Impacts of Aerosol Copper on Marine Phytoplankton: A Review
Abstract
1. Introduction
2. Perspectives
2.1. Physiological Functions and Toxicity of Cu
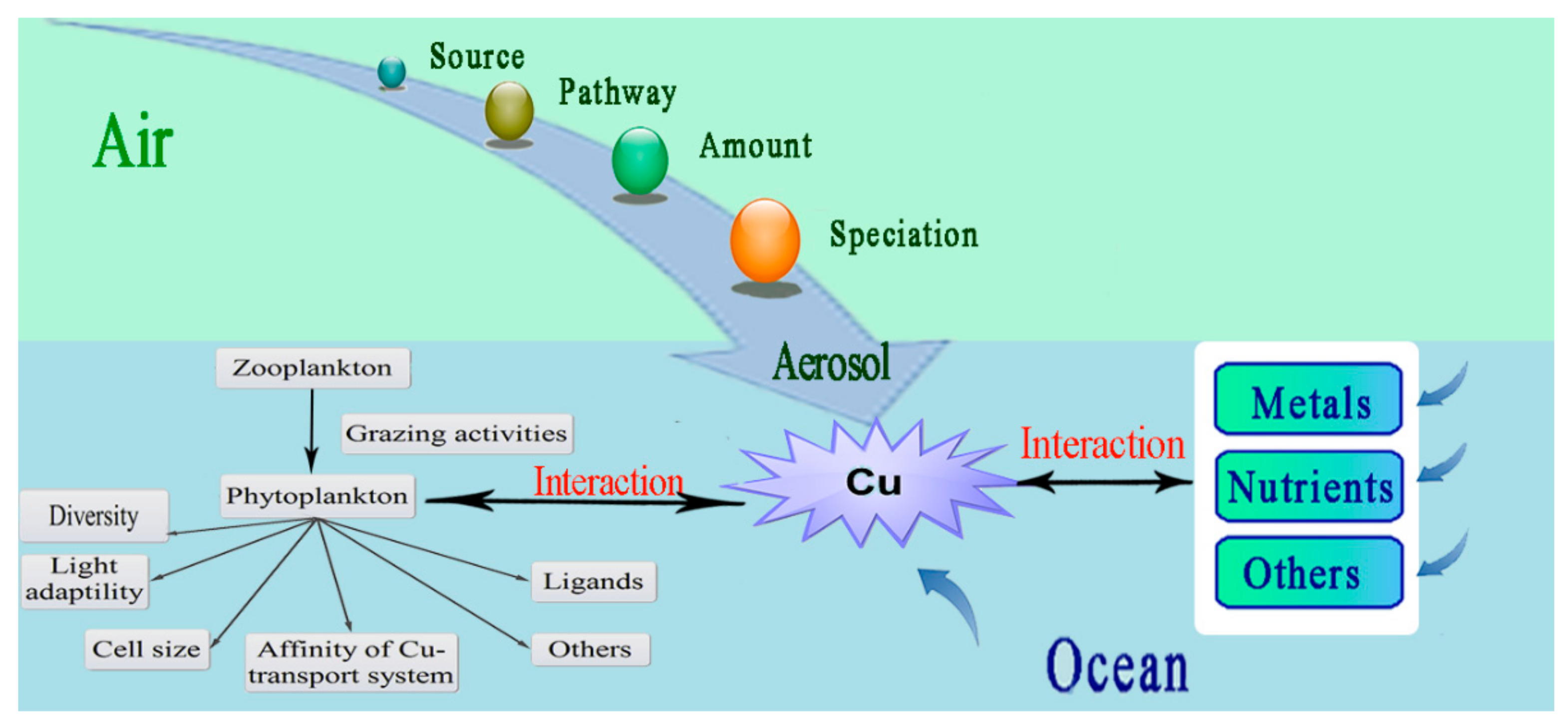
2.2. Interactions between Cu and Other Metals and Nutrients
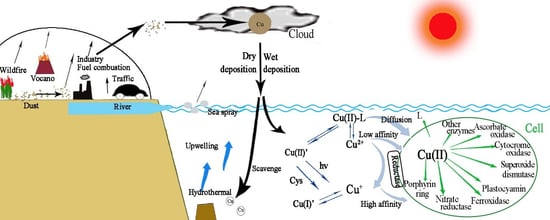
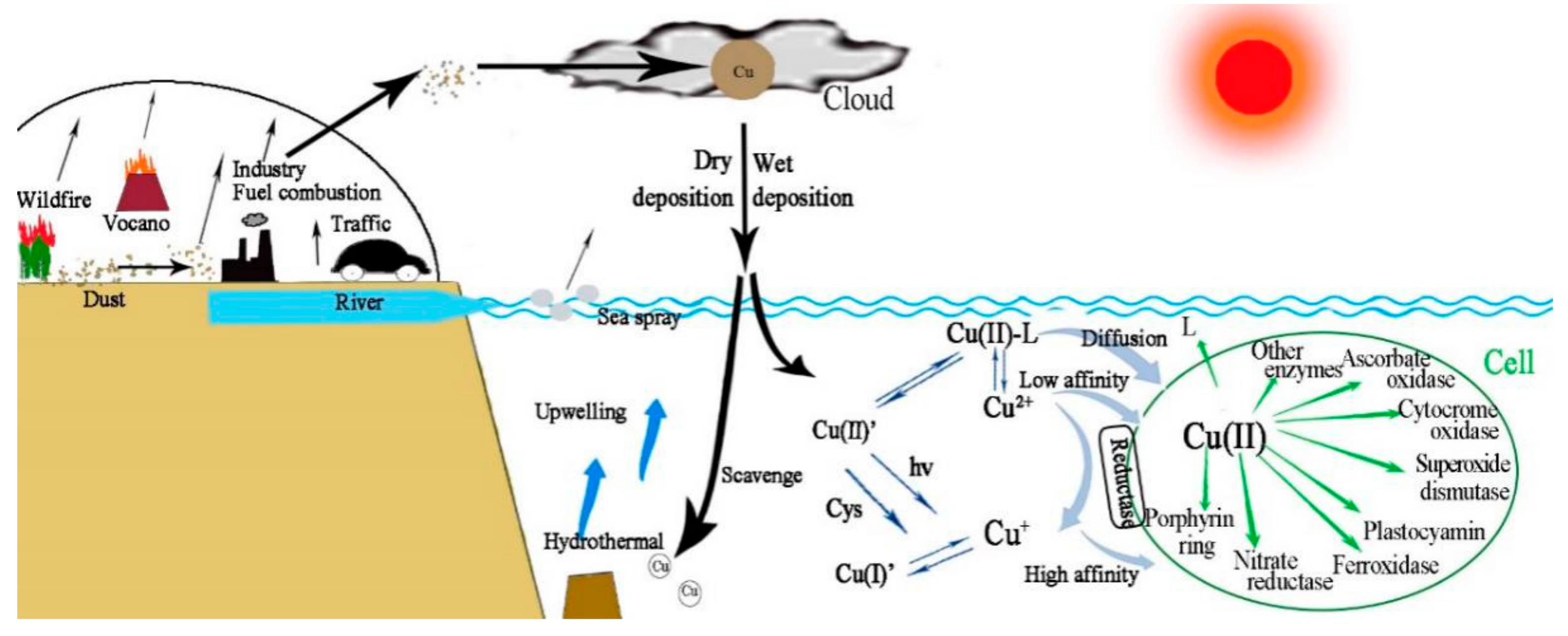
2.3. Bioavailability and Uptake of Cu
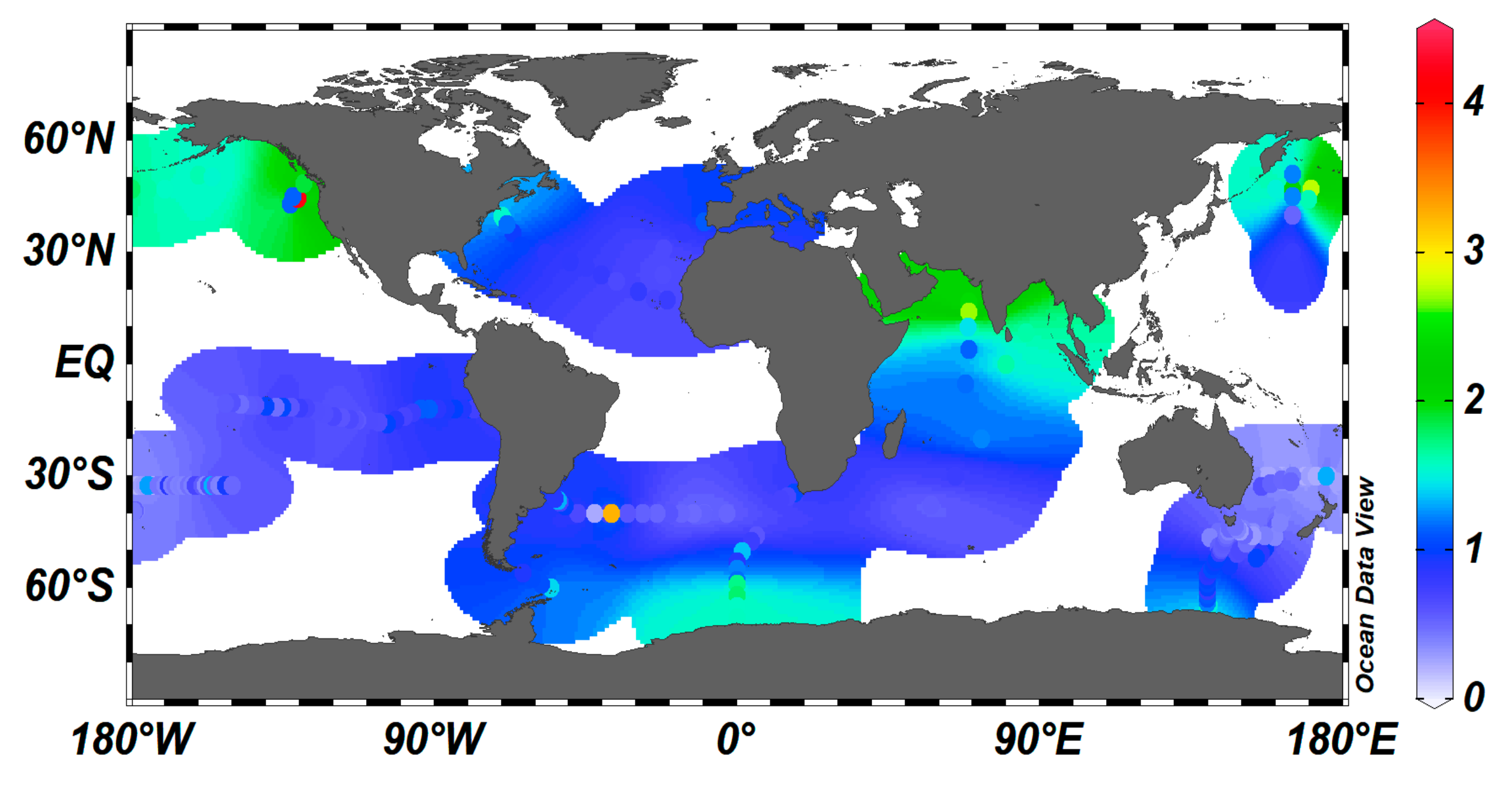
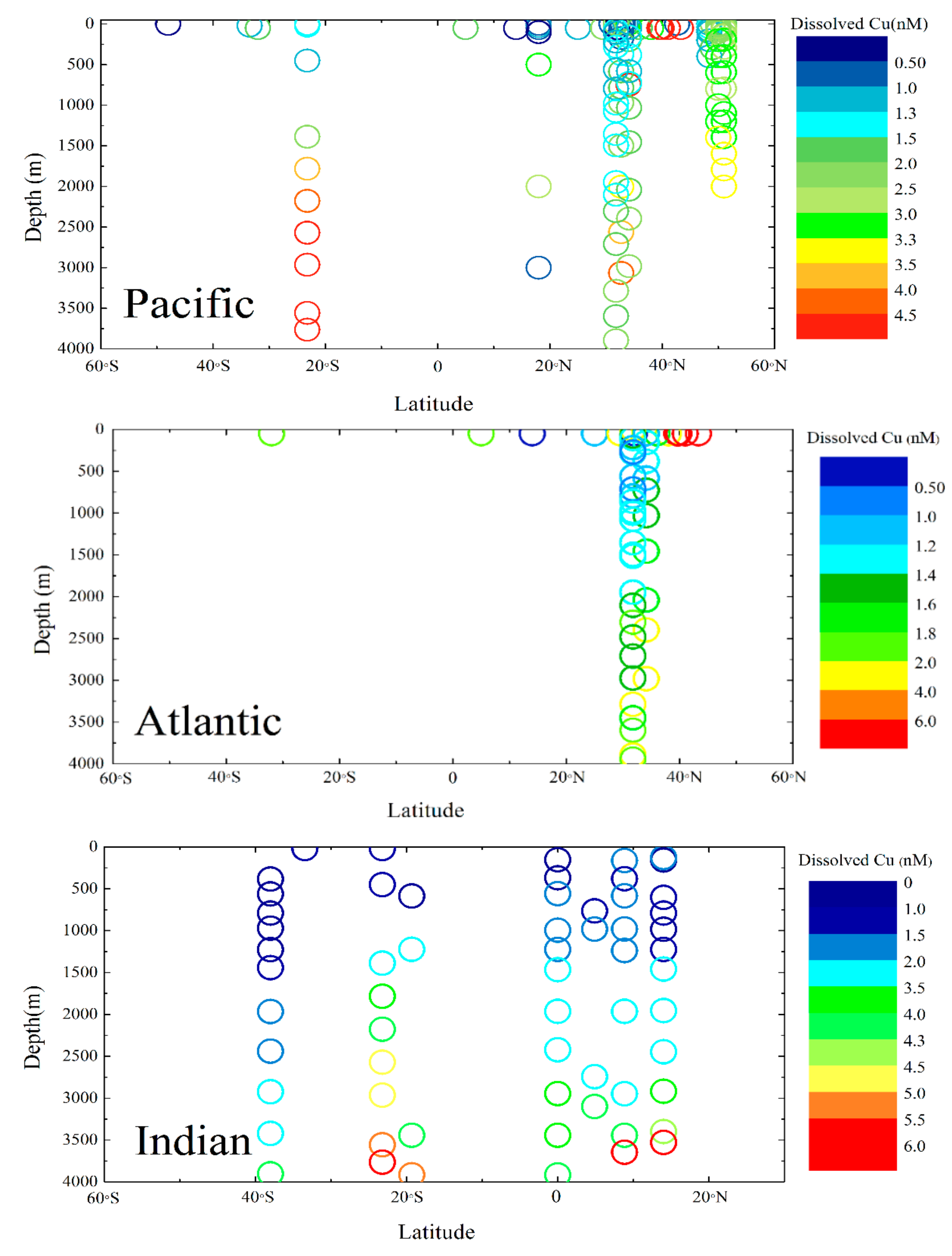
2.4. Distribution of Dissolved Cu in the Ocean
2.5. Copper Speciation in the Seawater
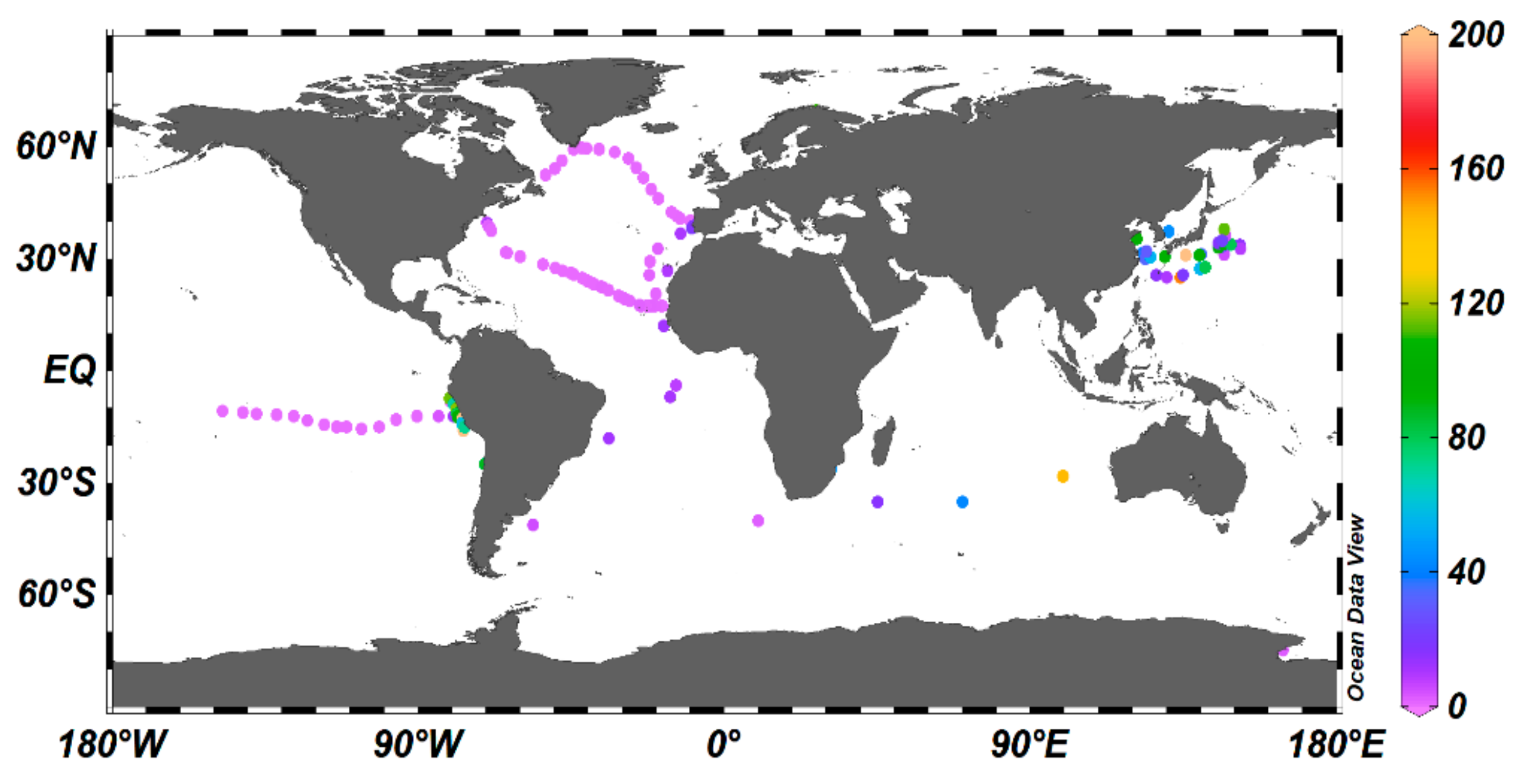
2.6. Atmospheric Contribution to Oceanic Cu
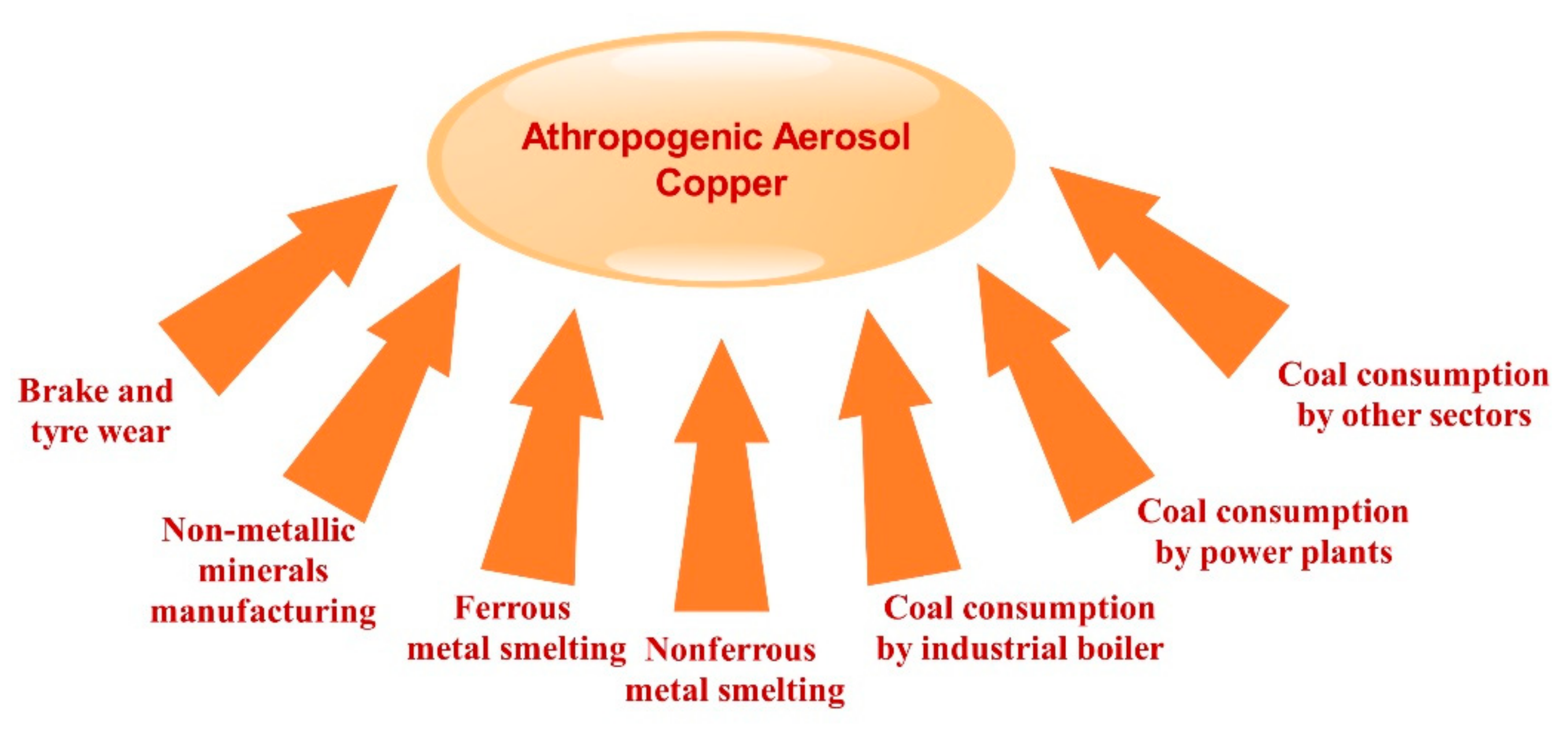
2.7. Sources, Solubility, and Deposition of Atmospheric Cu
3. Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallisai, R.; Peters, F.; Volpe, G.; Basart, S.; Baldasano, J.M. Saharan Dust Deposition May Affect Phytoplankton Growth in the Mediterranean Sea at Ecological Time Scales. PLoS ONE 2014, 9, e110762. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, N.M.; Hamilton, D.S.; Mackey, K.R.M.; Moore, J.K.; Baker, A.R.; Scanza, R.A.; Zhang, Y. Aerosol Trace Metal Leaching and Impacts on Marine Microorganisms. Nat. Commun. 2018, 9, 2614. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.N.; Lee, K.; Gruber, N.; Karl, D.M.; Bullister, J.L.; Yang, S.; Kim, T.W. Increasing Anthropogenic Nitrogen in the North Pacific Ocean. Science 2014, 346, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Jordi, A.; Basterretxea, G.; Tovar-Sanchez, A.; Alastuey, A.; Querol, X. Copper Aerosols Inhibit Phytoplankton Growth in the Mediterranean Sea. Proc. Natl. Acad. Sci. USA 2012, 109, 21246–21249. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.J.; Breitbarth, E.; Boyd, P.W.; Hunter, K.A. Influence of Ocean Warming and Acidification on Trace Metal Biogeochemistry. Mar. Ecol. Prog. Ser. 2012, 470, 191–205. [Google Scholar] [CrossRef]
- Mackey, K.R.; Buck, K.N.; Casey, J.R.; Cid, A.; Lomas, M.W.; Sohrin, Y.; Paytan, A. Phytoplankton Responses to Atmospheric Metal Deposition in the Coastal and Open-Ocean Sargasso Sea. Front. Microbiol. 2012, 3, 359. [Google Scholar] [CrossRef] [PubMed]
- Duggen, S.; Croot, P.; Schacht, U.; Hoffmann, L. Subduction Zone Volcanic Ash Can Fertilize the Surface Ocean and Stimulate Phytoplankton Growth: Evidence from Biogeochemical Experiments and Satellite Data. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Walsh, J.J.; Steidinger, K.A. Saharan dust and Florida red tides: The cyanophyte connection. J. Geophys. Res. Space Phys. 2001, 106, 11597–11612. [Google Scholar] [CrossRef]
- Chien, C.; Mackey, K.R.; Dutkiewicz, S.; Mahowald, N.M.; Prospero, J.; Paytan, A. Effects of African dust deposition on phytoplankton in the western tropical Atlantic Ocean off Barbados. Glob. Biogeochem. Cycles 2016, 30, 716–734. [Google Scholar] [CrossRef]
- Paytan, A.; Chen, Y.; Mahowald, N.; Labiosa, R.; Mackey, K.R.M.; Lima, I.D.; Doney, S.C.; Post, A.F. Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 4601–4605. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Moore, J.K.; Mahowald, N.; Luo, C.; Doney, S.C.; Lindsay, K.; Zender, C.S. Impacts of increasing anthropogenic soluble iron and nitrogen deposition on ocean biogeochemistry. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Sedwick, P.N.; Church, T.M. Influence of anthropogenic combustion emissions on the deposition of soluble aerosol iron to the ocean: Empirical estimates for island sites in the North Atlantic. Geochim. Cosmochim. Acta 2009, 73, 3981–4003. [Google Scholar] [CrossRef]
- Jickells, T. Atmospheric inputs of metals and nutrients to the oceans: Their magnitude and effects. Mar. Chem. 1995, 48, 199–214. [Google Scholar] [CrossRef]
- Eichler, A.; Tobler, L.; Eyrikh, S.; Malygina, N.; Papina, T.; Schwikowski, M. Ice-Core Based Assessment of Historical Anthropogenic Heavy Metal (Cd, Cu, Sb, Zn) Emissions in the Soviet Union. Environ. Sci. Technol. 2014, 48, 2635–2642. [Google Scholar] [CrossRef]
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Hao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. Discuss. 2015, 15, 10127–10147. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Guerzoni, S.; Chester, R.; Dulac, F.; Herut, B.; Loÿe-Pilot, M.-D.; Measures, C.; Migon, C.; Molinaroli, E.; Moulin, C.; Rossini, P.; et al. The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog. Oceanogr. 1999, 44, 147–190. [Google Scholar] [CrossRef]
- Hooper, J.; Mayewski, P.; Marx, S.; Henson, S.; Potocki, M.; Sneed, S.; Handley, M.; Gassó, S.; Fischer, M.; Saunders, K.M. Examining links between dust deposition and phytoplankton response using ice cores. Aeolian Res. 2019, 36, 45–60. [Google Scholar] [CrossRef]
- Wang, R.; Balkanski, Y.; Bopp, L.; Aumont, O.; Boucher, O.; Ciais, P.; Gehlen, M.; Peñuelas, J.; Ethé, C.; Hauglustaine, D.; et al. Influence of anthropogenic aerosol deposition on the relationship between oceanic productivity and warming. Geophys. Res. Lett. 2015, 42, 10745–10754. [Google Scholar] [CrossRef]
- Yoon, J.E.; Yoo, K.C.; Macdonald, A.M.; Yoon, H.I.; Park, K.T.; Yang, E.J.; Kim, H.C.; Lee, J.I.; Lee, M.K.; Jung, J.; et al. Reviews and Syntheses: Ocean Iron Fertilization Experiments-Past, Present, and Future Looking to a Future Korean Iron Fertilization Experiment in the Southern Ocean (Kskies Project). Biogeosciences 2018, 15, 5847–5889. [Google Scholar] [CrossRef]
- Coale, K.H. Effects of iron, manganese, copper, and zinc enrichments on productivity and biomass in the subarctic Pacific. Limnol. Oceanogr. 1991, 36, 1851–1864. [Google Scholar] [CrossRef]
- Zou, H.-X.; Pang, Q.-Y.; Zhang, A.-Q.; Lin, L.-D.; Li, N.; Yan, X.-F. Excess copper induced proteomic changes in the marine brown algae Sargassum fusiforme. Ecotoxicol. Environ. Saf. 2015, 111, 271–280. [Google Scholar] [CrossRef]
