidative potential to support policies that protect human health.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4433/11/10/1086/s1, Figure S1: DTT consumed (nM DTT/min) per Filter, Table S1: ICP-MS Instrumental Parameters, Table S2: Element Concentration (ppb), Table S3: Element Concentrations (ppb/µg), Table S4: Element Concentrations (µg/m3), Table S5: PM2.5 sample mass, Table S6: p-values for Pearson correlations, Table S7: p-values for Pearson correlations.
Author Contributions
Conceptualization, A.V. and C.R.; methodology, A.V., O.B.; validation, A.V. and O.B.; formal analysis, A.S.; investigation, A.V.; resources, C.R.; writing—original draft preparation, A.V. and A.S.; writing—review and editing, C.R. and O.B.; visualization, A.S.; supervision, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Mississippi (UM), UM Department of BioMolecular Sciences, and UM School of Pharmacy.
Acknowledgments
All PM2.5 samples were donated by the Lane Regional Air Protection Agency (LRAPA) in Lane County Oregon, USA. The ICP-MS facility at the University of Mississippi, University, MS, USA was utilized for all chemical analyses. Graphical abstract created through biorender.com.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential—The RAPTES project. Part Fibre Toxicol. 2011, 8, 26. [Google Scholar] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 30 June 2020).
- European Commission. Standards—Air Quality—Environment—European Commission. Available online: https://ec.europa.eu/environment/air/quality/standards.htm (accessed on 9 July 2020).
- US EPA O. National Ambient Air Quality Standards (NAAQS) for PM. US EPA, 2020. Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 9 July 2020).
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [PubMed]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zhen, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef]
- Peixoto, M.S.; de Oliveira Galvão, M.F.; de Medeiros, S.R.B. Cell death pathways of particulate matter toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- Cheung, K.; Daher, N.; Kam, W.; Shafer, M.M.; Ning, Z.; Schauer, J.J.; Sioutas, C. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10–2.5) in the Los Angeles area. Atmos. Environ. 2011, 45, 2651–2662. [Google Scholar] [CrossRef]
- Demerjian, K.L.; Mohnen, V.A. Synopsis of the Temporal Variation of Particulate Matter Composition and Size. J. Air Waste Manag. Assoc. 2008, 58, 216–233. [Google Scholar]
- Röösli, M.; Theis, G.; Künzli, N.; Staehelin, J.; Mathys, P.; Oglesby, L.; Camenzind, M.; Braun-Fahrländer, C. Temporal and spatial variation of the chemical composition of PM10 at urban and rural sites in the Basel area, Switzerland. Atmos. Environ. 2001, 35, 3701–3713. [Google Scholar]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Bell, M.L. HEI Health Review Committee. Assessment of the health impacts of particulate matter characteristics. Res. Rep. Health Eff. Inst. 2012, 161, 5–38. [Google Scholar]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Boogaard, H.; Janssen, N.A.H.; Fischer, P.H.; Kos, G.P.A.; Weijers, E.P.; Cassee, F.R.; van der Zee, S.C.; de Hartog, J.J.; Brunekreef, B.; Hoek, G. Contrasts in Oxidative Potential and Other Particulate Matter Characteristics Collected Near Major Streets and Background Locations. Environ. Health Perspect. 2012, 120, 185–191. [Google Scholar] [PubMed]
- Dagher, Z.; Garçon, G.; Billet, S.; Gosset, P.; Ledoux, F.; Courcot, D.; Aboukais, A.; Shirali, P. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology 2006, 225, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 1–13. [Google Scholar]
- Shi, T.; Schins, R.P.F.; Knaapen, A.M.; Kuhlbusch, T.; Pitz, M.; Heinrich, J.; Borm, P.J.A. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monit. 2003, 5, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Kelly, F.; Künzli, N.; Schins, R.P.F.; Donaldson, K. Oxidant generation by particulate matter: From biologically effective dose to a promising, novel metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef]
- Hedayat, F.; Stevanovic, S.; Miljevic, B.; Bottle, S.; Ristovski, Z.D. Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Eng. Q. 2015, 21, 201–210. [Google Scholar] [CrossRef]
- Roper, C.; Perez, A.; Barrett, D.; Hystad, P.; Massey Simonich, S.L.; Tanguay, R.L. Workflow for comparison of chemical and biological metrics of filter collected PM2.5. Atmos. Environ. 2020, 226, 117379. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef]
- Wang, J.; Lin, X.; Lu, L.; Wu, Y.; Zhang, H.; Lv, Q.; Liu, W.; Zhang, Y.; Zhuang, S. Temporal variation of oxidative potential of water soluble components of ambient PM2.5 measured by dithiothreitol (DTT) assay. Sci. Total Environ. 2019, 649, 969–978. [Google Scholar] [CrossRef]
- Shao, L.; Hu, Y.; Shen, R.; Schäfer, K.; Wang, J.; Wang, J.; Schnelle-Kreis, J.; Zimmermann, R.; BéruBé, K.; Suppan, P. Seasonal variation of particle-induced oxidative potential of airborne particulate matter in Beijing. Sci. Total Environ. 2017, 579, 1152–1160. [Google Scholar] [PubMed]
- Cheung, K.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Diurnal Trends in Oxidative Potential of Coarse Particulate Matter in the Los Angeles Basin and Their Relation to Sources and Chemical Composition. Environ. Sci. Technol. 2012, 46, 3779–3787. [Google Scholar] [PubMed]
- Szigeti, T.; Óvári, M.; Dunster, C.; Kelly, F.J.; Lucarelli, F.; Záray, G. Changes in chemical composition and oxidative potential of urban PM2.5 between 2010 and 2013 in Hungary. Sci. Total Environ. 2015, 518–519, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef]
- LRAPA. Air Quality Sensors | Lane Regional Air Protection Agency, OR. Available online: http://www.lrapa.org/307/Air-Quality-Sensors (accessed on 7 September 2020).