- Takano, S.; Tanimizu, M.; Hirata, T.; Sohrin, Y. Isotopic constraints on biogeochemical cycling of copper in the ocean. Nat. Commun. 2014, 5, 5663. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Sedwick, P.N.; Church, T.M. 24. On the fractional solubility of copper in marine aerosols: Toxicity of aeolian copper revisited. Geophys. Res. Lett. 2010, 37, 20601. [Google Scholar] [CrossRef]
- Chen, Y.; Street, J.; Paytan, A. Comparison between pure-water- and seawater-soluble nutrient concentrations of aerosols from the Gulf of Aqaba. Mar. Chem. 2006, 101, 141–152. [Google Scholar] [CrossRef]
- Chester, R.; Murphy, K.; Lin, F.; Berry, A.; Bradshaw, G.; Corcoran, P. Factors controlling the solubilities of trace metals from non-remote aerosols deposited to the sea surface by the ‘dry’ deposition mode. Mar. Chem. 1993, 42, 107–126. [Google Scholar] [CrossRef]
- Brand, L.E.; Sunda, W.G.; Guillard, R.R. Reduction of marine phytoplankton reproduction rates by copper and cadmium. J. Exp. Mar. Boil. Ecol. 1986, 96, 225–250. [Google Scholar] [CrossRef]
- Wang, F.J.; Chen, Y.; Guo, Z.G.; Gao, H.W.; Mackey, K.R.; Yao, X.H.; Zhuang, G.S.; Paytan, A. Combined effects of iron and copper from atmospheric dry deposition on ocean productivity. Geophys. Res. Lett. 2017, 44, 2546–2555. [Google Scholar] [CrossRef]
- Irving, H.; William, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 637, 3192–3210. [Google Scholar] [CrossRef]
- Sunda, W.G. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 2012, 3, 204. [Google Scholar] [CrossRef]
- Duce, R.A.; Liss, P.S.; Merrill, J.T.; Atlas, E.L.; Buat-Menard, P.; Hicks, B.B.; Miller, J.M.; Prospero, J.M.; Arimoto, R.; Church, T.M.; et al. The atmospheric input of trace species to the world ocean. Glob. Biogeochem. Cycles 1991, 5, 193–259. [Google Scholar] [CrossRef]
- Mahowald, N.M.; Baker, A.R.; Bergametti, G.; Brooks, N.; Duce, R.A.; Jickells, T.D.; Kubilay, N.; Prospero, J.M.; Tegen, I. Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Echeveste, P.; Croot, P.; von Dassow, P. Differences in the sensitivity to Cu and ligand production of coastal vs offshore strains of Emiliania huxleyi. Sci. Total Environ. 2018, 625, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Biswas, H.; Bandyopadhyay, D. Physiological responses of coastal phytoplankton (Visakhapatnam, SW Bay of Bengal, India) to experimental copper addition. Mar. Environ. Res. 2017, 131, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.L.; Ahlgren, N.; Moffett, J.W.; Chisholm, S.W. Copper toxicity and cyanobacteria ecology in the Sargasso Sea. Limnol. Oceanogr. 2002, 47, 976–988. [Google Scholar] [CrossRef]
- Stuart, R.K.; Dupont, C.L.; Johnson, D.A.; Paulsen, I.T.; Palenik, B. Coastal strains of marine Synechococcus species exhibit increased tolerance to copper shock and a distinctive transcriptional response relative to those of open-ocean strains. Appl. Environ. Microb. 2009, 75, 5047–5057. [Google Scholar] [CrossRef] [PubMed]
- Twining, B.S.; Baines, S.B. The trace metal composition of marine phytoplankton. Annu. Rev. Mar. Sci. 2013, 5, 191–215. [Google Scholar] [CrossRef]
- Ridge, P.G.; Zhang, Y.; Gladyshev, V.N. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE 2008, 3, e1378. [Google Scholar] [CrossRef]
- Harrison, W.; Eppley, R.; Renger, E. Phytoplankton nitrogen metabolism, nitrogen budgets, and observations on copper toxicity: Controlled ecosystem pollution experiment. Bull. Mar. Sci. 1977, 27, 44–57. [Google Scholar]
- Magalhães, C.M.; Machado, A.; Matos, P.; Bordalo, A.A. Impact of copper on the diversity, abundance and transcription of nitrite and nitrous oxide reductase genes in an urban European estuary. FEMS Microbiol. Ecol. 2011, 77, 274–284. [Google Scholar] [CrossRef]
- Lopez, J.S.; Lee, L.; Mackey, K.R.M. The toxicity of copper to Crocosphaera watsonii and other marine phytoplankton: A systematic review. Front. Mar. Sci. 2018, 5, 511. [Google Scholar] [CrossRef]
- Baron, M.; Arellano, J.B.; Gorge, J.L. Copper and photosystem-II: A controversial relationship. Physiol. Plant. 1995, 94, 174–180. [Google Scholar] [CrossRef]
- Ritter, A.; Ubertini, M.; Romac, S.; Gaillard, F.; Delage, L.; Mann, A.; Cock, J.M.; Tonon, T.; Correa, J.A.; Potin, P. Copper stress proteomics highlights local adaptation of two strains of the model brown alga Ectocarpus siliculosus. Proteomics 2010, 10, 2074–2088. [Google Scholar] [CrossRef]
- Perez, P.; Estevez-Blanco, P.; Beiras, R.; Fernandez, E. Effect of copper on the photochemical efficiency, growth, and chlorophyll a biomass of natural phytoplankton assemblages. Environ. Toxicol. Chem. 2006, 25, 137–143. [Google Scholar] [CrossRef]
- Miao, A.J.; Wang, W.X.; Juneau, P. Comparison of Cd, Cu, and Zn toxic effects on four marine phytoplankton by pulse-amplitude-modulated fluorometry. Environ. Toxicol. Chem. 2005, 24, 2603–2611. [Google Scholar] [CrossRef]
- Moffett, J.W.; Brand, L.E.; Croot, P.L.; Barbeau, K.A. Cu speciation and cyanobacterial distribution in harbors subject to anthropogenic Cu inputs. Limnol. Oceanogr. 1997, 42, 789–799. [Google Scholar] [CrossRef]
- Levy, J.L.; Stauber, J.L.; Jolley, D.F. Sensitivity of marine microalgae to copper: The effect of biotic factors on copper adsorption and toxicity. Sci. Total Environ. 2007, 387, 141–154. [Google Scholar] [CrossRef]
- Semeniuk, D.M.; Taylor, R.L.; Bundy, R.M.; Johnson, W.K.; Cullen, J.T.; Robert, M.; Barbeau, K.A.; Maldonado, M.T. Iron-copper interactions in iron-limited phytoplankton in the northeast subarctic Pacific Ocean. Limnol. Oceanogr. 2016, 61, 279–297. [Google Scholar] [CrossRef]
- Peers, G.; Quesnel, S.A.; Price, N.M. Copper requirements for iron acquisition and growth of coastal and oceanic diatoms. Limnol. Oceanogr. 2005, 50, 1149–1158. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Santana-Casiano, J.M.; González, A.G.; González-Dávila, M. Phenolic profile of Dunaliella tertiolecta growing under high levels of copper and iron. Environ. Sci. Pollut. Res. 2015, 22, 14820–14828. [Google Scholar] [CrossRef]
- Hall, J.; Healey, F.P.; Robinson, G.G.C. The interaction of chronic copper toxicity with nutrient limitation in two chlorophytes in batch culture. Aquat. Toxicol. 1989, 14, 1–13. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Relationships among growth rate, cellular manganese concentrations and manganese transport knetics in estuarine and oceanic species of the diatom thalassiosir. J. Phycol. 1986, 22, 259–270. [Google Scholar] [CrossRef]
- Fisher, N.S.; Jones, G.J.; Nelson, D.M. Effects of copper and zinc on growth, morphology, and metabolism of Asterionella japonica. J. Exp. Mar. Biol. Ecol. 1981, 51, 37–56. [Google Scholar] [CrossRef]
- Guo, C.; Yu, J.; Ho, T.Y.; Wang, L.; Song, S.; Kong, L.; Liu, H. Dynamics of phytoplankton community structure in the South China Sea in response to the East Asian aerosol input. Biogeosciences 2012, 9, 1519–1536. [Google Scholar] [CrossRef]
- Sunda, W.; Tester, P.; Huntsman, S. Effects of cupric and zinc ion activities on the survival and reproduction of marine copepods. Mar. Biol. 1987, 94, 203–210. [Google Scholar] [CrossRef]
- Schenck, R.C. Copper deficiency and toxicity in Gonyaulax-Tamarensis (Lebour). Mar. Biol. Lett. 1984, 5, 13–19. [Google Scholar]
- Anderson, D.M.; Morel, F.M.M. Copper sensitivity of Gonyaulax-Tamarensis. Limnol. Oceanogr. 1978, 23, 283–295. [Google Scholar] [CrossRef]
- Pistocchi, R.; Mormile, M.A.; Guerrini, F.; Isani, G.; Boni, L. Increased production of extra- and intracellular metal-ligands in phytoplankton exposed to copper and cadmium. J. Appl. Phycol. 2000, 12, 469–477. [Google Scholar] [CrossRef]
- Cid, A.; Herrero, C.; Torres, E.; Abalde, J. Copper toxicity on the marine microalga Phaeodactylum tricornutum: Effects on Photosynthesis and Related Parameters. Aquat. Toxicol. 1995, 31, 165–174. [Google Scholar] [CrossRef]
- Shi, W.; Jin, Z.; Hu, S.; Fang, X.; Li, F. Dissolved organic matter affects the bioaccumulation of copper and lead in Chlorella pyrenoidosa: A case of long-term exposure. Chemosphere 2017, 174, 447–455. [Google Scholar] [CrossRef]
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef]
- Morel, F.M.; Price, N.M. The biogeochemical cycles of trace metals in the oceans. Science 2003, 300, 944–947. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Allen, A.E.; Chong, J.S.; Lin, K.; Leus, D.; Karpenko, N.; Harris, S.L. Copper-dependent iron transport in coastal and oceanic diatoms. Limnol. Oceanogr. 2006, 51, 1729–1743. [Google Scholar] [CrossRef]
- Annett, A.L.; Lapi, S.; Ruth, T.J.; Maldonado, M.T. The effects of Cu and Fe availability on the growth and Cu: C ratios of marine diatoms. Limnol. Oceanogr. 2008, 53, 2451–2461. [Google Scholar] [CrossRef]
- Rueter, J.G.; Morel, F.M.M. The interaction between zinc deficiency and copper toxicity as it affects the silicic acid uptake mechanisms of Thalassiosira pseudonana. Limnol. Oceanogr. 1981, 26, 67–73. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Effect of competitive interactions between manganese and copper on cellular manganese and growth in estuarine and oceanic species of the diatom Thalassiosira. Limnol. Oceanogr. 1983, 28, 924–934. [Google Scholar] [CrossRef]
- Rocha, G.S.; Lombardi, A.T.; Melao Mda, G. Influence of phosphorus on copper toxicity to Selenastrum gracile (Reinsch) Korshikov. Ecotoxicol. Environ. Saf. 2016, 128, 30–35. [Google Scholar] [CrossRef]
- Hall, J.; Healey, F.; Robinson, G. The interaction of chronic copper toxicity with nutrient limitation in chemostat cultures of Chlorella. Aquat. Toxicol. 1989, 14, 15–26. [Google Scholar] [CrossRef]
- Rijstenbil, J.W.; Dehairs, F.; Ehrlich, R.; Wijnholds, J.A. Effect of the nitrogen status on copper accumulation and pools of metal-binding peptides in the planktonic diatom Thalassiosira pseudonana. Aquat. Toxicol. 1998, 42, 187–209. [Google Scholar] [CrossRef]
- Roshan, S.; Wu, J.F. Dissolved and colloidal copper in the tropical South Pacific. Geochim. Cosmochim. Acta 2018, 233, 81–94. [Google Scholar] [CrossRef]
- Kuss, J.; Kremling, K. Spatial variability of particle associated trace elements in near-surface waters of the North Atlantic (30 N/60 W to 60 N/2 W), derived by large volume sampling. Mar. Chem. 1999, 68, 71–86. [Google Scholar] [CrossRef]
- Tovar-Sanchez, A.; Sanudo-Wilhelmy, S.A.; Kustka, A.B.; Agustí, S.; Dachs, J.; Hutchins, D.A.; Capone, D.G.; Duarte, C.M. Effects of dust deposition and river discharges on trace metal composition of Trichodesmium spp. in the tropical and subtropical North Atlantic Ocean. Limnol. Oceanogr. 2006, 51, 1755–1761. [Google Scholar] [CrossRef]
- Nuester, J.; Vogt, S.; Newville, M.; Kustka, A.B.; Twining, B.S. The unique biogeochemical signature of the marine diazotroph Trichodesmium. Front. Microbiol. 2012, 3, 150. [Google Scholar] [CrossRef]
- Semeniuk, D.M.; Cullen, J.T.; Johnson, W.K.; Gagnon, K.; Ruth, T.J.; Maldonado, M.T. Plankton copper requirements and uptake in the subarctic Northeast Pacific Ocean. Deep Sea Res. Oceanogr. Res. Pap. 2009, 56, 1130–1142. [Google Scholar] [CrossRef]
- Semeniuk, D.M.; Bundy, R.M.; Posacka, A.M.; Robert, M.; Barbeau, K.A.; Maldonado, M.T. Using 67Cu to study the biogeochemical cycling of copper in the northeast subarctic pacific ocean. Front. Mar. Sci. 2016, 3, 78. [Google Scholar] [CrossRef]