- US EPA. List of Designated Reference and Equivalent Methods. 2020. Available online: www.epa.gov/ttn/amtic/criteria.html (accessed on 12 October 2020).
- Gorai, A.K.; Tchounwou, P.B.; Biswal, S.; Tuluri, F. Spatio-Temporal Variation of Particulate Matter (PM2.5) Concentrations and Its Health Impacts in a Mega City, Delhi in India. Environ. Health Insights 2018, 12. [Google Scholar] [CrossRef]
- Ho, K.; Lee, S.; Cao, J.; Chow, J.; Watson, J.; Chan, C. Seasonal variations and mass closure analysis of particulate matter in Hong Kong. Sci. Total Environ. 2006, 355, 276–287. [Google Scholar] [CrossRef]
- Ledoux, F.; Courcot, L.; Courcot, D.; Aboukaïs, A.; Puskaric, E. A summer and winter apportionment of particulate matter at urban and rural areas in northern France. Atmos. Res. 2006, 82, 633–642. [Google Scholar] [CrossRef]
- Lyman, S.; Tran, T. Inversion structure and winter ozone distribution in the Uintah Basin, Utah, USA. Atmos. Environ. 2015, 123, 156–165. [Google Scholar] [CrossRef]
- Vitasse, Y.; Klein, G.; Kirchner, J.W.; Rebetez, M. Intensity, frequency and spatial configuration of winter temperature inversions in the closed La Brevine valley, Switzerland. Theor. Appl. Climatol. 2017, 130, 1073–1083. [Google Scholar] [CrossRef]
- USDA. USDA—National Agricultural Statistics Service—Mississippi—2017–2020 County Estimates. Available online: https://www.nass.usda.gov/Statistics_by_State/Mississippi/Publications/County_Estimates/index.php (accessed on 30 June 2020).
- Dall’Osto, M.; Querol, X.; Amato, F.; Karanasiou, A.; Lucarelli, F.; Nava, S.; Calzolai, G.; Chiari, M. Hourly elemental concentrations in PM2.5 aerosols sampled simultaneously at urban background and road site. Atmos. Chem. Phys. Discuss 2012, 12, 20135–20180. [Google Scholar] [CrossRef]
- Sanders, P.G.; Xu, N.; Dalka, T.M.; Maricq, M.M. Airborne Brake Wear Debris: Size Distributions, Composition, and a Comparison of Dynamometer and Vehicle Tests. Environ. Sci. Technol. 2003, 37, 4060–4069. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M. Iron metabolism of grasses: I. Effect of iron supply on some inorganic and organic constituents. Plant Soil. 1969, 31, 451–462. [Google Scholar] [CrossRef]
- Bozlaker, A.; Peccia, J.; Chellam, S. Indoor/Outdoor Relationships and Anthropogenic Elemental Signatures in Airborne PM2.5 at a High School: Impacts of Petroleum Refining Emissions on Lanthanoid Enrichment. Environ. Sci. Technol. 2017, 51, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Titler, R.V. Chemical Analysis of Major Constituents and Trace Contaminants of Rock Salt. 2011. Available online: http://files.dep.state.pa.us/Water/Wastewater%20Management/WastewaterPortalFiles/Rock%20Salt%20Paper%20final%20052711.pdf (accessed on 10 July 2020).
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef]
- Charrier, J.G.; Richards-Henderson, N.K.; Bein, K.J.; McFall, A.S.; Wexler, A.S.; Anastasio, C. Oxidant production from source-oriented particulate matter—Part 1: Oxidative potential using the dithiothreitol (DTT) assay. Atmos. Chem. Phys. 2015, 15, 2327–2340. [Google Scholar]
- Yatkin, S.; Bayram, A. Elemental composition and sources of particulate matter in the ambient air of a Metropolitan City. Atmos. Res. 2007, 85, 126–139. [Google Scholar] [CrossRef]
- Asano, H.; Aoyama, T.; Mizuno, Y.; Shiraishi, Y. Highly Time-Resolved Atmospheric Observations Using a Continuous Fine Particulate Matter and Element Monitor. ACS Earth Space Chem. 2017, 1, 580–590. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Gao, D.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmos. Environ. 2020, 224, 117327. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Wang, Y.; Zhang, L.; Li, Y.; Han, Y. Oxidative Potential of Water-Soluble Matter Associated with Chromophoric Substances in PM2.5 over Xi’an, China. Environ. Sci. Technol. 2019, 53, 8574–8584. [Google Scholar] [CrossRef]
- Wei, J.; Yu, H.; Wang, Y.; Verma, V. Complexation of Iron and Copper in Ambient Particulate Matter and Its Effect on the Oxidative Potential Measured in a Surrogate Lung Fluid. Environ. Sci. Technol. 2019, 53, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, L.; Froines, J.R.; Cho, A.K.; Sioutas, C. Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Part. Fibre Toxicol. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Olson, M.R.; Shelton, B.; Schauer, J.J.; Sioutas, C. Seasonal and spatial variations of individual organic compounds of coarse particulate matter in the Los Angeles Basin. Atmos. Environ. 2012, 59, 1–10. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).