- Cullen, J.T.; Chase, Z.; Coale, K.H.; Fitzwater, S.E.; Sherrell, R.M. Effect of iron limitation on the cadmium to phosphorus ratio of natural phytoplankton assemblages from the Southern Ocean. Limnol. Oceanogr. 2003, 48, 1079–1087. [Google Scholar] [CrossRef]
- Guo, J.; Lapi, S.; Ruth, T.J.; Maldonado, M.T. The effects of iron and copper availability on the copper stoichiometry of marine phytoplankton. J. Phycol. 2012, 48, 312–325. [Google Scholar] [CrossRef]
- Liao, W.-H.; Yang, S.-C.; Ho, T.-Y. Trace metal composition of size-fractionated plankton in the Western Philippine Sea: The impact of anthropogenic aerosol deposition. Limnol. Oceanogr. 2017, 62, 2243–2259. [Google Scholar] [CrossRef]
- Buck, K.N.; Ross, J.R.M.; Russell Flegal, A.; Bruland, K.W. A review of total dissolved copper and its chemical speciation in San Francisco Bay, California. Environ. Res. 2007, 105, 5–19. [Google Scholar] [CrossRef]
- Phinney, J.T.; Bruland, K.W. Uptake of lipophilic organic Cu, Cd, and Pb complexes in the coastal diatom Thalassiosira weissflogii. Environ. Sci. Technol. 1994, 28, 1781–1790. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci. Total Environ. 1998, 219, 165–181. [Google Scholar] [CrossRef]
- Moffett, J.W.; Brand, L.E. Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol. Oceanogr. 1996, 41, 388–395. [Google Scholar] [CrossRef]
- Gordon, A.S.; Donat, J.R.; Kango, R.A.; Dyer, B.J.; Stuart, L.M. Dissolved copper-complexing ligands in cultures of marine bacteria and estuarine water. Mar. Chem. 2000, 70, 149–160. [Google Scholar] [CrossRef]
- Semeniuk, D.M.; Bundy, R.M.; Payne, C.D.; Barbeau, K.A.; Maldonado, M.T. Acquisition of organically complexed copper by marine phytoplankton and bacteria in the northeast subarctic Pacific Ocean. Mar. Chem. 2015, 173, 222–233. [Google Scholar] [CrossRef]
- Walsh, M.J.; Goodnow, S.D.; Vezeau, G.E.; Richter, L.V.; Ahner, B.A. Cysteine enhances bioavailability of copper to marine phytoplankton. Environ. Sci. Technol. 2015, 49, 12145–12152. [Google Scholar] [CrossRef]
- Guo, J.; Annett, A.L.; Taylor, R.L.; Lapi, S.; Ruth, T.J.; Maldonado, M.T. Copper-uptake kinetics of coastal and oceanic diatoms. J. Phycol. 2010, 46, 1218–1228. [Google Scholar] [CrossRef]
- Hudson, R.J.M. Which aqueous species control the rates of trace metal uptake by aquatic biota. Sci. Total Environ. 1998, 219, 95–115. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Merchant, S.S. The ins and outs of algal metal transport. BBA Mol. Cell Res. 2012, 1823, 1531–1552. [Google Scholar] [CrossRef]
- Quigg, A.; Reinfelder, J.R.; Fisher, N.S. Copper uptake kinetics in diverse marine phytoplankton. Limnol. Oceanogr. 2006, 51, 893–899. [Google Scholar] [CrossRef]
- Gonzalez-Davila, M.; Santana-Casiano, J.M.; Laglera, L.M. Copper adsorption in diatom cultures. Mar. Chem. 2000, 70, 161–170. [Google Scholar] [CrossRef]
- Tien, C.-J.; Sigee, D.C.; White, K.N. Copper adsorption kinetics of cultured algal cells and freshwater phytoplankton with emphasis on cell surface characteristics. J. Appl. Phycol. 2005, 17, 379–389. [Google Scholar] [CrossRef]
- Gonzalez-Davila, M.; Santana-Casiano, J.M.; Perez-Pena, J.; Millero, F.J. Binding of Cu (II) to the surface and exudates of the alga Dunaliella tertiolecta in seawater. Environ. Sci. Technol. 1995, 29, 289–301. [Google Scholar] [CrossRef]
- Zirino, A.; Clavell, C.; Seligman, P.F.; Barber, R.T. Copper and pH in the suface waters of the eastern tropical Pacifc Oocean and the Peruvian upwelling system. Mar. Chem. 1983, 12, 25–42. [Google Scholar] [CrossRef]
- Martinez-Soto, M.C.; Tovar-Sanchez, A.; Sanchez-Quiles, D.; Rodellas, V.; Garcia-Orellana, J.; Basterretxea, G. Seasonal variation and sources of dissolved trace metals in Mao Harbour, Minorca Island. Sci. Total Environ. 2016, 565, 191–199. [Google Scholar] [CrossRef]
- Posacka, A.M.; Semeniuk, D.M.; Whitby, H.; van den Berg, C.M.; Cullen, J.T.; Orians, K.; Maldonado, M.T. Dissolved Copper (Dcu) Biogeochemical Cycling in the Subarctic Northeast Pacific and a Call for Improving Methodologies. Mar. Chem. 2017, 196, 47–61. [Google Scholar] [CrossRef]
- Blake, A.; Chadwick, D.; Zirino, A.; Rivera-Duarte, I. Spatial and temporal variations in copper speciation in San Diego Bay. Estuaries 2004, 27, 437–447. [Google Scholar] [CrossRef]
- Braungardt, C.B.; Achterberg, E.P.; Elbaz-Poulichet, F.; Morley, N.H. Metal geochemistry in a mine-polluted estuarine system in Spain. Appl. Geochem. 2003, 18, 1757–1771. [Google Scholar] [CrossRef]
- Chase, Z.; Paytan, A.; Beck, A.; Biller, D.; Bruland, K.; Measures, C.; Sanudo-Wilhelmy, S. Evaluating the impact of atmospheric deposition on dissolved trace-metals in the Gulf of Aqaba, Red Sea. Mar. Chem. 2011, 126, 256–268. [Google Scholar] [CrossRef]
- Peng, S. The nutrient, total petroleum hydrocarbon and heavy metal contents in the seawater of Bohai Bay, China: Temporal-spatial variations, sources, pollution statuses, and ecological risks. Mar. Pollut. Bull. 2015, 95, 445–451. [Google Scholar] [CrossRef]
- Schlitzer, R.; Anderson, R.F.; Dodas, E.M.; Lohan, M.; Geibert, W.; Tagliabue, A.; Bowie, A.; Jeandel, C.; Maldonado, M.T.; Landing, W.M.; et al. The Geotraces Intermediate Data Product 2017. Chem. Geol. 2018, 493, 210–223. [Google Scholar] [CrossRef]
- Chen, Y.; Paytan, A.; Chase, Z.; Measures, C.; Beck, A.J.; Sanudo-Wilhelmy, S.A.; Post, A.F. Sources and fluxes of atmospheric trace elements to the Gulf of Aqaba, Red Sea. J. Geophys. Res. Atmos. 2008, 113, D05306. [Google Scholar] [CrossRef]
- Boyle, E.A.; Sclater, F.R.; Edmond, J.M. The Distribution of Dissolved Copper in the Pacific. Earth Planet. Sci. Lett. 1977, 37, 38–54. [Google Scholar] [CrossRef]
- Little, S.H.; Vance, D.; McManus, J.; Severmann, S.; Lyons, T.W. Copper Isotope Signatures in Modern Marine Sediments. Geochim. Cosmochim. Acta 2017, 212, 253–273. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Spatial and Temporal Variability in Copper Complexation in the North Pacific. Deep Sea Res. 1990, 37, 317–336. [Google Scholar] [CrossRef]
- Chen, Y. Sources and Fate of Atmospheric Nutrients over the Remote Oceans and Their Role on Controlling Marine Diazotrophic Microorganisms. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2004. [Google Scholar]
- Wen, L.S.; Jiann, K.T.; Santschi, P.H. Physicochemical speciation of bioactive trace metals (Cd, Cu, Fe, Ni) in the oligotrophic South China Sea. Mar. Chem. 2006, 101, 104–129. [Google Scholar] [CrossRef]
- Vance, D.; Archer, C.; Bermin, J.; Perkins, J.; Statham, P.J.; Lohan, M.C.; Ellwood, M.J.; Mills, R.A. The copper isotope geochemistry of rivers and the oceans. Earth Planet. Sci. Lett. 2008, 274, 204–213. [Google Scholar] [CrossRef]
- Vu, H.T.D.; Sohrin, Y. Diverse stoichiometry of dissolved trace metals in the Indian Ocean. Sci. Rep. 2013, 3, 1745. [Google Scholar]
- Lagerström, M.E.; Field, M.P.; Séguret, M.; Fischer, L.; Hann, S.; Sherrell, R.M. Automated on-line flow-injection ICP-MS determination of trace metals (Mn, Fe, Co, Ni, Cu and Zn) in open ocean seawater: Application to the GEOTRACES program. Mar. Chem. 2013, 155, 71–80. [Google Scholar] [CrossRef]
- Pinedo-Gonzalez, P.; West, A.J.; Rivera-Duarte, I.; Sanudo-Wilhelmy, S.A. Diel changes in trace metal concentration and distribution in coastal waters: Catalina Island as a study case. Environ. Sci. Technol. 2014, 48, 7730–7737. [Google Scholar] [CrossRef]
- Middag, R.; Seferian, R.; Conway, T.M.; John, S.G.; Bruland, K.W.; de Baar, H.J.W. Intercomparison of dissolved trace elements at the Bermuda Atlantic Time Series station. Mar. Chem. 2015, 177, 476–489. [Google Scholar] [CrossRef]
- Whitby, H.; Posacka, A.M.; Maldonado, M.T.; van den Berg, C.M. Copper-binding ligands in the NE Pacific. Mar. Chem. 2018, 204, 36–48. [Google Scholar] [CrossRef]
- Ebling, A.M.; Landing, W.M. Sampling and analysis of the sea surface microlayer for dissolved and particulate trace elements. Mar. Chem. 2015, 177, 134–142. [Google Scholar] [CrossRef]
- Cunliffe, M.; Engel, A.; Frka, S.; Gasparovic, B.; Guitart, C.; Murrell, J.C.; Salter, M.; Stolle, C.; Upstill-Goddard, R.; Wurl, O. Sea surface microlayers: A unified physicochemical and biological perspective of the air-ocean interface. Prog. Oceanogr. 2013, 109, 104–116. [Google Scholar] [CrossRef]
- Wurl, O.; Wurl, E.; Miller, L.; Johnson, K.; Vagle, S. Formation and global distribution of sea-surface microlayers. Biogeosciences 2011, 8, 121–135. [Google Scholar] [CrossRef]
- Antonowicz, J.P.; Mudryk, Z.; Zdanowicz, M. A relationship between accumulation of heavy metals and microbiological parameters in the surface microlayer and subsurface water of a coastal Baltic lake. Hydrobiologia 2015, 762, 65–80. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Arrieta, J.M.; Duarte, C.M.; Sañudo-Wilhelmy, S.A. Spatial gradients in trace metal concentrations in the surface microlayer of the Mediterranean Sea. Front. Mar. Sci. 2014, 1, 79. [Google Scholar] [CrossRef]
- Gašparović, B.; Plavšić, M.; Ćosović, B.; Saliot, A. Organic Matter Characterization in the Sea Surface Microlayers in the Subarctic Norwegian Fjords Region. Mar. Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Plavšić, M.; Gašparović, B.; Ćosović, B. Copper Complexation and Surfactant Activity of Organic Matter in Coastal Seawater and Surface Microlayer Samples of North Norwegian Fjords and Mediterranean. Fresenius Environ. Bull. 2007, 16, 372–378. [Google Scholar]
- Karavoltsos, S.; Kalambokis, E.; Sakellari, A.; Plavšić, M.; Dotsika, E.; Karalis, P.; Leondiadis, L.; Dassenakis, M.; Scoullos, M. Organic Matter Characterization and Copper Complexing Capacity in the Sea Surface Microlayer of Coastal Areas of the Eastern Mediterranean. Mar. Chem. 2015, 173, 234–243. [Google Scholar] [CrossRef]
- Ebling, A.M.; Landing, W.M. Trace Elements in the Sea Surface Microlayer: Rapid Responses to Changes in Aerosol Deposition. Elem. Sci. Anthr. 2017, 5, 42. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Copper complexation in the Northeast Pacific. Limnol. Oceanogr. 1988, 33, 1084–1101. [Google Scholar] [CrossRef]
- Moffett, J.W.; Zika, R.G. Oxidation kinetics of Cu(I) in seawater: Implications for its existence in the marine environment. Mar. Chem. 1983, 13, 239–251. [Google Scholar] [CrossRef]
- Voelker, B.M.; Sedlak, D.L.; Zafiriou, O.C. Chemistry of superoxide radical in seawater: Reactions with organic Cu complexes. Environ. Sci. Technol. 2000, 34, 1036–1042. [Google Scholar] [CrossRef]
- González-Dávila, M.; Santana-Casiano, J.M.; González, A.; Pérez, N.; Millero, F. Oxidation of copper (I) in seawater at nanomolar levels. Mar. Chem. 2009, 115, 118–124. [Google Scholar] [CrossRef]
- Millero, F.J.; Woosley, R.; Ditrolio, B.; Waters, J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 2009, 22, 72–85. [Google Scholar] [CrossRef]
- Pérez-Almeida, N.; González-Dávila, M.; Santana-Casiano, J.M.; González, A.G.; Suárez de Tangil, M. Oxidation of Cu (I) in seawater at low oxygen concentrations. Environ. Sci. Technol. 2013, 47, 1239–1247. [Google Scholar] [CrossRef]
- Sharma, V.K.; Millero, F.J. Oxidation of copper (I) in seawater. Environ. Sci. Technol. 1988, 22, 768–771. [Google Scholar] [CrossRef]
- González, A.G.; Pérez-Almeida, N.; Santana-Casiano, J.M.; Millero, F.J.; González-Dávila, M. Redox interactions of Fe and Cu in seawater. Mar. Chem. 2016, 179, 12–22. [Google Scholar] [CrossRef]
- Pérez-Almeida, N.; González, A.G.; Santana-Casiano, J.M.; González-Dávila, M. Iron and copper redox interactions in UV-seawater: A kinetic model approach. Chem. Geol. 2019, 506, 149–161. [Google Scholar] [CrossRef]
- Shi, J.; Abid, A.D.; Kennedy, I.M.; Hristova, K.R.; Silk, W.K. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ. Pollut. 2011, 159, 1277–1282. [Google Scholar] [CrossRef]
- Moffett, J.W.; Zika, R.G.; Brand, L.E. Distribution and potential sources and sinks of copper chelators in the Sargasso Sea. Deep Sea Res. 1990, 37, 27–36. [Google Scholar] [CrossRef]
- Mackey, K.R.; Chien, C.-T.; Post, A.F.; Saito, M.A.; Paytan, A. Rapid and gradual modes of aerosol trace metal dissolution in seawater. Front. Microbiol. 2015, 5, 794. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Balboni, V.; Boni, L. Copper toxicity and carbohydrate production in the microalgae Cylindrotheca fusiformis and Gymnodinium sp. Eur. J. Phycol. 1997, 32, 125–132. [Google Scholar] [CrossRef]
- Croot, P.L.; Moffett, J.W.; Brand, L.E. Production of extracellular Cu complexing ligands by eucaryotic phytoplankton in response to Cu stress. Limnol. Oceanogr. 2000, 45, 619–627. [Google Scholar] [CrossRef]
- Vasconcelos, M.T.S.D.; Leal, M.F.C.; van den Berg, C.M.G. Influence of the nature of the exudates released by different marine algae on the growth, trace metal uptake, and exudation of Emiliania huxleyi in natural seawater. Mar. Chem. 2002, 77, 187–210. [Google Scholar] [CrossRef]
- Leal, M.F.C.; Vasconcelos, M.; van den Berg, C.M. Copper-induced release of complexing ligands similar to thiols by Emiliania huxleyi in seawater cultures. Limnol. Oceanogr. 1999, 44, 1750–1762. [Google Scholar] [CrossRef]
- Croot, P.L. Seasonal cycle of copper speciation in Gullmar Fjord, Sweden. Limnol. Oceanogr. 2003, 48, 764–776. [Google Scholar] [CrossRef]
- Whitby, H.; van den Berg, C.M. Evidence for copper-binding humic substances in seawater. Mar. Chem. 2015, 173, 282–290. [Google Scholar] [CrossRef]
- Sedlak, D.L.; Phinney, J.T.; Bedsworth, W.W. Strongly complexed Cu and Ni in wastewater effluents and surface runoff. Environ. Sci. Technol. 1997, 31, 3010–3016. [Google Scholar] [CrossRef]
- Yang, R.; Van den Berg, C.M. Metal complexation by humic substances in seawater. Environ. Sci. Technol. 2009, 43, 7192–7197. [Google Scholar] [CrossRef]
- Town, R.M.; Filella, M. Dispelling the myths: Is the existence of L1 and L2 ligands necessary to explain metal ion speciation in natural waters? Limnol. Oceanogr. 2000, 45, 1341–1357. [Google Scholar] [CrossRef]
- Hurst, M.P.; Bruland, K.W. The use of Nafion-coated thin mercury film electrodes for the determination of the dissolved copper speciation in estuarine water. Anal. Chim. Acta 2005, 546, 68–78. [Google Scholar] [CrossRef]
- Little, S.; Vance, D.; Walker-Brown, C.; Landing, W. The oceanic mass balance of copper and zinc isotopes, investigated by analysis of their inputs, and outputs to ferromanganese oxide sediments. Geochim. Cosmochim. Acta 2014, 125, 673–693. [Google Scholar] [CrossRef]
- Sander, S.G.; Koschinsky, A. Metal flux from hydrothermal vents increased by organic complexation. Nat. Geosci. 2011, 4, 145. [Google Scholar] [CrossRef]
- Williams, M.; Millward, G.; Nimmo, M.; Fones, G. Fluxes of Cu, Pb and Mn to the North-Eastern Irish Sea: The importance of sedimental and atmospheric inputs. Mar. Pollut. Bull. 1998, 36, 366–375. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Duan, L. Distribution and contamination of heavy metals in surface sediments of the South Yellow Sea. Mar Pollut. Bull. 2012, 64, 2151–2159. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.L. Riverine composition and estuarine geochemistry of particulate metals in China—Weathering features, anthropogenic impact and chemical fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Guieu, C.; Loÿe-Pilot, M.D.; Benyahya, L.; Dufour, A. Spatial variability of atmospheric fluxes of metals (Al, Fe, Cd, Zn and Pb) and phosphorus over the whole Mediterranean from a one-year monitoring experiment: Biogeochemical implications. Mar. Chem. 2010, 120, 164–178. [Google Scholar] [CrossRef]
- Calabrese, S.; Aiuppa, A.; Allard, P.; Bagnato, E.; Bellomo, S.; Brusca, L.; D’Alessandro, W.; Parello, F. Atmospheric sources and sinks of volcanogenic elements in a basaltic volcano (Etna, Italy). Geochim. Cosmochim. Acta 2011, 75, 7401–7425. [Google Scholar] [CrossRef]
- Cattell, F.C.; Scott, W.D. Copper in aerosol particles produced by the ocean. Science 1978, 202, 429–430. [Google Scholar] [CrossRef]
- Weisel, C.; Duce, R.; Fasching, J.; Heaton, R. Estimates of the transport of trace metals from the ocean to the atmosphere. J. Geophys. Res. Atmos. 1984, 89, 11607–11618. [Google Scholar] [CrossRef]
- VanCuren, R.A. Asian aerosols in North America: Extracting the chemical composition and mass concentration of the Asian continental aerosol plume from long-term aerosol records in the western United States. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Perry, K.D.; Cahill, T.A.; Schnell, R.C.; Harris, J.M. Long-range transport of anthropogenic aerosols to the National Oceanic and Atmospheric Administration baseline station at Mauna Loa Observatory, Hawaii. J. Geophys. Res. Atmos. 1999, 104, 18521–18533. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J. Atmospheric heavy metals and arsenic in China: Situation, sources and control policies. Atmos. Environ. 2013, 74, 93–101. [Google Scholar] [CrossRef]
- Sternbeck, J.; Sjödin, Å.; Andréasson, K. Metal emissions from road traffic and the influence of resuspension—Results from two tunnel studies. Atmos. Environ. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Bhanarkar, A.; Rao, P.; Gajghate, D.; Nema, P. Inventory of SO2, PM and toxic metals emissions from industrial sources in Greater Mumbai, India. Atmos. Environ. 2005, 39, 3851–3864. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Schulz, M.; Prospero, J.M.; Baker, A.R.; Dentener, F.; Ickes, L.; Liss, P.S.; Mahowald, N.M.; Nickovic, S.; Garcia-Pando, C.P.; Rodriguez, S.; et al. Atmospheric transport and deposition of mineral dust to the ocean: Implications for research needs. Environ. Sci. Technol. 2012, 46, 10390–10404. [Google Scholar] [CrossRef]
- Kubilay, N.; Nickovic, S.; Moulin, C.; Dulac, F. An illustration of the transport and deposition of mineral dust onto the eastern Mediterranean. Atmos. Environ. 2000, 34, 1293–1303. [Google Scholar] [CrossRef]
- Prenni, A.J.; Petters, M.D.; Kreidenweis, S.M.; Heald, C.L.; Martin, S.T.; Artaxo, P.; Garland, R.M.; Wollny, A.G.; Pöschl, U. Relative roles of biogenic emissions and Saharan dust as ice nuclei in the Amazon basin. Nat. Geosci. 2009, 2, 402. [Google Scholar] [CrossRef]
- Guieu, C.; Chester, R.; Nimmo, M.; Martin, J.M.; Guerzoni, S.; Nicolas, E.; Mateu, J.; Keyse, S. Atmospheric input of dissolved and particulate metals to the northwestern Mediterranean. Deep Sea Res. Top. Stud. Oceanogr. 1997, 44, 655–674. [Google Scholar] [CrossRef]
- Mao, J.; Fan, S.; Jacob, D.J.; Travis, K.R. Radical loss in the atmosphere from Cu-Fe redox coupling in aerosols. Atmos. Chem. Phys. 2013, 13, 509–519. [Google Scholar] [CrossRef]
- Deguillaume, L.; Leriche, M.; Desboeufs, K.; Mailhot, G.; George, C.; Chaumerliac, N. Transition metals in atmospheric liquid phases: Sources, reactivity, and sensitive parameters. Chem. Rev. 2005, 105, 3388–3431. [Google Scholar] [CrossRef]
- Weber, R.J.; Guo, H.; Russell, A.G.; Nenes, A. High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nat. Geosci. 2016, 9, 282. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly acidic ambient particles, soluble metals, and oxidative potential: A link between sulfate and aerosol toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Desboeufs, K.V.; Sofikitis, A.; Losno, R.; Colin, J.L.; Ausset, P. Dissolution and solubility of trace metals from natural and anthropogenic aerosol particulate matter. Chemosphere 2005, 58, 195–203. [Google Scholar] [CrossRef]
- Lim, B.; Jickells, T.D.; Colin, J.L.; Losno, R. Solubilities of Al, Pb, Cu, and Zn in rain sampled in the marine environment over the North Atlantic Ocean and Mediterranean Sea. Glob. Biogeochem. Cycles 1994, 8, 349–362. [Google Scholar] [CrossRef]
- Hsu, S.C.; Wong, G.T.F.; Gong, G.C.; Shiah, F.K.; Huang, Y.T.; Kao, S.J.; Tsai, F.J.; Lung, S.C.C.; Lin, F.J.; Lin, I.I.; et al. Sources, solubility, and dry deposition of aerosol trace elements over the East China Sea. Mar. Chem. 2010, 120, 116–127. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lin, F.J.; Jeng, W.L. Seawater solubility of natural and anthropogenic metals within ambient aerosols collected from Taiwan coastal sites. Atmos. Environ. 2005, 39, 3989–4001. [Google Scholar] [CrossRef]
- Kersten, M.; Kriews, M.; Forstner, U. Partitioning of trace metals released from polluted marine aerosols in coastal seawater. Mar. Chem. 1991, 36, 165–182. [Google Scholar] [CrossRef]
- Chester, R.; Nimmo, M.; Corcoran, P.A. Rain water aerosol trace metal relationships at Cap Ferrat: A coastal site in the western Mediterranean. Mar. Chem. 1997, 58, 293–312. [Google Scholar] [CrossRef]
- Williams, R.M. A model for the dry deposition of deposition of particles to natural water surfaces. Atmos. Environ. 1982, 16, 1933–1938. [Google Scholar]
- Kadko, D.; Landing, W.M.; Shelley, R.U. A novel tracer technique to quantify the atmospheric flux of trace elements to remote ocean regions. J. Geophys. Res. Oceans 2015, 120, 848–858. [Google Scholar] [CrossRef]
- Fu, J.P.; Wang, B.; Chen, Y.; Ma, Q.W. The influence of continental air masses on the aerosols and nutrients deposition over the western North Pacific. Atmos. Environ. 2018, 172, 1–11. [Google Scholar] [CrossRef]
- Witt, M.L.I.; Mather, T.A.; Baker, A.R.; De Hoog, J.C.M.; Pyle, D.M. Atmospheric trace metals over the south-west Indian Ocean: Total gaseous mercury, aerosol trace metal concentrations and lead isotope ratios. Mar. Chem. 2010, 121, 2–16. [Google Scholar] [CrossRef]
- Maenhaut, W.; Cafmeyer, J. Long-term atmospheric aerosol study at urban and rural sites in Belgium using multi-elemental analysis by particle-induced X-ray emission spectrometry and short-irradiation instrumental neutron activation analysis. X Ray Spectrom. 1998, 27, 236–246. [Google Scholar] [CrossRef]
- Virkkula, A.; Aurela, M.; Hillamo, R.; Mäkelä, T.; Pakkanen, T.; Kerminen, V.M.; Maenhaut, W.; François, F.; Cafmeyer, J. Chemical composition of atmospheric aerosol in the European subarctic: Contribution of the Kola Peninsula smelter areas, central Europe, and the Arctic Ocean. J. Geophys. Res. Atmos. 1999, 104, 23681–23696. [Google Scholar] [CrossRef]
- Witt, M.; Baker, A.R.; Jickells, T.D. Atmospheric trace metals over the Atlantic and South Indian Oceans: Investigation of metal concentrations and lead isotope ratios in coastal and remote marine aerosols. Atmos. Environ. 2006, 40, 5435–5451. [Google Scholar] [CrossRef]
- Chand, D.; Hegg, D.A.; Wood, R.; Shaw, G.E.; Wallace, D.; Covert, D.S. Source attribution of climatically important aerosol properties measured at Paposo (Chile) during VOCALS. Atmos. Chem. Phys. 2010, 10, 10789–10801. [Google Scholar] [CrossRef]
- Kang, J.; Choi, M.-S.; Yi, H.-I.; Song, Y.-H.; Lee, D.; Cho, J.-H. A five-year observation of atmospheric metals on Ulleung Island in the East/Japan Sea: Temporal variability and source identification. Atmos. Environ. 2011, 45, 4252–4262. [Google Scholar] [CrossRef]
- Laing, J.R.; Hopke, P.K.; Hopke, E.F.; Husain, L.; Dutkiewicz, V.A.; Paatero, J.; Viisanen, Y. Long-term particle measurements in Finnish Arctic: Part I—Chemical composition and trace metal solubility. Atmos. Environ. 2014, 88, 275–284. [Google Scholar] [CrossRef]
- Chance, R.; Jickells, T.D.; Baker, A.R. Atmospheric trace metal concentrations, solubility and deposition fluxes in remote marine air over the south-east Atlantic. Mar. Chem. 2015, 177, 45–56. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Libani, G.; Mantini, C.; Scarponi, G. Determination of water:soluble, acid-extractable and inert fractions of Cd, Pb and Cu in Antarctic aerosol by square wave anodic stripping voltammetry after sequential extraction and microwave digestion. J. Electroanal. Chem. 2015, 755, 182–196. [Google Scholar] [CrossRef]
- Baker, A.R.; Thomas, M.; Bange, H.W.; Sanchez, E.P. Soluble trace metals in aerosols over the tropical south-east Pacific offshore of Peru. Biogeosciences 2016, 13, 817–825. [Google Scholar] [CrossRef]
- Campbell, A.L.; Mangan, S.; Ellis, R.P.; Lewis, C. Ocean acidification increases copper toxicity to the early life history stages of the polychaete Arenicola marina in artificial seawater. Environ. Sci. Technol. 2014, 48, 9745–9753. [Google Scholar] [CrossRef]
- Lewis, C.; Ellis, R.P.; Vernon, E.; Elliot, K.; Newbatt, S.; Wilson, R.W. Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid-base responses. Sci. Rep. 2016, 6, 21554. [Google Scholar] [CrossRef]
| Phytoplankton | Threshold | Speciation | Indicator | Reference |
|---|---|---|---|---|
| Pyrrophyta | (nM) | |||
| Gonyaulax tamarensis | 0.0001 | Cu2+ ions | Inhibited growth | [56] |
| Peridinium sp. (A1572) | 0.001 | Cu2+ ions | Reduced reproduction rates | [27] |
| Prorocentrum sp. (R1568) | 0.001 | Cu2+ ions | Reduced reproduction rates | [27] |
| Gonyaulax tamarensis | 0.04 | Cu2+ ions | 50% nonmotile | [57] |
| Gonyaulax tamarensis | 0.2 | Cu2+ ions | 100% nonmotile | [57] |
| Cyanobacteria | (nM) | |||
| Cyanobacteria | 0.001 | Cu2+ ions | Reduced reproduction rates | [27] |
| Synechococcus bacilaris | 0.003 | Cu2+ ions | 50% inhibition of reproduction rate | [27] |
| Synchrococcus | 0.112 | Cu2+ ions | Reduced cell division rate | [35] |
| Synechrococcus (Red sea) | 0.2-2* | Total Cu | Impaired cell growth | [10] |
| Bacillariophyta | (μM) | |||
| Asterionella glacialis | 0.1 | Cu2+ ions | Dead | [27] |
| Bacteriastrum delicatulum | 0.1 | Cu2+ ions | Dead | [27] |
| Hentiuulus sinensi | 0.1 | Cu2+ ions | Dead | [27] |
| Rhizosolenia setigera | 0.1 | Cu2+ ions | Dead | [27] |
| Thalassiosira oceanica (Bering Sea) | 0.001 | Dissolved Cu | unable to grow | [49] |
| Thalassiosira sp. (Adriatic Sea) | 0.31–0.78 | Dissolved Cu | Inhibited growth | [58] |
| Thalassiosira decipiens (SW Bay) | 1.00 | Dissolved Cu | Abundance | [34] |
| Phaeodactylum tricornutum | 1.6 | Dissolved Cu | 50% growth reduction | [59] |
| 15.7 | Dissolved Cu | Inhibited growth | [59] | |
| Cylindrotheca closterium (Adriatic Sea) | 3.13–7.81 | Dissolved Cu | Inhibited growth | [58] |
| Achnanthes brevipes | 3.13–7.81 | Dissolved Cu | Inhibited growth | [58] |
| Skeleonema costatum | 0.0002 | Cu2+ ions | Cell division rates reduced | [27] |
| Chlorophyta | (μM) | |||
| Chlorella pyrenoidosa | 4.13 | Dissolved Cu | Biosorption capacities | [60] |
| Chlamydomonas geitleri Ettl | 10 | Cu2+ ions | 50% reduction in growth rate | [51] |
| Chlorella vulgaris Beyerinck | 10 | Cu2+ ions | 50% reduction in growth rate | [51] |
| Ochrophyta | (μM) | |||
| Ectocarpus siliculosus (Southern Peru) | 0.78 | Dissolved Cu | Chlorophyll drop to 70% of chlorophyll autofluorescence | [43] |
| Ectocarpus siliculosus (Northern Chile) | 3.91 | Dissolved Cu | Chlorophyll decay of cell-autofluorescence | [43] |
| Haptophyta | (μM) | |||
| Hymenomonus corterae | 0.0007 | Cu2+ ions | Dead | [27] |
| Emiliania huxleyi | 0.3 | Dissolved Cu | Inhibited growth | [33] |
| Emiliania huxleyi (Mediterranean strain) | 0.32 | Dissolved Cu | EC50 | [33] |
| Gephyrocapsa oceanica | 0.4 | Dissolved Cu | EC50 | [33] |
| Ligand Producer | Taxa | Class | Reference |
|---|---|---|---|
| Cylindrotheca fusiformis | Diatom | Strong ligands | [134] |
| Amnphidiniumn carterae | Dinoflagellate | Strong ligands | [135] |
| Synechococcu. spp | Cyanobacteria | Strong ligands | [82,135] |
| Emiliania huxleyi | Haptophyta | Both strong and weak ligands | [136,137] |
| Hymnenoinonas carterae | Coccolithophorid | Weak ligands | [135] |
| Ocean | Seawater | Pure Water | Sample Types | References |
|---|---|---|---|---|
| East China Sea | 51% | Non-dust event days | [169] | |
| An island in Taiwan Strait | 42% | Aerosol samples | [170] | |
| Coastal site in Taiwan Strait | 27% | Aerosol samples | [170] | |
| North Atlantic (Bermuda) | 84% | Rain samples | [168] | |
| Atlantic | 40% | Aerosol samples | [10] | |
| German Bight | 41% | Aerosol samples | [171] | |
| Gulf of Aqaba | 49% | 66% | Aerosol samples | [25] |
| Mediterranean Sea (Corsica) | 48% | Rain samples | [168] | |
| Western Mediterranean | 76% | Rain samples | [172] | |
| Sargasso Sea and Bermuda | 1–7% | Dust source | [24] | |
| Sargasso Sea | 10–100% | Anthropogenic source | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Chen, Y.; Zhou, S.; Li, H. Impacts of Aerosol Copper on Marine Phytoplankton: A Review. Atmosphere 2019, 10, 414. https://doi.org/10.3390/atmos10070414
Yang T, Chen Y, Zhou S, Li H. Impacts of Aerosol Copper on Marine Phytoplankton: A Review. Atmosphere. 2019; 10(7):414. https://doi.org/10.3390/atmos10070414
Chicago/Turabian StyleYang, Tianjiao, Ying Chen, Shengqian Zhou, and Haowen Li. 2019. "Impacts of Aerosol Copper on Marine Phytoplankton: A Review" Atmosphere 10, no. 7: 414. https://doi.org/10.3390/atmos10070414
APA StyleYang, T., Chen, Y., Zhou, S., & Li, H. (2019). Impacts of Aerosol Copper on Marine Phytoplankton: A Review. Atmosphere, 10(7), 414. https://doi.org/10.3390/atmos10070414